Antibacterial Properties of Polymeric Membranes Containing Doxycycline for Potential Applications in Foot Ulcer Treatment
Abstract
:1. Introduction
2. Results and Discussion
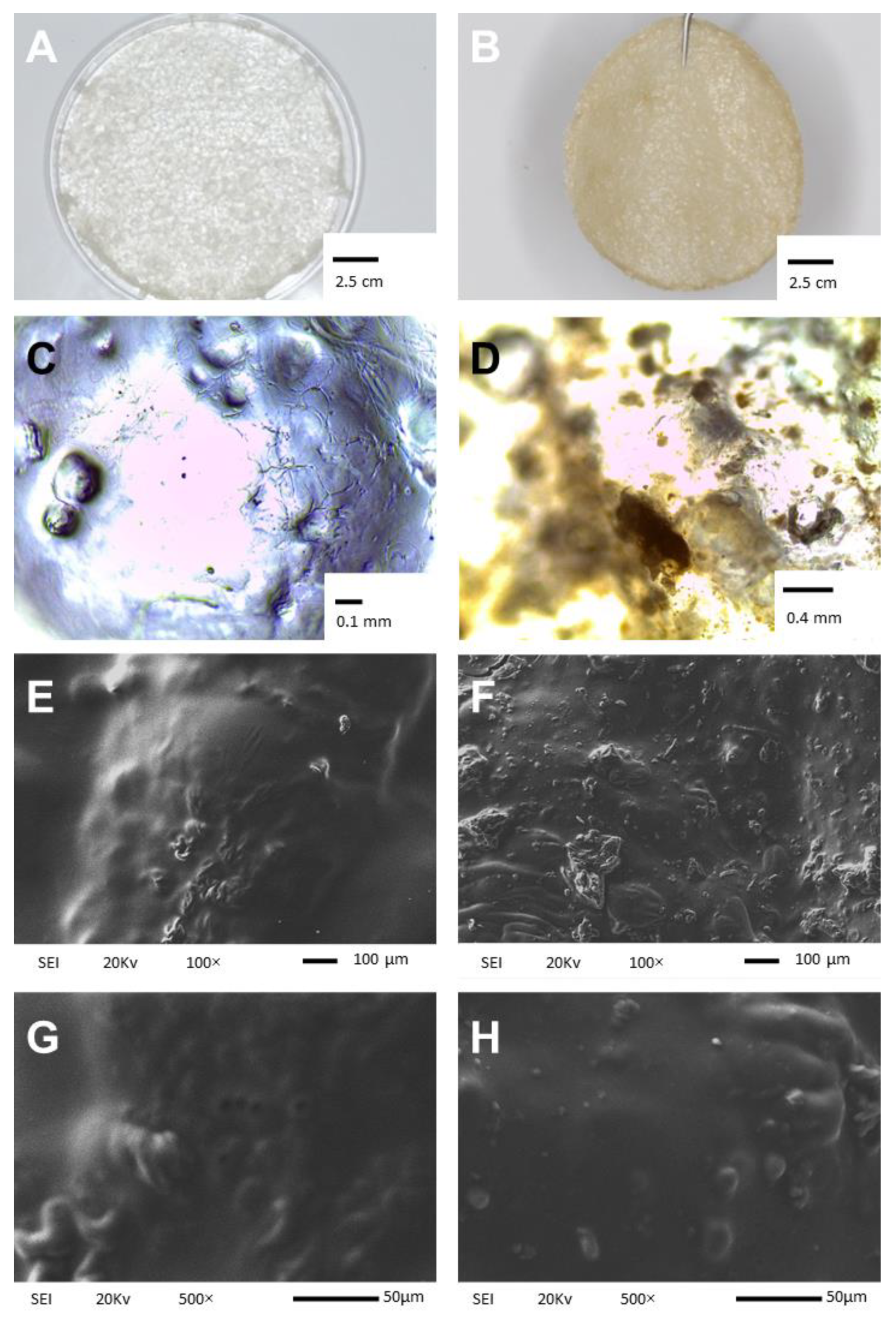
2.1. Physical Appearance and Scanning Electron Microscopy Analysis
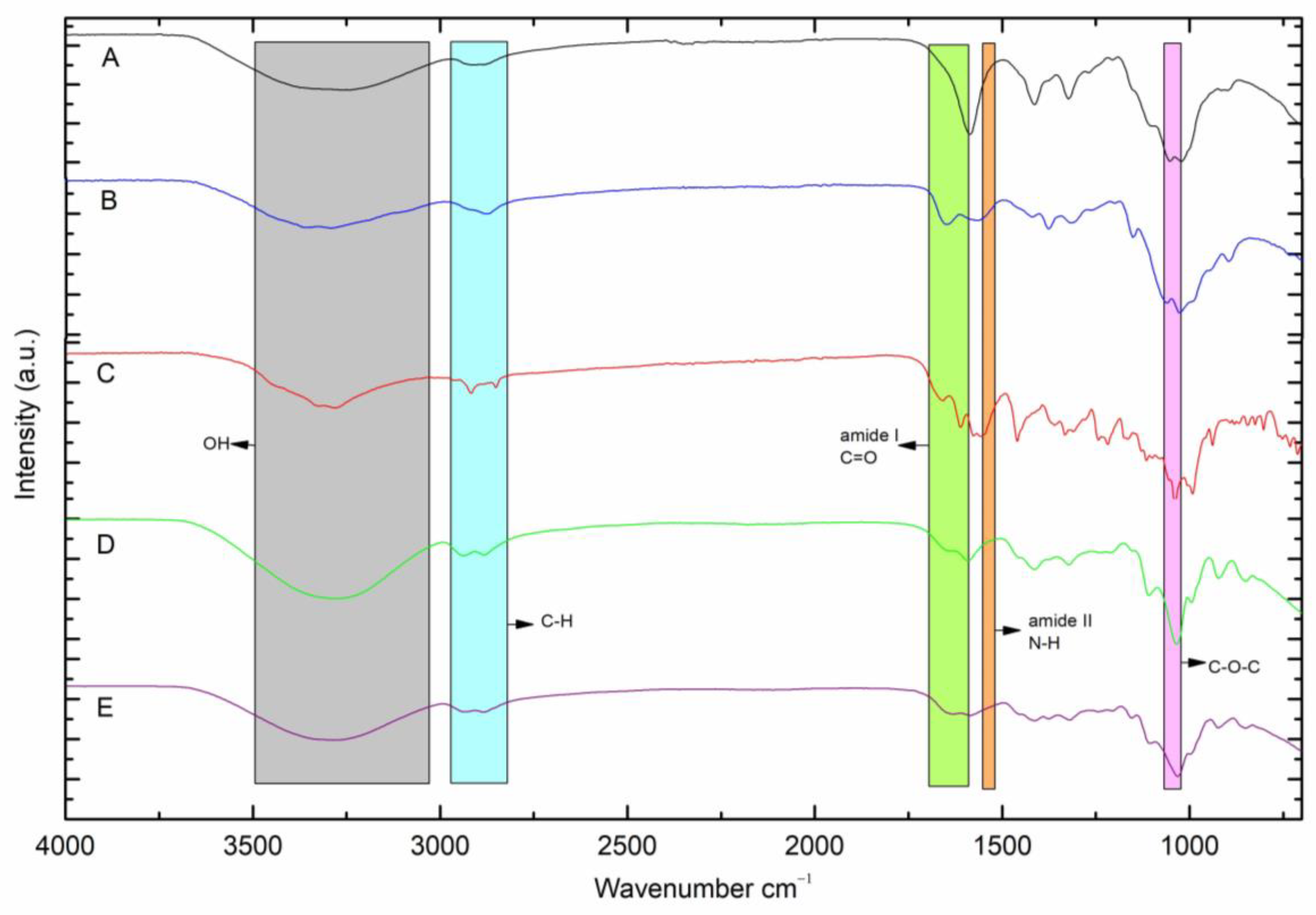
2.2. FTIR−ATR Analysis
2.3. Tensile Properties
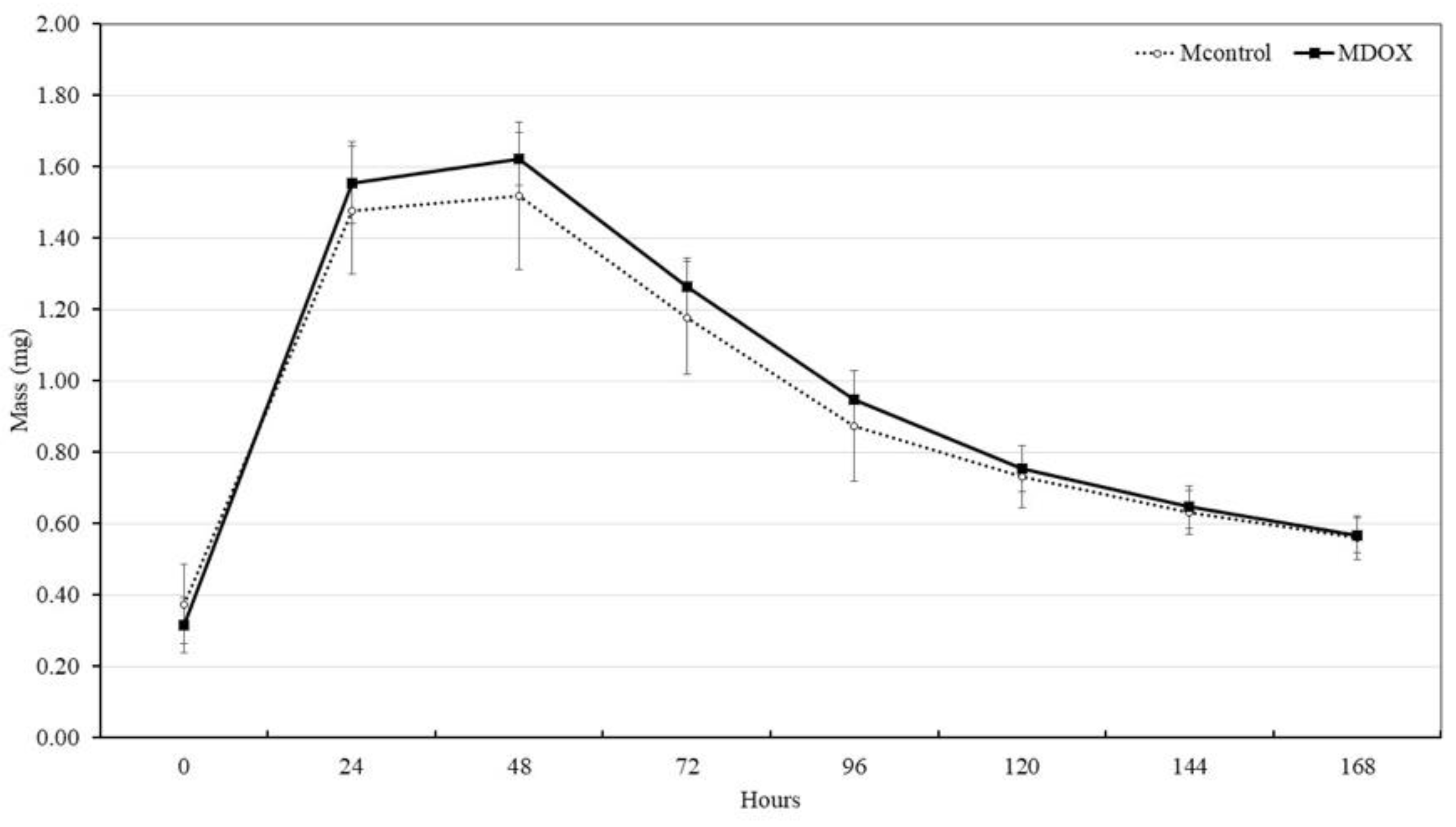
2.4. Mass, Thickness and Roughness of the Membrane
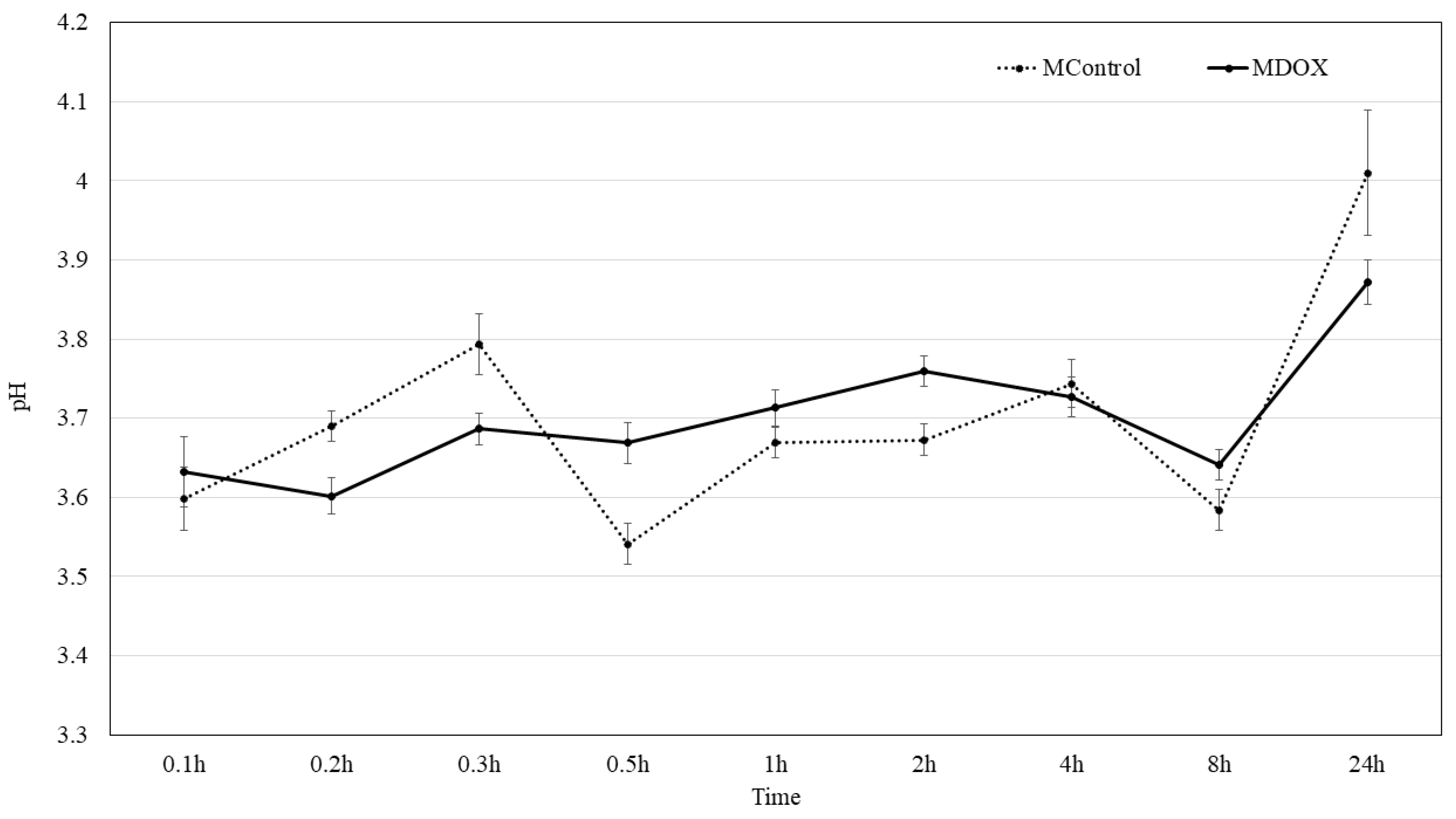
2.5. Surface pH Kinetic
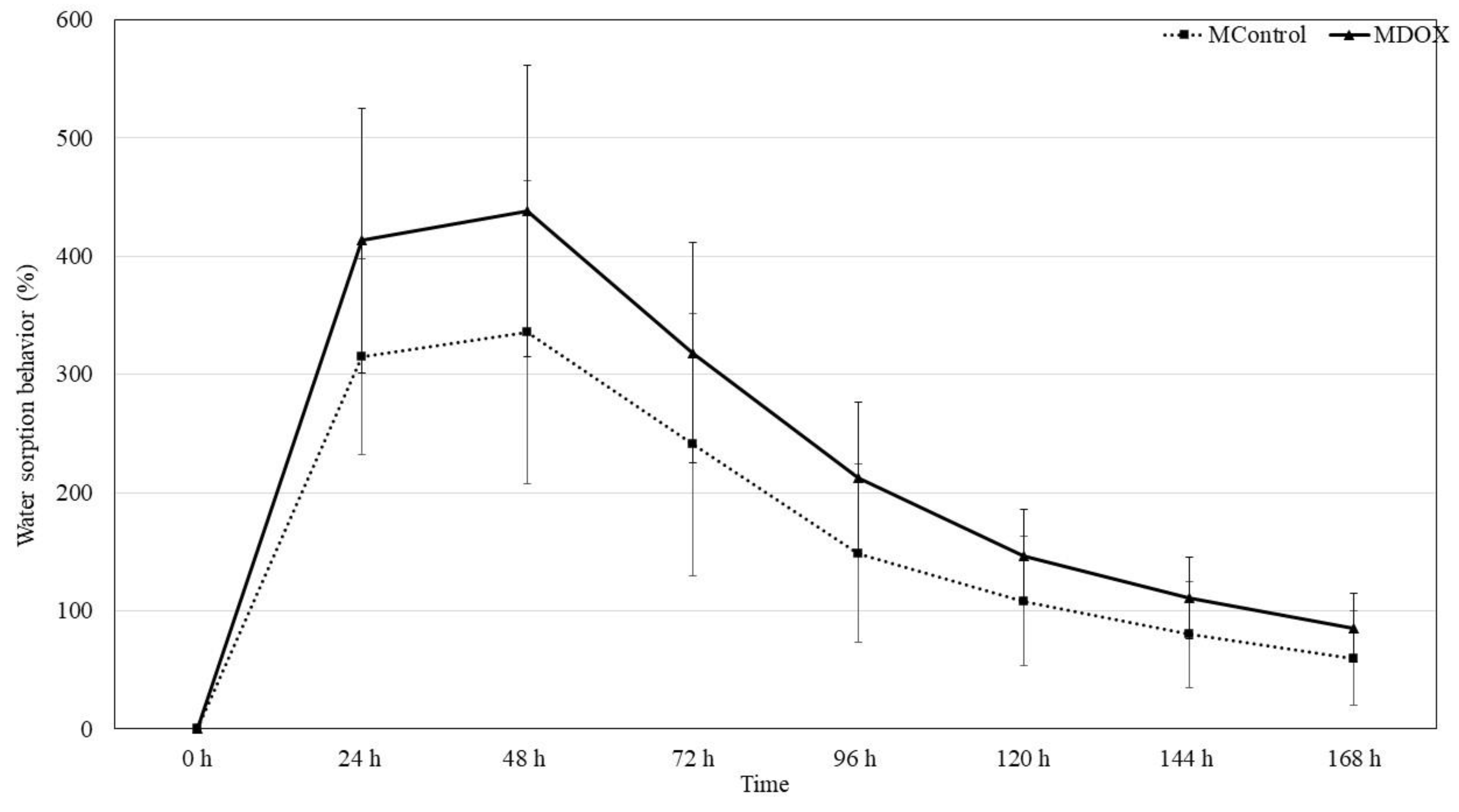
2.6. Moisture Sorption Water Sorption Behavior and Swelling Capacity
2.7. Disintegration or Biodegradability
2.8. Blood Coagulation
2.9. Hemolytic Properties
2.10. Antibacterial Activity of CMC/CHS/G/DOX Membranes
3. Material and Methods
3.1. Formulation and Synthesis of Membranes
Preparation of CMC/CHS Membrane
3.2. In Vitro Characterization of the Membranes
3.2.1. Visual Inspection and Evaluation of Content Uniformity in the Membranes
3.2.2. Scanning Electron Microscopy Analysis
3.2.3. Fourier Transform Infrared (FTIR-ATR) Spectra Analysis
3.2.4. Tensile Strength and Elongation
3.2.5. Mass and Thickness of Membranes
3.2.6. Roughness
3.2.7. Surface pH
3.2.8. Moisture Sorption
3.2.9. Water Sorption Behavior
3.2.10. Swelling Capacity
3.2.11. Evaluation of Disintegration or Biodegradability
3.2.12. Blood Coagulation Time: Prothrombin Time and Activated Partial Thromboplastin Time
3.2.13. Hemolysis Test
3.3. Assessing the Antibacterial Activity of CMC/CHS/G/DOX Membranes
Bacterial Growth
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkus, G.; Sert, M. Diabetic Foot Ulcers: A Devastating Complication of Diabetes Mellitus Continues Non-Stop in Spite of New Medical Treatment Modalities. World J. Diabetes 2022, 13, 1106–1121. [Google Scholar] [CrossRef]
- Boulton, A.J.M. The Pathway to Foot Ulceration in Diabetes. Med. Clin. N. Am. 2013, 97, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Leton, N.; Nayak, N.; Jha, A.; Anand, N.; Thompson, K.; Boothe, D.; Cromer, A.; Garcia, Y.; Al-Islam, A.; et al. A Systematic Review of Diabetic Foot Infections: Pathogenesis, Diagnosis, and Management Strategies. Front. Clin. Diabetes Healthc. 2024, 5, 1393309. [Google Scholar]
- Saliy, O.; Popova, M.; Tarasenko, H.; Getalo, O. Development Strategy of Novel Drug Formulations for the Delivery of Doxycycline in the Treatment of Wounds of Various Etiologies. Eur. J. Pharm. Sci. 2024, 195, 106636. [Google Scholar] [CrossRef] [PubMed]
- Samari, M.; Kashanian, S.; Zinadini, S.; Derakhshankhah, H. Designing of a New Transdermal Antibiotic Delivery Polymeric Membrane Modified by Functionalized SBA-15 Mesoporous Filler. Sci. Rep. 2024, 14, 10418. [Google Scholar] [CrossRef]
- Aldaghi, N.; Kamalabadi-Farahani, M.; Alizadeh, M.; Salehi, M. Doxycycline-Loaded Carboxymethyl Cellulose/Sodium Alginate/Gelatin Hydrogel: An Approach for Enhancing Pressure Ulcer Healing in a Rat Model. J. Biomed. Mater. Res. Part A 2024, 112, 2289–2300. [Google Scholar] [CrossRef]
- Ambrosch, A.; Haefner, S.; Jude, E.; Lobmann, R. Diabetic Foot Infections: Microbiological Aspects, Current and Future Antibiotic Therapy Focusing on Methicillin-Resistant Staphylococcus aureus. Int. Wound J. 2011, 8, 567–577. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent Advances in Carboxymethyl Chitosan-Based Materials for Biomedical Applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Pandian, M.; Reshma, G.; Arthi, C.; Másson, M.; Rangasamy, J. Biodegradable Polymeric Scaffolds and Hydrogels in the Treatment of Chronic and Infectious Wound Healing. Eur. Polym. J. 2023, 198, 112390. [Google Scholar] [CrossRef]
- Hachity-Ortega, J.A.; Jerezano-Domínguez, A.V.; Pazos-Rojas, L.A.; Flores-Ledesma, A.; Pazos-Guarneros, D.d.C.; Parra-Solar, K.A.; Reyes-Cervantes, E.; Juárez-Díaz, I.; Medina, M.E.; González-Martínez, M.; et al. Effect of Glycerol on Properties of Chitosan/Chlorhexidine Membranes and Antibacterial Activity against Streptococcus mutans. Front. Microbiol. 2024, 15, 1430954. [Google Scholar] [CrossRef]
- Ulu, A.; Aygün, T.; Birhanlı, E.; Ateş, B. Preparation, Characterization, and Evaluation of Multi–Biofunctional Properties of a Novel Chitosan–Carboxymethylcellulose–Pluronic P123 Hydrogel Membranes Loaded with Tetracycline Hydrochloride. Int. J. Biol. Macromol. 2022, 222, 2670–2682. [Google Scholar] [CrossRef] [PubMed]
- Dinte, E.; Muntean, D.M.; Andrei, V.; Boșca, B.A.; Dudescu, C.M.; Barbu-Tudoran, L.; Borodi, G.; Andrei, S.; Gal, A.F.; Rus, V.; et al. In Vitro and In Vivo Characterisation of a Mucoadhesive Buccal Film Loaded with Doxycycline Hyclate for Topical Application in Periodontitis. Pharmaceutics 2023, 15, 580. [Google Scholar] [CrossRef]
- Tang, R.; Wang, Z.; Muhammad, Y.; Shi, H.; Liu, K.; Ji, J.; Zhu, Y.; Tong, Z.; Zhang, H. Fabrication of Carboxymethyl Cellulose and Chitosan Modified Magnetic Alkaline Ca-Bentonite for the Adsorption of Hazardous Doxycycline. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125730. [Google Scholar] [CrossRef]
- Tort, S.; Acartürk, F.; Beşikci, A. Evaluation of Three-Layered Doxycycline-Collagen Loaded Nanofiber Wound Dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Ehtisham-ul-Haque, S.; Ahmad, S.; Arif, K.; Hussain, E.A.; Iqbal, M.; Alshawwa, S.Z.; Abbas, M.; Amjed, N.; Nazir, A. Enhanced Antibacterial Activity of Chitosan, Guar Gum and Polyvinyl Alcohol Blend Matrix Loaded with Amoxicillin and Doxycycline Hyclate Drugs. Arab. J. Chem. 2021, 14, 103156. [Google Scholar] [CrossRef]
- Hassan, A.; Bilal Khan Niazi, M.; Hussain, A.; Farrukh, S.; Ahmad, T. Development of Anti-Bacterial PVA/Starch Based Hydrogel Membrane for Wound Dressing. J. Polym. Environ. 2018, 26, 235–243. [Google Scholar] [CrossRef]
- Yıldırım, Y.; İnce, İ.; Gümüştaş, B.; Vardar, Ö.; Yakar, N.; Munjaković, H.; Özdemir, G.; Emingil, G. Development of Doxycycline and Atorvastatin-Loaded Chitosan Nanoparticles for Local Delivery in Periodontal Disease. J. Drug Deliv. Sci. Technol. 2023, 82, 104322. [Google Scholar] [CrossRef]
- Sander, E.A.; Lynch, K.A.; Boyce, S.T. Development of the Mechanical Properties of Engineered Skin Substitutes After Grafting to Full-Thickness Wounds. J. Biomech. Eng. 2014, 136, 0510081. [Google Scholar] [CrossRef]
- Lin, X.; Filppula, A.M.; Zhao, Y.; Shang, L.; Zhang, H. Mechanically Regulated Microcarriers with Stem Cell Loading for Skin Photoaging Therapy. Bioact. Mater. 2025, 46, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, P.; Chandika, P.; Khan, F.; Kim, T.-H.; Kim, S.-C.; Kim, Y.-M.; Jung, W.-K. PVA/Gelatin Nanofibrous Scaffold with High Anti-Bacterial Activity for Skin Wound Healing Applications. Mater. Today Commun. 2024, 40, 109356. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, N.; Liu, L.; Shang, H.; Wei, D.; Ji, Z.; Wang, R.; Ding, Y. Advances in the Study of Polysaccharide-Based Hydrogel Wound Dressings. Int. J. Biol. Macromol. 2025, 307, 142134. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Ding, L.; Qi, Q.; Zhang, S.; Ren, C.; Deng, M.; Sun, Z.; Zhang, R.; Liu, Q.; Duan, S.; Wang, X.; et al. Hydroxypropyl Methylcellulose Reinforced Collagen/PVA Composite Hydrogel Wound Dressing with Self-Adaptive, Hemostasis and Antibacterial Ability for Wound Healing. Int. J. Biol. Macromol. 2025, 304, 140811. [Google Scholar] [CrossRef]
- Feitosa, R.C.; Ishikawa, E.S.A.; Silva, M.F.A.d.; da Silva-Júnior, A.A.; Oliveira-Nascimento, L. Five Decades of Doxycycline: Does Nanotechnology Improve Its Properties? Int. J. Pharm. 2022, 618, 121655. [Google Scholar] [CrossRef]
- Tinku; Prajapati, A.K.; Choudhary, S. Physicochemical Insights into the Micellar Delivery of Doxycycline and Minocycline to the Carrier Protein in Aqueous Environment. J. Mol. Liq. 2023, 379, 121675. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, B. Synthesis of Hydrogels Based on Sterculia Gum-Co-Poly(Vinyl Pyrrolidone)-Co-Poly(Vinyl Sulfonic Acid) for Wound Dressing and Drug-Delivery Applications. RSC Sustain. 2024, 2, 2693–2708. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef]
- Triana-Ricci, R.; Martinez-de-Jesús, F.; Aragón-Carreño, M.P.; Saurral, R.; Tamayo-Acosta, C.A.; García-Puerta, M.; Vicente-Bernal, P.; Silva-Quiñonez, K.; Felipe-Feijo, D.; Reyes, C.; et al. Vista de Recomendaciones de Manejo Del Paciente Con Pie Diabético. Curso de Instrucción. Rev. Colomb. Ortop. Traumatol. 2022, 35, 303–329. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, C.; Huang, Y.; Quan, G.; Huang, L.; Wu, L.; Pan, X.; Li, G.; Wu, C. Improvement of the Stability of Doxycycline Hydrochloride Pellet-Containing Tablets through a Novel Granulation Technique and Proper Excipients. Powder Technol. 2015, 270, 221–229. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications; InTech: London, UK, 2011; ISBN 978-953-307-268-5. [Google Scholar]
- Wu, X.; Xia, D.; Shi, T.; Li, B.; Wang, D.; Liang, C.; Dong, M. Thermo-Responsive Microneedles Patch for Transdermal Drug Delivery via Squeezing in Diabetic Foot Ulcers. J. Mater. Sci. Technol. 2025, 205, 299–314. [Google Scholar] [CrossRef]
- Pan, S.; Li, Y.; Tong, X.; Chen, L.; Wang, L.; Li, T.; Zhang, Q. Strongly-Adhesive Easily-Detachable Carboxymethyl Cellulose Aerogel for Noncompressible Hemorrhage Control. Carbohydr. Polym. 2023, 301, 120324. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Rezaei kolarijani, N.; Naeiji, M.; Vaez, A.; Maghsoodifar, H.; Sadeghi douki, S.A.H.; Salehi, M. Development of Carboxymethyl Cellulose/Gelatin Hydrogel Loaded with Omega-3 for Skin Regeneration. J. Biomater. Appl. 2024, 39, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Y.; Li, W.; Li, D.; Li, H. Preparation of Fully Bio-Based Multilayers Composed of Heparin-like Carboxymethylcellulose Sodium and Chitosan to Functionalize Poly (L-Lactic Acid) Film for Cardiovascular Implant Applications. Int. J. Biol. Macromol. 2023, 231, 123285. [Google Scholar] [CrossRef]
- Cerda-Cristerna, B.I.; Flores, H.; Pozos-Guillén, A.; Pérez, E.; Sevrin, C.; Grandfils, C. Hemocompatibility Assessment of Poly(2-Dimethylamino Ethylmethacrylate) (PDMAEMA)-Based Polymers. J. Control. Release 2011, 153, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Boonkong, W.; Petsom, A.; Thongchul, N. Rapidly Stopping Hemorrhage by Enhancing Blood Clotting at an Opened Wound Using Chitosan/Polylactic Acid/Polycaprolactone Wound Dressing Device. J. Mater. Sci. Mater. Med. 2013, 24, 1581–1593. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers 2023, 15, 396. [Google Scholar] [CrossRef]
- Zhang, F.; Ramachandran, G.; Mothana, R.A.; Noman, O.M.; Alobaid, W.A.; Rajivgandhi, G.; Manoharan, N. Anti-Bacterial Activity of Chitosan Loaded Plant Essential Oil against Multi Drug Resistant K. pneumoniae. Saudi J. Biol. Sci. 2020, 27, 3449–3455. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Heidari, F.; Raoufi, Z.; Abdollahi, S.; Asl, H.Z. Antibiotic Delivery in the Presence of Green AgNPs Using Multifunctional Bilayer Carrageenan Nanofiber/Sodium Alginate Nanohydrogel for Rapid Control of Wound Infections. Int. J. Biol. Macromol. 2024, 277, 134109. [Google Scholar] [CrossRef]
- Raval, J.P.; Chejara, D.R.; Ranch, K.; Joshi, P. Development of Injectable in Situ Gelling Systems of Doxycycline Hyclate for Controlled Drug Delivery System. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Sawston, UK, 2018; pp. 149–162. [Google Scholar] [CrossRef]
- Fluit, A.C.; Van Gorkum, S.; Vlooswijk, J. Minimal Inhibitory Concentration of Omadacycline and Doxycycline against Bacterial Isolates with Known Tetracycline Resistance Determinants. Diagn. Microbiol. Infect. Dis. 2018, 94, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Kusmono; Abdurrahim, I. Water Sorption, Antimicrobial Activity, and Thermal and Mechanical Properties of Chitosan/Clay/Glycerol Nanocomposite Films. Heliyon 2019, 5, e02342. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Wiącek, A.E. Physicochemical Characteristics of Chitosan-TiO2 Biomaterial. 2. Wettability and Biocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127546. [Google Scholar] [CrossRef]
- Korelc, K.; Larsen, B.S.; Gašperlin, M.; Tho, I. Water-Soluble Chitosan Eases Development of Mucoadhesive Buccal Films and Wafers for Children. Int. J. Pharm. 2023, 631, 122544. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; Chavarría-Bolaños, D.; Pozos-Guillén, A.d.J.; Escobar-Barrios, V.A.; Cerda-Cristerna, B.I. Synthesis of a PVA Drug Delivery System for Controlled Release of a Tramadol-Dexketoprofen Combination. J. Mater. Sci. Mater. Med. 2021, 32, 56. [Google Scholar] [CrossRef]
- ASTM Designation F 756-00; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Corral-Lugo, A.; Morales-García, Y.E.; Pazos-Rojas, L.A.; Ramirez-Valverde, A.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. Quantification of Cultivable Bacteria by the “Massive Stamping Drop Plate” Method. Rev. Colomb. Biotecnol. 2012, 14, 147–156. [Google Scholar]
- The Jamovi Project (2025). Jamovi (Version 2.6) R Core Team. Project for Statistical. (Computer Software). Available online: https://www.jamovi.org/ (accessed on 2 February 2025).
| MControl CMC/CHS/G | MDOX CMC/CHS/G/DOX | p Value | ||
|---|---|---|---|---|
| Tensile strength (MPa ± SD) | 0.09 ± 0.03 | 0.07 ± 0.02 | 0.096 | |
| Elongation at break (% ± SD) | 75.29 ± 2.73 | 73.52 ± 5.12 | 0.348 | |
| Thickness (µm ± SD) | 604.00 ± 182 | 833.00 ± 134 | <0.005 | |
| Mass (mg ± SD) | 6.05 ± 0.31 | 9.25 ± 0.69 | <0.001 | |
| Roughness | Ra (µm ± SD) | 0.49 ± 0.25 | 0.359 ± 0.237 | 0.241 |
| Rz (µm ± SD) | 3.58 ± 1.658 | 1.543 ± 1.014 | <0.004 | |
| Surface pH (mean ± SD) | 4.01 ± 0.08 | 3.87 ± 0.03 | <0.002 | |
| Moisture sorption (% ± SD) | 37.52 ± 6.54 | 205.01 ± 66.94 | <0.002 | |
| Water sorption at 48 h (% ± SD) | 335.71 ± 128.51 | 437.81 ± 123.32 | 0.087 | |
| Swelling index (mean ± SD) | 0.52 ± 0.05 | 0.53 ± 0.03 | <0.01 | |
| Blood coagulation time | PT (s ± SD) | 11.50 ± 0.40 | 11.7 ± 0.70 | 0.443 |
| TT (s ± SD) | 25.60 ± 3.60 | 30.1 ± 1.90 | <0.002 | |
| Hemolysis (% ± SD) | 16.80 ± 0.03 | 14.72 ± 0.05 | <0.001 | |
| Membrane | S. mutans | S. aureus | E. coli |
|---|---|---|---|
| Control b | 7.75 ± 0.16 | 8.35 ± 0.44 | 8.93 ± 0.68 |
| MControl | 7.25 mm ± 0.33 | 8.24 ± 0.23 | 8.36 ± 0.30 |
| MDOX | 0.00 | 0.00 | 6.21 ± 0.21 |
| Sample | MControl (%) * | MDOX (%) * |
|---|---|---|
| CMC (p/p) | 7.56 | 7.56 |
| CHS (p/p) | 3.78 | 3.78 |
| G (v/v) | 7 | 7 |
| DOX (p/p) | 0 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Gutiérrez, S.; Ramírez-Enciso, J.Á.; Pazos-Rojas, L.A.; Flores-Ledesma, A.; Reyes-Cervantes, E.; Pazos-Guarneros, D.d.C.; Juárez-Díaz, I.; Gordillo-Guerra, P.G.; Cerda-Cristerna, B.I.; Suárez-Franco, J.L.; et al. Antibacterial Properties of Polymeric Membranes Containing Doxycycline for Potential Applications in Foot Ulcer Treatment. Int. J. Mol. Sci. 2025, 26, 3274. https://doi.org/10.3390/ijms26073274
Pérez-Gutiérrez S, Ramírez-Enciso JÁ, Pazos-Rojas LA, Flores-Ledesma A, Reyes-Cervantes E, Pazos-Guarneros DdC, Juárez-Díaz I, Gordillo-Guerra PG, Cerda-Cristerna BI, Suárez-Franco JL, et al. Antibacterial Properties of Polymeric Membranes Containing Doxycycline for Potential Applications in Foot Ulcer Treatment. International Journal of Molecular Sciences. 2025; 26(7):3274. https://doi.org/10.3390/ijms26073274
Chicago/Turabian StylePérez-Gutiérrez, Stevaly, Jesús Ángel Ramírez-Enciso, Laura Abisai Pazos-Rojas, Abigailt Flores-Ledesma, Eric Reyes-Cervantes, Diana del C. Pazos-Guarneros, Ismael Juárez-Díaz, Paola G. Gordillo-Guerra, Bernardino Isaac Cerda-Cristerna, José Luis Suárez-Franco, and et al. 2025. "Antibacterial Properties of Polymeric Membranes Containing Doxycycline for Potential Applications in Foot Ulcer Treatment" International Journal of Molecular Sciences 26, no. 7: 3274. https://doi.org/10.3390/ijms26073274
APA StylePérez-Gutiérrez, S., Ramírez-Enciso, J. Á., Pazos-Rojas, L. A., Flores-Ledesma, A., Reyes-Cervantes, E., Pazos-Guarneros, D. d. C., Juárez-Díaz, I., Gordillo-Guerra, P. G., Cerda-Cristerna, B. I., Suárez-Franco, J. L., Samano-Valencia, C., Castillo-Silva, B. E., Martínez-Guerrero, A. G., de Celis-Quintana, G. N. R., & Jerezano-Domínguez, A. V. (2025). Antibacterial Properties of Polymeric Membranes Containing Doxycycline for Potential Applications in Foot Ulcer Treatment. International Journal of Molecular Sciences, 26(7), 3274. https://doi.org/10.3390/ijms26073274







