Broad Vitamin B6-Related Metabolic Disturbances in a Zebrafish Model of Hypophosphatasia (TNSALP-Deficiency)
Abstract
1. Introduction
2. Results
2.1. Generation of the alpl Knockout Zebrafish
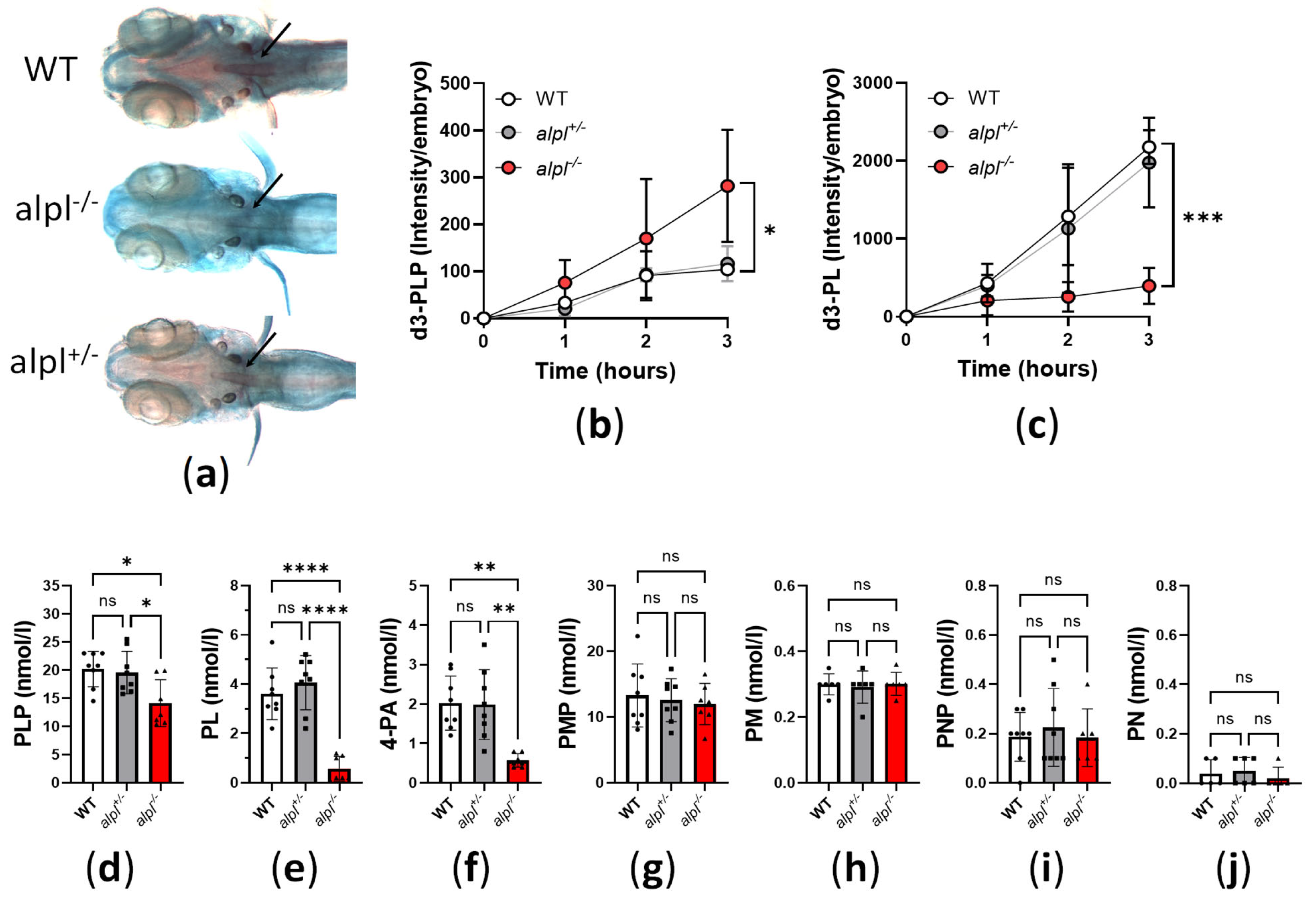
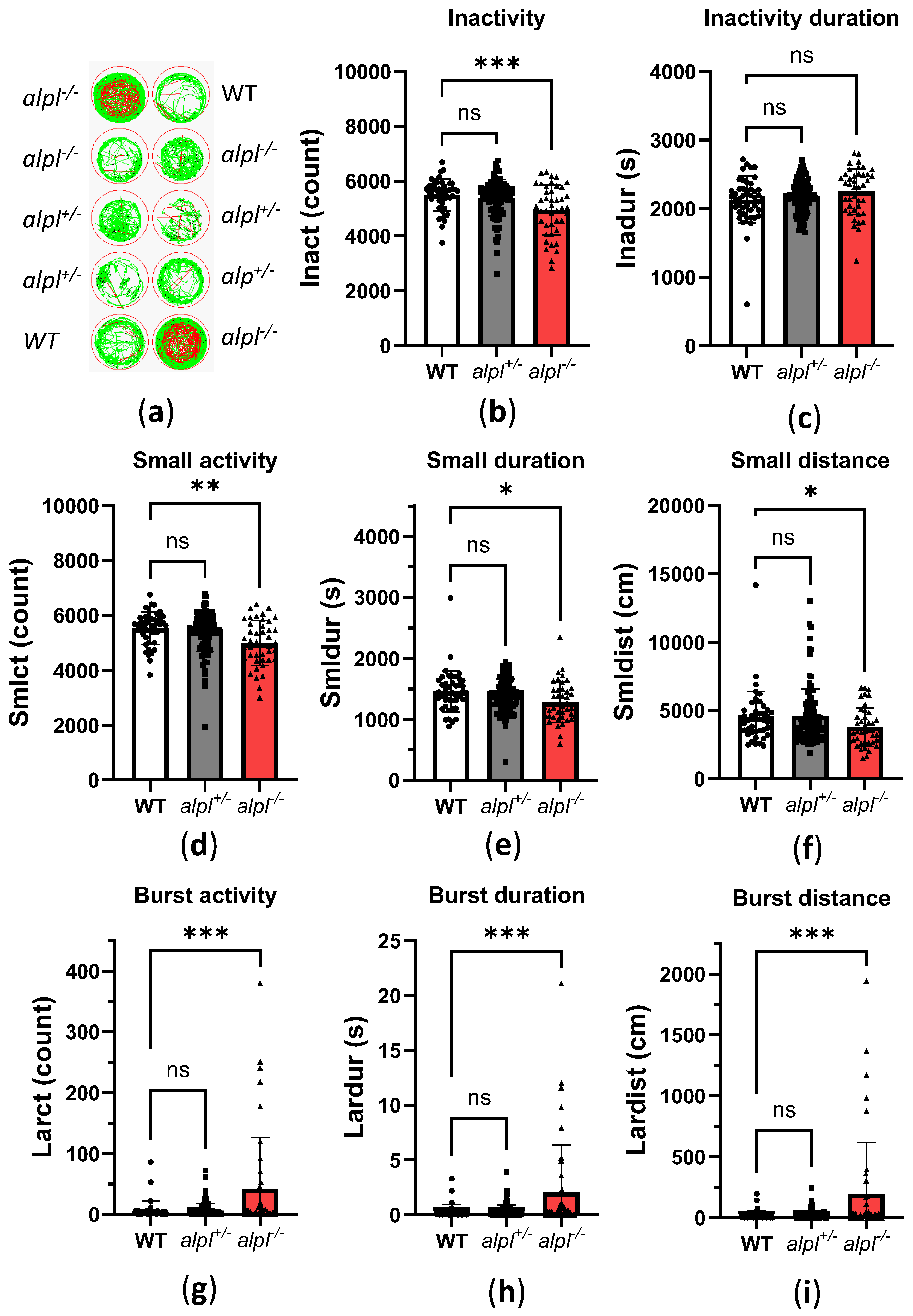
2.2. Abnormal Bone Mineralization, Vitamin B6 Metabolism and Locomotion in alpl-/- Embryos
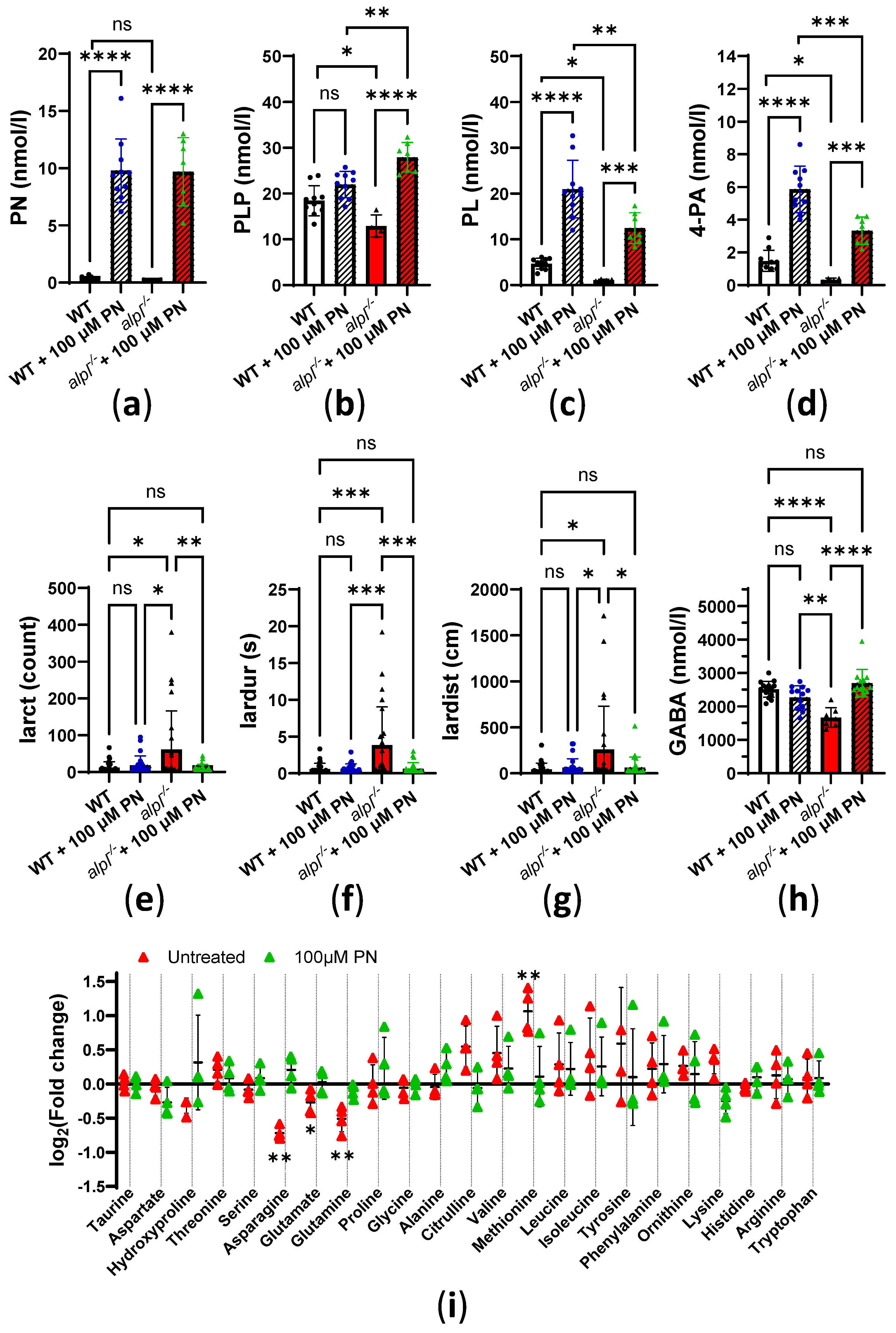
2.3. Pyridoxine Treatment Normalizes Locomotion and Some Metabolic Abnormalities in alpl-/- Zebrafish Embryos
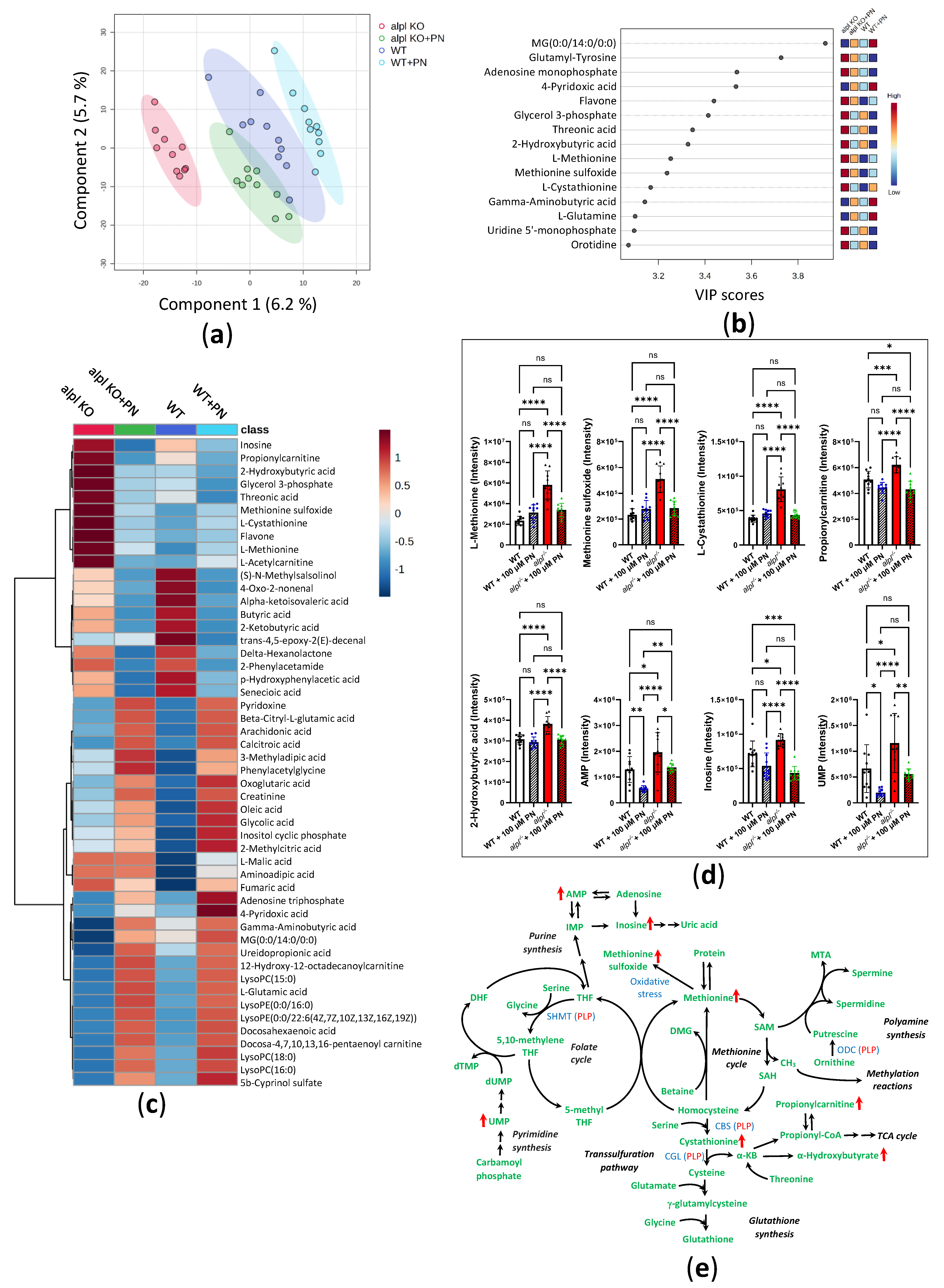
2.4. Progression of Metabolic Abnormalities and Decreased Survival in alpl-/- Zebrafish Larvae
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance and Treatment Protocols
4.2. sgRNA and Cas9 mRNA Design and Synthesis
4.3. Generation of alpl Knockout Zebrafish
4.4. DNA Extraction and Genotyping
4.5. RNA Isolation and Real-Time PCR
4.6. Alizarin Red and Alcian Blue Staining
4.7. Alkaline Phosphatase Enzyme Activity
4.8. Western Blotting
4.9. B6 Vitamer Analysis
4.10. Non-Quantitative Direct-Infusion High-Resolution Mass Spectrometry (DI-HRMS)
4.11. Amino Acid and γ-Aminobutyric Acid (GABA) Analysis
4.12. Locomotion Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathbun, J.C. Hypophosphatasia; a new developmental anomaly. Am. J. Dis. Child. 1948, 75, 822–831. [Google Scholar] [CrossRef]
- Farman, M.R.; Rehder, C.; Malli, T.; Rockman-Greenberg, C.; Dahir, K.; Martos-Moreno, G.A.; Linglart, A.; Ozono, K.; Seefried, L.; Del Angel, G.; et al. The Global ALPL gene variant classification project: Dedicated to deciphering variants. Bone 2024, 178, 116947. [Google Scholar] [CrossRef]
- Zurutuza, L.; Muller, F.; Gibrat, J.F.; Taillandier, A.; Simon-Bouy, B.; Serre, J.L.; Mornet, E. Correlations of genotype and phenotype in hypophosphatasia. Hum. Mol. Genet. 1999, 8, 1039–1046. [Google Scholar] [CrossRef]
- Brun-Heath, I.; Ermonval, M.; Chabrol, E.; Xiao, J.; Palkovits, M.; Lyck, R.; Miller, F.; Couraud, P.O.; Mornet, E.; Fonta, C. Differential expression of the bone and the liver tissue non-specific alkaline phosphatase isoforms in brain tissues. Cell Tissue Res. 2011, 343, 521–536. [Google Scholar] [CrossRef]
- Millan, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Int. 2016, 98, 398–416. [Google Scholar] [CrossRef]
- Russell, R.G. Excretion of Inorganic Pyrophosphate in Hypophosphatasia. Lancet 1965, 2, 461–464. [Google Scholar] [CrossRef]
- Russell, R.G.; Bisaz, S.; Donath, A.; Morgan, D.B.; Fleisch, H. Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J. Clin. Investig. 1971, 50, 961–969. [Google Scholar] [CrossRef]
- Fedde, K.N.; Whyte, M.P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-5′-phosphate ectophosphatase: Normal and hypophosphatasia fibroblast study. Am. J. Hum. Genet. 1990, 47, 767–775. [Google Scholar]
- Whyte, M.P.; Mahuren, J.D.; Vrabel, L.A.; Coburn, S.P. Markedly increased circulating pyridoxal-5′-phosphate levels in hypophosphatasia. Alkaline phosphatase acts in vitamin B6 metabolism. J. Clin. Investig. 1985, 76, 752–756. [Google Scholar] [CrossRef]
- Whyte, M.P.; Mahuren, J.D.; Fedde, K.N.; Cole, F.S.; McCabe, E.R.; Coburn, S.P. Perinatal hypophosphatasia: Tissue levels of vitamin B6 are unremarkable despite markedly increased circulating concentrations of pyridoxal-5′-phosphate. Evidence for an ectoenzyme role for tissue-nonspecific alkaline phosphatase. J. Clin. Investig. 1988, 81, 1234–1239. [Google Scholar] [CrossRef]
- McCance, R.A.; Morrison, A.B.; Dent, C.E. The excretion of phosphoethanolamine and hypophosphatasia. Lancet 1955, 268, 131. [Google Scholar] [CrossRef]
- Fraser, D.; Yendt, E.R.; Christie, F.H. Metabolic abnormalities in hypophosphatasia. Lancet 1955, 268, 286. [Google Scholar] [CrossRef]
- Orimo, H.; Girschick, H.J.; Goseki-Sone, M.; Ito, M.; Oda, K.; Shimada, T. Mutational analysis and functional correlation with phenotype in German patients with childhood-type hypophosphatasia. J. Bone Miner. Res. 2001, 16, 2313–2319. [Google Scholar] [CrossRef]
- Nunes, M.E. Hypophosphatasia. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Meyer, J.L. Can biological calcification occur in the presence of pyrophosphate? Arch. Biochem. Biophys. 1984, 231, 1–8. [Google Scholar] [CrossRef]
- Collmann, H.; Mornet, E.; Gattenlohner, S.; Beck, C.; Girschick, H. Neurosurgical aspects of childhood hypophosphatasia. Childs Nerv. Syst. 2009, 25, 217–223. [Google Scholar] [CrossRef]
- Bacchetta, J. Renal impairment in hypophosphatasia. Arch. Pediatr. 2017, 24, 5S93–5S95. [Google Scholar] [CrossRef]
- Baumgartner-Sigl, S.; Haberlandt, E.; Mumm, S.; Scholl-Burgi, S.; Sergi, C.; Ryan, L.; Ericson, K.L.; Whyte, M.P.; Hogler, W. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677T>C, p.M226T; c.1112C>T, p.T371I) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007, 40, 1655–1661. [Google Scholar] [CrossRef]
- Basura, G.J.; Hagland, S.P.; Wiltse, A.M.; Gospe, S.M. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: Review of 63 North American cases submitted to a patient registry. Eur. J. Pediatr. 2009, 168, 697–704. [Google Scholar] [CrossRef]
- Taketani, T. Neurological Symptoms of Hypophosphatasia. Subcell. Biochem. 2015, 76, 309–322. [Google Scholar] [CrossRef]
- Demirbilek, H.; Alanay, Y.; Alikasifoglu, A.; Topcu, M.; Mornet, E.; Gonc, N.; Ozon, A.; Kandemir, N. Hypophosphatasia presenting with pyridoxine-responsive seizures, hypercalcemia, and pseudotumor cerebri: Case report. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 34–38. [Google Scholar] [CrossRef]
- Lefever, E.; Witters, P.; Gielen, E.; Vanclooster, A.; Meersseman, W.; Morava, E.; Cassiman, D.; Laurent, M.R. Hypophosphatasia in Adults: Clinical Spectrum and Its Association With Genetics and Metabolic Substrates. J. Clin. Densitom. 2020, 23, 340–348. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef]
- Whyte, M.P.; Zhang, F.; Mack, K.E.; Wenkert, D.; Gottesman, G.S.; Ericson, K.L.; Cole, J.T.; Coburn, S.P. Pyridoxine challenge reflects pediatric hypophosphatasia severity and thereby examines tissue-nonspecific alkaline phosphatase’s role in vitamin B(6) metabolism. Bone 2024, 181, 117033. [Google Scholar] [CrossRef]
- Colazo, J.M.; Hu, J.R.; Dahir, K.M.; Simmons, J.H. Correction to: Neurological Symptoms in Hypophosphatasia. Osteoporos. Int. 2019, 30, 535. [Google Scholar] [CrossRef]
- Whyte, M.P.; Greenberg, C.R.; Salman, N.J.; Bober, M.B.; McAlister, W.H.; Wenkert, D.; Van Sickle, B.J.; Simmons, J.H.; Edgar, T.S.; Bauer, M.L.; et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 2012, 366, 904–913. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Abe, M.; Matsuda, M. A correlation between changes in gamma-aminobutyric acid metabolism and seizures induced by antivitamin B6. J. Biochem. 1976, 80, 1165–1171. [Google Scholar] [CrossRef]
- Liedtke, D.; Hofmann, C.; Jakob, F.; Klopocki, E.; Graser, S. Tissue-Nonspecific Alkaline Phosphatase-A Gatekeeper of Physiological Conditions in Health and a Modulator of Biological Environments in Disease. Biomolecules 2020, 10, 1648. [Google Scholar] [CrossRef]
- Van Belle, H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin. Chem. 1976, 22, 972–976. [Google Scholar] [CrossRef]
- Bensimon-Brito, A.; Cardeira, J.; Cancela, M.L.; Huysseune, A.; Witten, P.E. Distinct patterns of notochord mineralization in zebrafish coincide with the localization of Osteocalcin isoform 1 during early vertebral centra formation. BMC Dev. Biol. 2012, 12, 28. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Pena, I.A.; Roussel, Y.; Daniel, K.; Mongeon, K.; Johnstone, D.; Weinschutz Mendes, H.; Bosma, M.; Saxena, V.; Lepage, N.; Chakraborty, P.; et al. Pyridoxine-Dependent Epilepsy in Zebrafish Caused by Aldh7a1 Deficiency. Genetics 2017, 207, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Ciapaite, J.; Albersen, M.; Savelberg, S.M.C.; Bosma, M.; Tessadori, F.; Gerrits, J.; Lansu, N.; Zwakenberg, S.; Bakkers, J.P.W.; Zwartkruis, F.J.T.; et al. Pyridox(am)ine 5′-phosphate oxidase (PNPO) deficiency in zebrafish results in fatal seizures and metabolic aberrations. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165607. [Google Scholar] [CrossRef]
- Afrikanova, T.; Serruys, A.S.; Buenafe, O.E.; Clinckers, R.; Smolders, I.; de Witte, P.A.; Crawford, A.D.; Esguerra, C.V. Validation of the zebrafish pentylenetetrazol seizure model: Locomotor versus electrographic responses to antiepileptic drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef]
- Haijes, H.A.; Willemsen, M.; Van der Ham, M.; Gerrits, J.; Pras-Raves, M.L.; Prinsen, H.; Van Hasselt, P.M.; De Sain-van der Velden, M.G.M.; Verhoeven-Duif, N.M.; Jans, J.J.M. Direct Infusion Based Metabolomics Identifies Metabolic Disease in Patients’ Dried Blood Spots and Plasma. Metabolites 2019, 9, 12. [Google Scholar] [CrossRef]
- Baggott, J.E.; Tamura, T. Metabolism of 10-formyldihydrofolate in humans. Biomed. Pharmacother. 2001, 55, 454–457. [Google Scholar] [CrossRef]
- Brun, L.; Ngu, L.H.; Keng, W.T.; Ch’ng, G.S.; Choy, Y.S.; Hwu, W.L.; Lee, W.T.; Willemsen, M.A.; Verbeek, M.M.; Wassenberg, T.; et al. Clinical and biochemical features of aromatic L-amino acid decarboxylase deficiency. Neurology 2010, 75, 64–71. [Google Scholar] [CrossRef]
- Nawrocki, L.; BreMiller, R.; Streisinger, G.; Kaplan, M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vision. Res. 1985, 25, 1569–1576. [Google Scholar] [CrossRef]
- Yang, Y.; Wandler, A.M.; Postlethwait, J.H.; Guillemin, K. Dynamic Evolution of the LPS-Detoxifying Enzyme Intestinal Alkaline Phosphatase in Zebrafish and Other Vertebrates. Front. Immunol. 2012, 3, 314. [Google Scholar] [CrossRef]
- Ohlebusch, B.; Borst, A.; Frankenbach, T.; Klopocki, E.; Jakob, F.; Liedtke, D.; Graser, S. Investigation of alpl expression and Tnap-activity in zebrafish implies conserved functions during skeletal and neuronal development. Sci. Rep. 2020, 10, 13321. [Google Scholar] [CrossRef]
- Millan, J.L.; Narisawa, S.; Lemire, I.; Loisel, T.P.; Boileau, G.; Leonard, P.; Gramatikova, S.; Terkeltaub, R.; Camacho, N.P.; McKee, M.D.; et al. Enzyme replacement therapy for murine hypophosphatasia. J. Bone Miner. Res. 2008, 23, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Waymire, K.G.; Mahuren, J.D.; Jaje, J.M.; Guilarte, T.R.; Coburn, S.P.; MacGregor, G.R. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat. Genet. 1995, 11, 45–51. [Google Scholar] [CrossRef]
- Fedde, K.N.; Blair, L.; Silverstein, J.; Coburn, S.P.; Ryan, L.M.; Weinstein, R.S.; Waymire, K.; Narisawa, S.; Millan, J.L.; MacGregor, G.R.; et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999, 14, 2015–2026. [Google Scholar] [CrossRef]
- Narisawa, S.; Frohlander, N.; Millan, J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 1997, 208, 432–446. [Google Scholar] [CrossRef]
- Belachew, D.; Kazmerski, T.; Libman, I.; Goldstein, A.C.; Stevens, S.T.; Deward, S.; Vockley, J.; Sperling, M.A.; Balest, A.L. Infantile hypophosphatasia secondary to a novel compound heterozygous mutation presenting with pyridoxine-responsive seizures. JIMD Rep. 2013, 11, 17–24. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Bowling, F.; Carpenter, K.; Earl, J.; Chaitow, J.; Pitt, J.; Mornet, E.; Sillence, D.; Ellaway, C. Perinatal hypophosphatasia presenting as neonatal epileptic encephalopathy with abnormal neurotransmitter metabolism secondary to reduced co-factor pyridoxal-5′-phosphate availability. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S25–S33. [Google Scholar] [CrossRef]
- Narisawa, S.; Wennberg, C.; Millan, J.L. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J. Pathol. 2001, 193, 125–133. [Google Scholar] [CrossRef]
- Cruz, T.; Gleizes, M.; Balayssac, S.; Mornet, E.; Marsal, G.; Millan, J.L.; Malet-Martino, M.; Nowak, L.G.; Gilard, V.; Fonta, C. Identification of altered brain metabolites associated with TNAP activity in a mouse model of hypophosphatasia using untargeted NMR-based metabolomics analysis. J. Neurochem. 2017, 140, 919–940. [Google Scholar] [CrossRef]
- Street, S.E.; Kramer, N.J.; Walsh, P.L.; Taylor-Blake, B.; Yadav, M.C.; King, I.F.; Vihko, P.; Wightman, R.M.; Millan, J.L.; Zylka, M.J. Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord. J. Neurosci. 2013, 33, 11314–11322. [Google Scholar] [CrossRef]
- Kantor, O.; Varga, A.; Kovacs-Oller, T.; Enzsoly, A.; Balogh, L.; Baksa, G.; Szepessy, Z.; Fonta, C.; Roe, A.W.; Nitschke, R.; et al. TNAP activity is localized at critical sites of retinal neurotransmission across various vertebrate species. Cell Tissue Res. 2014, 358, 85–98. [Google Scholar] [CrossRef]
- Laue, K.; Pogoda, H.M.; Daniel, P.B.; van Haeringen, A.; Alanay, Y.; von Ameln, S.; Rachwalski, M.; Morgan, T.; Gray, M.J.; Breuning, M.H.; et al. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am. J. Hum. Genet. 2011, 89, 595–606. [Google Scholar] [CrossRef]
- Zhong, M.; Kawaguchi, R.; Costabile, B.; Tang, Y.; Hu, J.; Cheng, G.; Kassai, M.; Ribalet, B.; Mancia, F.; Bok, D.; et al. Regulatory mechanism for the transmembrane receptor that mediates bidirectional vitamin A transport. Proc. Natl. Acad. Sci. USA 2020, 117, 9857–9864. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, Y.; Kweon, J.; Kim, H.S.; Bae, S.; Kim, J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Brocal, I.; White, R.J.; Dooley, C.M.; Carruthers, S.N.; Clark, R.; Hall, A.; Busch-Nentwich, E.M.; Stemple, D.L.; Kettleborough, R.N. Efficient identification of CRISPR/Cas9-induced insertions/deletions by direct germline screening in zebrafish. BMC Genom. 2016, 17, 259. [Google Scholar] [CrossRef]
- Gagnon, J.A.; Valen, E.; Thyme, S.B.; Huang, P.; Akhmetova, L.; Pauli, A.; Montague, T.G.; Zimmerman, S.; Richter, C.; Schier, A.F. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 2014, 9, e98186. [Google Scholar] [CrossRef]
- Tessadori, F.; Roessler, H.I.; Savelberg, S.M.C.; Chocron, S.; Kamel, S.M.; Duran, K.J.; van Haelst, M.M.; van Haaften, G.; Bakkers, J. Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Dis. Model. Mech. 2018, 11, dmm035469. [Google Scholar] [CrossRef]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef]
- Graser, S.; Mentrup, B.; Schneider, D.; Klein-Hitpass, L.; Jakob, F.; Hofmann, C. Overexpression of tissue-nonspecific alkaline phosphatase increases the expression of neurogenic differentiation markers in the human SH-SY5Y neuroblastoma cell line. Bone 2015, 79, 150–161. [Google Scholar] [CrossRef]
- van der Ham, M.; Albersen, M.; de Koning, T.J.; Visser, G.; Middendorp, A.; Bosma, M.; Verhoeven-Duif, N.M.; de Sain-van der Velden, M.G. Quantification of vitamin B6 vitamers in human cerebrospinal fluid by ultra performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2012, 712, 108–114. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Prinsen, H.; Schiebergen-Bronkhorst, B.G.M.; Roeleveld, M.W.; Jans, J.J.M.; de Sain-van der Velden, M.G.M.; Visser, G.; van Hasselt, P.M.; Verhoeven-Duif, N.M. Rapid quantification of underivatized amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass-spectrometry. J. Inherit. Metab. Dis. 2016, 39, 651–660. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciapaite, J.; Albersen, M.; Savelberg, S.M.C.; Bosma, M.; Meijer, N.W.F.; Tessadori, F.; Bakkers, J.P.W.; van Haaften, G.; Jans, J.J.; Verhoeven-Duif, N.M. Broad Vitamin B6-Related Metabolic Disturbances in a Zebrafish Model of Hypophosphatasia (TNSALP-Deficiency). Int. J. Mol. Sci. 2025, 26, 3270. https://doi.org/10.3390/ijms26073270
Ciapaite J, Albersen M, Savelberg SMC, Bosma M, Meijer NWF, Tessadori F, Bakkers JPW, van Haaften G, Jans JJ, Verhoeven-Duif NM. Broad Vitamin B6-Related Metabolic Disturbances in a Zebrafish Model of Hypophosphatasia (TNSALP-Deficiency). International Journal of Molecular Sciences. 2025; 26(7):3270. https://doi.org/10.3390/ijms26073270
Chicago/Turabian StyleCiapaite, Jolita, Monique Albersen, Sanne M. C. Savelberg, Marjolein Bosma, Nils W. F. Meijer, Federico Tessadori, Jeroen P. W. Bakkers, Gijs van Haaften, Judith J. Jans, and Nanda M. Verhoeven-Duif. 2025. "Broad Vitamin B6-Related Metabolic Disturbances in a Zebrafish Model of Hypophosphatasia (TNSALP-Deficiency)" International Journal of Molecular Sciences 26, no. 7: 3270. https://doi.org/10.3390/ijms26073270
APA StyleCiapaite, J., Albersen, M., Savelberg, S. M. C., Bosma, M., Meijer, N. W. F., Tessadori, F., Bakkers, J. P. W., van Haaften, G., Jans, J. J., & Verhoeven-Duif, N. M. (2025). Broad Vitamin B6-Related Metabolic Disturbances in a Zebrafish Model of Hypophosphatasia (TNSALP-Deficiency). International Journal of Molecular Sciences, 26(7), 3270. https://doi.org/10.3390/ijms26073270








_Verhoeven-Duif.png)
