Mycobacterium abscessus Virulence Factors: An Overview of Un-Explored Therapeutic Options
Abstract
:1. Introduction
2. Overview of Known M. abscessus’ Virulence Factors
2.1. Surface Elements
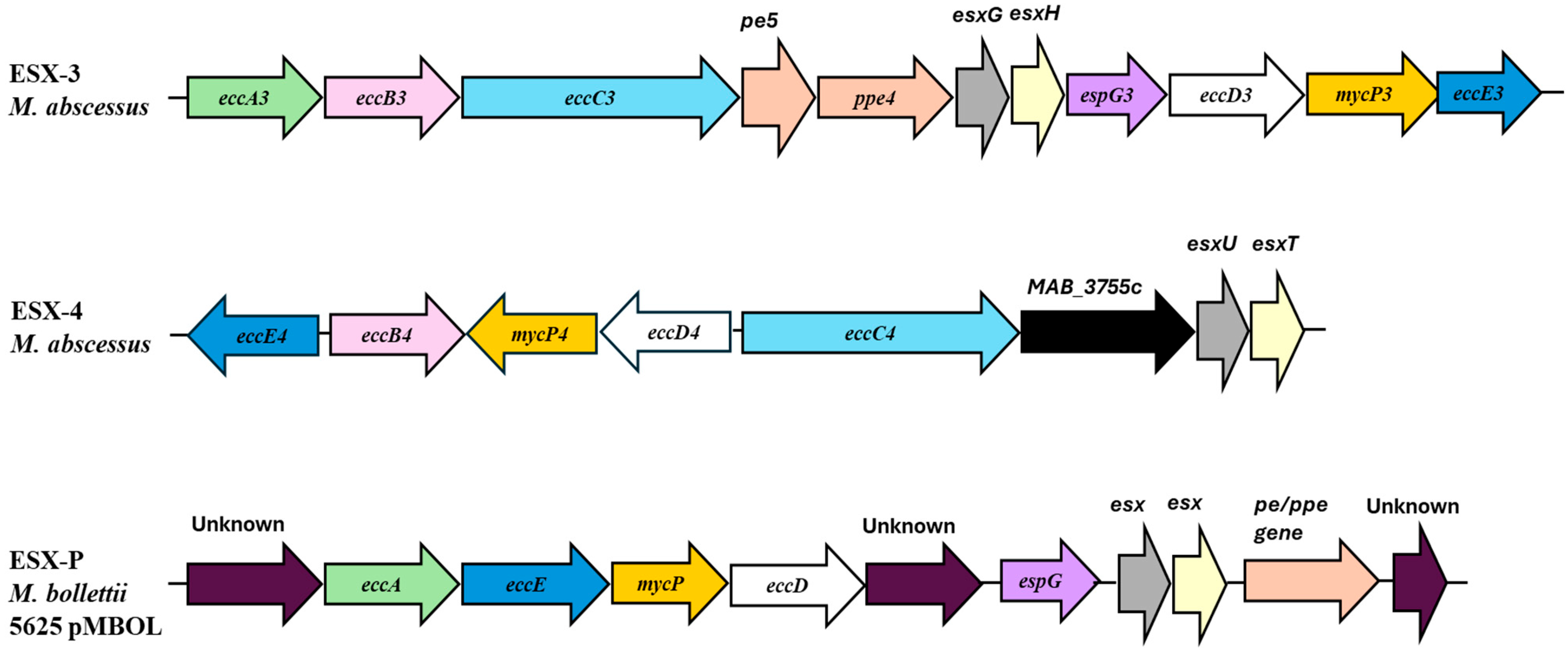
2.2. Secretion Systems and Efflux Pumps
2.3. Iron Acquisition
2.4. Other Virulence Factors
2.5. Biofilm and Implication
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pereira, A.C.; Ramos, B.; Reis, A.C.; Cunha, M.V. Non-tuberculous mycobacteria: Molecular and physiological bases of virulence and adaptation to ecological niches. Microorganisms 2020, 8, 1380. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, H.F.M.; Chan, E.D.; Young, L.; Baldwin, S.L.; Coler, R.N. Mycobacterium abscessus: It’s complex. Microorganisms 2022, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Recchia, D.; Stelitano, G.; Stamilla, A.; Gutierrez, D.L.; Degiacomi, G.; Chiarelli, L.R.; Pasca, M.R. Mycobacterium abscessus infections in cystic fibrosis individuals: A review on therapeutic options. Int. J. Mol. Sci. 2023, 24, 4635. [Google Scholar] [CrossRef] [PubMed]
- Byrd, T.F.; Lyons, C.R. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 1999, 67, 4700–4707. [Google Scholar] [CrossRef]
- Ripoll, F.; Pasek, S.; Schenowitz, C.; Dossat, C.; Barbe, V.; Rottman, M.; Macheras, E.; Heym, B.; Herrmann, J.L.; Daffe, M.; et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS ONE 2009, 4, e5660. [Google Scholar] [CrossRef]
- Byrd, T.F.; Chan, E.D. Editorial: Mycobacterium abscessus; the paradox of low pathogenicity and high virulence. Front. Microbiol. 2022, 13, 943694. [Google Scholar] [CrossRef]
- Dubois, V.; Pawlik, A.; Bories, A.; Le Moigne, V.; Sismeiro, O.; Legendre, R.; Varet, H.; Rodriguez-Ordonez, M.D.P.; Gaillard, J.L.; Coppee, J.Y.; et al. Mycobacterium abscessus virulence traits unraveled by transcriptomic profiling in amoeba and macrophages. PLoS Pathog. 2019, 15, e1008069. [Google Scholar] [CrossRef]
- Aung, T.T.; Chor, W.H.J.; Yam, J.K.H.; Givskov, M.; Yang, L.; Beuerman, R.W. Discovery of novel antimycobacterial drug therapy in biofilm of pathogenic nontuberculous mycobacterial keratitis. Ocul. Surf. 2017, 15, 770–783. [Google Scholar] [CrossRef]
- Cocorullo, M.; Bettoni, C.; Foiadelli, S.; Stelitano, G. Moles of molecules against Mycobacterium abscessus: A review of current research. Future Pharmacol. 2023, 3, 637–663. [Google Scholar] [CrossRef]
- Egorova, A.; Jackson, M.; Gavrilyuk, V.; Makarov, V. Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities. Med. Res. Rev. 2021, 41, 2350–2387. [Google Scholar] [CrossRef]
- Degiacomi, G.; Chiarelli, L.R.; Riabova, O.; Lore, N.I.; Munoz-Munoz, L.; Recchia, D.; Stelitano, G.; Postiglione, U.; Saliu, F.; Griego, A.; et al. The novel drug candidate VOMG kills Mycobacterium abscessus and other pathogens by inhibiting cell division. Int. J. Antimicrob. Agents 2024, 64, 107278. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, S.; Voos, K.; Haupenthal, J.; Wichelhaus, T.A.; Frank, D.; Weizel, L.; Rotter, M.; Brunst, S.; Kramer, J.S.; Proschak, E.; et al. N-Aryl mercaptoacetamides as potential multi-target inhibitors of metallo-beta-lactamases (MBLs) and the virulence factor LasB from Pseudomonas aeruginosa. RSC Med. Chem. 2021, 12, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Colmer-Hamood, J.A.; Kopel, J.; San Francisco, M.J.; Hamood, A.N. Anti-Pseudomonas aeruginosa vaccines and therapies: An assessment of clinical trials. Microorganisms 2023, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Kane, T.L.; Carothers, K.E.; Lee, S.W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug Targets 2018, 19, 111–127. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General overview of nontuberculous mycobacteria opportunistic pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Ribeiro, G.M.; Matsumoto, C.K.; Real, F.; Teixeira, D.; Duarte, R.S.; Mortara, R.A.; Leao, S.C.; de Souza Carvalho-Wodarz, C. Increased survival and proliferation of the epidemic strain Mycobacterium abscessus subsp. massiliense CRM0019 in alveolar epithelial cells. BMC Microbiol. 2017, 17, 195. [Google Scholar] [CrossRef]
- Chung, E.S.; Johnson, W.C.; Aldridge, B.B. Types and functions of heterogeneity in mycobacteria. Nat. Rev. Microbiol. 2022, 20, 529–541. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Daher, W.; Leclercq, L.D.; Johansen, M.D.; Hamela, C.; Karam, J.; Trivelli, X.; Nigou, J.; Guerardel, Y.; Kremer, L. Glycopeptidolipid glycosylation controls surface properties and pathogenicity in Mycobacterium abscessus. Cell Chem. Biol. 2022, 29, 910–924.e7. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.L.; Kissa, K.; Dubremetz, J.F.; Gaillard, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef]
- Viljoen, A.; Viela, F.; Kremer, L.; Dufrene, Y.F. Fast chemical force microscopy demonstrates that glycopeptidolipids define nanodomains of varying hydrophobicity on mycobacteria. Nanoscale Horiz. 2020, 5, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Nessar, R.; Reyrat, J.M.; Davidson, L.B.; Byrd, T.F. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology 2011, 157 (Pt 4), 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, M.; Zhao, L.; Guo, Q.; Chen, J.; Xu, B.; Zhan, M.; Zhang, Y.; Zhang, Z.; Chu, H. Glycopeptidolipid genotype correlates with the severity of Mycobacterium abscessus lung disease. J. Infect. Dis. 2020, 221 (Suppl. S2), S257–S262. [Google Scholar] [CrossRef] [PubMed]
- Kreutzfeldt, K.M.; McAdam, P.R.; Claxton, P.; Holmes, A.; Seagar, A.L.; Laurenson, I.F.; Fitzgerald, J.R. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS ONE 2013, 8, e63237. [Google Scholar] [CrossRef]
- Daher, W.; Leclercq, L.D.; Viljoen, A.; Karam, J.; Dufrene, Y.F.; Guerardel, Y.; Kremer, L. O-methylation of the glycopeptidolipid acyl chain defines surface hydrophobicity of Mycobacterium abscessus and macrophage invasion. ACS Infect. Dis. 2020, 6, 2756–2770. [Google Scholar] [CrossRef]
- Wiersma, C.J.; Belardinelli, J.M.; Avanzi, C.; Angala, S.K.; Everall, I.; Angala, B.; Kendall, E.; de Moura, V.C.N.; Verma, D.; Benoit, J.; et al. Cell surface remodeling of Mycobacterium abscessus under cystic fibrosis airway growth conditions. ACS Infect. Dis. 2020, 6, 2143–2154. [Google Scholar] [CrossRef]
- Roux, A.L.; Viljoen, A.; Bah, A.; Simeone, R.; Bernut, A.; Laencina, L.; Deramaudt, T.; Rottman, M.; Gaillard, J.L.; Majlessi, L.; et al. The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 2016, 6, 160185. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, B.J.; Kook, Y.H.; Kim, B.J. Phagosome escape of rough Mycobacterium abscessus strains in murine macrophage via phagosomal rupture can lead to Type I interferon production and their cell-to-cell spread. Front. Immunol. 2019, 10, 125. [Google Scholar] [CrossRef]
- Burbaud, S.; Laval, F.; Lemassu, A.; Daffe, M.; Guilhot, C.; Chalut, C. Trehalose polyphleates are produced by a glycolipid biosynthetic pathway conserved across phylogenetically distant mycobacteria. Cell Chem. Biol. 2016, 23, 278–289. [Google Scholar] [CrossRef]
- Thouvenel, L.; Prevot, G.; Chiaradia, L.; Parra, J.; Mouton-Barbosa, E.; Locard-Paulet, M.; Marcoux, J.; Tropis, M.; Burlet-Schiltz, O.; Daffe, M.; et al. The final assembly of trehalose polyphleates takes place within the outer layer of the mycobacterial cell envelope. J. Biol. Chem. 2020, 295, 11184–11194. [Google Scholar] [CrossRef]
- Llorens-Fons, M.; Perez-Trujillo, M.; Julian, E.; Brambilla, C.; Alcaide, F.; Byrd, T.F.; Luquin, M. Trehalose polyphleates, external cell wall lipids in Mycobacterium abscessus, are associated with the formation of clumps with cording morphology, which have been associated with virulence. Front. Microbiol. 2017, 8, 1402. [Google Scholar] [CrossRef]
- Julian, E.; Roldan, M.; Sanchez-Chardi, A.; Astola, O.; Agusti, G.; Luquin, M. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J. Bacteriol. 2010, 192, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, K.S.; Illouz, M.; Abad, L.; Aull, H.G.; Russell, D.A.; Garlena, R.A.; Cristinziano, M.; Malmsheimer, S.; Chalut, C.; Hatfull, G.F.; et al. Therapeutically useful mycobacteriophages BPs and Muddy require trehalose polyphleates. Nat. Microbiol. 2023, 8, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Madani, A.; Santucci, P.; Martin, B.P.; Paudel, R.R.; Delattre, S.; Herrmann, J.L.; Spilling, C.D.; Kremer, L.; Canaan, S.; et al. Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases. Int. J. Antimicrob. Agents 2018, 51, 651–654. [Google Scholar] [CrossRef]
- Nguyen, P.C.; Delorme, V.; Benarouche, A.; Martin, B.P.; Paudel, R.; Gnawali, G.R.; Madani, A.; Puppo, R.; Landry, V.; Kremer, L.; et al. Cyclipostins and Cyclophostin analogs as promising compounds in the fight against tuberculosis. Sci. Rep. 2017, 7, 11751. [Google Scholar] [CrossRef]
- Stelitano, G.; Sammartino, J.C.; Chiarelli, L.R. Multitargeting compounds: A promising strategy to overcome multi-drug resistant tuberculosis. Molecules 2020, 25, 1239. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczynska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Honda, J.R.; Hess, T.; Malcolm, K.C.; Ovrutsky, A.R.; Bai, X.; Irani, V.R.; Dobos, K.M.; Chan, E.D.; Flores, S.C. Pathogenic nontuberculous mycobacteria resist and inactivate cathelicidin: Implication of a novel role for polar mycobacterial lipids. PLoS ONE 2015, 10, e0126994. [Google Scholar] [CrossRef]
- Honda, J.R.; Hess, T.; Carlson, R.; Kandasamy, P.; Nieto Ramirez, L.M.; Norton, G.J.; Virdi, R.; Islam, M.N.; Mehaffy, C.; Hasan, N.A.; et al. Nontuberculous Mycobacteria Show Differential Infectivity and Use Phospholipids to Antagonize LL-37. Am. J. Respir. Cell Mol. Biol. 2020, 62, 354–363. [Google Scholar] [CrossRef]
- Becker, K.; Haldimann, K.; Selchow, P.; Reinau, L.M.; Dal Molin, M.; Sander, P. Lipoprotein Glycosylation by Protein-O-Mannosyltransferase (MAB_1122c) Contributes to Low Cell Envelope Permeability and Antibiotic Resistance of Mycobacterium abscessus. Front. Microbiol. 2017, 8, 2123. [Google Scholar] [CrossRef]
- Pal, R.; Bisht, M.K.; Mukhopadhyay, S. Secretory proteins of Mycobacterium tuberculosis and their roles in modulation of host immune responses: Focus on therapeutic targets. FEBS J. 2022, 289, 4146–4171. [Google Scholar] [CrossRef] [PubMed]
- Bar-Oz, M.; Meir, M.; Barkan, D. Virulence-associated secretion in Mycobacterium abscessus. Front. Immunol. 2022, 13, 938895. [Google Scholar] [CrossRef]
- McDonough, J.A.; Hacker, K.E.; Flores, A.R.; Pavelka, M.S., Jr.; Braunstein, M. The twin-arginine translocation pathway of Mycobacterium smegmatis is functional and required for the export of mycobacterial beta-lactamases. J. Bacteriol. 2005, 187, 7667–7679. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Bensing, B.A.; Sullam, P.M. The two distinct types of SecA2-dependent export systems. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Braunstein, M.; Espinosa, B.J.; Chan, J.; Belisle, J.T.; Jacobs, W.R., Jr. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 453–464. [Google Scholar] [CrossRef]
- Zulauf, K.E.; Sullivan, J.T.; Braunstein, M. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog. 2018, 14, e1007011. [Google Scholar] [CrossRef]
- Feltcher, M.E.; Gunawardena, H.P.; Zulauf, K.E.; Malik, S.; Griffin, J.E.; Sassetti, C.M.; Chen, X.; Braunstein, M. Label-free Quantitative Proteomics Reveals a Role for the Mycobacterium tuberculosis SecA2 Pathway in Exporting Solute Binding Proteins and Mce Transporters to the Cell Wall. Mol. Cell Proteom. 2015, 14, 1501–1516. [Google Scholar] [CrossRef]
- Lagune, M.; Petit, C.; Sotomayor, F.V.; Johansen, M.D.; Beckham, K.S.H.; Ritter, C.; Girard-Misguich, F.; Wilmanns, M.; Kremer, L.; Maurer, F.P.; et al. Conserved and specialized functions of Type VII secretion systems in non-tuberculous mycobacteria. Microbiology 2021, 167, 001054. [Google Scholar] [CrossRef]
- Houben, E.N.; Korotkov, K.V.; Bitter, W. Take five—Type VII secretion systems of Mycobacteria. Biochim. Biophys. Acta 2014, 1843, 1707–1716. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, C.S.; Nguyen, L.T.; Kim, J.K.; Jin, H.S.; Choe, J.H.; Kim, S.Y.; Lee, H.M.; Jung, M.; Kim, J.M.; et al. Mycobacterium abscessus ESX-3 plays an important role in host inflammatory and pathological responses during infection. Microbes Infect. 2017, 19, 5–17. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Tan, Z.; Li, A.; Fan, J.; Zhao, L.; Zhang, Z.; Chu, H. Impaired ESX-3 induces bedaquiline persistence in Mycobacterium abscessus growing under iron-limited conditions. Small Methods 2023, 7, e2300183. [Google Scholar] [CrossRef] [PubMed]
- Laencina, L.; Dubois, V.; Le Moigne, V.; Viljoen, A.; Majlessi, L.; Pritchard, J.; Bernut, A.; Piel, L.; Roux, A.L.; Gaillard, J.L.; et al. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl. Acad. Sci. USA 2018, 115, E1002–E1011. [Google Scholar] [CrossRef] [PubMed]
- Lagune, M.; Le Moigne, V.; Johansen, M.D.; Vasquez Sotomayor, F.; Daher, W.; Petit, C.; Cosentino, G.; Paulowski, L.; Gutsmann, T.; Wilmanns, M.; et al. The ESX-4 substrates, EsxU and EsxT, modulate Mycobacterium abscessus fitness. PLoS Pathog. 2022, 18, e1010771. [Google Scholar] [CrossRef]
- Bar-Oz, M.; Martini, M.C.; Alonso, M.N.; Meir, M.; Lore, N.I.; Miotto, P.; Riva, C.; Angala, S.K.; Xiao, J.; Masiello, C.S.; et al. The small non-coding RNA B11 regulates multiple facets of Mycobacterium abscessus virulence. PLoS Pathog. 2023, 19, e1011575. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Aull, H.G.; Jacobs-Sera, D.; Garlena, R.A.; Russell, D.A.; Smith, B.E.; Mahalingam, V.; Abad, L.; Gauthier, C.H.; Hatfull, G.F. The prophage and plasmid mobilome as a likely driver of Mycobacterium abscessus diversity. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Boudehen, Y.M.; Tasrini, Y.; Aguilera-Correa, J.J.; Alcaraz, M.; Kremer, L. Silencing essential gene expression in Mycobacterium abscessus during infection. Microbiol. Spectr. 2023, 11, e0283623. [Google Scholar] [CrossRef]
- Melly, G.; Purdy, G.E. MmpL proteins in physiology and pathogenesis of M. tuberculosis. Microorganisms 2019, 7, 70. [Google Scholar] [CrossRef]
- Pawlik, A.; Garnier, G.; Orgeur, M.; Tong, P.; Lohan, A.; Le Chevalier, F.; Sapriel, G.; Roux, A.L.; Conlon, K.; Honore, N.; et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol. Microbiol. 2013, 90, 612–629. [Google Scholar] [CrossRef]
- Dubois, V.; Viljoen, A.; Laencina, L.; Le Moigne, V.; Bernut, A.; Dubar, F.; Blaise, M.; Gaillard, J.L.; Guerardel, Y.; Kremer, L.; et al. MmpL8MAB controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc. Natl. Acad. Sci. USA 2018, 115, E10147–E10156. [Google Scholar] [CrossRef]
- Wong, D.; Chao, J.D.; Av-Gay, Y. Mycobacterium tuberculosis-secreted phosphatases: From pathogenesis to targets for TB drug development. Trends Microbiol. 2013, 21, 100–109. [Google Scholar] [CrossRef]
- Viljoen, A.; Alsteens, D.; Dufrene, Y. Mechanical forces between mycobacterial antigen 85 complex and fibronectin. Cells 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Natural anti-adhesive components against pathogenic bacterial adhesion and infection in gastrointestinal tract: Case studies of Helicobacter pylori, Salmonella enterica, Clostridium difficile, and diarrheagenic Escherichia coli. In Critical Reviews in Food Science and Nutrition; Taylor & Francis: Oxfordshire, UK, 2024; pp. 1–46. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Feng, Z.; Wang, K.; Shi, Z.; Zhang, L. Surface acoustic waves-enabled shielding fluid layers inhibit bacterial adhesion. Langmuir 2024, 40, 26203–26211. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.; Chakraborty, S.; Chen, R.; Palms, D.; Katsifis, G.; Li, Z.; Farajikhah, S.; Massedupally, V.; Hui, A.; Wong, E.H.H.; et al. The effect of immobilisation strategies on the ability of peptoids to reduce the adhesion of P. aeruginosa strains to contact lenses. Exp. Eye Res. 2025, 250, 110149. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Mori, M.; Stelitano, G.; Cazzaniga, G.; Gelain, A.; Tresoldi, A.; Cocorullo, M.; Roversi, M.; Chiarelli, L.R.; Tomaiuolo, M.; Del Re, P.; et al. Targeting siderophore-mediated iron uptake in M. abscessus: A new strategy to limit the virulence of non-tuberculous mycobacteria. Pharmaceutics 2023, 15, 502. [Google Scholar] [CrossRef]
- Foreman, M.; Kolodkin-Gal, I.; Barkan, D. A pivotal role for mycobactin/mbtE in growth and adaptation of Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0262322. [Google Scholar] [CrossRef]
- Mori, M.; Cocorullo, M.; Tresoldi, A.; Cazzaniga, G.; Gelain, A.; Stelitano, G.; Chiarelli, L.R.; Tomaiuolo, M.; Delre, P.; Mangiatordi, G.F.; et al. Structural basis for specific inhibition of salicylate synthase from Mycobacterium abscessus. Eur. J. Med. Chem. 2024, 265, 116073. [Google Scholar] [CrossRef]
- Tan, C.G.; Oberlag, N.M.; McGowan, A.E.; Dawrs, S.N.; Chan, Y.L.; Strong, M.; Hasan, N.A.; Honda, J.R. Genomic and microbiological analyses of iron acquisition pathways among respiratory and environmental nontuberculous mycobacteria from Hawai’i. Front. Microbiol. 2023, 14, 1268963. [Google Scholar] [CrossRef]
- Wang, L.; Asare, E.; Shetty, A.C.; Sanchez-Tumbaco, F.; Edwards, M.R.; Saranathan, R.; Weinrick, B.; Xu, J.; Chen, B.; Benard, A.; et al. Multiple genetic paths including massive gene amplification allow Mycobacterium tuberculosis to overcome loss of ESX-3 secretion system substrates. Proc. Natl. Acad. Sci. USA 2022, 119, e2112608119. [Google Scholar] [CrossRef]
- Quadri, L.E.; Sello, J.; Keating, T.A.; Weinreb, P.H.; Walsh, C.T. Identification of a Mycobacterium tuberculosis gene cluster encoding the biosynthetic enzymes for assembly of the virulence-conferring siderophore mycobactin. Chem. Biol. 1998, 5, 631–645. [Google Scholar] [CrossRef]
- Shyam, M.; Shilkar, D.; Verma, H.; Dev, A.; Sinha, B.N.; Brucoli, F.; Bhakta, S.; Jayaprakash, V. The mycobactin biosynthesis pathway: A prospective therapeutic target in the battle against tuberculosis. J. Med. Chem. 2021, 64, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Hatfull, G.F. The role of iron in Mycobacterium smegmatis biofilm formation: The exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 2007, 66, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Szekely, R.; Cole, S.T. Mechanistic insight into mycobacterial MmpL protein function. Mol. Microbiol. 2016, 99, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.; Puri, R.V.; Chauhan, P.; Kar, R.; Rohilla, A.; Khera, A.; Tyagi, A.K. Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J. Infect. Dis. 2013, 208, 1255–1265. [Google Scholar] [CrossRef]
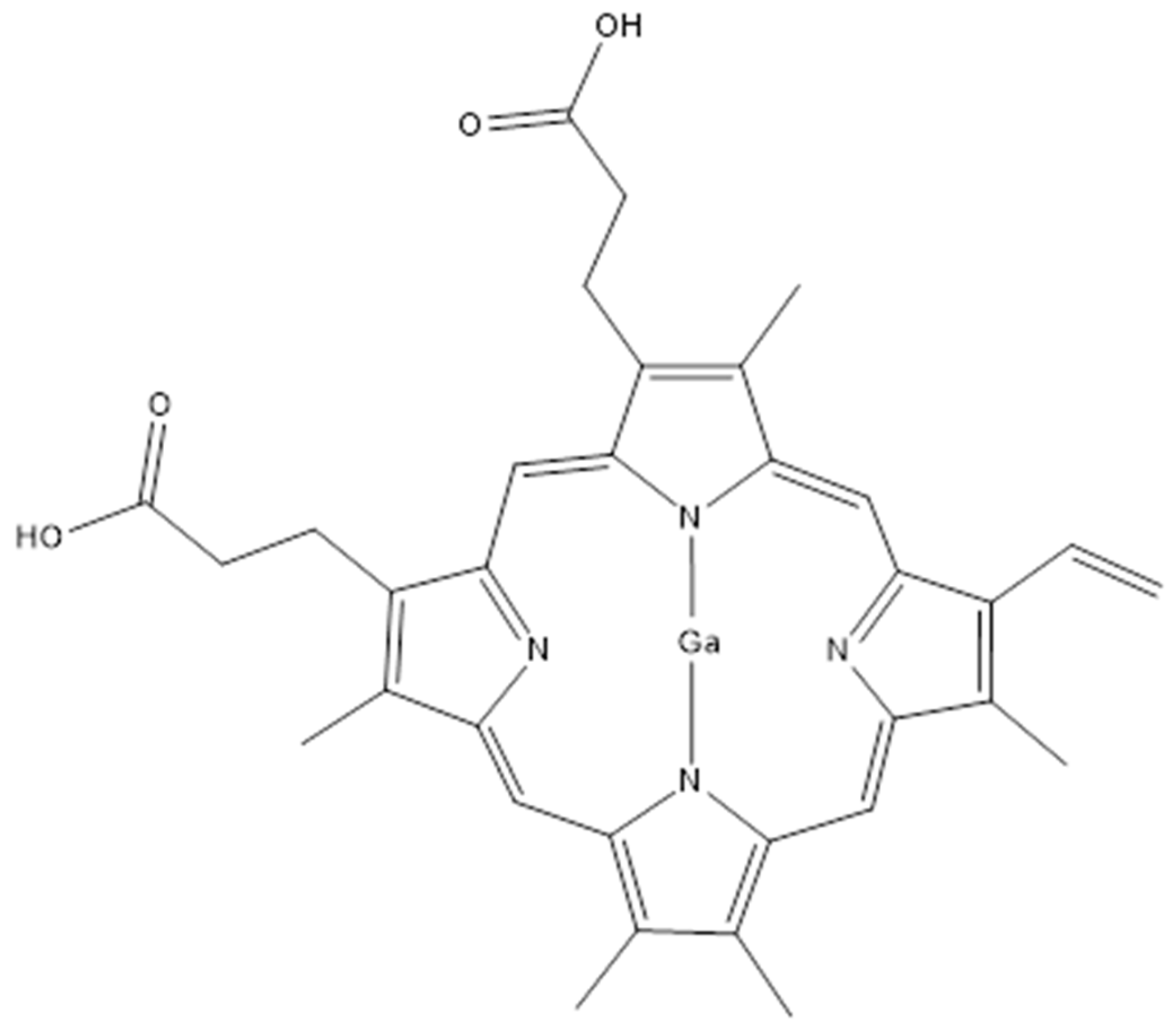
- Choi, S.R.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium porphyrin and gallium nitrate synergistically inhibit mycobacterial species by targeting different aspects of iron/heme metabolism. ACS Infect. Dis. 2020, 6, 2582–2591. [Google Scholar] [CrossRef]
- Feizi, S.; Awad, M.; Ramezanpour, M.; Cooksley, C.; Murphy, W.; Prestidge, C.A.; Psaltis, A.J.; Wormald, P.J.; Barry, S.; Vreugde, S. Promoting the efficacy of deferiprone-gallium-protoporphyrin (IX) against Mycobacterium abscessus intracellular infection with lipid liquid crystalline nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 70274–70283. [Google Scholar] [CrossRef]
- Dyett, B.P.; Yu, H.; Strachan, J.; Drummond, C.J.; Conn, C.E. Fusion dynamics of cubosome nanocarriers with model cell membranes. Nat. Commun. 2019, 10, 4492. [Google Scholar] [CrossRef]
- Bartek, I.L.; Woolhiser, L.K.; Baughn, A.D.; Basaraba, R.J.; Jacobs, W.R., Jr.; Lenaerts, A.J.; Voskuil, M.I. Mycobacterium tuberculosis Lsr2 is a global transcriptional regulator required for adaptation to changing oxygen levels and virulence. mBio 2014, 5, e01106-14. [Google Scholar] [CrossRef]
- Gerges, E.; Rodriguez-Ordonez Md, P.; Durand, N.; Herrmann, J.L.; Cremazy, F. Lsr2, a pleiotropic regulator at the core of the infectious strategy of Mycobacterium abscessus. Microbiol. Spectr. 2024, 12, e0352823. [Google Scholar] [CrossRef]
- Le Moigne, V.; Bernut, A.; Cortes, M.; Viljoen, A.; Dupont, C.; Pawlik, A.; Gaillard, J.L.; Misguich, F.; Cremazy, F.; Kremer, L.; et al. Lsr2 is an important determinant of intracellular growth and virulence in Mycobacterium abscessus. Front. Microbiol. 2019, 10, 905. [Google Scholar] [CrossRef]
- Liu, J.; Gordon, B.R. Targeting the global regulator Lsr2 as a novel approach for anti-tuberculosis drug development. Expert. Rev. Anti Infect. Ther. 2012, 10, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Pinault, L.; Han, J.S.; Kang, C.M.; Franco, J.; Ronning, D.R. Zafirlukast inhibits complexation of Lsr2 with DNA and growth of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ju, Y.; Li, L.; Hameed, A.; Yusuf, B.; Gao, Y.; Fang, C.; Tian, X.; Ding, J.; Ma, W.; et al. MtrAB two-component system is crucial for the intrinsic resistance and virulence of Mycobacterium abscessus. Int. J. Antimicrob. Agents 2024, 65, 107442. [Google Scholar] [CrossRef]
- van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Mouton, J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updat. 2012, 15, 149–161. [Google Scholar] [CrossRef]
- Peterson, E.J.R.; Brooks, A.N.; Reiss, D.J.; Kaur, A.; Do, J.; Pan, M.; Wu, W.J.; Morrison, R.; Srinivas, V.; Carter, W.; et al. MtrA modulates Mycobacterium tuberculosis cell division in host microenvironments to mediate intrinsic resistance and drug tolerance. Cell Rep. 2023, 42, 112875. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Arora, D.; Avanzi, C.; Wheat, W.H.; Bryant, J.M.; Spencer, J.S.; Blundell, T.L.; Parkhill, J.; Floto, R.A.; Jackson, M. Clinically relevant mutations in the PhoR sensor kinase of host-adapted Mycobacterium abscessus isolates impact response to acidic pH and virulence. Microbiol. Spectr. 2023, 11, e0158823. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Li, W.; Avanzi, C.; Angala, S.K.; Lian, E.; Wiersma, C.J.; Palcekova, Z.; Martin, K.H.; Angala, B.; de Moura, V.C.N.; et al. Unique features of Mycobacterium abscessus biofilms formed in synthetic cystic fibrosis medium. Front. Microbiol. 2021, 12, 743126. [Google Scholar] [CrossRef]
- Dokic, A.; Peterson, E.; Arrieta-Ortiz, M.L.; Pan, M.; Di Maio, A.; Baliga, N.; Bhatt, A. Mycobacterium abscessus biofilms produce an extracellular matrix and have a distinct mycolic acid profile. Cell Surf. 2021, 7, 100051. [Google Scholar] [CrossRef]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152 (Pt 6), 1581–1590. [Google Scholar] [CrossRef]
- Gloag, E.S.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Mycobacterium abscessus biofilms have viscoelastic properties which may contribute to their recalcitrance in chronic pulmonary infections. Sci. Rep. 2021, 11, 5020. [Google Scholar] [CrossRef]
- Clary, G.; Sasindran, S.J.; Nesbitt, N.; Mason, L.; Cole, S.; Azad, A.; McCoy, K.; Schlesinger, L.S.; Hall-Stoodley, L. Mycobacterium abscessus smooth and rough orphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Pourmoshtagh, H.; Sabour, S.; Hadi, N.; Azimi, T.; Soleiman-Meigooni, S. Biofilm Formation in Mycobacterial Genus; Mechanism of Biofilm Formation and Anti-Mycobacterial Biofilm Agents. Curr. Pharm. Biotechnol. 2024, 26, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Kumar, A. The extracellular matrix of mycobacterial biofilms: Could we shorten the treatment of mycobacterial infections? Microb. Cell 2019, 6, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Meliefste, H.M.; Mudde, S.E.; Ammerman, N.C.; de Steenwinkel, J.E.M.; Bax, H.I. A laboratory perspective on Mycobacterium abscessus biofilm culture, characterization and drug activity testing. Front. Microbiol. 2024, 15, 1392606. [Google Scholar] [CrossRef]
- Faria, S.; Joao, I.; Jordao, L. General overview on Nontuberculous Mycobacteria, biofilms, and human infection. J. Pathog. 2015, 2015, 809014. [Google Scholar] [CrossRef]
- Novotny, L.A.; Jurcisek, J.A.; Goodman, S.D.; Bakaletz, L.O. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016, 10, 33–44. [Google Scholar] [CrossRef]
- Jurcisek, J.A.; Kurbatfinski, N.; Wilbanks, K.Q.; Rhodes, J.D.; Goodman, S.D.; Bakaletz, L.O. Mycobacterium abscessus biofilm cleared from murine lung by monoclonal antibody against bacterial DNABII proteins. J. Cyst. Fibros. 2025, 24, 374–381. [Google Scholar] [CrossRef]
- Aung, T.T.; Yam, J.K.; Lin, S.; Salleh, S.M.; Givskov, M.; Liu, S.; Lwin, N.C.; Yang, L.; Beuerman, R.W. Biofilms of pathogenic nontuberculous mycobacteria targeted by new therapeutic approaches. Antimicrob. Agents Chemother. 2016, 60, 24–35. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocorullo, M.; Stamilla, A.; Recchia, D.; Marturano, M.C.; Maci, L.; Stelitano, G. Mycobacterium abscessus Virulence Factors: An Overview of Un-Explored Therapeutic Options. Int. J. Mol. Sci. 2025, 26, 3247. https://doi.org/10.3390/ijms26073247
Cocorullo M, Stamilla A, Recchia D, Marturano MC, Maci L, Stelitano G. Mycobacterium abscessus Virulence Factors: An Overview of Un-Explored Therapeutic Options. International Journal of Molecular Sciences. 2025; 26(7):3247. https://doi.org/10.3390/ijms26073247
Chicago/Turabian StyleCocorullo, Mario, Alessandro Stamilla, Deborah Recchia, Maria Concetta Marturano, Ludovica Maci, and Giovanni Stelitano. 2025. "Mycobacterium abscessus Virulence Factors: An Overview of Un-Explored Therapeutic Options" International Journal of Molecular Sciences 26, no. 7: 3247. https://doi.org/10.3390/ijms26073247
APA StyleCocorullo, M., Stamilla, A., Recchia, D., Marturano, M. C., Maci, L., & Stelitano, G. (2025). Mycobacterium abscessus Virulence Factors: An Overview of Un-Explored Therapeutic Options. International Journal of Molecular Sciences, 26(7), 3247. https://doi.org/10.3390/ijms26073247







