The Therapeutic Potential of ADSC-Secreted LEFTY2 in Treating Alzheimer’s Disease
Abstract
1. Introduction
2. Results
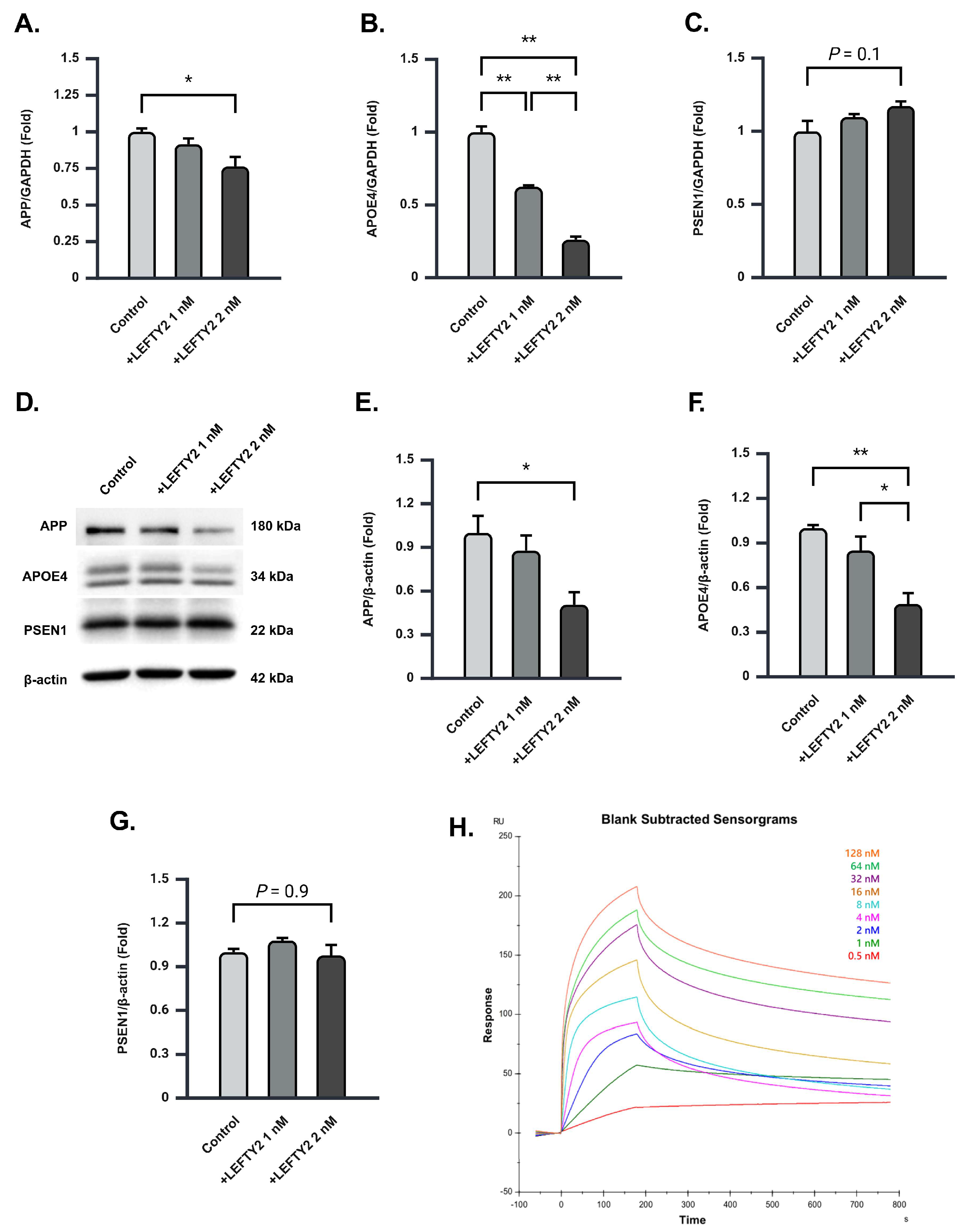
2.1. Co-Culture of Ts21 Neurons with ADSCs Significantly Enhances the Population of TS21 Neurons and Downregulates the Expression of the APOE Gene and Protein
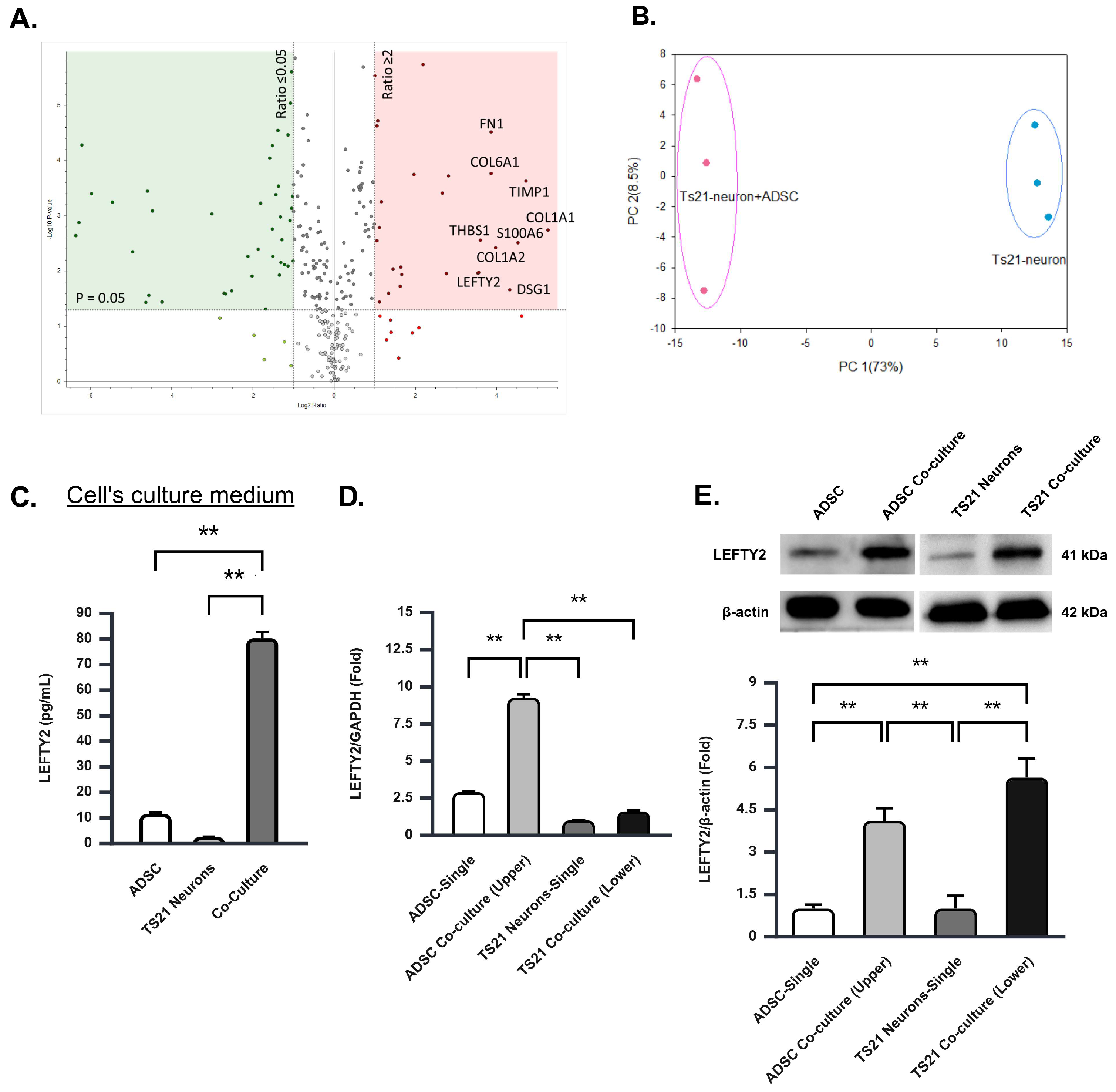
2.2. The Co-Culture of TS21 Neurons and ADSCs Significantly Elevates LEFTY2 Protein
2.3. Upregulation of LEFTY2 Protein in the Co-Culture Is Primarily Driven by ADSCS Secretion
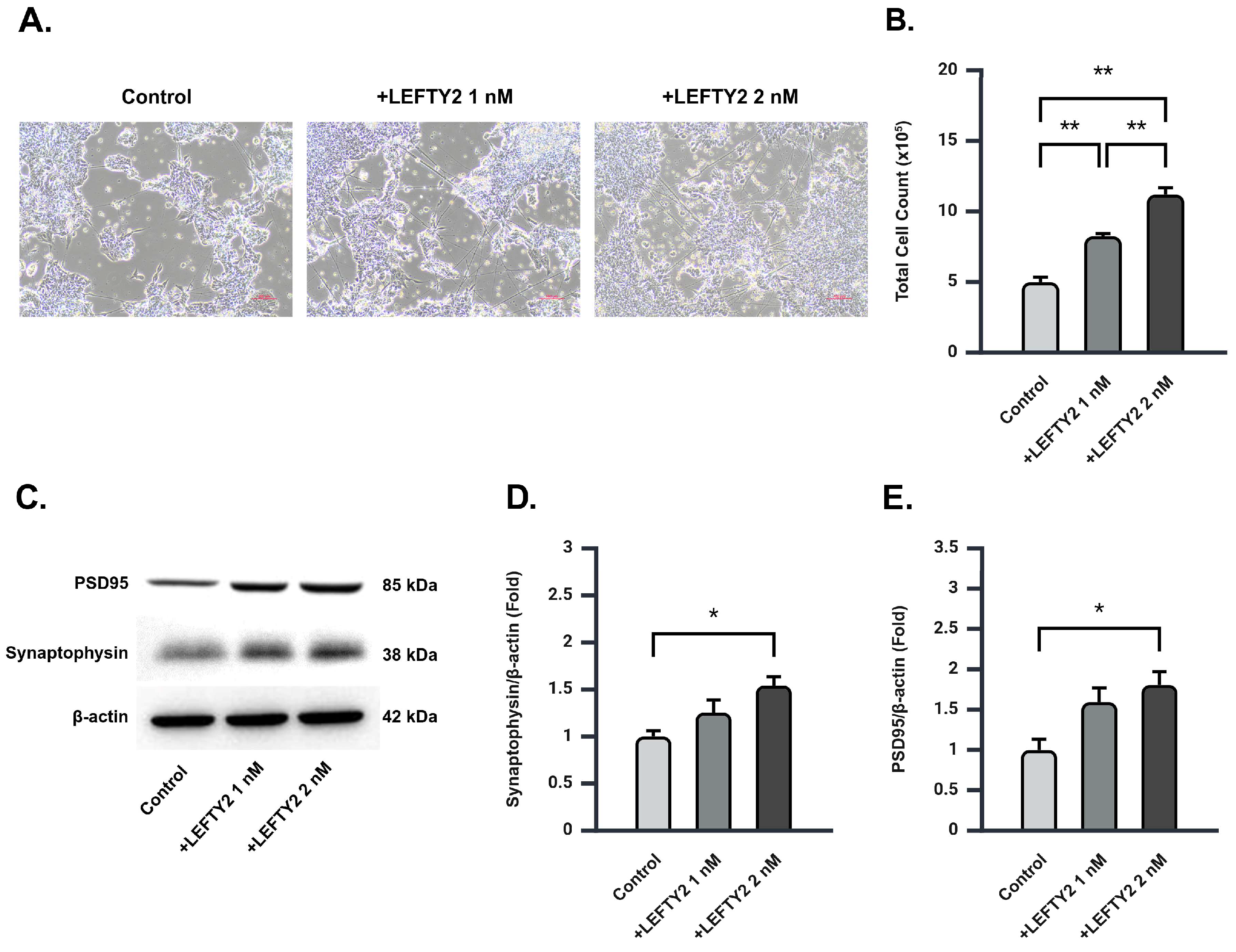
2.4. LEFTY2 Supplementation Stimulates Neuronal Growth and Upregulates Synaptic Protein Expression in Ts21 Neurons
2.5. LEFTY2 Supplementation Attenuates the Expression of Amyloid Beta 1–42 in Ts21 Neurons
2.6. LEFTY2 Strongly Binds to APOE4 and Downregulates Its Protein Expression in Ts21 Neurons
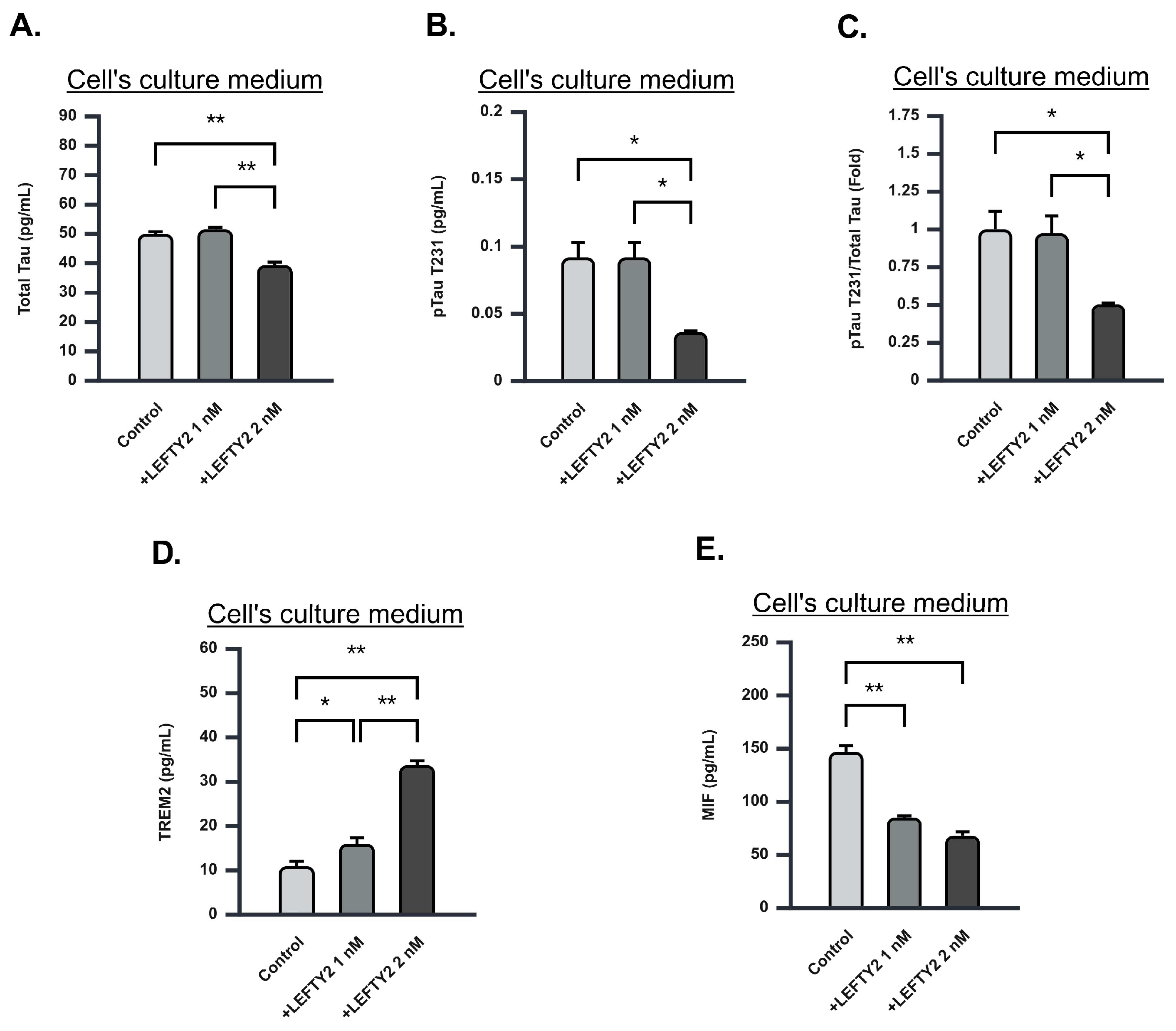
2.7. LEFTY2 Supplementation Attenuates Phosphorylated Tau-231 Levels
2.8. LEFTY2 Supplementation Enhances Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) Expression While Downregulating Macrophage Migration Inhibitory Factor (MIF) Expression
3. Discussion
4. Experimental Procedures
4.1. Human Adipose Stem Cell Culture and Subculture
4.2. Ts21-iPSCs
4.3. Neuronal Differentiation of Ts21-Derived Induced Pluripotent Stem Cells
4.4. Ts21 Neurons Co-Culture with ADSCs
4.5. Immunofluorescence Staining
4.6. RT-qPCR
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Western Blot
4.9. Surface Plasmon Resonance
4.10. Protein Digestion Using Filter-Aided Sample Preparation Method
4.11. MS/MS Protein Identification and Quantification
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, A.; Norton, N.; Fast, T.; Frolich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA 2021, 118, e2102191118. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The beta-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Wuli, W.; Lin, S.Z.; Chen, S.P.; Tannous, B.A.; Huang, W.S.; Woon, P.Y.; Wu, Y.C.; Yang, H.H.; Chen, Y.C.; Fleming, R.L.; et al. Targeting PSEN1 by lnc-CYP3A43-2/miR-29b-2-5p to Reduce beta Amyloid Plaque Formation and Improve Cognition Function. Int. J. Mol. Sci. 2022, 23, 554. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, T.; Gong, K.; Ao, Q.; Zhang, X.; Gong, Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 2014, 9, 798–805. [Google Scholar] [CrossRef]
- Yu, S.; Cheng, Y.; Zhang, L.; Yin, Y.; Xue, J.; Li, B.; Gong, Z.; Gao, J.; Mu, Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther. 2019, 10, 333. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Ralvenius, W.T.; Tsai, L.H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali-Ellis, Z.; Galat, V.; Galat, Y.; Gilgur, A.; Seftor, E.A.; Hendrix, M.J.C. Lefty Glycoproteins in Human Embryonic Stem Cells: Extracellular Delivery Route and Posttranslational Modification in Differentiation. Stem Cells Dev. 2016, 25, 1681–1690. [Google Scholar] [CrossRef]
- Yang, Y.R.; Bu, F.T.; Yang, Y.; Li, H.; Huang, C.; Meng, X.M.; Zhang, L.; Lv, X.W.; Li, J. LEFTY2 alleviates hepatic stellate cell activation and liver fibrosis by regulating the TGF-beta1/Smad3 pathway. Mol. Immunol. 2020, 126, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.B.; Dowling, A.L.; Lianekhammy, J.; Lott, I.T.; Doran, E.; Murphy, M.P.; Beckett, T.L.; Schmitt, F.A.; Head, E. Synaptophysin and synaptojanin-1 in Down syndrome are differentially affected by Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 767–775. [Google Scholar] [CrossRef]
- Xu, L.; Long, J.; Su, Z.; Xu, B.; Lin, M.; Chen, Y.; Long, D. Restored presynaptic synaptophysin and cholinergic inputs contribute to the protective effects of physical running on spatial memory in aged mice. Neurobiol. Dis. 2019, 132, 104586. [Google Scholar] [CrossRef]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 protects synapses from beta-amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Dickson, M.R.; Perry, R.T.; Wiener, H.; Go, R.C. Association studies of transforming growth factor-beta 1 and Alzheimer’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 139B, 38–41. [Google Scholar] [CrossRef]
- Yin, Z.; Rosenzweig, N.; Kleemann, K.L.; Zhang, X.; Brandao, W.; Margeta, M.A.; Schroeder, C.; Sivanathan, K.N.; Silveira, S.; Gauthier, C.; et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFbeta-mediated checkpoints. Nat. Immunol. 2023, 24, 1839–1853. [Google Scholar] [CrossRef]
- Ulloa, L.; Tabibzadeh, S. Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. J. Biol. Chem. 2001, 276, 21397–21404. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.M.; Heinsinger, N.M.; Rebeck, G.W. Alzheimer’s Disease Genetic Risk Factor APOE-epsilon4 Also Affects Normal Brain Function. Curr. Alzheimer Res. 2016, 13, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Shao, C.Y.; Mirra, S.S.; Sait, H.B.; Sacktor, T.C.; Sigurdsson, E.M. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Abeta and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 2011, 122, 285–292. [Google Scholar] [CrossRef]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Li, S.Q.; Yu, Y.; Han, J.Z.; Wang, D.; Liu, J.; Qian, F.; Fan, G.H.; Bucala, R.; Ye, R.D. Deficiency of macrophage migration inhibitory factor attenuates tau hyperphosphorylation in mouse models of Alzheimer’s disease. J. Neuroinflammation 2015, 12, 177. [Google Scholar] [CrossRef]
- Bacher, M.; Deuster, O.; Aljabari, B.; Egensperger, R.; Neff, F.; Jessen, F.; Popp, J.; Noelker, C.; Reese, J.P.; Al-Abed, Y.; et al. The role of macrophage migration inhibitory factor in Alzheimer’s disease. Mol. Med. 2010, 16, 116–121. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, S.M.; Lu, H.E.; Lai, S.M.; Lai, P.S.; Shen, P.W.; Chen, P.Y.; Shen, C.I.; Harn, H.J.; Lin, S.Z.; et al. N-butylidenephthalide attenuates Alzheimer’s disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons. Sci. Rep. 2015, 5, 8744. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-W.; Yang, H.-H.; Chiou, T.-W.; Woon, P.-Y.; Xu, Y.-X.; Tjandra, C.; Wijaya, I.; Harn, H.-J.; Lin, S.-Z. The Therapeutic Potential of ADSC-Secreted LEFTY2 in Treating Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 3382. https://doi.org/10.3390/ijms26073382
Li W-W, Yang H-H, Chiou T-W, Woon P-Y, Xu Y-X, Tjandra C, Wijaya I, Harn H-J, Lin S-Z. The Therapeutic Potential of ADSC-Secreted LEFTY2 in Treating Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(7):3382. https://doi.org/10.3390/ijms26073382
Chicago/Turabian StyleLi, Wei-Wu, Hsueh-Hui Yang, Tzyy-Wen Chiou, Peng-Yeong Woon, Yue-Xuan Xu, Cynthia Tjandra, Ivan Wijaya, Horng-Jyh Harn, and Shinn-Zong Lin. 2025. "The Therapeutic Potential of ADSC-Secreted LEFTY2 in Treating Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 7: 3382. https://doi.org/10.3390/ijms26073382
APA StyleLi, W.-W., Yang, H.-H., Chiou, T.-W., Woon, P.-Y., Xu, Y.-X., Tjandra, C., Wijaya, I., Harn, H.-J., & Lin, S.-Z. (2025). The Therapeutic Potential of ADSC-Secreted LEFTY2 in Treating Alzheimer’s Disease. International Journal of Molecular Sciences, 26(7), 3382. https://doi.org/10.3390/ijms26073382







