Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice
Abstract
:1. Introduction
2. Results
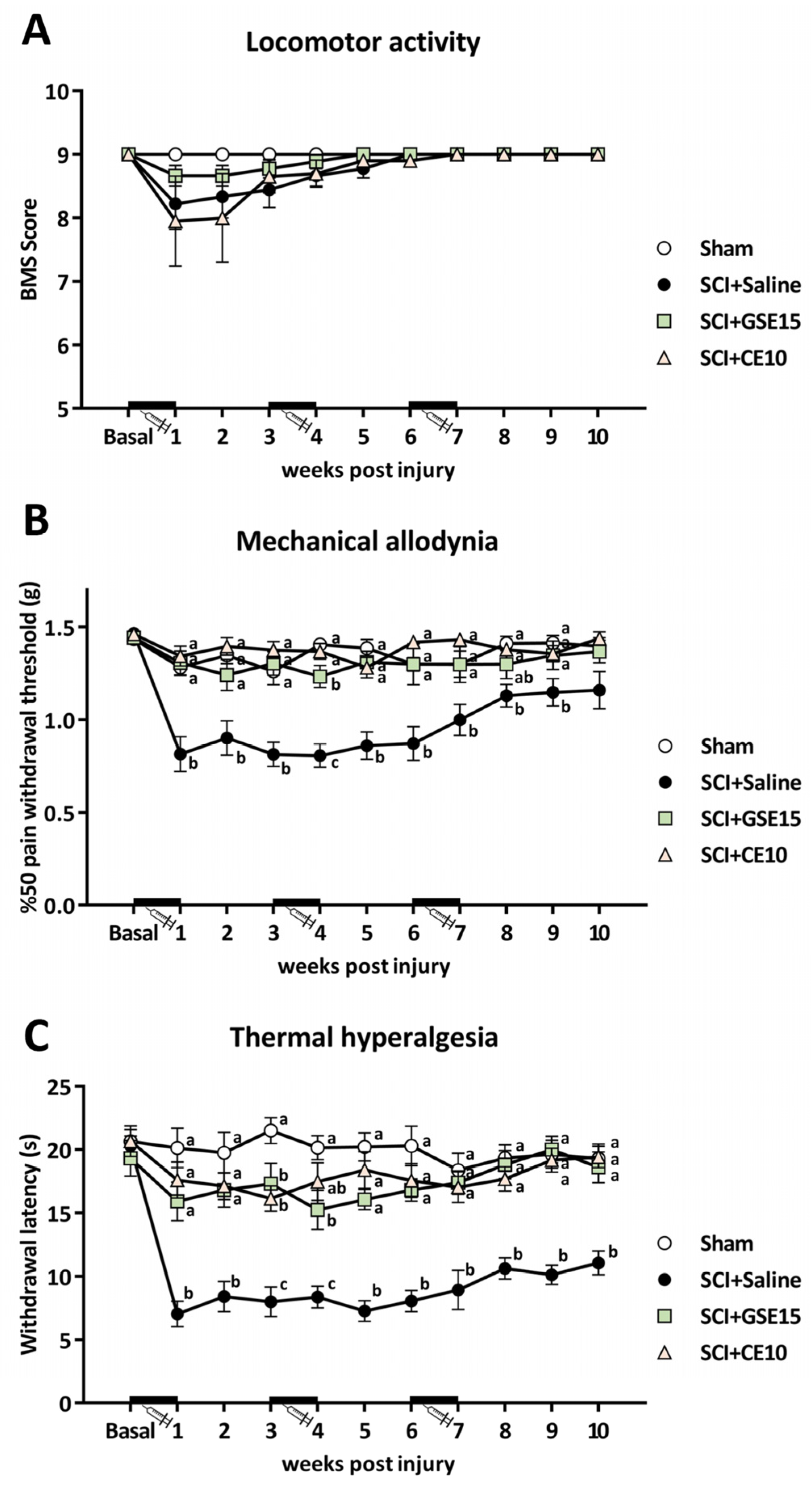
2.1. Repeated Administration of GSE15 or CE10 During the First, Third, and Sixth Weeks Following Mild Spinal Cord Injury Prevents the Development of Both Mechanical Allodynia and Thermal Hyperalgesia
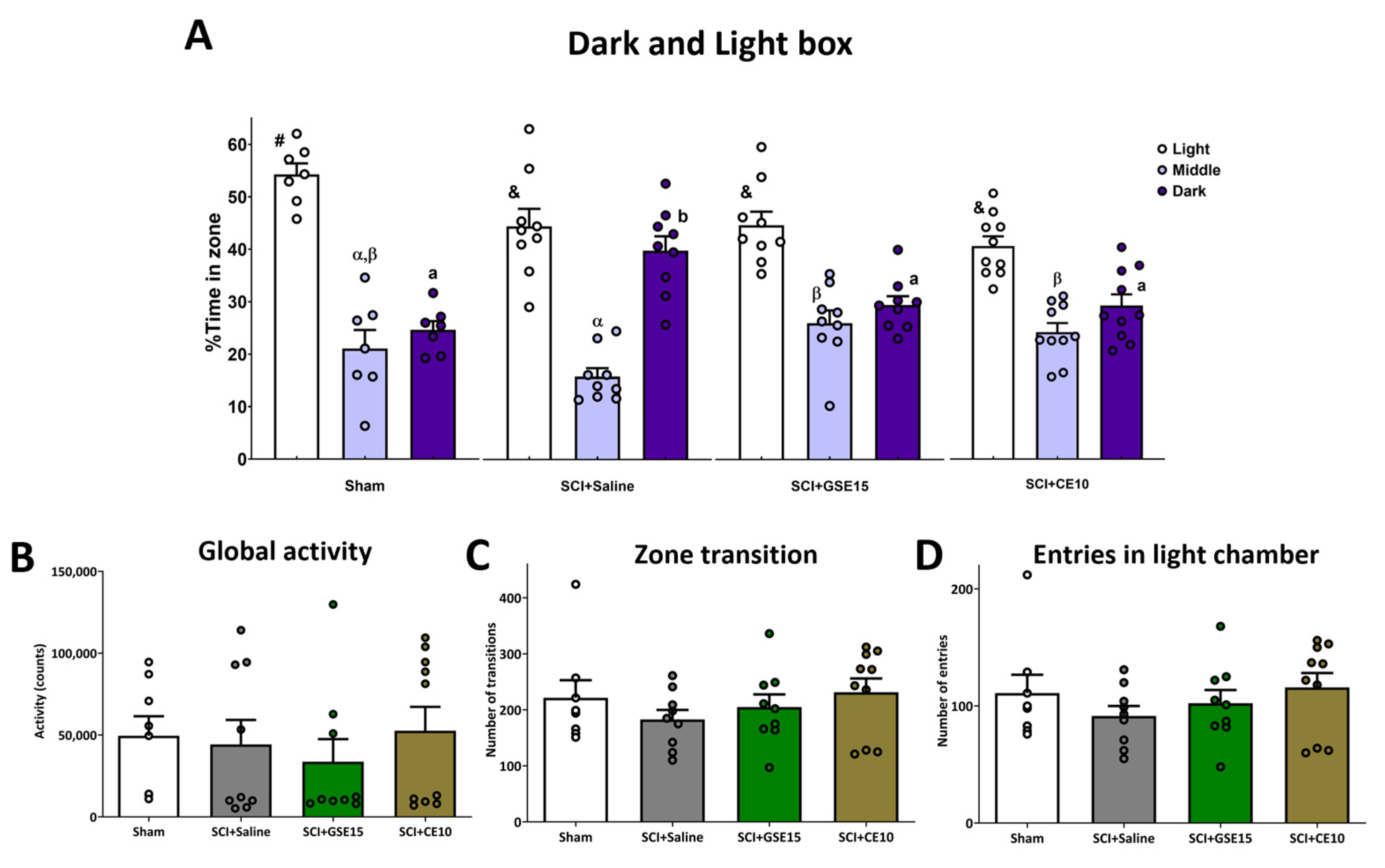
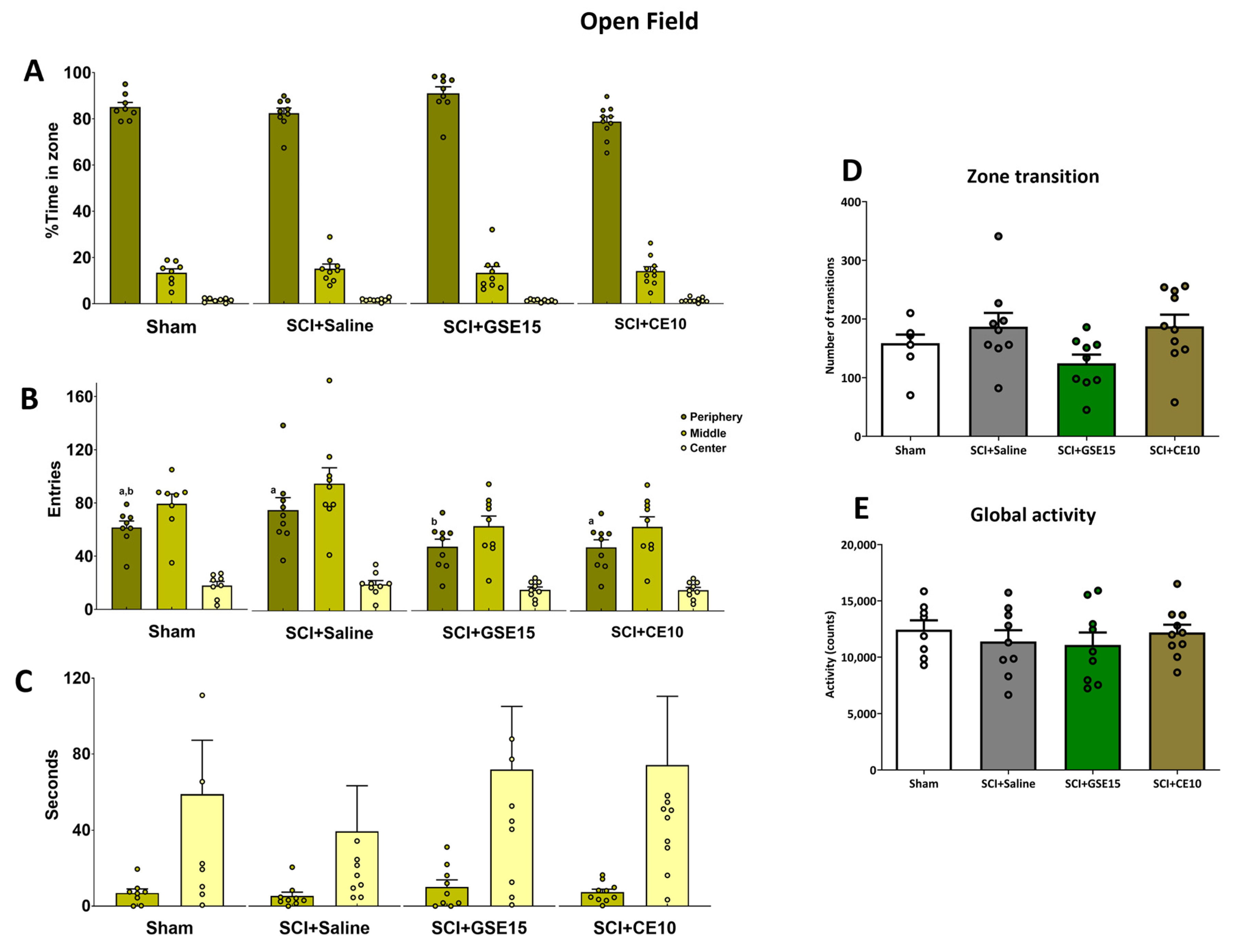
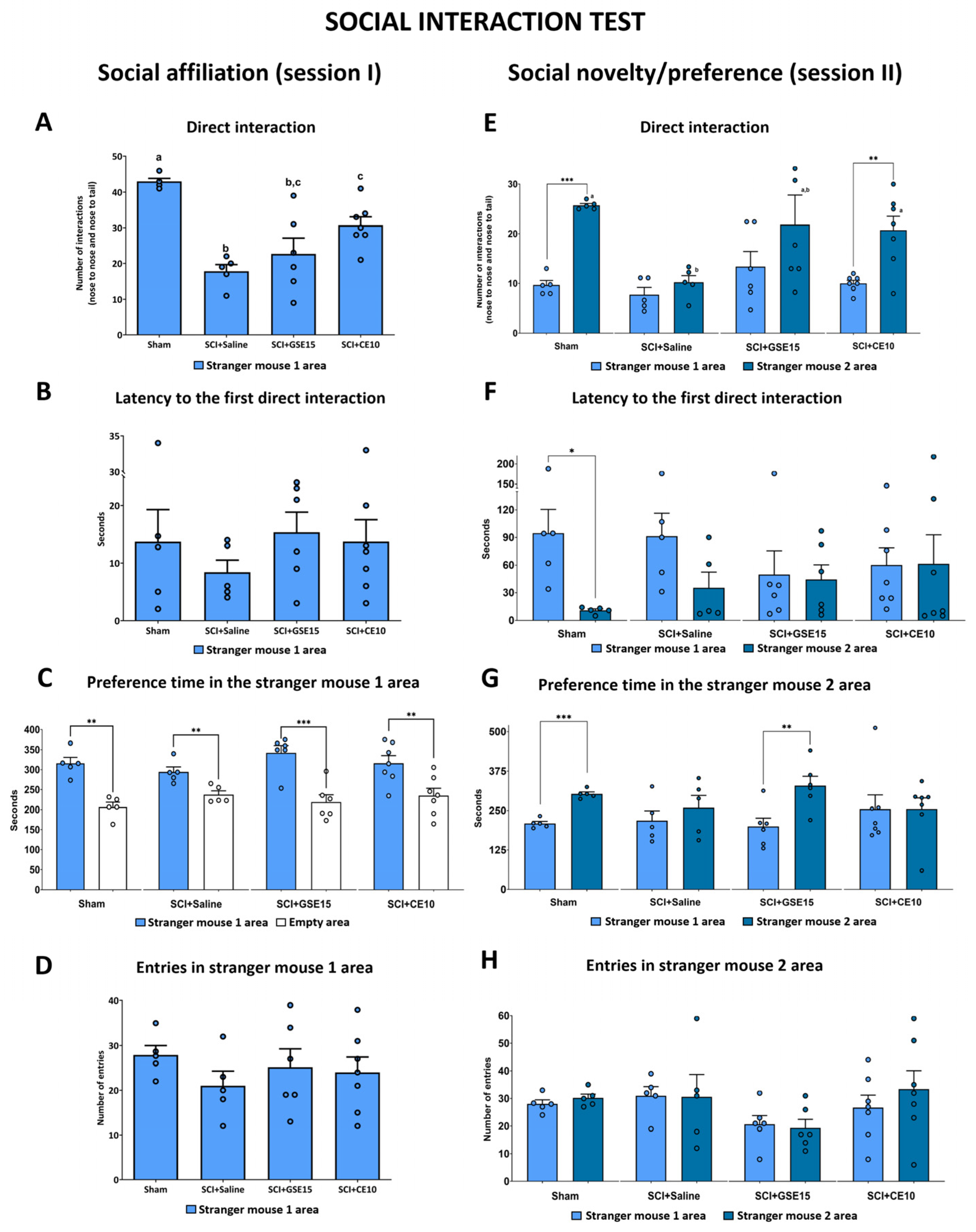
2.2. Repeated GSE15 or CE10 Administration During the First, Third and Sixth Week Post-Injury Modulates Non-Reflexive Pain Behaviors Induced by Mild Spinal Cord Injury at 10 Weeks Post-Injury
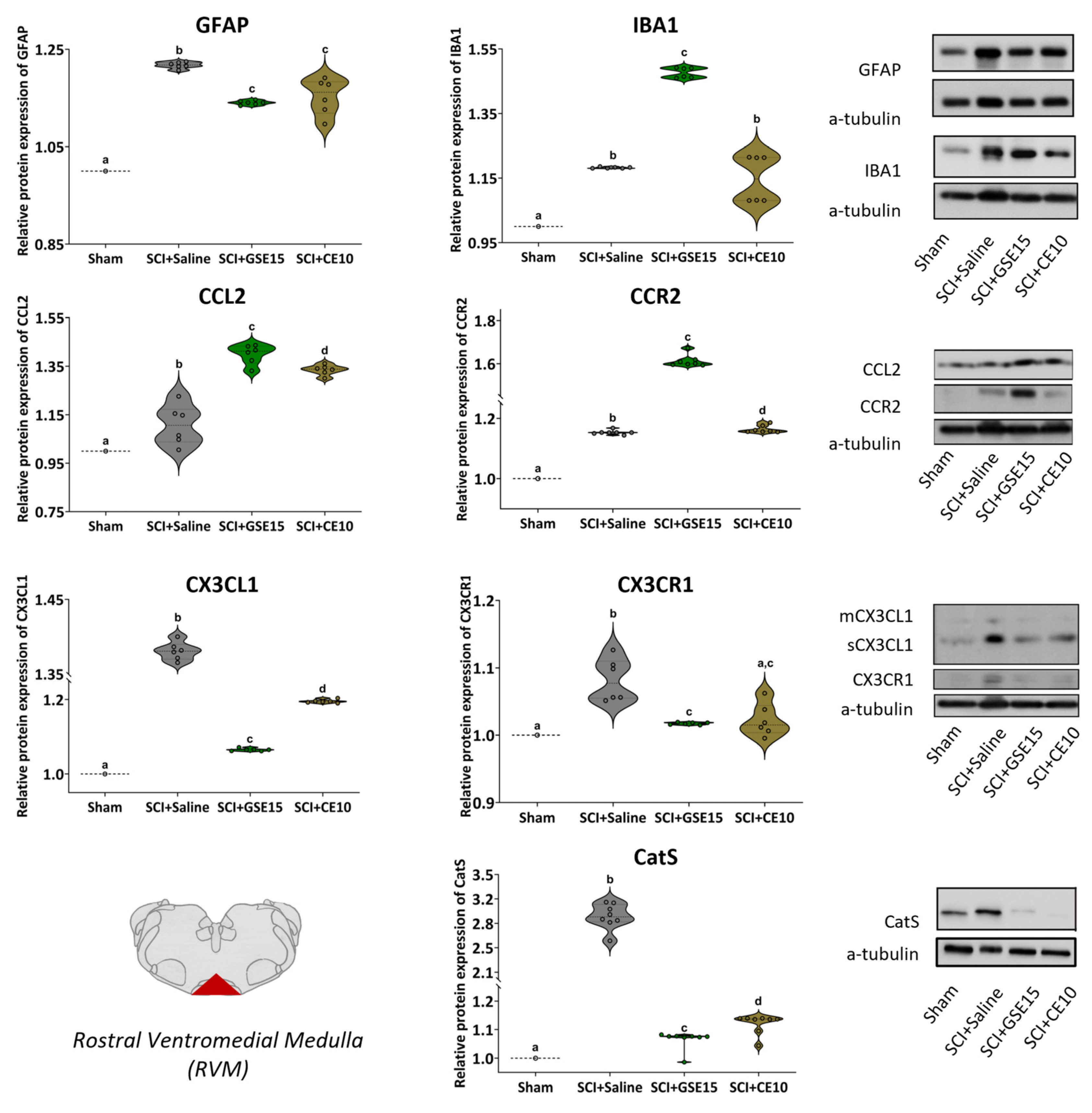
2.3. Repeated GSE15 or CE10 Administration During the First, Third and Sixth Week Post-Injury Reduces Astrogliosis and Modulates CCL2/CCR2 and CX3CL1/CX3CR1 Signaling in PAG and RVM at 10 Weeks Post-SCI
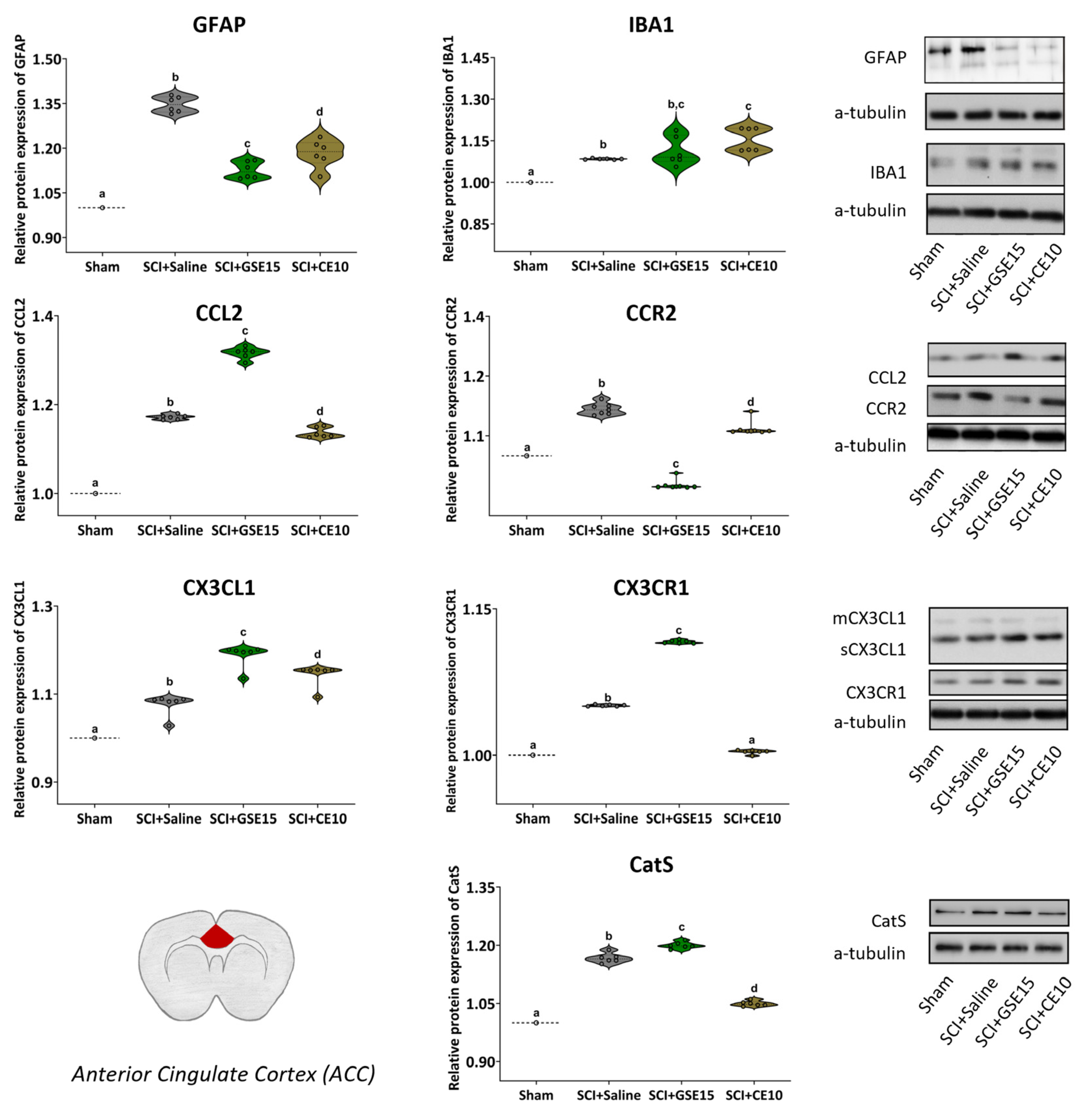
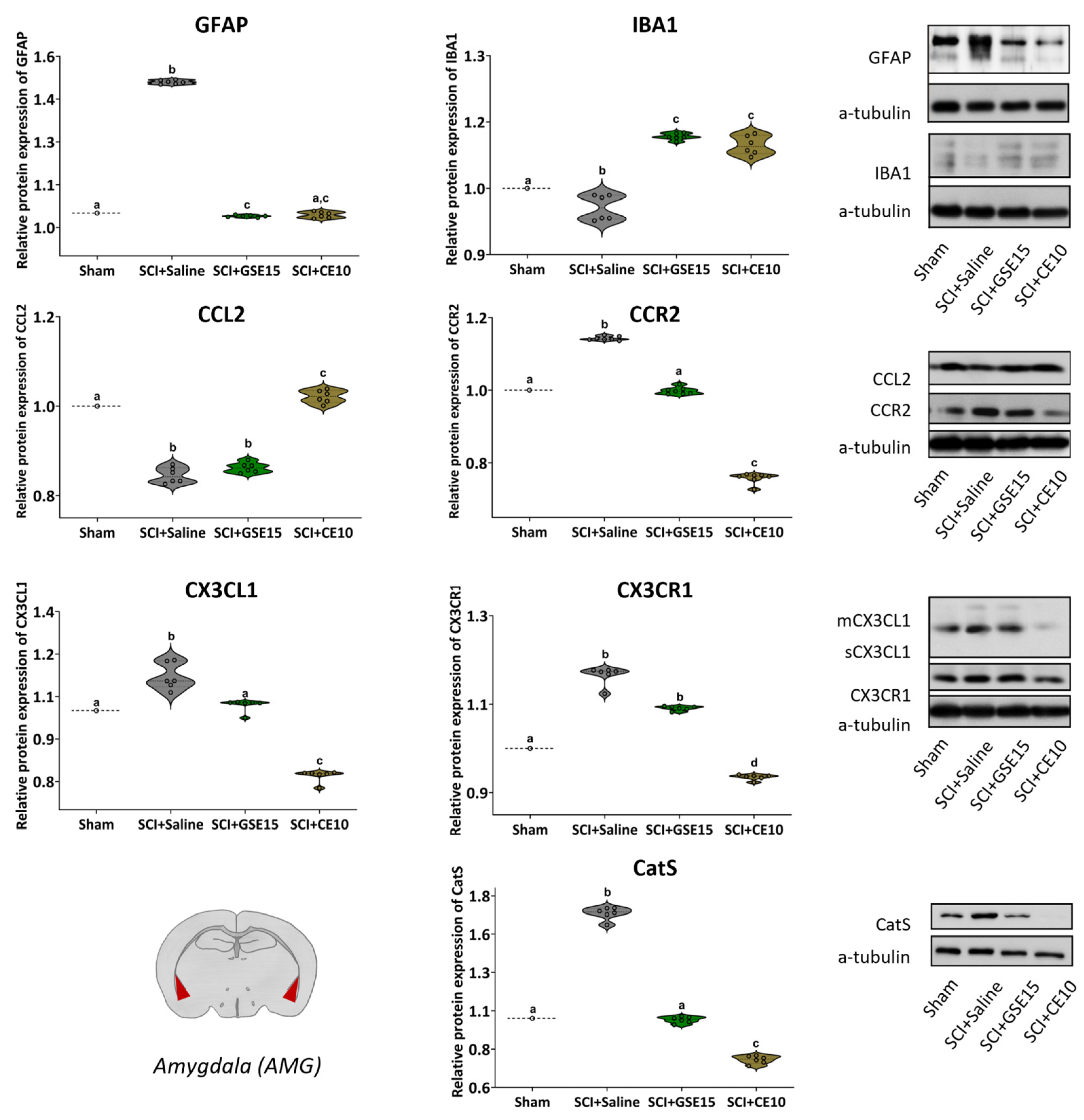
2.4. Repeated GSE15 or CE10 Administration During the First, Third and Sixth Week Post-Injury Reduces Astrogliosis and Modulates CCL2/CCR2 and CX3CL1/CX3CR1 Signaling in the ACC and Amygdala at 10 Weeks Post-SCI
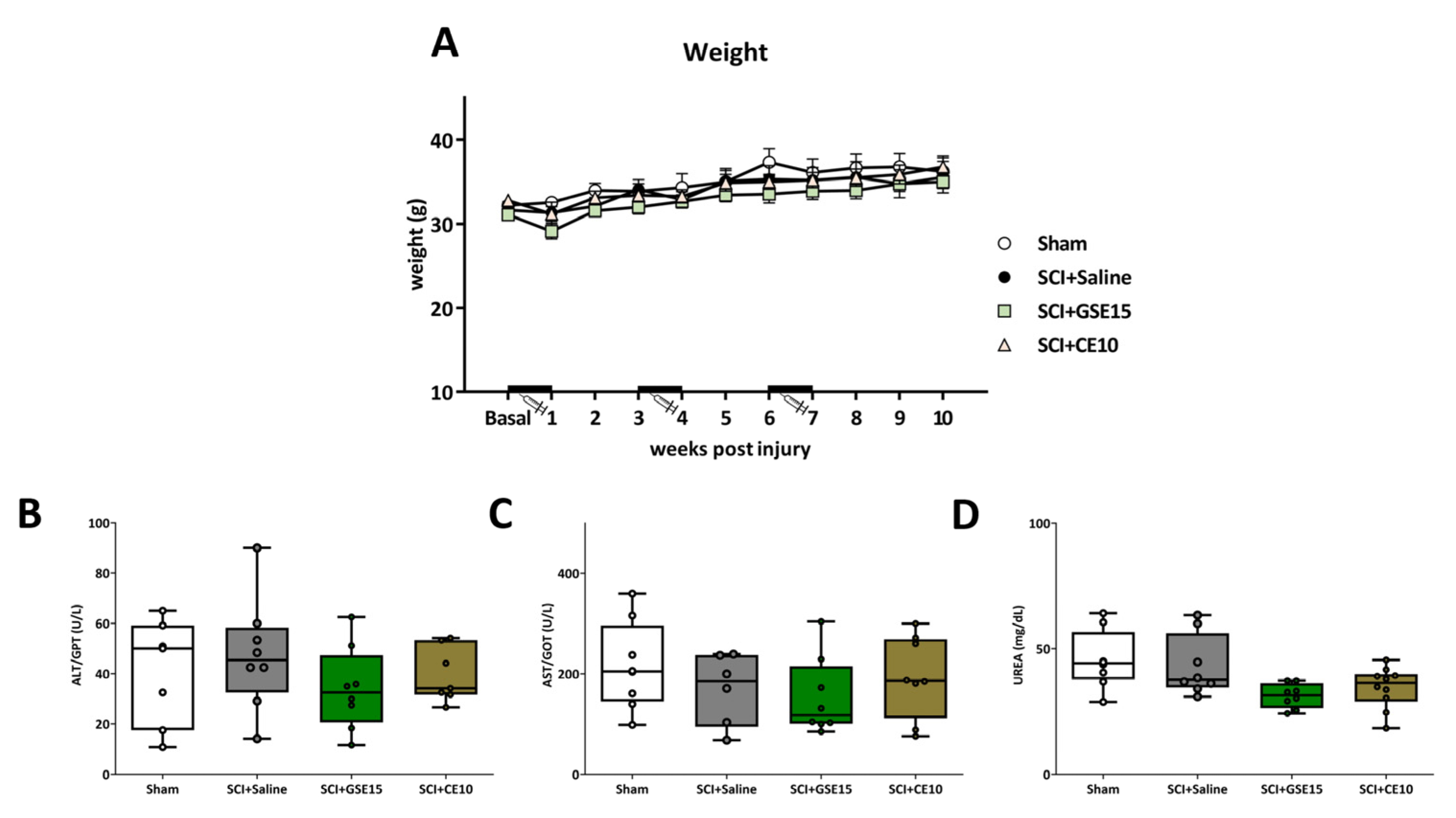
2.5. Repeated GSE15 or CE10 Administration During the First, Third and Sixth Week Post-Injury Does Not Trigger Weight Loss or Hepatotoxic or Nephrotoxic Effects in Spinal Cord-Injured Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Animals
4.3. Surgical Procedure and Pharmacological Treatment with Polyphenolic Extracts
4.4. Treatment with Polyphenolic Extracts
4.5. Locomotor Activity Evaluation
4.6. Reflexive Pain Response Assessment
4.7. Non-Reflexive Pain Responses Assessments
4.8. Biological Sample Collection
4.9. Western Blotting Analysis
4.10. Biochemical Analysis of Hepato- and Nephrotoxicity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| ALT | Alanine aminotransferase |
| AMG | Amygdala |
| AST | Aspartate aminotransferase |
| BMS | Basso mouse scale |
| CatS | Cathepsin S |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCR2 | Chemokine (C-C motif) ligand 2 receptor |
| CE | Coffee extract |
| CNP | Central neuropathic pain |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| CX3CR1 | Chemokine (C-X3-C motif) ligand 1 receptor |
| dlPAG | Dorsolateral periaqueductal gray |
| GFAP | Glial fibrillary acidic protein |
| GSE | Grape stalk extract |
| RVM | Rostroventromedial medulla |
| SCI | Spinal cord injury |
| vlPAG | Ventrolateral periaqueductal gray |
| wpi | Weeks post injury |
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 441408. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic Pain Prevalence Following Spinal Cord Injury: A Systematic Review and Meta-Analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Warner, F.M.; Cragg, J.J.; Jutzeler, C.R.; Finnerup, N.B.; Werhagen, L.; Weidner, N.; Maier, D.; Kalke, Y.B.; Curt, A.; Kramer, J.L.K. Progression of Neuropathic Pain after Acute Spinal Cord Injury: A Meta-Analysis and Framework for Clinical Trials. J. Neurotrauma 2018, 36, 1461–1468. [Google Scholar] [CrossRef]
- Hunt, C.; Moman, R.; Peterson, A.; Wilson, R.; Covington, S.; Mustafa, R.; Murad, M.H.; Hooten, W.M. Prevalence of Chronic Pain after Spinal Cord Injury: A Systematic Review and Meta-Analysis. Reg. Anesth. Pain Med. 2021, 46, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Hashimoto, R.E.; Dettori, J.R.; Fehlings, M.G. Spinal Cord Injury and Quality of Life: A Systematic Review of Outcome Measures. Evid. Based Spine Care J. 2011, 2, 37–44. [Google Scholar] [CrossRef]
- Rivers, C.S.; Fallah, N.; Noonan, V.K.; Whitehurst, D.G.; Schwartz, C.E.; Finkelstein, J.A.; Craven, B.C.; Ethans, K.; O’Connell, C.; Truchon, B.C.; et al. Health Conditions: Effect on Function, Health-Related Quality of Life, and Life Satisfaction After Traumatic Spinal Cord Injury. A Prospective Observational Registry Cohort Study. Arch. Phys. Med. Rehabil. 2018, 99, 443–451. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Neuropathic Pain: An Overview of the Current Treatment and Future Therapeutic Approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Villalobos, M.; Tortorici, V.; Brito, B.E.; Ryskamp, D.; Uribe, A.; Weaver, T. The Role of Neuroinflammation in the Transition of Acute to Chronic Pain and the Opioid-Induced Hyperalgesia and Tolerance. Front. Pharmacol. 2023, 14, 1297931. [Google Scholar] [CrossRef]
- Ramos, D.; Cruz, C.D. Involvement of Microglia in Chronic Neuropathic Pain Associated with Spinal Cord Injury—A Systematic Review. Rev. Neurosci. 2023, 34, 933–950. [Google Scholar] [CrossRef]
- Shiao, R.; Lee-Kubli, C.A. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics 2018, 15, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.T. Neuroinflammatory Contributions to Pain after SCI: Roles for Central Glial Mechanisms and Nociceptor-Mediated Host Defense. Exp. Neurol. 2014, 258, 48–61. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, M.; Han, F.; Liao, W.; Duan, X. Natural Polyphenols for Drug Delivery and Tissue Engineering Construction: A Review. Eur. J. Med. Chem. 2024, 266, 116141. [Google Scholar] [CrossRef]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiopathology of Neuropathic Pain. Curr. Drug Targets 2016, 18, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Antonopoulos, S.R. Benefit of Dietary Supplementation of Nutraceuticals as an Integrative Approach for Management of Migraine: Evidence From Preclinical and Clinical Studies. Curr. Pain Headache Rep. 2024, 28, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Soler-Martínez, R.; Deulofeu, M.; Bagó-Mas, A.; Dubový, P.; Verdú, E.; Fiol, N.; Boadas-Vaello, P. Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice. Biology 2022, 11, 1617. [Google Scholar] [CrossRef] [PubMed]
- Bagó-Mas, A.; Korimová, A.; Deulofeu, M.; Verdú, E.; Fiol, N.; Svobodová, V.; Dubový, P.; Boadas-Vaello, P. Polyphenolic Grape Stalk and Coffee Extracts Attenuate Spinal Cord Injury-Induced Neuropathic Pain Development in ICR-CD1 Female Mice. Sci. Rep. 2022, 12, 14980. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, B.; Homs, J.; Bosch-Mola, M.; Puig, T.; Reina, F.; Verdú, E.; Boadas-Vaello, P. Epigallocatechin-3-Gallate Treatment Reduces Thermal Hyperalgesia after Spinal Cord Injury by down-Regulating RhoA Expression in Mice. Eur. J. Pain 2016, 20, 341–352. [Google Scholar] [CrossRef]
- Becker, S.; Gandhi, W.; Schweinhardt, P. Cerebral Interactions of Pain and Reward and Their Relevance for Chronic Pain. Neurosci. Lett. 2012, 520, 182–187. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Castany, S.; Bagó-Mas, A.; Vela, J.M.; Verdú, E.; Bretová, K.; Svobodová, V.; Dubový, P.; Boadas-Vaello, P. Transient Reflexive Pain Responses and Chronic Affective Nonreflexive Pain Responses Associated with Neuroinflammation Processes in Both Spinal and Supraspinal Structures in Spinal Cord-Injured Female Mice. Int. J. Mol. Sci. 2023, 24, 1761. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Martos, R.; Bagó-Mas, A.; Deulofeu, M.; Homs, J.; Fiol, N.; Verdú, E.; Boadas-Vaello, P. Natural Polyphenolic Coffee Extract Administration Relieves Chronic Nociplastic Pain in a Reserpine-Induced Fibromyalgia-like Female Mouse Model. Brain Behav. 2024, 14, e3386. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Stoica, B.A.; Luo, T.; Sabirzhanov, B.; Zhao, Z.; Guanciale, K.; Nayar, S.K.; Foss, C.A.; Pomper, M.G.; Faden, A.I. Isolated Spinal Cord Contusion in Rats Induces Chronic Brain Neuroinflammation, Neurodegeneration, and Cognitive Impairment. Cell Cycle 2014, 13, 2446–2458. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Kumar, A.; Lipinski, M.M.; Loane, D.J.; Stoica, B.A.; Faden, A.I. Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury. J. Neurotrauma 2016, 33, 1919–1935. [Google Scholar] [CrossRef]
- Wang, L.; Gunduz, M.A.; Semeano, A.T.; Yılmaz, E.C.; Alanazi, F.A.H.; Imir, O.B.; Yener, U.; Arbelaez, C.A.; Usuga, E.; Teng, Y.D. Coexistence of Chronic Hyperalgesia and Multilevel Neuroinflammatory Responses after Experimental SCI: A Systematic Approach to Profiling Neuropathic Pain. J. Neuroinflamm. 2022, 19, 264. [Google Scholar] [CrossRef]
- Taoka, Y.; Okajima, K. Spinal Cord Injury in the Rat. Prog. Neurobiol. 1998, 56, 341–358. [Google Scholar] [CrossRef]
- Young, W. Chapter 17 Spinal Cord Contusion Models. Prog. Brain Res. 2002, 137, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.A.; Hagg, T. Anti-Inflammatory Treatments during the Chronic Phase of Spinal Cord Injury Improve Locomotor Function in Adult Mice. J. Neurotrauma 2011, 28, 1995–2002. [Google Scholar] [CrossRef]
- Bisaz, R.; Boadas-Vaello, P.; Genoux, D.; Sandi, C. Age-Related Cognitive Impairments in Mice with a Conditional Ablation of the Neural Cell Adhesion Molecule. Learn. Mem. 2013, 20, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending Control of Nociception: Specificity, Recruitment and Plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D. Central Nervous System Mechanisms for Pain Modulation. Appl. Neurophysiol. 1985, 48, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending Pain Modulation and Chronification of Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- McMullan, S.; Lumb, B.M. Midbrain Control of Spinal Nociception Discriminates between Responses Evoked by Myelinated and Unmyelinated Heat Nociceptors in the Rat. Pain 2006, 124, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Eidson, L.N.; Murphy, A.Z. Persistent Peripheral Inflammation Attenuates Morphine-Induced Periaqueductal Gray Glial Cell Activation and Analgesic Tolerance in the Male Rat. J. Pain 2013, 14, 393–404. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of Spinal Microglial Cathepsin S for the Reversal of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ren, W.H.; Zhang, Y.Q.; Zhao, Z.Q. Contributions of the Anterior Cingulate Cortex and Amygdala to Pain- and Fear-Conditioned Place Avoidance in Rats. Pain 2004, 110, 343–353. [Google Scholar] [CrossRef]
- Veinante, P.; Yalcin, I.; Barrot, M. The Amygdala between Sensation and Affect: A Role in Pain. J. Mol. Psychiatry 2013, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Griffiths, P.H. Guidelines on the Recognition of Pain, Distress and Discomfort in Experimental Animals and an Hypothesis for Assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The Evidence for Pharmacological Treatment of Neuropathic Pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological Treatments of Neuropathic Pain: The Latest Recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Ketchum, J.M.; Starkweather, A.; Nicholls, E.; Wilk, A.R. Factors Predicting Depression among Persons with Spinal Cord Injury 1 to 5 Years Post Injury. NeuroRehabilitation 2011, 29, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Tran, Y.; Wijesuriya, N.; Craig, A. Central Correlates of Impaired Information Processing in People with Spinal Cord Injury. J. Clin. Neurophysiol. 2013, 30, 59–65. [Google Scholar] [CrossRef]
- Rao, P.N.; Mainkar, O.; Bansal, N.; Rakesh, N.; Haffey, P.; Urits, I.; Orhurhu, V.; Kaye, A.D.; Urman, R.D.; Gulati, A.; et al. Flavonoids in the Treatment of Neuropathic Pain. Curr. Pain Headache Rep. 2021, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Pirvulescu, I.; Biskis, A.; Candido, K.D.; Knezevic, N.N. Overcoming Clinical Challenges of Refractory Neuropathic Pain. Expert Rev. Neurother. 2022, 22, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Q.; Nie, L.Y.; Qian, L.N.; Zhao, K. Efficacy and Safety of Polyphenols for Osteoarthritis Treatment: A Meta-Analysis. Clin. Ther. 2021, 43, e241–e253.e2. [Google Scholar] [CrossRef] [PubMed]
- Coletro, H.N.; Diniz, A.P.; Guimarães, N.S.; Carraro, J.C.C.; Mendonça, R.d.D.; Meireles, A.L. Polyphenols for Improvement of Inflammation and Symptoms in Rheumatic Diseases: Systematic Review. Sao Paulo Med. J. 2021, 139, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Budh, C.N.; Lund, I.; Ertzgaard, P.; Holtz, A.; Hultling, C.; Levi, R.; Werhagen, L.; Lundeberg, T. Pain in a Swedish Spinal Cord Injury Population. Clin. Rehabil. 2003, 17, 685–690. [Google Scholar] [CrossRef]
- Cardenas, D.D.; Bryce, T.N.; Shem, K.; Richards, J.S.; Elhefni, H. Gender and Minority Differences in the Pain Experience of People with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2004, 85, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.L.K.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.K.S.; Liu, L.J.W.; Ramer, M.S. Neuropathic Pain Following Traumatic Spinal Cord Injury: Models, Measurement, and Mechanisms. J. Neurosci. Res. 2017, 95, 1295–1306. [Google Scholar] [CrossRef]
- Miller, L.R.; Cano, A. Comorbid Chronic Pain and Depression: Who Is at Risk? J. Pain 2009, 10, 619–627. [Google Scholar] [CrossRef]
- Goesling, J.; Clauw, D.J.; Hassett, A.L. Pain and Depression: An Integrative Review of Neurobiological and Psychological Factors. Curr. Psychiatry Rep. 2013, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of Nociception I: Responses of 11 Inbred Mouse Strains on 12 Measures of Nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; Straetemans, R.; D’Hooge, R.; Meert, T. Differences in Nociceptive Behavioral Performance between C57BL/6J, 129S6/SvEv, B6 129 F1 and NMRI Mice. Behav. Brain Res. 2008, 190, 233–242. [Google Scholar] [CrossRef]
- Bailey, K.R.; Crawley, J.N. Chapter 5—Anxiety-Related Behaviors in Mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; pp. 77–101. [Google Scholar]
- Castany, S.; Gris, G.; Vela, J.M.; Verdú, E.; Boadas-Vaello, P. Critical Role of Sigma-1 Receptors in Central Neuropathic Pain-Related Behaviours after Mild Spinal Cord Injury in Mice. Sci. Rep. 2018, 8, 3873. [Google Scholar] [CrossRef]
- Castany, S.; Codony, X.; Zamanillo, D.; Merlos, M.; Verdú, E.; Boadas-Vaello, P. Repeated Sigma-1 Receptor Antagonist MR309 Administration Modulates Central Neuropathic Pain Development after Spinal Cord Injury in Mice. Front. Pharmacol. 2019, 10, 433451. [Google Scholar] [CrossRef]
- Sandkühler, J. Models and Mechanisms of Hyperalgesia and Allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef]
- King, T.; Porreca, F. Preclinical Assessment of Pain: Improving Models in Discovery Research. Curr. Top. Behav. Neurosci. 2014, 20, 101–120. [Google Scholar] [CrossRef]
- Williams, R.; Murray, A. Prevalence of Depression After Spinal Cord Injury: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Dorstyn, D. Anxiety Prevalence Following Spinal Cord Injury: A Meta-Analysis. Spinal Cord. 2016, 54, 570–578. [Google Scholar] [CrossRef]
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- do Espírito Santo, C.C.; da Silva Fiorin, F.; Ilha, J.; Duarte, M.M.M.F.; Duarte, T.; Santos, A.R.S. Spinal Cord Injury by Clip-Compression Induces Anxiety and Depression-like Behaviours in Female Rats: The Role of the Inflammatory Response. Brain Behav. Immun. 2019, 78, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.S.; Kemp, B.; Coker, J. Depression after Spinal Cord Injury: Relation to Gender, Ethnicity, Aging, and Socioeconomic Indicators. Arch. Phys. Med. Rehabil. 2000, 81, 1099–1109. [Google Scholar] [CrossRef]
- Saurí, J.; Chamarro, A.; Gilabert, A.; Gifre, M.; Rodriguez, N.; Lopez-Blazquez, R.; Curcoll, L.; Benito-Penalva, J.; Soler, D. Depression in Individuals with Traumatic and Nontraumatic Spinal Cord Injury Living in the Community. Arch. Phys. Med. Rehabil. 2017, 98, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.C.; Hull, T.C.L.; Delaney, G.A.; Potter, P.J.; Sequeira, K.A.J.; Campbell, K.; Popovich, P.G. Elevated Serum Titers of Proinflammatory Cytokines and CNS Autoantibodies in Patients with Chronic Spinal Cord Injury. J. Neurotrauma 2004, 19, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical Correlates of Elevated Serum Concentrations of Cytokines and Autoantibodies in Patients with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The Paradox of Chronic Neuroinflammation, Systemic Immune Suppression and Autoimmunity after Traumatic Chronic Spinal Cord Injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2015, 16, 22–34. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. Targeting Inflammation to Influence Mood Following Spinal Cord Injury: A Randomized Clinical Trial. J. Neuroinflamm. 2015, 12, 204. [Google Scholar] [CrossRef]
- Maldonado-Bouchard, S.; Peters, K.; Woller, S.A.; Madahian, B.; Faghihi, U.; Patel, S.; Bake, S.; Hook, M.A. Inflammation Is Increased with Anxiety- and Depression-like Signs in a Rat Model of Spinal Cord Injury. Brain Behav. Immun. 2016, 51, 176–195. [Google Scholar] [CrossRef]
- Austin, P.J.; Fiore, N.T. Supraspinal Neuroimmune Crosstalk in Chronic Pain States. Curr. Opin. Physiol. 2019, 11, 7–15. [Google Scholar] [CrossRef]
- Mor, D.; Bembrick, A.L.; Austin, P.J.; Keay, K.A. Evidence for Cellular Injury in the Midbrain of Rats Following Chronic Constriction Injury of the Sciatic Nerve. J. Chem. Neuroanat. 2011, 41, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.K.; Nilsson-Todd, L.; Cleren, C.; Léna, I.; Garcia, R.; Finn, D.P. Molecular and Electrophysiological Changes in the Prefrontal Cortex–Amygdala–Dorsal Periaqueductal Grey Pathway during Persistent Pain State and Fear-Conditioned Analgesia. Physiol. Behav. 2011, 104, 1075–1081. [Google Scholar] [CrossRef]
- Roberts, J.; Ossipov, M.H.; Porreca, F. Glial Activation in the Rostroventromedial Medulla Promotes Descending Facilitation to Mediate Inflammatory Hypersensitivity. Eur. J. Neurosci. 2009, 30, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Guo, W.; Zou, S.; Ren, K.; Dubner, R. Supraspinal Glial–Neuronal Interactions Contribute to Descending Pain Facilitation. J. Neurosci. 2008, 28, 10482–10495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bu, H.; Liu, C.; Gao, F.; Yang, H.; Tian, X.; Xu, A.; Chen, Z.; Cao, F.; Tian, Y. Inhibition of Glial Activation in Rostral Ventromedial Medulla Attenuates Mechanical Allodynia in a Rat Model of Cancer-Induced Bone Pain. J. Huazhong Univ. Sci. Technol.—Med. Sci. 2012, 32, 291–298. [Google Scholar] [CrossRef] [PubMed]
- White, F.A.; Jung, H.; Miller, R.J. Chemokines and the Pathophysiology of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 20151–20158. [Google Scholar] [CrossRef] [PubMed]
- Hulsebosch, C.E.; Hains, B.C.; Crown, E.D.; Carlton, S.M. Mechanisms of Chronic Central Neuropathic Pain after Spinal Cord Injury. Brain Res. Rev. 2009, 60, 202–213. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. Chemokines, Neuronal–Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.T.; Lee, C.M.; Day, Y.J. The Immune Aspect in Neuropathic Pain: Role of Chemokines. Acta Anaesthesiol. Taiwanica 2013, 51, 127–132. [Google Scholar] [CrossRef]
- Knerlich-Lukoschus, F.; Noack, M.; Von Der Ropp-Brenner, B.; Lucius, R.; Mehdorn, H.M.; Held-Feindt, J. Spinal Cord Injuries Induce Changes in CB1 Cannabinoid Receptor and C-C Chemokine Expression in Brain Areas Underlying Circuitry of Chronic Pain Conditions. J. Neurotrauma 2011, 28, 619–634. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Zou, S.; Dubner, R.; Ren, K. Chemokine Signaling Involving Chemokine (C-C Motif) Ligand 2 Plays Arole in Descending Pain Facilitation. Neurosci. Bull. 2012, 28, 193–207. [Google Scholar] [CrossRef]
- Chen, X.; Geller, E.B.; Rogers, T.J.; Adler, M.W. The Chemokine CX3CL1/Fractalkine Interferes with the Antinociceptive Effect Induced by Opioid Agonists in the Periaqueductal Grey of Rats. Brain Res. 2007, 1153, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bornhövd, K.; Quante, M.; Glauche, V.; Bromm, B.; Weiller, C.; Büchel, C. Painful Stimuli Evoke Different Stimulus–Response Functions in the Amygdala, Prefrontal, Insula and Somatosensory Cortex: A Single-trial FMRI Study. Brain 2002, 125, 1326–1336. [Google Scholar] [CrossRef]
- Paulson, P.E.; Casey, K.L.; Morrow, T.J. Long-Term Changes in Behavior and Regional Cerebral Blood Flow Associated with Painful Peripheral Mononeuropathy in the Rat. Pain 2002, 95, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The Amygdala and Persistent Pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. CHAPTER 15. Amygdala Pain Mechanisms. Handb. Exp. Pharmacol. 2015, 227, 261–284. [Google Scholar]
- Gonçalves, L.; Silva, R.; Pinto-Ribeiro, F.; Pêgo, J.M.; Bessa, J.M.; Pertovaara, A.; Sousa, N.; Almeida, A. Neuropathic Pain Is Associated with Depressive Behaviour and Induces Neuroplasticity in the Amygdala of the Rat. Exp. Neurol. 2008, 213, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional Processing in Anterior Cingulate and Medial Prefrontal Cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Barthas, F.; Sellmeijer, J.; Hugel, S.; Waltisperger, E.; Barrot, M.; Yalcin, I. The Anterior Cingulate Cortex Is a Critical Hub for Pain-Induced Depression. Biol. Psychiatry 2015, 77, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Berendse, H.W.; Groenewegen, H.J. Restricted Cortical Termination Fields of the Midline and Intralaminar Thalamic Nuclei in the Rat. Neuroscience 1991, 42, 73–102. [Google Scholar] [CrossRef]
- Hsu, M.M.; Shyu, B.C. Electrophysiological Study of the Connection between Medial Thalamus and Anterior Cingulate Cortex in the Rat. Neuroreport 1997, 8, 2701–2707. [Google Scholar] [CrossRef]
- Mcdonald, A.J.; Mascagni, F.; Guo, L. Projections of the Medial and Lateral Prefrontal Cortices to the Amygdala: A Phaseolus Vulgaris Leucoagglutinin Study in the Rat. Neuroscience 1996, 71, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Marcello, L.; Cavaliere, C.; Colangelo, A.M.; Bianco, M.R.; Cirillo, G.; Alberghina, L.; Papa, M. Remodelling of Supraspinal Neuroglial Network in Neuropathic Pain Is Featured by a Reactive Gliosis of the Nociceptive Amygdala. Eur. J. Pain 2013, 17, 799–810. [Google Scholar] [CrossRef]
- Sawada, A.; Niiyama, Y.; Ataka, K.; Nagaishi, K.; Yamakage, M.; Fujimiya, M. Suppression of Bone Marrow-Derived Microglia in the Amygdale Improves Anxiety-like Behavior Induced by Chronic Partial Sciatic Nerve Ligation in Mice. Pain 2014, 155, 1762–1772. [Google Scholar] [CrossRef]
- Cho, I.; Kim, J.M.; Kim, E.J.; Kim, S.Y.; Kam, E.H.; Cheong, E.; Suh, M.; Koo, B.N. Orthopedic Surgery-Induced Cognitive Dysfunction Is Mediated by CX3CL1/R1 Signaling. J. Neuroinflamm. 2021, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gao, Y.J.; Ren, W.H.; Li, T.T.; Duan, K.Z.; Cui, Y.H.; Cao, X.H.; Zhao, Z.Q.; Ji, R.R.; Zhang, Y.Q. Activation of Extracellular Signal-Regulated Kinase in the Anterior Cingulate Cortex Contributes to the Induction and Expression of Affective Pain. J. Neurosci. 2009, 29, 3307–3321. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.L.; Wei, R.; Zhou, P.; Luo, Y.W.; Wang, X.Q.; Duan, J.; Bi, F.F.; Zhang, J.Y.; Li, C.Q.; Dai, R.P.; et al. Activation of Anterior Cingulate Cortex Extracellular Signal-Regulated Kinase-1 and -2 (ERK1/2) Regulates Acetic Acid-Induced, Pain-Related Anxiety in Adult Female Mice. Acta Histochem. Cytochem. 2012, 45, 219–225. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.L.; Luo, W.; Zhou, F.H.; Li, C.Q.; Xu, J.M.; Dai, R.P. Differential Effects of General Anesthetics on Anxiety-like Behavior in Formalin-Induced Pain: Involvement of ERK Activation in the Anterior Cingulate Cortex. Psychopharmacology 2015, 232, 4433–4444. [Google Scholar] [CrossRef]
- Semenova, S.; Contet, C.; Roberts, A.J.; Markou, A. Mice Lacking the Β4 Subunit of the Nicotinic Acetylcholine Receptor Show Memory Deficits, Altered Anxiety- and Depression-like Behavior, and Diminished Nicotine-Induced Analgesia. Nicotine Tob. Res. 2012, 14, 1346–1355. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Montero-Atalaya, M.; Expósito, S.; Muñoz-Arnaiz, R.; Makarova, J.; Bartolom, B.; Martín, E.; Moreno-Arribas, M.V.; Herreras, O. A Dietary Polyphenol Metabolite Alters CA1 Excitability Ex Vivo and Mildly Affects Cortico-Hippocampal Field Potential Generators in Anesthetized Animals. Cereb. Cortex 2023, 33, 10411–10425. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Avila, S.; Diaz, N.F.; Gómez-Pinedo, U.; Canales-Aguirre, A.A.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; Marquez-Aguirre, A.L.; Díaz-Martínez, N.E. Neuroprotective Effects of Phytochemicals on Dopaminergic Neuron Cultures. Neurologia 2019, 34, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Parepally, J.M.R.; Mandula, H.; Smith, Q.R. Brain Uptake of Nonsteroidal Anti-Inflammatory Drugs: Ibuprofen, Flurbiprofen, and Indomethacin. Pharm. Res. 2006, 23, 873–881. [Google Scholar] [PubMed]
- Bjarnason, I. Gastrointestinal Safety of NSAIDs and Over-the-Counter Analgesics. Int. J. Clin. Pract. 2013, 67, 37–42. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-Term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for Locomotion Detects Differences in Recovery after Spinal Cord Injury in Five Common Mouse Strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A New and Sensitive Method for Measuring Thermal Nociception in Cutaneous Hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Jakobsson, J.; Cordero, M.I.; Bisaz, R.; Groner, A.C.; Busskamp, V.; Bensadoun, J.C.; Cammas, F.; Losson, R.; Mansuy, I.M.; Sandi, C.; et al. KAP1-Mediated Epigenetic Repression in the Forebrain Modulates Behavioral Vulnerability to Stress. Neuron 2008, 60, 818–831. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary Report of a Simple Animal Behavior Model for the Anxiolytic Effects of Benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Hascoët, M. The Mouse Light/Dark Box Test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Behavioral Phenotyping of Rodents—PubMed. Comp. Med. 2003, 53, 140–146. [Google Scholar] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 1997; ISBN 0123741211. [Google Scholar]
- Keay, K.A.; Clement, C.I.; Depaulis, A.; Bandler, R. Different Representations of Inescapable Noxious Stimuli in the Periaqueductal Gray and Upper Cervical Spinal Cord of Freely Moving Rats. Neurosci. Lett. 2001, 313, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123910578. [Google Scholar]



| Grape Stalk Extract—GSE | 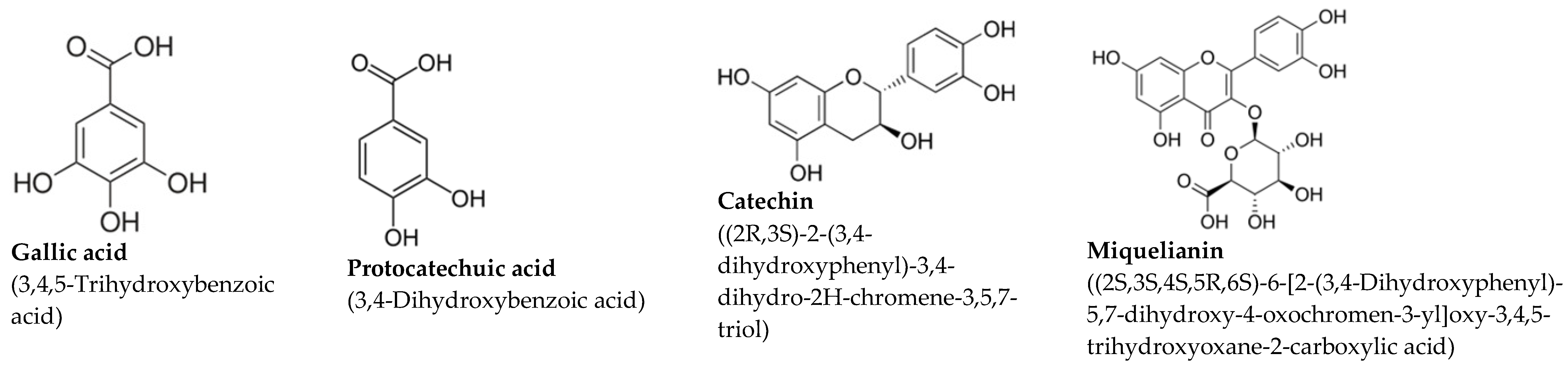 | ||
| Polyphenol | [mg/L] | SEM | |
| Gallic acid | 39.55 | 1.3 | |
| Protocatechuic acid | 30.5 | 0.9 | |
| Catechin | 29.2 | 0.7 | |
| Miquelianin | 18.12 | 1.6 | |
| Caftaric acid | 11.5 | 0.7 | |
| Coffee Extract—CE | 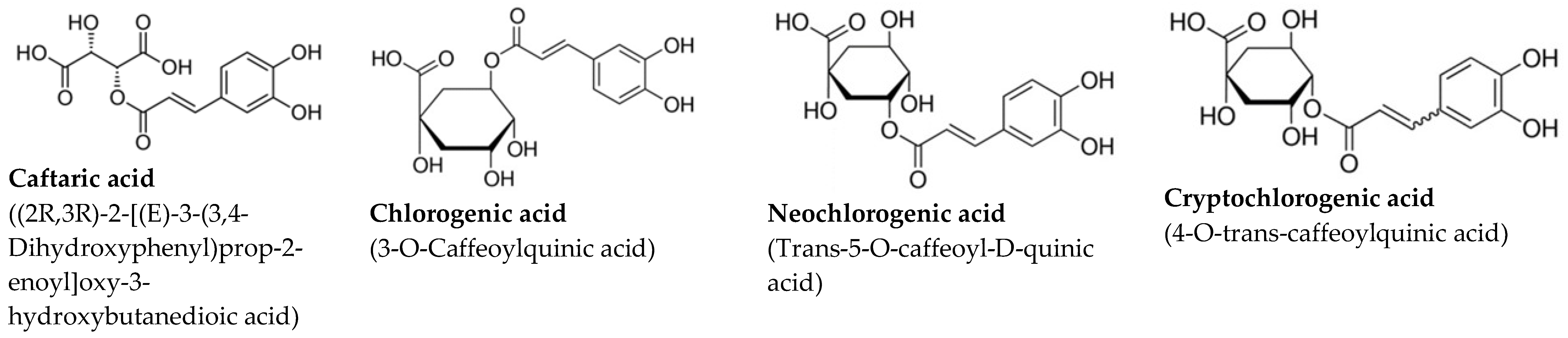 | ||
| Polyphenol | [mg/L] | SEM | |
| Chlorogenic acid | 339.2 | 12.9 | |
| Neochlorogenic acid | 308.1 | 14.3 | |
| Cryptochlorogenic acid | 307.6 | 18.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagó-Mas, A.; Korimová, A.; Bretová, K.; Deulofeu, M.; Verdú, E.; Fiol, N.; Dubový, P.; Boadas-Vaello, P. Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice. Int. J. Mol. Sci. 2025, 26, 3325. https://doi.org/10.3390/ijms26073325
Bagó-Mas A, Korimová A, Bretová K, Deulofeu M, Verdú E, Fiol N, Dubový P, Boadas-Vaello P. Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice. International Journal of Molecular Sciences. 2025; 26(7):3325. https://doi.org/10.3390/ijms26073325
Chicago/Turabian StyleBagó-Mas, Anna, Andrea Korimová, Karolína Bretová, Meritxell Deulofeu, Enrique Verdú, Núria Fiol, Petr Dubový, and Pere Boadas-Vaello. 2025. "Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice" International Journal of Molecular Sciences 26, no. 7: 3325. https://doi.org/10.3390/ijms26073325
APA StyleBagó-Mas, A., Korimová, A., Bretová, K., Deulofeu, M., Verdú, E., Fiol, N., Dubový, P., & Boadas-Vaello, P. (2025). Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice. International Journal of Molecular Sciences, 26(7), 3325. https://doi.org/10.3390/ijms26073325








