Comparative Study of Placental Allografts with Distinct Layer Composition
Abstract
1. Introduction
2. Results
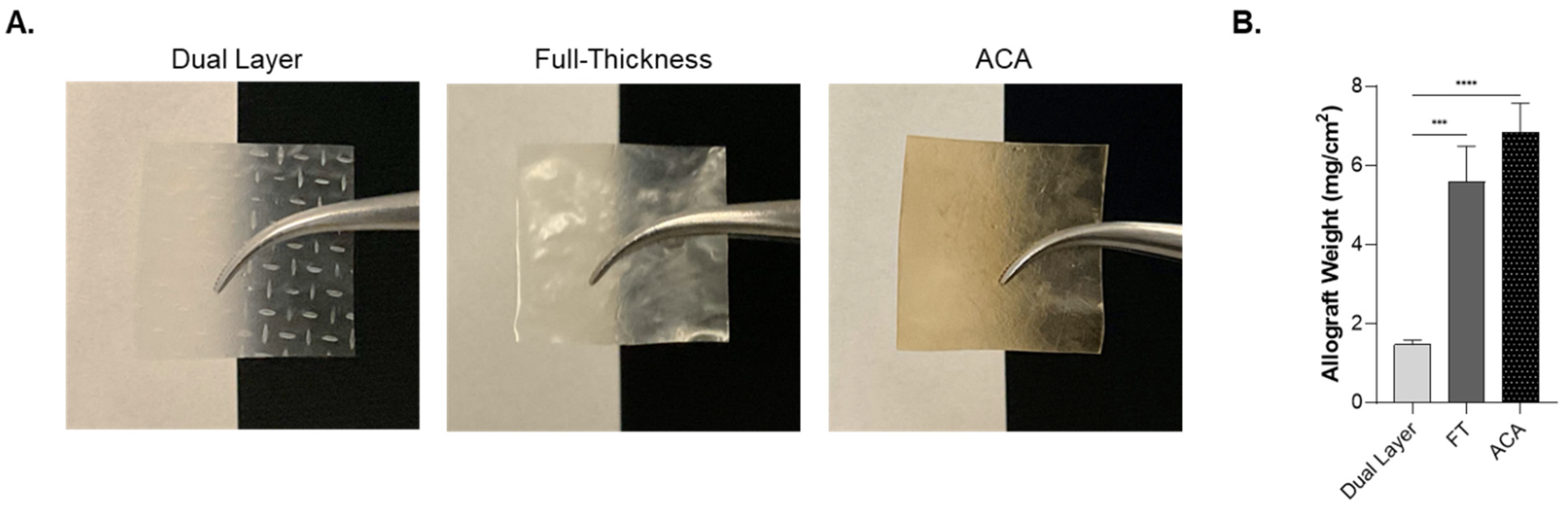
2.1. Physical Characteristics of Dual Layer, Full-Thickness, and ACA
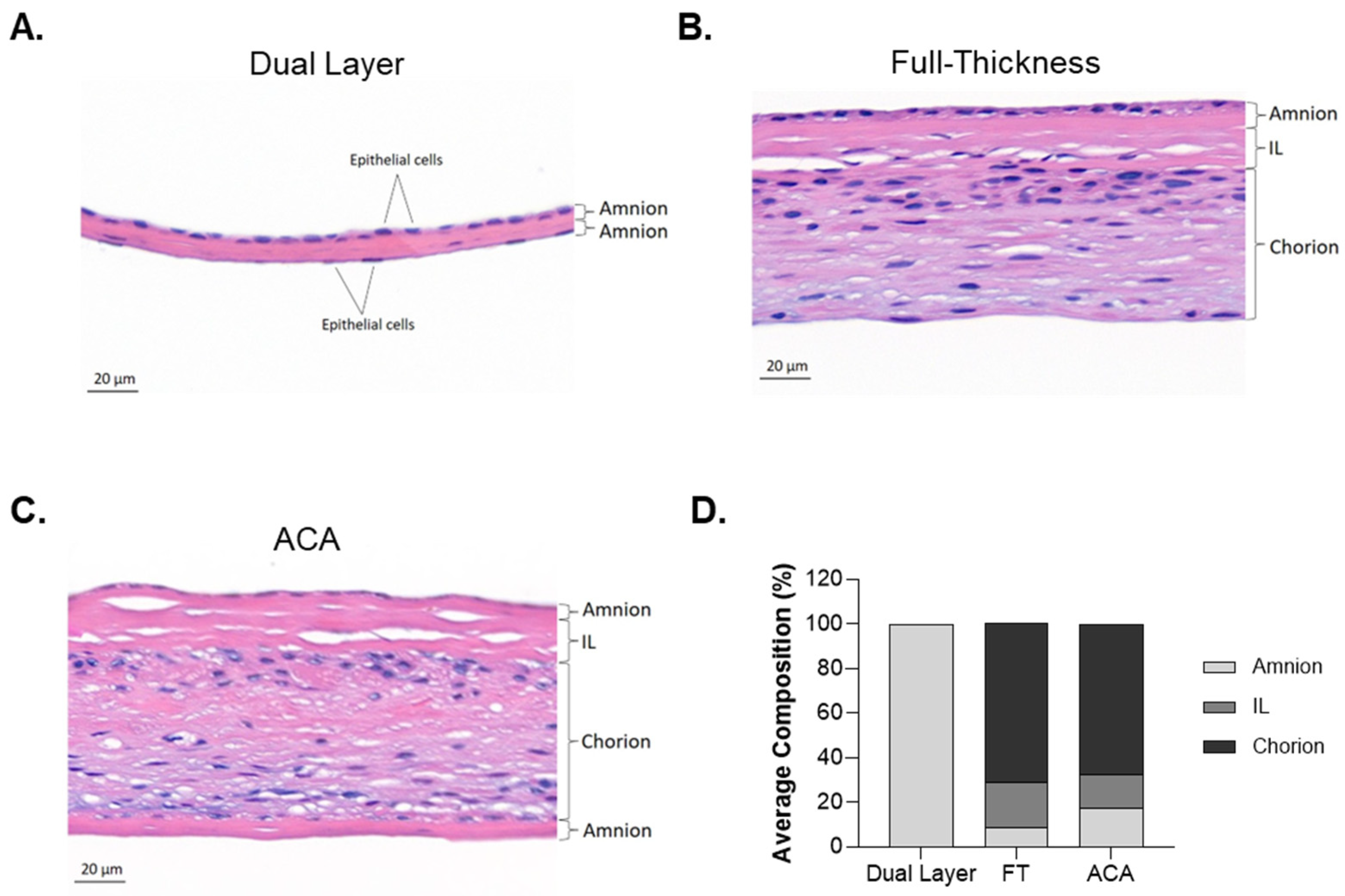
2.2. Differences in Structural Architecture Between Dual Layer, Full-Thickness, and ACA
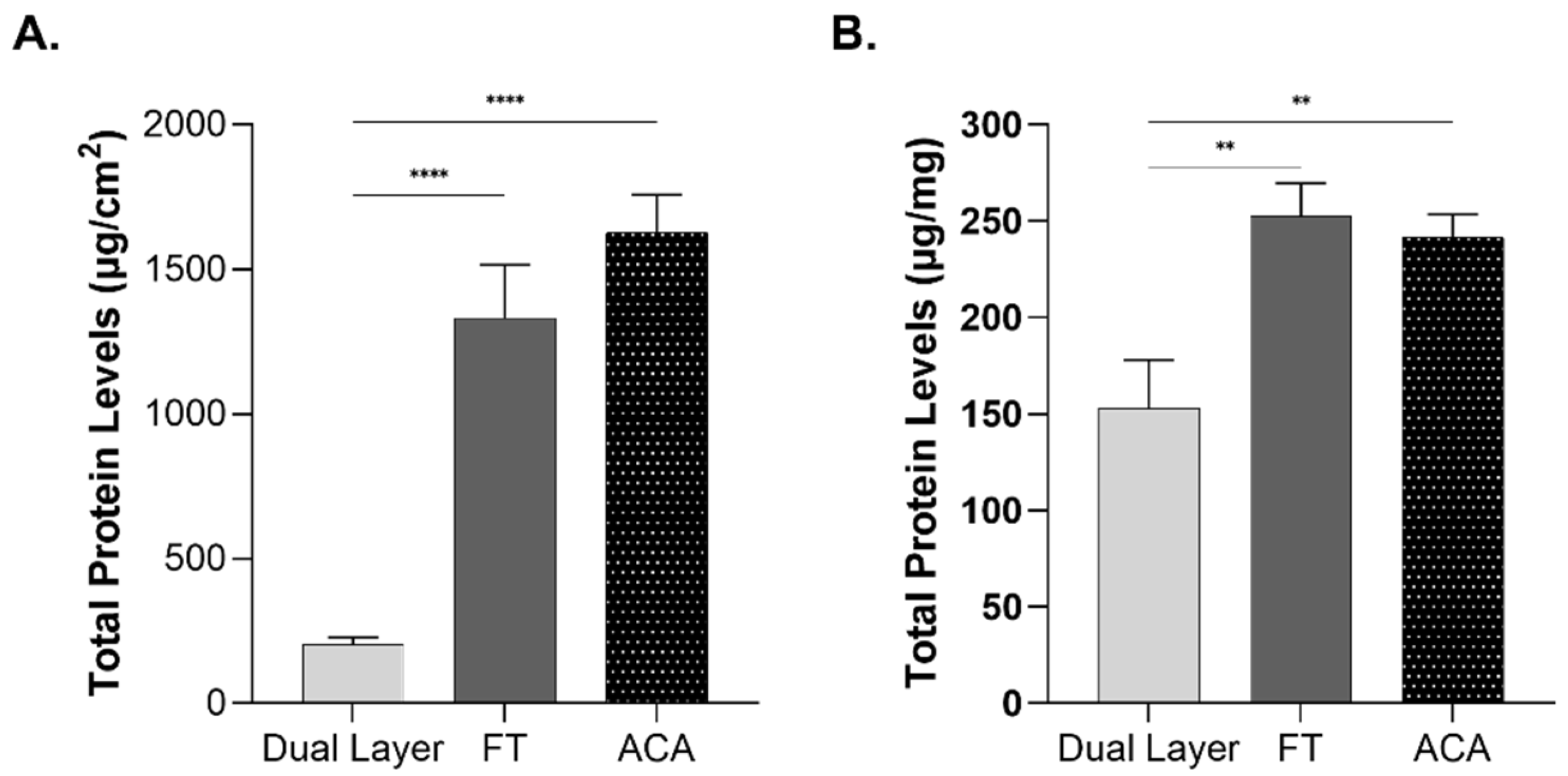
2.3. Influence of Compositional Layering on Total Protein Content
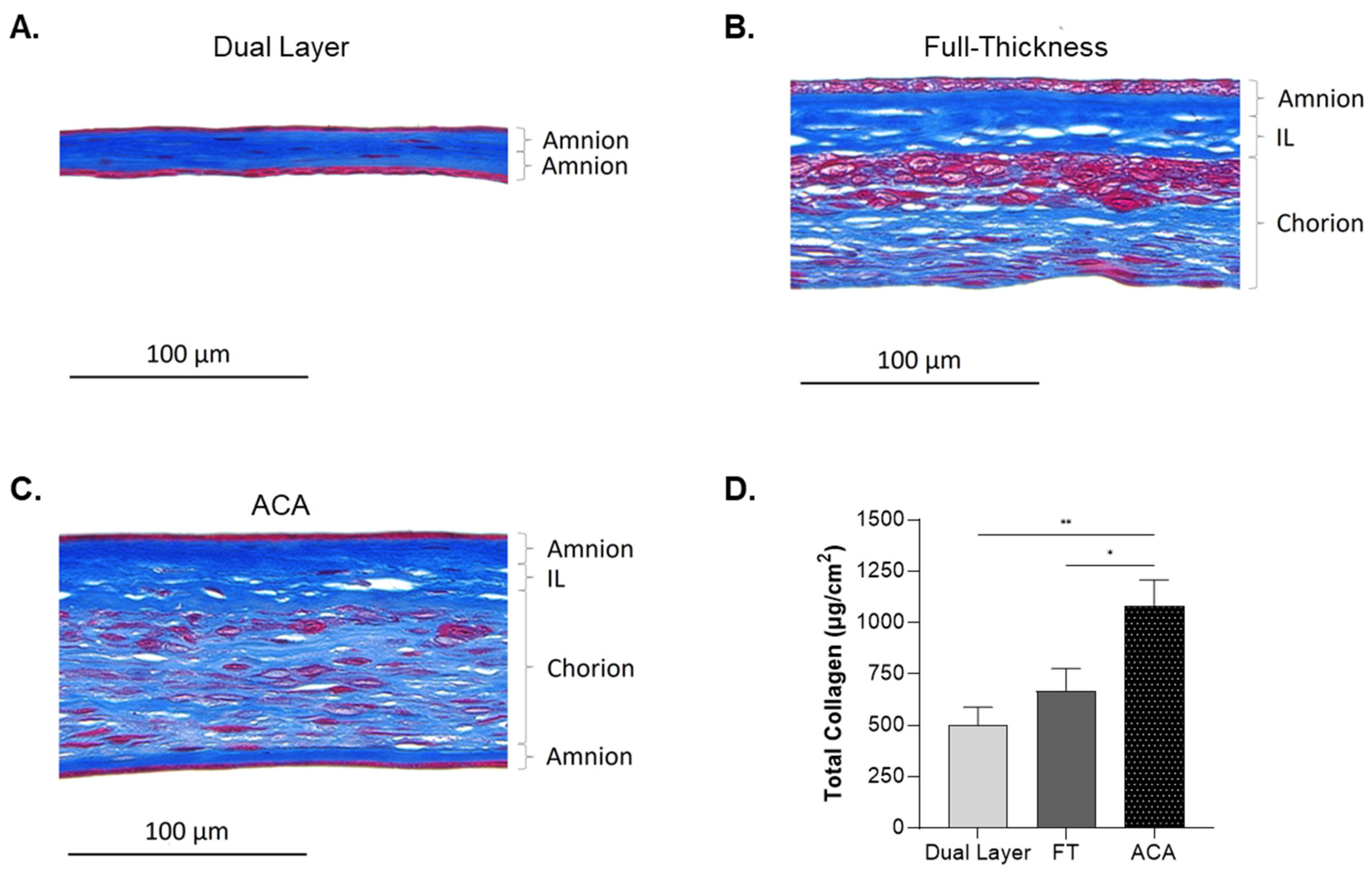
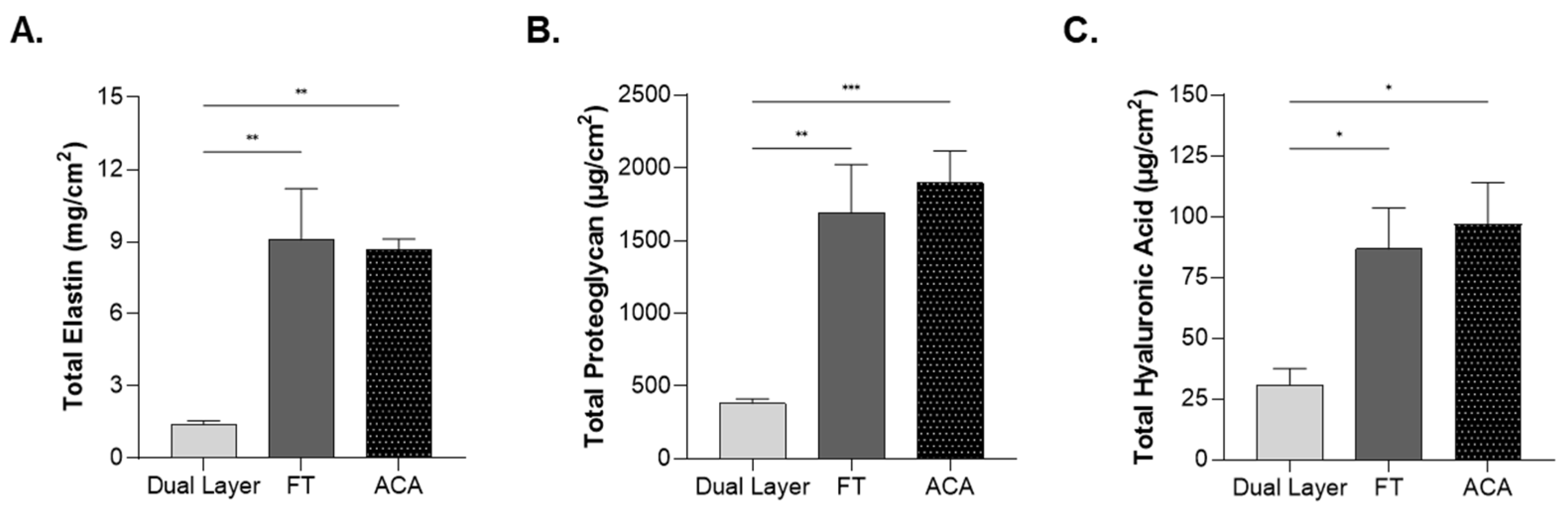
2.4. Impact of Compositional Layering on ECM Content
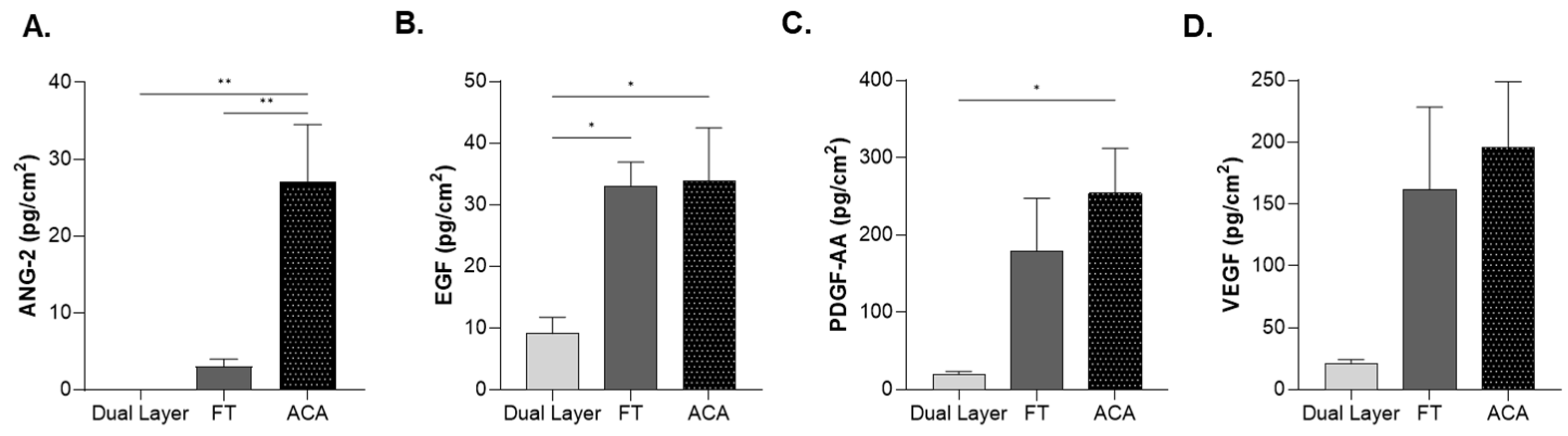
2.5. Influence of Compositional Layering on Growth Factors Levels
3. Discussion
4. Materials and Methods
4.1. Preparation of Placental Allograft
4.2. Histological Analysis
4.3. Compositional Layer Analysis
4.4. Quantification of Extracellular Matrix Components
4.4.1. Collagen
4.4.2. Elastin
4.4.3. Hyaluronic Acid
4.4.4. Proteoglycans
4.5. Total Protein Extraction and Quantification
4.6. Growth Factor Levels Analysis
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AATB | American Association of Tissue Banks |
| ACA | Amnion–Intermediate–Chorion–Amnion |
| ANG-2 | Angiopoietin-2 |
| ANOVA | Analysis of Variance |
| CAMPs | Cellular, Acellular, Matrix-like Products |
| CRF | Code of Federal Regulation |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FDA | Food and Drug Administration |
| FT | Full-Thickness |
| HA | Hyaluronic Acid |
| HCl | Hydrochloric Acid |
| hDACM | Dehydrated Human Amnion/Chorion Membrane |
| H&E | Hematoxylin and Eosin |
| IL | Intermediate Layer |
| IND | Investigational New Drug |
| IP | Intellectual Property |
| IRB | Institutional Review Board |
| LLC | Limited Liability Company |
| MDPI | Multidisciplinary Digital Publishing Institute |
| microBCA | Micro Bicinchoninic Acid |
| NaCl | Sodium Chloride |
| nm | Nanometers |
| PDGF-AA | Platelet-Derived Growth Factor-AA |
| RIPA Buffer | Radioimmunoprecipitation Assay Buffer |
| ROUT | Robust Regression and Outlier Removal |
| rpm | Rotations Per Minute |
| RT | Room Temperature |
| SD | Standard Deviation |
| SEM | Standard Error Mean |
| sGAG | Sulfated Glycosaminoglycan |
| μg/cm2 | Micrograms per Square Centimeter |
| μg/mg | Micrograms per Milligram |
| μm | Micrometer |
| VEGF | Vascular Endothelial Growth Factor |
References
- O’Connor, B.B.; Pope, B.D.; Peters, M.M.; Ris-Stalpers, C.; Parker, K.K. The Role of Extracellular Matrix in Normal and Pathological Pregnancy: Future Applications of Microphysiological Systems in Reproductive Medicine. Exp. Biol. Med. 2020, 245, 1163–1174. [Google Scholar] [CrossRef]
- Bryant-Greenwood, G.D. The Extracellular Matrix of the Human Fetal Membranes: Structure and Function. Placenta 1998, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Carter, M.; Cole, W.; Crombie, R.; Kapp, D.L.; Kim, P.; Milne, C.; Molnar, J.; Niezgoda, J.; Woo, K.; et al. Best Practice for Wound Repair and Regeneration Use of Cellular, Acellular and Matrix-like Products (CAMPs). J. Wound Care 2023, 32, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Griffiths, S. Intermediate Layer Contribution in Placental Membrane Allografts. J. Tissue Eng. Regen. Med. 2020, 14, 1126–1135. [Google Scholar] [CrossRef]
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental Tissues as Biomaterials in Regenerative Medicine. Biomed. Res. Int. 2022, 2022, 6751456. [Google Scholar] [CrossRef]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denozière, G. Properties of Dehydrated Human Amnion/Chorion Composite Grafts: Implications for Wound Repair and Soft Tissue Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1353–1362. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the Amniotic Membrane for Potential Use in Tissue Engineering. Eur. Cell Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Derby, A.; Abd Elkodous, M.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the Amniotic Membrane in Tissue Engineering and Regeneration: The Hundred-Year Challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.-S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Protzman, N.M.; Mao, Y.; Long, D.; Sivalenka, R.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering 2023, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Lim, J.J.; Zabek, N.; Massee, M. Cytokines in Single Layer Amnion Allografts Compared to Multilayer Amnion/Chorion Allografts for Wound Healing. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Bonvallet, P.P.; Damaraju, S.M.; Modi, H.N.; Stefanelli, V.L.; Lin, Q.; Saini, S.; Gandhi, A. Biophysical Characterization of a Novel Tri-Layer Placental Allograft Membrane. Adv. Wound Care 2022, 11, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sabol, T.J.; Tran, G.S.; Matuszewski, J.; Weston, W.W. Standardized Reporting of Amnion and Amnion/Chorion Allograft Data for Wound Care. Health Sci. Rep. 2022, 5, e794. [Google Scholar] [CrossRef]
- Wetzell, B.; Ork, B.; Softic, D.; Morse, J.; Hutchens, W.; Meng, F.; McLean, J.B.; Moore, M.A.; Qin, X. Characterization of a Full-thickness Decellularized and Lyophilized Human Placental Membrane for Clinical Applications. Int. Wound J. 2024, 21, e14888. [Google Scholar] [CrossRef]
- Maquart, F.X.; Monboisse, J.C. Extracellular Matrix and Wound Healing. Pathol. Biol. 2014, 62, 91–95. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Zou, Y.; Lu, G.; Ronca, A.; D’Amora, U.; Liang, J.; Fan, Y.; Zhang, X.; Sun, Y. Advanced Application of Collagen-Based Biomaterials in Tissue Repair and Restoration. J. Leather Sci. Eng. 2022, 4, 30. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Antonicelli, F.; Bellon, G.; Lorimier, S.; Hornebeck, W. Role of the Elastin Receptor Complex (S-Gal/Cath-A/Neu-1) in Skin Repair and Regeneration. Wound Repair. Regen. 2009, 17, 631–638. [Google Scholar] [CrossRef]
- Sicari, B.M.; Londono, R.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix as a Bioscaffold for Tissue Engineering. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–172. [Google Scholar] [CrossRef]
- Koob, T.J.; Rennert, R.; Zabek, N.; Massee, M.; Lim, J.J.; Temenoff, J.S.; Li, W.W.; Gurtner, G. Biological Properties of Dehydrated Human Amnion/Chorion Composite Graft: Implications for Chronic Wound Healing. Int. Wound J. 2013, 10, 493–500. [Google Scholar] [CrossRef] [PubMed]
- McQuilling, J.P.; Kammer, M.; Kimmerling, K.A.; Mowry, K.C. Characterisation of Dehydrated Amnion Chorion Membranes and Evaluation of Fibroblast and Keratinocyte Responses in Vitro. Int. Wound J. 2019, 16, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Cazzell, S.; Vayser, D.; Reyzelman, A.M.; Dosluoglu, H.; Tovmassian, G.; EpiFix VLU Study Group. A Multicentre Randomised Controlled Trial Evaluating the Efficacy of Dehydrated Human Amnion/Chorion Membrane (EpiFix®) Allograft for the Treatment of Venous Leg Ulcers. Int. Wound J. 2018, 15, 114–122. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Jacobs, A.M.; Zelen, C.M. Use of an Aseptically Processed, Dehydrated Human Amnion and Chorion Membrane Improves Likelihood and Rate of Healing in Chronic Diabetic Foot Ulcers: A Prospective, Randomised, Multi-centre Clinical Trial in 80 Patients. Int. Wound J. 2018, 15, 950–957. [Google Scholar] [CrossRef]
- Cazzell, S.M.; Caporusso, J.; Vayser, D.; Davis, R.D.; Alvarez, O.M.; Sabolinski, M.L. Dehydrated Amnion Chorion Membrane versus Standard of Care for Diabetic Foot Ulcers: A Randomised Controlled Trial. J. Wound Care 2024, 33, S4–S14. [Google Scholar] [CrossRef]
- Amenta, P.S.; Gay, S.; Vaheri, A.; Martinez-Hernandez, A. The Extracellular Matrix Is an Integrated Unit: Ultrastructural Localization of Collagen Types I, III, IV, V, VI, Fibronectin, and Laminin in Human Term Placenta. Coll. Relat. Res. 1986, 6, 125–152. [Google Scholar] [CrossRef]
- Hitscherich, P.G.; Chnari, E.; Deckwa, J.; Long, M.; Khalpey, Z. Human Placental Allograft Membranes: Promising Role in Cardiac Surgery and Repair. Front. Cardiovasc. Med. 2022, 9, 809960. [Google Scholar] [CrossRef]
- Lin, J.; Shi, Y.; Men, Y.; Wang, X.; Ye, J.; Zhang, C. Mechanical Roles in Formation of Oriented Collagen Fibers. Tissue Eng. Part B Rev. 2020, 26, 116–128. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern Collagen Wound Dressings: Function and Purpose. J. Am. Col. Certif. Wound Spec. 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Hieber, A.D.; Corcino, D.; Motosue, J.; Sandberg, L.B.; Roos, P.J.; Yeh Yu, S.; Csiszar, K.; Kagan, H.M.; Boyd, C.D.; Bryant-Greenwood, G.D. Detection of Elastin in the Human Fetal Membranes: Proposed Molecular Basis for Elasticity. Placenta 1997, 18, 301–312. [Google Scholar] [CrossRef]
- Rnjak, J.; Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Severe Burn Injuries and the Role of Elastin in the Design of Dermal Substitutes. Tissue Eng. Part B Rev. 2011, 17, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin Signaling in Wound Repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.B.; Grootveld, M.; Farrell, A.; Smith, E.C.; Thompson, P.W.; Blake, D.R. A Pathological Role for Damaged Hyaluronan in Synovitis. Ann. Rheum. Dis. 1991, 50, 196–200. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Halper, J. Proteoglycans and Diseases of Soft Tissues. In Progress in Heritable Soft Connective Tissue Diseases; Halper, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2014; Volume 802, pp. 49–58. ISBN 978-94-007-7892-4. [Google Scholar]
- Nazari Hashemi, P.; Chaventre, F.; Bisson, A.; Gueudry, J.; Boyer, O.; Muraine, M. Mapping of Proteomic Profile and Effect of the Spongy Layer in the Human Amniotic Membrane. Cell Tissue Bank. 2020, 21, 329–338. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound Dressings as Growth Factor Delivery Platforms for Chronic Wound Healing. Expert. Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Nagaraja, S.; Chen, L.; DiPietro, L.A.; Reifman, J.; Mitrophanov, A.Y. Predictive Approach Identifies Molecular Targets and Interventions to Restore Angiogenesis in Wounds With Delayed Healing. Front. Physiol. 2019, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in Surgical Applications of Growth Factors for Wound Healing. Burn. Trauma 2019, 7, s41038-019-0148-1. [Google Scholar] [CrossRef]
- Kim, H.; Kong, W.H.; Seong, K.-Y.; Sung, D.K.; Jeong, H.; Kim, J.K.; Yang, S.Y.; Hahn, S.K. Hyaluronate—Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules 2016, 17, 3694–3705. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between Extracellular Matrix and Growth Factors in Wound Healing. Wound Repair. Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H. Growth Factors and Cytokines in Wound Healing. Wound Repair. Regen. 2008, 16, 565–576. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.J.; Keller, J.; Li, W.W. A Prospective, Randomised, Controlled, Multi-centre Comparative Effectiveness Study of Healing Using Dehydrated Human Amnion/Chorion Membrane Allograft, Bioengineered Skin Substitute or Standard of Care for Treatment of Chronic Lower Extremity Diabetic Ulcers. Int. Wound J. 2015, 12, 724–732. [Google Scholar] [CrossRef]
- ISO 1137-2; Sterilization of Health Care Products-Radiation-Part 2: Establishing the Sterilization Dose. Association for the Advancement of Medical Instrumentation: Arlington, MA, USA, 2013.
- Standards for Tissue Banking 14th Edition; American Association of Tissue Banks: Arlington, MA, USA, 2016.
| Dual Layer | Full-Thickness | ACA | |
|---|---|---|---|
| Total Thickness (μm) 1 | 26.97 ± 16.89 | 133.23 ± 77.77 a | 153.19 ± 57.74 a |
| Amnion (%) 2 | 100% | 4–12% | 9–29% |
| Intermediate Layer (%) 2 | - | 11–33% | 7–36% |
| Chorion (%) 2 | - | 56–86% | 38–81% |
| Growth Factor Levels (pg/cm2) | Dual Layer | Full-Thickness | ACA |
|---|---|---|---|
| ANG-2 | 1.08 × 10−14 ± 0 | 3.05 ± 2.36 | 27.1 ± 20.9 a,b |
| EGF | 9.12 ± 6.95 | 33.1 ± 10.9 a | 33.9 ± 24.3 a |
| PDGF-AA | 22.3 ± 8.51 | 179 ± 194 | 205 ± 97 a |
| VEGF | 20.9 ± 9.24 | 162 ± 189 | 196 ± 151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Easley, A.; Menchaca, K.T.; Fanniel, V.; Gomez, R.; Marquez, J.; Hill, S. Comparative Study of Placental Allografts with Distinct Layer Composition. Int. J. Mol. Sci. 2025, 26, 3406. https://doi.org/10.3390/ijms26073406
Singh P, Easley A, Menchaca KT, Fanniel V, Gomez R, Marquez J, Hill S. Comparative Study of Placental Allografts with Distinct Layer Composition. International Journal of Molecular Sciences. 2025; 26(7):3406. https://doi.org/10.3390/ijms26073406
Chicago/Turabian StyleSingh, Pragya, Acarizia Easley, Karla Tapia Menchaca, Victor Fanniel, Raymond Gomez, Joanna Marquez, and Shauna Hill. 2025. "Comparative Study of Placental Allografts with Distinct Layer Composition" International Journal of Molecular Sciences 26, no. 7: 3406. https://doi.org/10.3390/ijms26073406
APA StyleSingh, P., Easley, A., Menchaca, K. T., Fanniel, V., Gomez, R., Marquez, J., & Hill, S. (2025). Comparative Study of Placental Allografts with Distinct Layer Composition. International Journal of Molecular Sciences, 26(7), 3406. https://doi.org/10.3390/ijms26073406




