CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis
Abstract
1. Introduction
2. Results
2.1. CsHY5 and ABGs Were Significantly Induced by Light
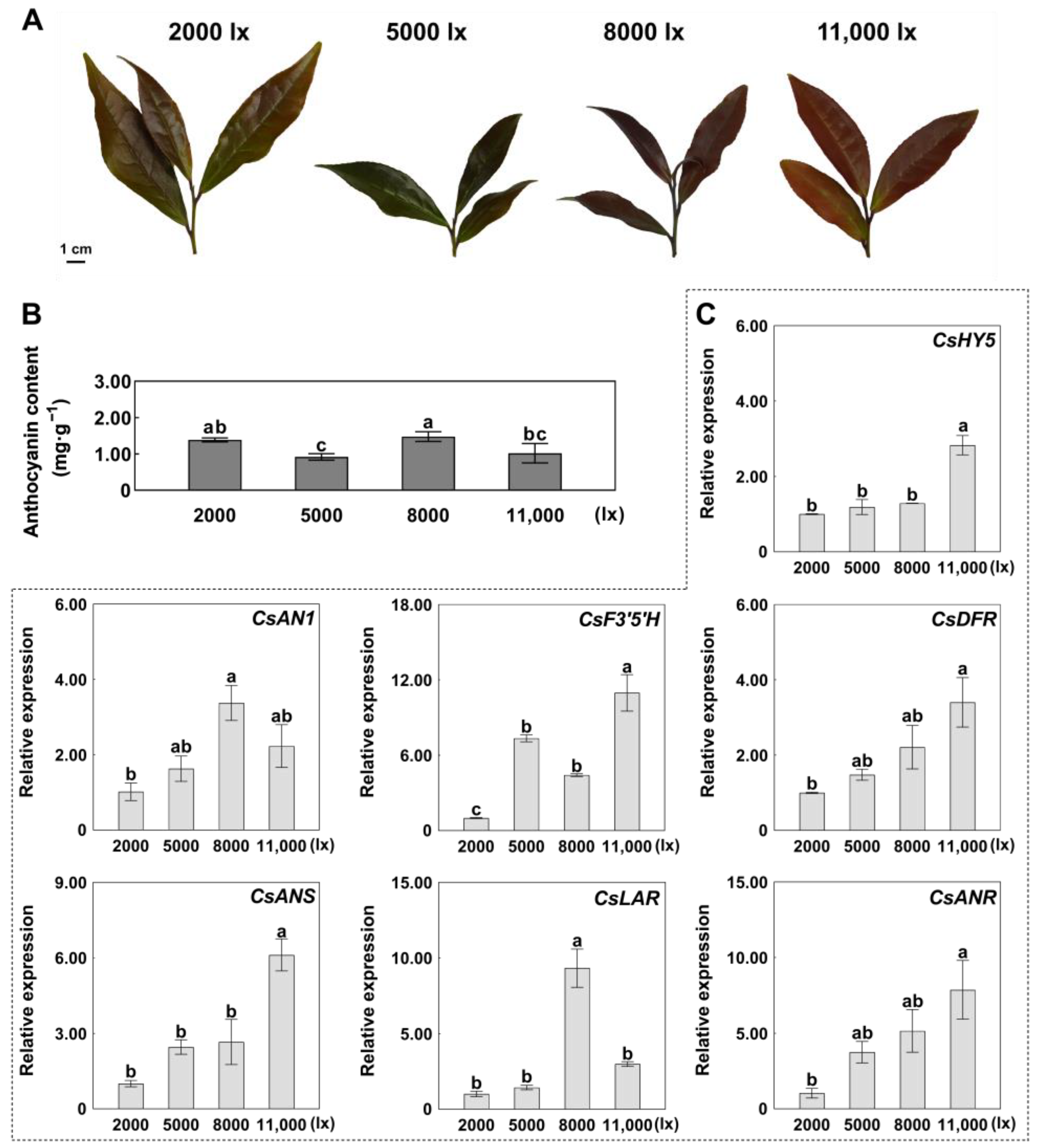
2.2. Expression Patterns of CsHY5 and ABGs in Response to Light Intensity
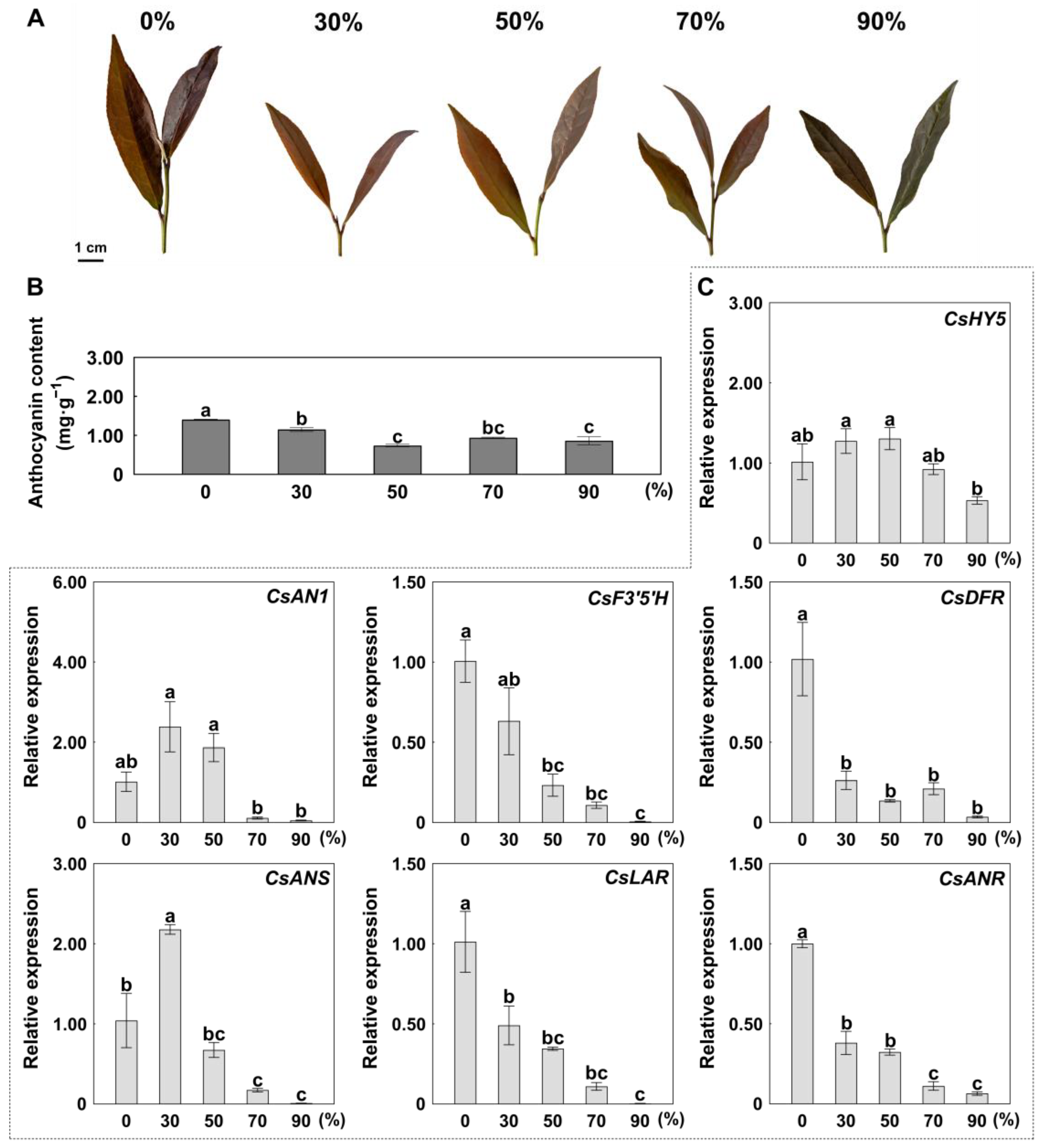
2.3. Shading-Mediated Suppression of Anthocyanin Accumulation
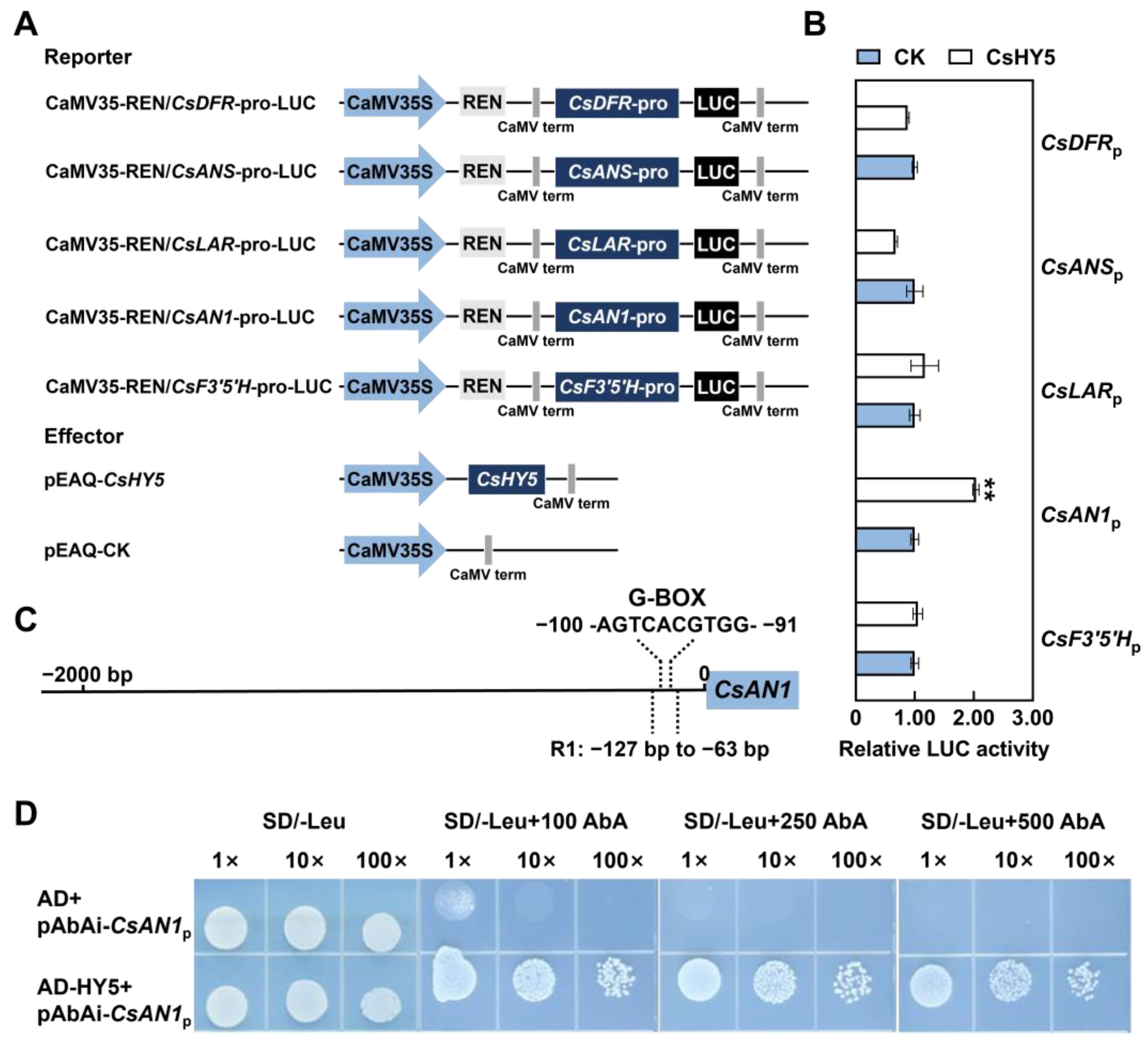
2.4. CsHY5 Controls Anthocyanin Biosynthesis by Directly Regulating CsAN1 Expression
3. Discussion
3.1. Light Influenced Anthocyanin Biosynthesis
3.2. Shading Could Be Used to Control the Anthocyanin Content of Tea Plants
3.3. CsHY5 Regulated Anthocyanin Biosynthesis via CsAN1 Expression
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Anthocyanin Extraction and Analysis
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Multiple Sequence Alignment and Phylogenetic Analysis
4.5. Subcellular Localization
4.6. Yeast One-Hybrid Assays (Y1H)
4.7. Dual-Luciferase Reporter Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, S.; Chen, P.; Cai, J.; Tang, S.; Yang, W.; Cao, F.; Zheng, P.; Sun, B. Systematic Analysis of the R2R3-MYB Family in Camellia Sinensis: Evidence for Galloylated Catechins Biosynthesis Regulation. Front. Plant Sci. 2022, 12, 2867. [Google Scholar] [CrossRef]
- Ramlagan, P.; Narrain, D.; Fawdar, S.; Bahorun, T. 1—Tea, the “Ambrosia” Beverage: Biochemical, Cellular, Molecular, and Clinical Evidences. In Non-Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–61. ISBN 978-0-12-815270-6. [Google Scholar]
- Sun, B.; Zhu, Z.; Cao, P.; Chen, H.; Chen, C.; Zhou, X.; Mao, Y.; Lei, J.; Jiang, Y.; Meng, W.; et al. Purple Foliage Coloration in Tea (Camellia sinensis L.) Arises from Activation of the R2R3-MYB Transcription Factor CsAN1. Sci. Rep. 2016, 6, 32534. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A Coupled Role for CsMYB75 and CsGSTF1 in Anthocyanin Hyperaccumulation in Purple Tea. Plant J. 2019, 97, 825–840. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, P.; Cui, D.; Yang, X.; Zhang, D.; Yang, Y.; Chen, W.; Tang, D.; Tang, Q.; Li, P. Multi-Omics Analysis Revealed Anthocyanin Accumulation Differences in Purple Tea Plants ‘Ziyan’, ‘Zijuan’ and Their Dark-Purple Hybrid. Sci. Hortic. 2023, 321, 112275. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, X.; Shoji, T.; Kanda, T.; Zhou, J.; Zhao, L. Characterization and Activity of Anthocyanins in Zijuan Tea (Camellia sinensis Var. Kitamura). J. Agric. Food Chem. 2013, 61, 3306–3310. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in Black Rice, Soybean and Purple Corn Increase Fecal Butyric Acid and Prevent Liver Inflammation in High Fat Diet-Induced Obese Mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar]
- Zhang, K.; Lin, C.; Chen, B.; Lin, Y.; Su, H.; Du, Y.; Zhang, H.; Zhou, H.; Ji, R.; Zhang, L. A Light Responsive Transcription Factor CsbHLH89 Positively Regulates Anthocyanidin Synthesis in Tea (Camellia sinensis). Sci. Hortic. 2024, 327, 112784. [Google Scholar] [CrossRef]
- Li, X.; Cao, L.; Jiao, B.; Yang, H.; Ma, C.; Liang, Y. The BHLH Transcription Factor AcB2 Regulates Anthocyanin Biosynthesis in Onion (Allium cepa L.). Hortic. Res. 2022, 9, uhac128. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB Transcription Factors That Colour Our Fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, W.; He, H.; Li, H.; Wang, P.; Wang, M.; Zhao, H.; Wang, Y.; Ni, D.; Guo, F. CsMYBPA1-CsGSTU18 Interaction Plays an Important Role in Anthocyanin Metabolism Regulation in Tea Plant (Camellia sinensis). Sci. Hortic. 2023, 321, 112338. [Google Scholar] [CrossRef]
- Shui, L.; Li, W.; Yan, M.; Li, H.; Guo, F. Characterization of the R2R3-MYB Transcription Factor CsMYB113 Regulates Anthocyanin Biosynthesis in Tea Plants (Camellia sinensis). Plant Mol. Biol. Rep. 2023, 41, 46–58. [Google Scholar] [CrossRef]
- Cai, J.; Lv, L.; Zeng, X.; Zhang, F.; Chen, Y.; Tian, W.; Li, J.; Li, X.; Li, Y. Integrative Analysis of Metabolomics and Transcriptomics Reveals Molecular Mechanisms of Anthocyanin Metabolism in the Zikui Tea Plant (Camellia sinensis Cv. Zikui). Int. J. Mol. Sci. 2022, 23, 4780. [Google Scholar] [CrossRef]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in Metabolites of Purple Corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A. Pigment Loss in Response to the Environment: A New Role for the WD/BHLH/MYB Anthocyanin Regulatory Complex. New Phytol. 2009, 182, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-Induced Vegetative Anthocyanin Pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Zuo, H.; Peng, A.; Lin, J.; Li, P.; Wang, K.; Tang, Q.; Tadege, M.; Liu, Z.; et al. CsMYBL2 Homologs Modulate the Light and Temperature Stress-Regulated Anthocyanin and Catechins Biosynthesis in Tea Plants (Camellia sinensis). Plant J. 2023, 115, 1051–1070. [Google Scholar] [CrossRef]
- Li, F.; Deng, X.; Huang, Z.; Zhao, Z.; Li, C.; Song, Q.; He, Y.; Niu, S. Integrated Transcriptome and Metabolome Provide Insights into Flavonoid Biosynthesis in “P113”, a New Purple Tea of Camellia tachangensis. Beverage Plant Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-Induced Expression of a MYB Gene Regulates Anthocyanin Biosynthesis in Red Apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Alabd, A.; Ahmad, M.; Zhang, X.; Gao, Y.; Peng, L.; Zhang, L.; Ni, J.; Bai, S.; Teng, Y. Light-Responsive Transcription Factor PpWRKY44 Induces Anthocyanin Accumulation by Regulating PpMYB10 Expression in Pear. Hortic. Res. 2022, 9, uhac199. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Singh, S.; Khurana, J.P.; Burman, N. HY5-COP1: The Central Module of Light Signaling Pathway. J. Plant Biochem. Biotechnol. 2020, 29, 590–610. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Jeong, C.Y.; Kang, G.H.; Yoo, S.D.; Hong, S.W.; Lee, H. MYBD Employed by HY5 Increases Anthocyanin Accumulation via Repression of MYBL2 in Arabidopsis. Plant J. 2015, 84, 1192–1205. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Osterlund, M.T.; Hardtke, C.S.; Ning, W.; Deng, X.W. Targeted Destabilization of HY5 during Light-Regulated Development of Arabidopsis. Nature 2000, 405, 462–466. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y. Il HY5 Regulates Anthocyanin Biosynthesis by Inducing the Transcriptional Activation of the MYB75/PAP1 Transcription Factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, X.; Lin, N.; Yu, S.; Fernie, A.R.; Zhao, J. CsbZIP1-CsMYB12 Mediates the Production of Bitter-Tasting Flavonols in Tea Plants (Camellia sinensis) through a Coordinated Activator–Repressor Network. Hortic. Res. 2021, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Elango, T.; Jeyaraj, A.; Dayalan, H.; Arul, S.; Govindasamy, R.; Prathap, K.; Li, X. Influence of Shading Intensity on Chlorophyll, Carotenoid and Metabolites Biosynthesis to Improve the Quality of Green Tea: A Review. Energy Nexus 2023, 12, 100241. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; Wang, W.; Ma, R.; Ding, C.; Wei, K.; Wang, L.; Ge, S.; Shi, Y.; Li, X. Transcriptomic and Metabolomic Insights into Temperature-Dependent Changes in Catechin and Anthocyanin Accumulation in Tea Plants with Different Leaf Colors. Plant Stress 2024, 14, 100705. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, W.; Sun, Y.; Li, J.; Zhang, J.; Wu, T.; Song, T.; Yao, Y.; Tian, J. MPK6-Mediated HY5 Phosphorylation Regulates Light-Induced Anthocyanin Accumulation in Apple Fruit. Plant Biotechnol. J. 2023, 21, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, J.; Zhang, S.; Chi, R.; Wang, C.; Wang, D.; Li, H. The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum Bungeanum. Int. J. Mol. Sci. 2022, 23, 2651. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bakshi, S.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.K.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection in Arabidopsis. Plant Physiol. 2019, 179, 1436–1452. [Google Scholar]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering Anthocyanin Biosynthesis in Plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar]
- Carbonell-Bejerano, P.; Diago, M.P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar Ultraviolet Radiation is Involved in Regulation of Anthocyanin Accumulation in Tempranillo Grape Skins. Front. Plant Sci. 2014, 5, 498. [Google Scholar]
- Zhang, Q.; Hu, J.; Liu, M.; Shi, Y.; De Vos, R.C.H.; Ruan, J. Stimulated Biosynthesis of Delphinidin-Related Anthocyanins in Tea Shoots Reducing the Quality of Green Tea in Summer. J. Sci. Food Agric. 2020, 100, 1505–1514. [Google Scholar] [CrossRef]
- Matsunaga, A.; Sano, T.; Hirono, Y.; Horie, H. Effects of Various Directly Covered Shading Levels on Chemical Components in Tea New Shoots of the First Flush. Chagyo Kenkyu Hokoku (Tea Res. J.) 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Ji, H.G.; Lee, Y.R.; Lee, M.S.; Hwang, K.H.; Park, C.Y.; Kim, E.H.; Park, J.S.; Hong, Y.S. Diverse Metabolite Variations in Tea (Camellia sinensis L.) Leaves Grown under Various Shade Conditions Revisited: A Metabolomics Study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Shan, Y.; Liu, Y.; Tian, Y.; Xia, T. Influence of Shade on Flavonoid Biosynthesis in Tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Fallahi, E.; Colt, W.M.; Baird, C.R.; Fallahi, B.; Chun, I.J. Influence of Nitrogen and Bagging on Fruit Quality and Mineral Concentrations of “BC-2 Fuji” Apple. Horttechnology 2001, 11, 462–466. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. The Effects of Bagging and Debagging on External Fruit Quality, Metabolites, and the Expression of Anthocyanin Biosynthetic Genes in “Jonagold” Apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Bursch, K.; Toledo-Ortiz, G.; Pireyre, M.; Lohr, M.; Braatz, C.; Johansson, H. Identification of BBX Proteins as Rate-Limiting Cofactors of HY5. Nat. Plants 2020, 6, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Manuela, D.; Xu, M. The R2R3-MYB Transcription Factor IbMYB1 Enhances Anthocyanin Accumulation and Salt Stress Tolerance in Transgenic Sweet Potato. Plant Biotechnol. J. 2023, 15, 693. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Liu, Y.; Zheng, P.; Liu, S.; Sun, B. A genus-speciffc R2R3 MYB transcription factor, CsMYB34, regulates galloylated catechin biosynthesis in Camellia sinensis. Plant Physiol. Biochem. 2025, 219, 109401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, Y.; Zhao, H.; Xu, J.; Zheng, P.; Liu, S.; Sun, B. CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis. Int. J. Mol. Sci. 2025, 26, 3253. https://doi.org/10.3390/ijms26073253
Chen J, Liu Y, Zhao H, Xu J, Zheng P, Liu S, Sun B. CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis. International Journal of Molecular Sciences. 2025; 26(7):3253. https://doi.org/10.3390/ijms26073253
Chicago/Turabian StyleChen, Jiahao, Yihao Liu, Hongbo Zhao, Jianmei Xu, Peng Zheng, Shaoqun Liu, and Binmei Sun. 2025. "CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis" International Journal of Molecular Sciences 26, no. 7: 3253. https://doi.org/10.3390/ijms26073253
APA StyleChen, J., Liu, Y., Zhao, H., Xu, J., Zheng, P., Liu, S., & Sun, B. (2025). CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis. International Journal of Molecular Sciences, 26(7), 3253. https://doi.org/10.3390/ijms26073253






