Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway
Abstract
1. Introduction
2. Results
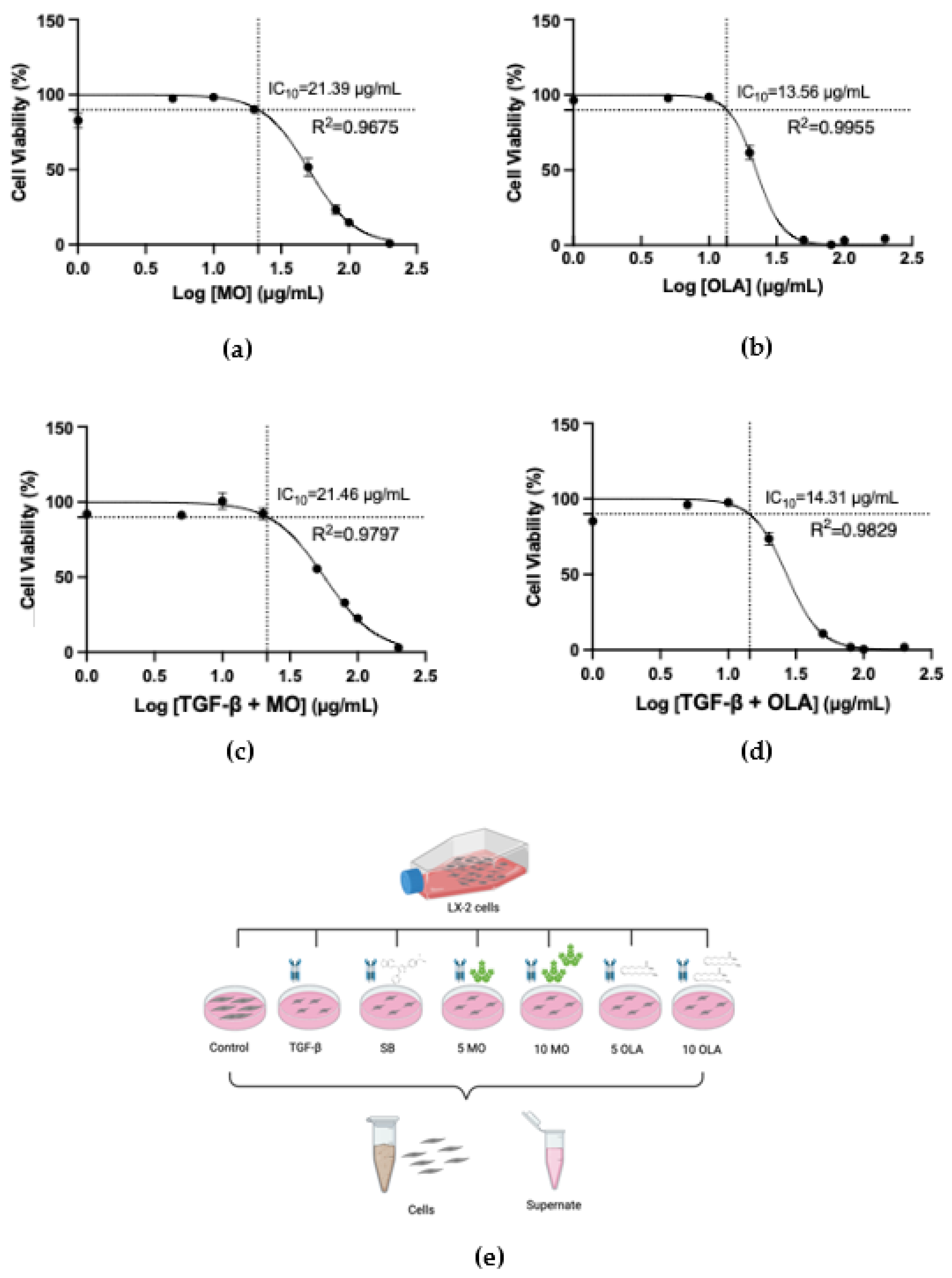
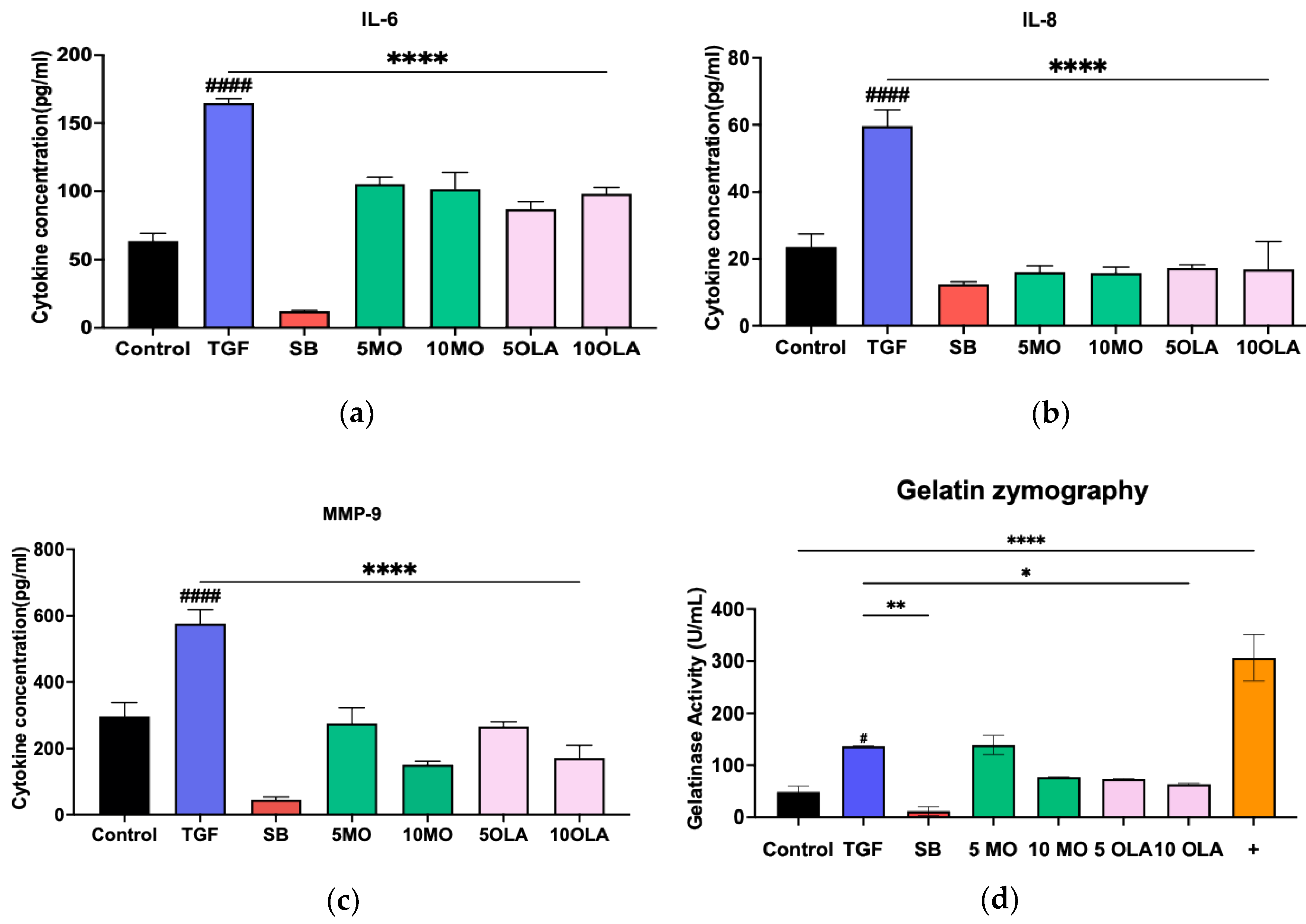
2.1. OLA Suppresses Activation of Hepatic Stellate Cells (HSCs) via TGF-β1
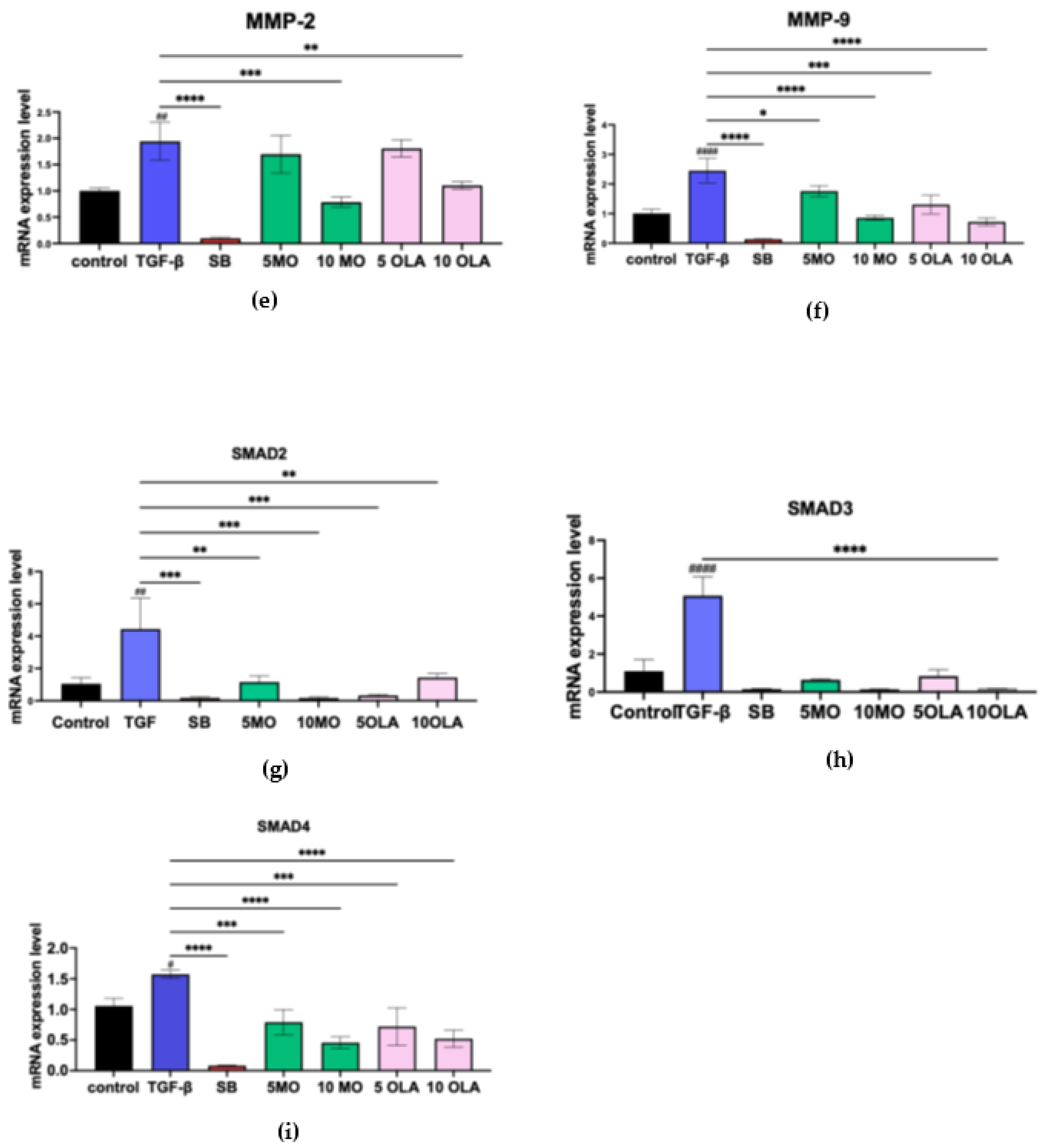
2.2. OLA Suppresses Fibrotic Markers and Inhibits HSCs Activation by Blocking the SMAD Signaling Pathway at the Gene Expression Level
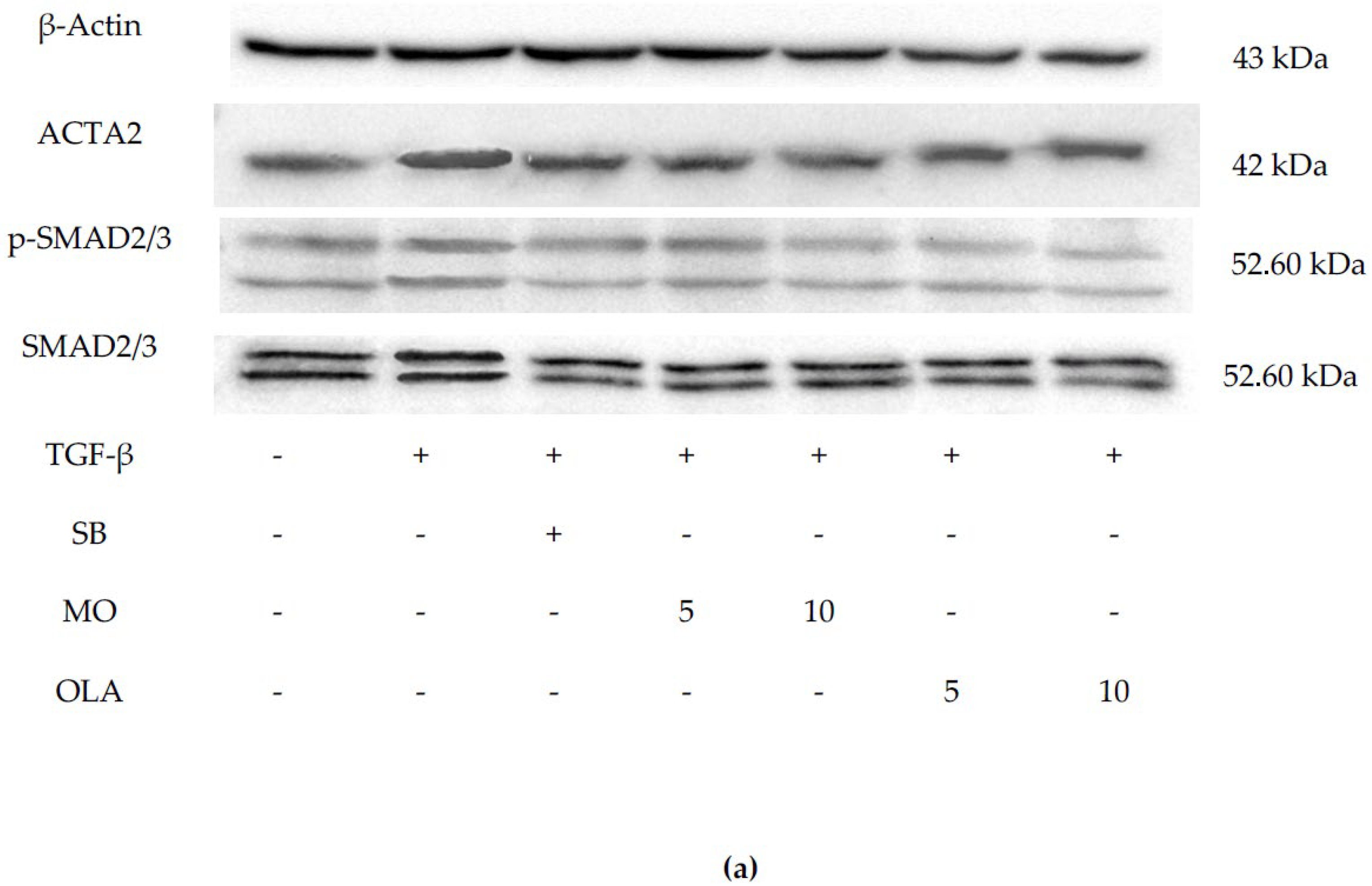
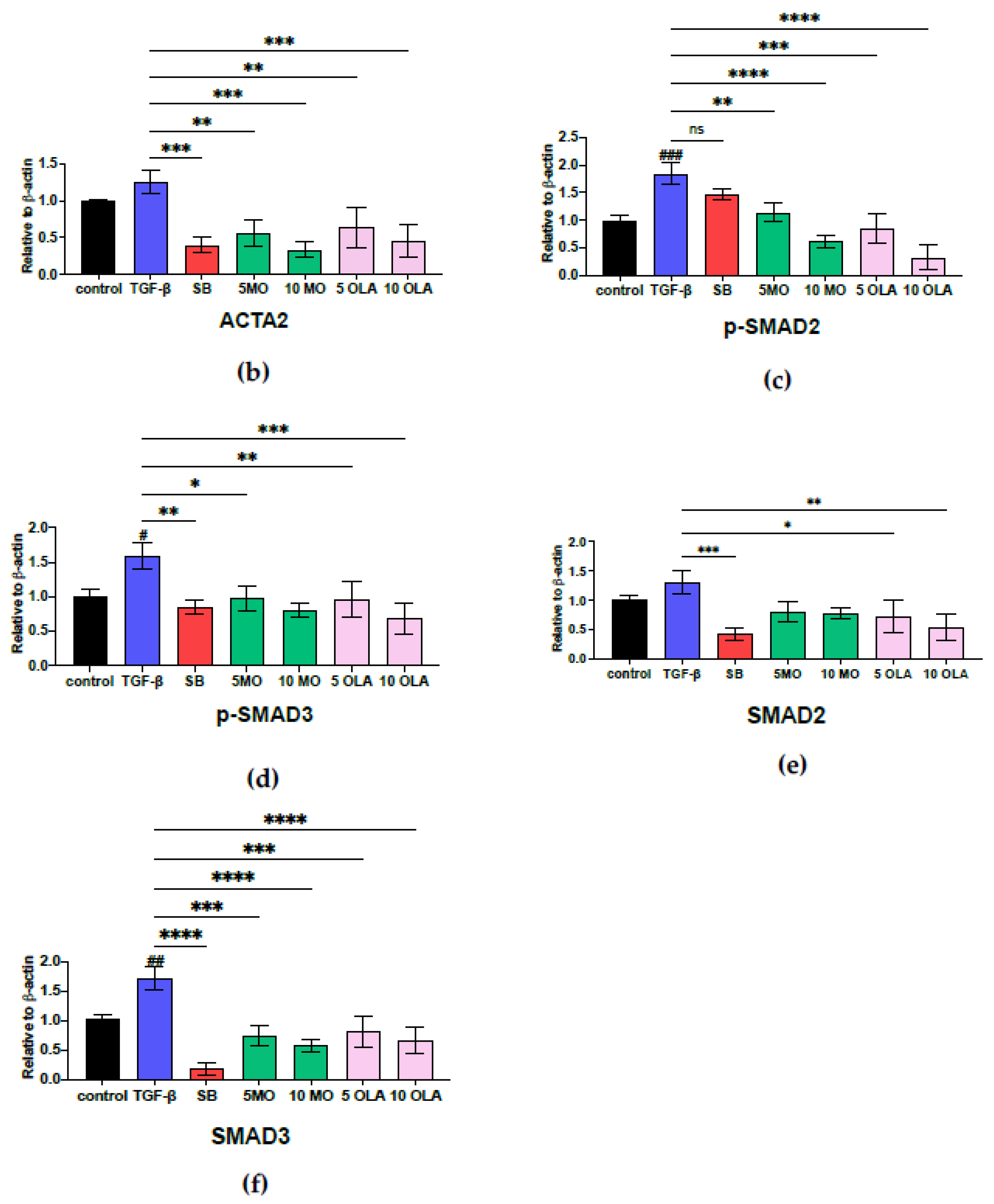
2.3. OLA Suppresses Fibrotic Markers via the SMAD2/3 Signaling Pathway at Translational Levels
2.4. In Silico Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Oleamide Identified from the Moringa oleifera Lam. Leaves
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Gelatin Degradation Assay
4.6. Real-Time Quantitative Reverse Transcription (qRT-PCR)
4.7. Molecular Docking
4.8. Western Blot Analysis
4.9. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, liver. In StatPearls—NCBI Bookshelf; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535438/ (accessed on 1 May 2023).
- Dolganiuc, A. Alcohol and Viral Hepatitis: Role of Lipid Rafts. PubMed 2015, 37, 299–309. Available online: https://pubmed.ncbi.nlm.nih.gov/26695752 (accessed on 12 March 2025).
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, A. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J. Gastroenterol. 2012, 18, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.; Hayes, P. Pathogenesis and treatment of hepatic fibrosis: Is cirrhosis reversible? Clin. Med. 2011, 11, 179–183. [Google Scholar] [CrossRef]
- Gitto, S.; Micco, L.; Conti, F.; Andreone, P.; Bernardi, M. Alcohol and viral hepatitis: A mini-review. Dig. Liver Dis. 2008, 41, 67–70. [Google Scholar] [CrossRef]
- Patel, R.; Mueller, M. Alcoholic Liver Disease. In StatPearls—NCBI Bookshelf; St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546632/ (accessed on 11 March 2025).
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef]
- Albillos, A.; Martin-Mateos, R.; Van Der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 112–134. [Google Scholar] [CrossRef]
- Campana, L.; Esser, H.; Huch, M.; Forbes, S. Liver regeneration and inflammation: From fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol. 2021, 22, 608–624. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2018, 65, 37–55. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Wiering, L.; Subramanian, P.; Hammerich, L. Hepatic Stellate Cells–Dictating outcome in non-alcoholic fatty liver disease. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.C.; Chen, J.J.; Xu, Q.X.; Zhou, M.H.; Lin, Y.; Zhang, Q.W.; Zhang, C.; Zhang, Z.G. Kinetin inhibits hepatic stellate cell activation and induces apoptosis via interactions with the TGF-β1/Smad signaling pathway. Toxicol. Appl. Pharmacol. 2023, 475, 116655. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, J.; Xing, L.; Li, C. Extra- and Intra-Cellular Mechanisms of Hepatic Stellate Cell Activation. Biomedicines 2021, 9, 1014. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Yoshida, K.; Murata, M.; Yamaguchi, T.; Zaki, K.M. TGF-β/Smad signaling during hepatic fibro-carcinogenesis (Review). Int. J. Oncol. 2014, 45, 1363–1371. [Google Scholar] [CrossRef]
- Su, X.; Lu, G.; Ye, L.; Shi, R.; Zhu, M.; Yu, X.; Li, Z.; Jia, X.; Feng, L. Moringa oleifera Lam.: A comprehensive review on active components, health benefits and application. RSC Adv. 2023, 13, 24353–24384. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 8571–8576. [Google Scholar] [CrossRef]
- Milla, P.G.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef] [PubMed]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.; Park, Y.; Suh, Y.; Choi, S.; Kim, M.; Cho, H.; Chang, Y.; Hong, B.; Kim, H.; Kim, E.; et al. Effects of Oleamide on Choline Acetyltransferase and Cognitive Activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, S.A.; Hong, J.H.; Kim, J.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Lee, J.H.; Kim, J.E.; Ha, J.; Kang, I. Oleamide suppresses lipopolysaccharide-induced expression of iNOS and COX-2 through inhibition of NF-κB activation in BV2 murine microglial cells. Neurosci. Lett. 2010, 474, 148–153. [Google Scholar] [CrossRef]
- Kita, M.; Ano, Y.; Inoue, A.; Aoki, J. Identification of P2Y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells. Sci. Rep. 2019, 9, 3135. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (accessed on 14 March 2024).
- Gatlin, A. Akero Therapeutics, 89bio Stocks Catapult on Promising MASH Treatment. Investor’s Business Daily. Available online: https://www.investors.com/news/technology/akero-therapeutics-89bio-stocks-mash-treatment/ (accessed on 28 January 2024).
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. New Drugs for Hepatic Fibrosis. Front. Pharmacol. 2022, 13, 874408. [Google Scholar] [CrossRef]
- Phaosri, M.; Jantrapirom, S.; Na Takuathung, M.; Soonthornchareonnon, N.; Sireeratawong, S.; Buacheen, P.; Pitchakarn, P.; Nimlamool, W.; Potikanond, S.; Salacia Chinensis, L. Stem extract exerts antifibrotic effects on human hepatic stellate cells through the inhibition of the TGF-β1-induced SMAD2/3 signaling pathway. Int. J. Mol. Sci. 2019, 20, 6314. [Google Scholar] [CrossRef]
- Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Khamto, N.; Sutana, P.; Potup, P.; Thongsri, Y.; Daowtak, K.; Ferrante, A.; Léon, C.; et al. In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells. Int. J. Mol. Sci. 2024, 25, 8995. [Google Scholar] [CrossRef]
- Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Potup, P.; Thongsri, Y.; Daowtak, K.; Ferrante, A.; Usuwanthim, K. Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model. Biomolecules 2024, 14, 800. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Zhang, X.; Xie, M.; Yang, C.; Tu, S.; Shen, H.; Hu, G.; Tao, L.; Yang, H. Fluorofenidone affects hepatic stellate cell activation in hepatic fibrosis by targeting the TGF-β1/Smad and MAPK signaling pathways. Exp. Ther. Med. 2019, 18, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kong, D.; Qiu, J.; Xie, Y.; Lu, Z.; Zhou, C.; Liu, X.; Zhang, R.; Wang, Y. Praziquantel ameliorates CCl4-induced liver fibrosis in mice by inhibiting TGF-β/Smad signalling via up-regulating Smad7 in hepatic stellate cells. Br. J. Pharmacol. 2019, 176, 4666–4680. [Google Scholar] [CrossRef] [PubMed]
- Samsuzzaman, M.; Kim, S.Y. Anti-fibrotic effects of DL-glyceraldehyde in hepatic stellate cells via activation of ERK-JNK-caspase-3 signaling axis. Biomol. Ther. 2023, 31, 425. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, O.S.; Attia, H.G.; Mohamed, B.M.; Elbaset, M.A.; Fayed, H.M. Current investigations for liver fibrosis treatment: Between repurposing the FDA-approved drugs and the other emerging approaches. J. Pharm. Pharm. Sci. 2023, 26, 11808. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]

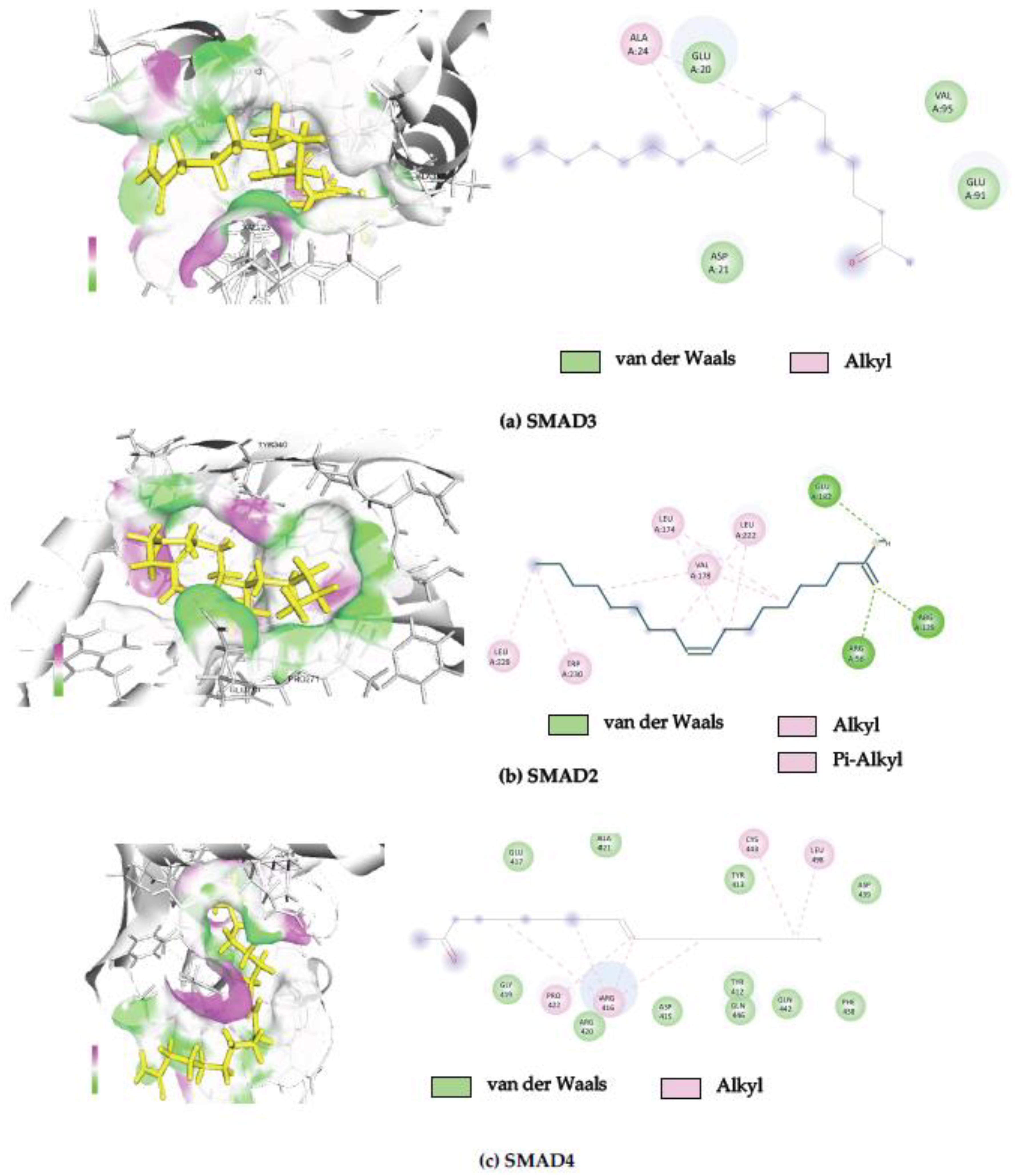
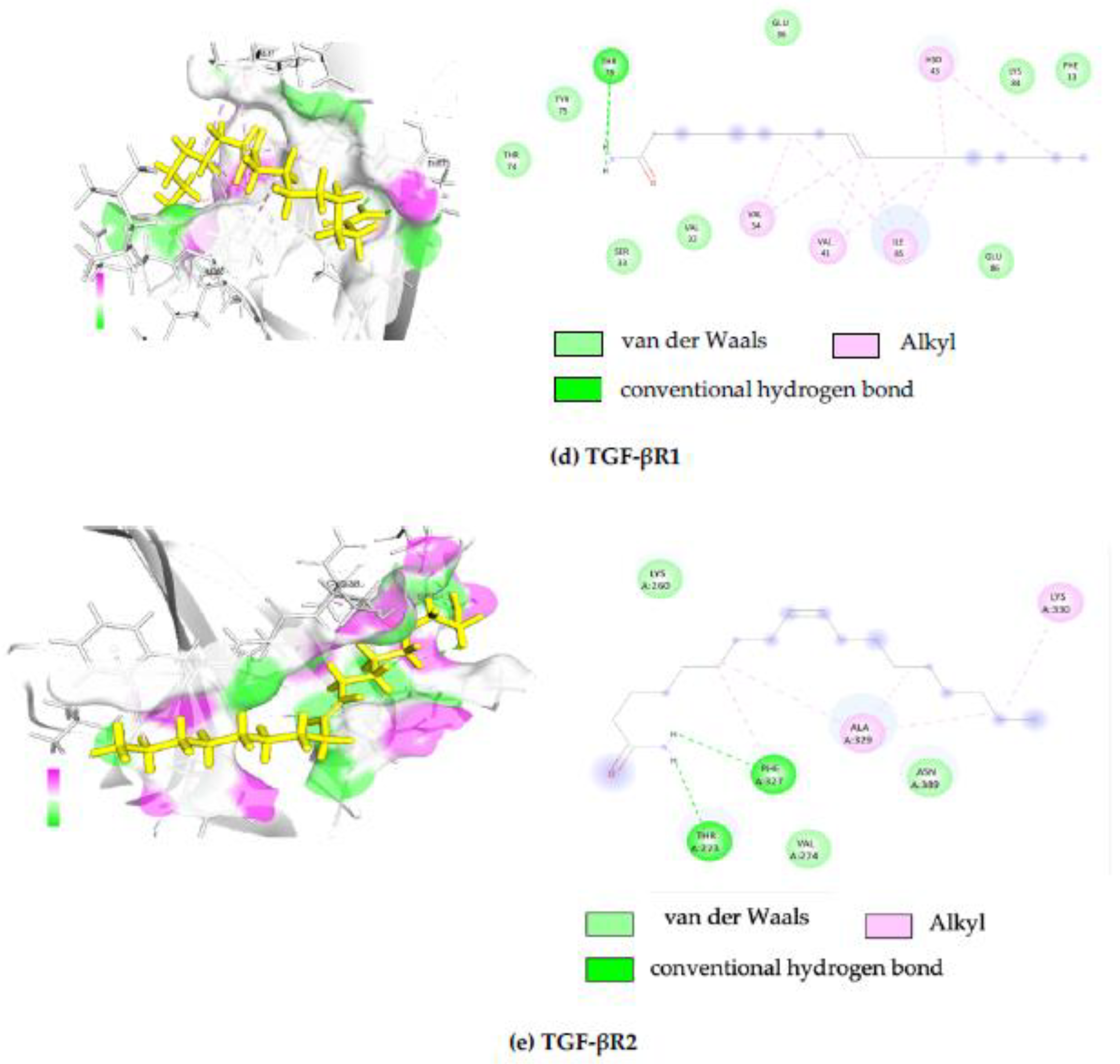
| PDB ID | Protein Name | Binding Free Energy (kcal/mol) | Interacting Amino Acid |
|---|---|---|---|
| 6YIB | Mothers against decapentaplegic homolog 3 | −8.27 | ALA24, GLU20, ASP21, VAL95, GLU91 |
| 6YIA | Mothers against decapentaplegic homolog 2 | −7.57 | LEU229, TRP230, VAL178, Leu174, LEU222, ARG56, ARG129, GLU182 |
| 1DD1 | Mothers against decapentaplegic homolog 4 | −7.42 | GLU417, GLY419, ALA421, PRO422, ARG416, ARG420, ASP415, TYR412, TYR413, GLN446, GLN442, PHE438, CYS443, LEU498, ASP439 |
| 5E8X | Transforming growth factor-beta (TGF-β) receptor type 1 | −7.16 | THR74, TYR75, THR73, SER33, VAL32, VAL34, VAL41, ILE85, GLU36, GLU86, HSD43, LYS84, PHE13 |
| 5E8V | Transforming growth factor-beta (TGF-β) receptor type 2 | −6.99 | LYS260, THR273, PHE327, VAL274, ALA329, ASN389, LYS330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongpiroon, C.; Buakaew, W.; Brindley, P.J.; Potikanond, S.; Daowtak, K.; Thongsri, Y.; Potup, P.; Usuwanthim, K. Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3388. https://doi.org/10.3390/ijms26073388
Khongpiroon C, Buakaew W, Brindley PJ, Potikanond S, Daowtak K, Thongsri Y, Potup P, Usuwanthim K. Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(7):3388. https://doi.org/10.3390/ijms26073388
Chicago/Turabian StyleKhongpiroon, Chavisa, Watunyoo Buakaew, Paul J. Brindley, Saranyapin Potikanond, Krai Daowtak, Yordhathai Thongsri, Pachuen Potup, and Kanchana Usuwanthim. 2025. "Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway" International Journal of Molecular Sciences 26, no. 7: 3388. https://doi.org/10.3390/ijms26073388
APA StyleKhongpiroon, C., Buakaew, W., Brindley, P. J., Potikanond, S., Daowtak, K., Thongsri, Y., Potup, P., & Usuwanthim, K. (2025). Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway. International Journal of Molecular Sciences, 26(7), 3388. https://doi.org/10.3390/ijms26073388










