Agronomic and Metabolic Responses of Citrus clementina to Long-Term Irrigation with Saline Reclaimed Water as Abiotic Factor
Abstract
:1. Introduction
2. Results and Discussion
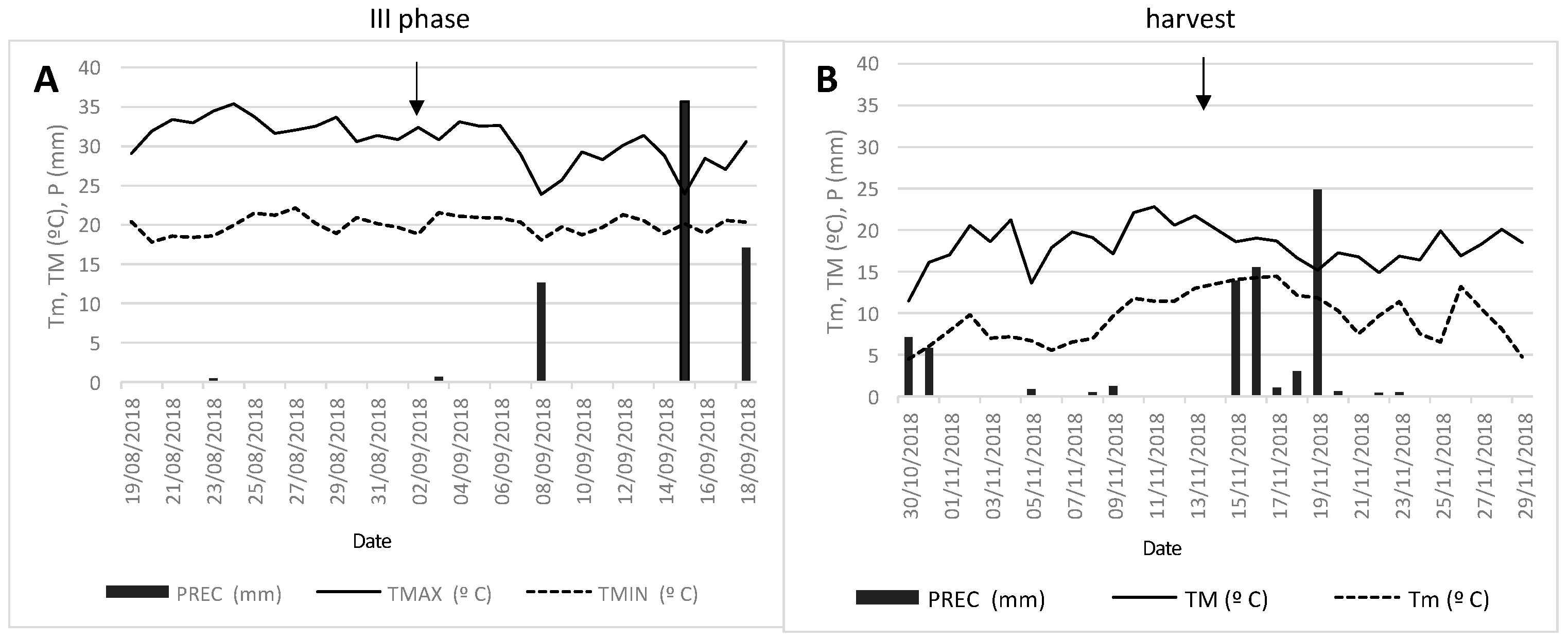
2.1. Meteorological Conditions and Irrigation Water Analysis
2.2. Response of Nutrient and Agrometabolites at Leaf Level to Saline-Reclaimed Water
2.2.1. Nutritional Status
2.2.2. Amino Acid Metabolism
2.2.3. Individually Characterizing Phytohormones
2.3. Response of Agronomic Parameters to Saline Reclaimed Water: Tree Canopy, Yield, and Fruit Quality
2.4. Correlation Between the Main Agrometabolic and Agronomic Parameters
3. Materials and Methods
3.1. Experimental Plot and Irrigation Treatments
3.2. Water Analyses
3.3. Chemicals
3.4. Nutrient and Agrometabolites at Leaf Level
3.4.1. Nutritional Status
3.4.2. Free Amino Acid Extraction and Chromatographic Determination
3.4.3. Qualitative and Quantitative Determination of Phytohormones
3.5. Agronomic Parameters: Growth Canopy, Yield, and Quality Fruit
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- O’Connell, E. Towards adaptation of water resource systems to climatic and socio-economic change. Water Resour. Manag. 2017, 31, 2965–2984. [Google Scholar]
- Martínez-Alvarez, V.; González-Real, M.M.; Baille, A.; Valero, J.M.; Elvira, B.G. Regional assessment of evaporation from agricultural irrigation reservoirs in a semiarid climate. Agric. Water Manag. 2008, 95, 1056–1066. [Google Scholar]
- Romero-Trigueros, C.; Alarcón-Cabañero, J.J.; Tortosa, P.A.N.; Gambín, J.M.G.; Maestre-Valero, J.F.; Nicolás, N.N. Medium-long term effects of saline reclaimed water and regulated deficit irrigation on fruit quality of citrus. J. Sci. Food Agric. 2020, 100, 1350–1357. [Google Scholar]
- Global Water Market. Meeting the World’s Water and Wastewater Needs Until 2020; Global Water Intelligence: Oxford, UK, 2017. [Google Scholar]
- EUR-Lex. Commission Notice Guidelines to Support the Application of Regulation 2020/741 on Minimum Requirements for Water Reuse 2022/C 298/01. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.C_.2022.298.01.0001.01.ENG&toc=OJ%3AC%3A2022%3A298%3ATOC#ntc3-C_2022298EN.01000101-E0003 (accessed on 13 February 2025).
- Gómez-Bellot, M.J.; Lorente, B.; Medina, S.; Gil-Izquierdo, A.; Durand, T.; Galano, J.M.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J. Acute and rapid response of Melissa officinalis and Mentha spicata to saline reclaimed water in terms of water relations, hormones, amino acids and plant oxylipins. Plants 2022, 11, 3427. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Lorente, B.; Ortuño, M.F.; Medina, S.; Gil-Izquierdo, A.; Bañón, S.; Sánchez-Blanco, M.J. Recycled wastewater and reverse osmosis brine use for halophytes irrigation: Differences in physiological, nutritional and hormonal responses of Crithmum maritimum and Atriplex halimus plants. Agronomy 2021, 11, 627. [Google Scholar] [CrossRef]
- Nicolás, E.; Alarcón, J.J.; Mounzer, O.; Pedrero, F.; Nortes, P.A.; Alcobendas, R.; Romero Trigueros, C.; Bayona, J.M.; Marstre-Valero, J.F. Long-term physiological and agronomic responses of mandarin trees to irrigation with saline reclaimed water. Agric. Water Manag. 2016, 166, 1–8. [Google Scholar]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Ruiz-Navarro, A.; Alarcón, J.J.; Garcia, C.; Nicolás, E. Comparing the impacts of drip irrigation by freshwater and reclaimed wastewater on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar]
- Adrover, M.; Farrús, E.; Moyà, G.; Vadell, J. Chemical properties and biological activity in soils of Mallorca following twenty years of treated wastewater irrigation. J. Environ. Manag. 2012, 95, 188–192. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Special Report on Climate Change, Desertification, Land Degradation, Sutainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Available online: https://www.ipcc.ch/srccl/ (accessed on 13 February 2025).
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Nicolás, E. Determination of 15N stable isotope natural abundances for assessing the use of saline reclaimed water in grapefruit. Environ. Eng. Manag. J. 2014, 13, 2525–2530. [Google Scholar]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Droogers, P.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on Citrus physiology assessed by UAV remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [PubMed]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar]
- Alva, A.C.; Syvertsen, J.P. Irrigation water salinity affects soil nutrient distribution root density and leaf nutrient levels of citrus under drip fertigation. J. Plant Nutr. 1991, 14, 715–727. [Google Scholar]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and salinity in citriculture: Optimal practices to alleviate salinity and water stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Balfagón Sanmartín, D. Participación del ABA en la respuesta de las plantas a la combinación de sequía y calor. Master’s Thesis, Universitat Jaume I, Castellón de la Plana, Spain, 2016. [Google Scholar]
- Hutton, M.J.; van Staden, J. Transport and Metabolism of 8(14C)Zeatin Zeatin Applied to Leaves of Citrus sinensis. Z. Pflanzenphysiol. 1983, 111, 75–83. [Google Scholar]
- Osorio Mora, O.; Zacarías, L. Efecto de las bajas temperaturas en la biosintesis de etileno en discos de flavedo de la mandarina “fortune”. Rev. Iber. Tecnol. Postcosecha 2000, 3, 53–64. [Google Scholar]
- Sonnino, A.; Ruane, J. La innovación en agricultura como herramienta de la política de seguridad alimentaria: El caso de las biotecnologías agrícolas. In Biotecnologías e Innovación: El Compromiso Social de la Ciencia; Editorial Pontificia Universidad Javeriana: Bogotà, Colombia, 2013; pp. 25–52. Available online: https://www.fao.org/4/ar635s/ar635s.pdf (accessed on 20 February 2025).
- Castillo Sánchez, E.J. Importancia de los aminoácidos en la agricultura bajo condiciones de estrés abiótico. Bachelor’s Thesis, Universidad Técnica de Babahoyo, Babahoyo, Ecuador, 2022. Available online: http://dspace.utb.edu.ec/handle/49000/11367 (accessed on 20 February 2025).
- Khoshbakht, D.; Asgharei, M.R. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica 2015, 53, 410–418. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Vincent, C.I.; Grosser, J.W. The response of salt-stressed Valencia sweet orange (Citrus sinensis) to salicylic acid and methyl jasmonate treatments. Plant Physiol. Rep. 2021, 26, 137–151. [Google Scholar]
- Boman, B.J. First-year response of ‘Ruby Red’ grapefruit on four rootstocks to fertilization and salinity. In Proceedings of the Florida-State Horticultural Society, Miami Beach, FL, USA, 19–21 October 1993; Volume 106, pp. 12–18. [Google Scholar]
- Romero-Trigueros, C.; Nortes, P.A.; Pedrero, F.; Mounzer, O.; Alarcón, J.J.; Bayona, J.M.; Nicolás, E. Assessment of the sustainability of using saline reclaimed water in grapefruit in medium to long term. Span. J. Agric. Res. 2014, 12, 1137–1148. [Google Scholar]
- Ferguson, L.; Grattan, S.R. How salinity damages citrus: Osmotic and specific ion effects. Hort. Technol. 2015, 15, 95–99. [Google Scholar]
- Pérez-Pérez, J.G.; García-Sánchez, F.; Robles, J.M.; Botía, P. ‘Star Ruby’ grapefruit and ‘Clemenules’ mandarin trees show different physiological and agronomic responses to irrigation with saline water. Irrig. Sci. 2015, 33, 191–204. [Google Scholar] [CrossRef]
- Vives-Peris, V.; López-Climent, M.F.; Moliner-Sabater, M.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Morphological, physiological, and molecular scion traits are determinant for salt-stress tolerance of grafted citrus plants. Front. Plant Sci. 2023, 14, 1145625. [Google Scholar] [CrossRef] [PubMed]
- Bradford, G.R. Lithium Toxicity in southern California citrus. Calif. Agric. 1961, 15, 14. [Google Scholar]
- Martin, J.P.; Helmkamp, G.K.; Ervin, J.O. Effect of Bromide from a Soil Fumigant and from CaBr2 on Growth and Chemical Composition of Citrus Plants. Soil Sci. Soc. Am. J. 1956, 20, 143–300. [Google Scholar]
- Cámara, J.M.; García-Sánchez, F.; Martínez, V.; Nieves, M.; Cerdá, A. Effect of NaCl on citrus cultivars. Agronomie 2004, 24, 155–160. [Google Scholar]
- Scholberg, J.M.; Parsons, L.R.; Wheaton, T.A.; McNeal, B.L.; Morgan, K.T. Soil temperature, nitrogen concentration, and residence time affect nitrogen uptake efficiency in citrus. J. Environ. Qual. 2002, 31, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Abou Seeda, M.A.; Abou El-Nour, E.A.A.; Yassen, A.A.; Gad Mervat, M.; Zaghloul, S.M. Importance of sulfur and its roles in Plants physiology: A Review. Curr. Sci. Int. 2020, 9, 98–231. [Google Scholar]
- Kaur, N.; Monga, P.K.; Arora, P.K.; Kumar, K. Effect of micronutrients on leaf composition, fruit quality and yield of Kinnow mandarin. J. Appl. Nat. Sci. 2015, 7, 639–643. [Google Scholar]
- Rabe, E.; Lovatt, C.J. De Novo Arginine Biosynthesis in Leaves of Phosphorus-Deficient Citrus and Poncirus Species. Plant Physiol. 1984, 76, 747–752. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Perez, J.L.; Kunta, M.; Patt, J.M.; Mangan, R.L. Changes in free amino acids and polyamine levels in Satsuma leaves in response to Asian citrus psyllid infestation and water stress. Insect Sci. 2014, 21, 707–716. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep. 2017, 36, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, C.; Delfine, S.; Pizzuto, R.; Loreto, F.; Fuggi, A. Free amino acids and glycine betaine in leaf osmoregulation spinach responding to Blackwell Publishing Ltd. increasing salt stress. New Phytol. 2003, 1, 455–463. [Google Scholar]
- Abadía, J.; Bastida, F.; Romero-Trigueros, C.; Bayona, J.M.; Vera, A.; García, C.; Alarcón, J.J.; Nicolás, E. Interactions between soil microbial communities and agronomic behaviour in a mandarin crop subjected to water deficit and irrigated with reclaimed water. Agric. Water Manag. 2021, 247, 106749. [Google Scholar]
- Ros, R.; Muñóz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar]
- Li, Y.; Fan, K.; Shen, J.; Wang, Y.; Jeyaraj, A.; Hu, S.; Chen, X.; Ding, Z.; Li, X. Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant. Foods 2023, 12, 334. [Google Scholar] [CrossRef]
- Hernández-Miñana, F.M.; Primo-Millo, E. Studies on endogenous cytokinins in citrus. J. Hortic. Sci. 1990, 65, 595–601. [Google Scholar]
- Glass, A.D.M.; Dunlop, J. Influence of phenolic acids on ion uptake: IV. Depolarization of membrane potentials. Plant Physiol. 1974, 54, 855–858. [Google Scholar]
- Barkosky, R.R.; Einhellig, F.A. Effects of salicylic acid on plant-water relationships. J. Chem. Ecol. 1993, 19, 237–247. [Google Scholar]
- Aldesuquy, H.S.; Mankarios, A.T.; Awad, H.A. Effect of some antitranspirants on growth, metabolism and productivity of saline-treated wheat plants: Induction of stomatal closure, inhibiton of transpitration and improvement of leaf turgidity. Acta Bot. Hung. 1998, 40, 1–10. [Google Scholar]
- Leslie, C.A.; Romani, R.J. Inhibition of ethylene biosynthesis by salicylic acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 138, 87–96. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Moinuddin, A.S. Salicylic acid acts as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J. Crop Sci. Biotechnol. 2010, 13, 183–188. [Google Scholar]
- Raskin, I. Role of salicylic acid in plants. Ann. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar]
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; Javed, H.U.; Altaf, M.A.; Tu, P.; et al. Influence of Citrus Rootstocks on Scion Growth, Hormone Levels, and Metabolites Profile of ‘Shatangju’ Mandarin (Citrus reticulata Blanco). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Berli, F.J.; Alonso, R.E.; Pharis, R.P.; Bottini, A.R. Applied GA5, GA4, and GA4/7 increase berry number per bunch, yield, and grape quality for winemaking in Vitis vinifera L. cv. Malbec. J. Sci. Food Agric. 2021, 11, 1–10. [Google Scholar]
- Tonetto de Freitas, S.; Cai-Zhong, J.; Mitcham, E.J. Mechanisms Involved in Calcium Deficiency Development in Tomato Fruit in Response to Gibberellins. J. Plant Growth Regul. 2012, 3, 221–234. [Google Scholar]
- Gómez-Cadenas, A.; Arbona, V.; Jacas, J.; Primo-Millo, E.; Talon, M. Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J. Plant Growth Regul. 2003, 21, 234–240. [Google Scholar]
- Xie, R.; Zhang, J.; Ma, Y.; Pan, X.; Dong, C.; Pang, S.; He, S.; Deng, L.; Yi, S.; Zheng, Y.; et al. Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots. Sci. Rep. 2017, 7, 42094. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Camañes, G.; García-Agustín, P.J. Ammonium enhances resistance to salinity stress in citrus plants. Plant Physiol. 2012, 169, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, E.; Romero Trigueros, C.; Nortes Tortosa, P.A.; Pedrero Salcedo, F.; Bayona Gambín, J.M.; Maestre Valero, J.F.; Alarcón Cabañero, J.J. Long-Term Physiological and Agronomic Responses of Citrus Irrigated with Saline Reclaimed Water. In Water Scarcity a Sustainable Agriculture in Semiarid Environmnet; Academic Press: Cambridge, MA, USA, 2018; Chapter 7; 582p, ISBN 9780128131657. [Google Scholar]
- Contreras, S.; Pérez-Cutillas, P.; Santoni, C.S.; Romero-Trigueros, C.; Pedrero, F.; Alarcón, J.J. Effects of reclaimed waters on spectral properties and leaf traits of citrus orchards. Water Environ. Res. 2014, 86, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.F.; He, Z.; Stoffella, P.J.; Montes, C.R.; Melfi, A.J.; Baligar, V.C. Nutrients and nonessential elements in soil after 11 years of wastewater irrigation. J. Environ. Qual. 2012, 41, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Embleton, T.W.; Jones, W.W. Effects of potassium on peel thickness and juiciness of lemon fruits. Hortic. Sci. 1966, 1, 25–26. [Google Scholar]
- Iglesias, D.J.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talon, M. In vivo sucrose stimulation of colour change in citrus fruit epicarps: Interactions between nutritional and hormonal signals. Physiol. Plant 2001, 112, 244–250. [Google Scholar]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Khan, M.N.; Hayat, F.; Asim, M.; Iqbal, S.; Ashraf, T.; Asghar, S. Influence of Citrus Rootstocks on Growth Performance and Leaf Mineral Nutrition of “Salustiana” Sweet Orange [Citrus sinensis (L.). Obsek]. J. Pure Appl. Agric. 2020, 5, 2617–2680. [Google Scholar]
- Soil Survey Staff. Illustrated Guide to Soil Taxonomy; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, Nebraska, 2014. Available online: https://www.secs.com.es/wp-content/uploads/2014/09/Illustrated-Guide-to-Soil-Taxonomy1.pdf (accessed on 1 April 2025).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Quidelines for Computing Crop Water Requirements; FAO Irrigation & Drainage Paper; FAO: Rome, Italy, 1998; p. 56. [Google Scholar]
- Watson, M.E.; Galliher, T.L. Comparison of Dumas and Kjeldahl methods with automatic analyzers on agricultural samples under routine rapid analysis conditions. Commun. Soil Sci. Plant 2006, 32, 2007–2019. [Google Scholar]
- Collado-González, J.; Cruz, Z.N.; Rodríguez, P.; Galindo, A.; Ferreres, F.; Medina, S.; Romojaro, F.; Egea, I.; Torrecillas, A.; Gil-Izquierdo, A. Effects of water deficit during maturation on amino acids and jujube fruit eating quality. Maced. J. Chem. Chem. Eng. 2014, 33, 105–119. [Google Scholar]
- Cerrillo, I.; Fernández-Pachón, M.S.; Collado-González, J.; Herrero-Martín, G.; Berná, G.; Escudero-López, B.; Ferreres, F.; Gil-Izquierdo, A. Effect of Fermentation and Subsequent Pasteurization Processes on Amino Acids Composition of Orange Juice. Plant Foods Hum. Nutr. 2015, 70, 153–159. [Google Scholar]
- Hutchinson, D.J. Influence of rootstock on the performance of Valencia sweet orange. In Proceedings of the International Socienty of Citriculture Congress, Orlando, FL, USA, 1–8 May 1977; Volume 2, pp. 523–525. [Google Scholar]
- Mc Guiere, R.G. Reporting of objective colour measurements. HortScience 1992, 27, 1254–1255. [Google Scholar]
| Parameter | Unit | FW | RW |
|---|---|---|---|
| ECw | dS m | 1.38 ± 0.12 | 3.41 ± 0.13 |
| SARw | (meq L−1)0.5 | 3.35 ± 0.69 | 6.09 ± 1.89 |
| pH | 8.10 ± 0.03 | 7.73 ± 0.06 | |
| Ca2+ | meq L−1 | 4.68 ± 0.70 | 7.35 ± 0.83 |
| Mg2+ | meq L−1 | 4.50 ± 5.02 | 8.27 ± 6.89 |
| K+ | mg L−1 | 15.76 ± 4.16 | 31.46 ± 3.37 |
| Na+ | meq L−1 | 7.19 ± 3.20 | 17.03 ± 4.10 |
| B | mg L−1 | 0.19 ± 0.04 | 0.60 ± 0.08 |
| Cl− | meq L− | 6.35 ± 3.98 | 17.10 ± 3.63 |
| NO3− | mg L−1 | 3.89 ± 0.73 | 10.50 ± 2.27 |
| PO43− | mg L−1 | 1.14 ± 1.58 | 2.86 ± 0.87 |
| SO42− | meq L−1 | 7.92 ± 5.10 | 14.90 ± 6.20 |
| Toxicity Elements | Macro Nutrients | Micronutrients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | FW | RW | Units | FW | RW | Units | FW | RW | |||
| Na+ | % dw | 0.05 ± 0.03 a | 0.05 ± 0.01 a | N total | % dw | 2.09 ± 0.08 a | 1.91 ± 0.07 a | Cu | mg kg−1 | 4.55 ± 0.68 a | 4.74 ± 0.61 a |
| Cl− | % dw | 0.40 ± 0.13 a | 0.98 ± 0.12 b | P | % dw | 0.09 ± 0 a | 0.08 ± 0 a | Zn2+ | mg kg−1 | 12.7 ± 0.83 a | 11.14 ± 0.55 a |
| B | mg kg−1 | 83.64 ± 11.54 a | 169.91 ± 6.70 b | K+ | % dw | 1.01 ± 0.11 a | 0.88 ± 0.03 a | Fe | mg kg−1 | 138.27 ± 5.67 a | 97.47 ± 17.12 a |
| Li+ | mg kg−1 | 6.81 ± 0.44 a | 10.48 ± 0.83 b | Ca2+ | % dw | 4.07 ± 0.06 a | 3.08 ± 0.11 a | Mn | mg kg−1 | 38.54 ± 1.31 a | 37.61 ± 1.22 a |
| Br− | % dw | 0.004 ± 0 a | 0.01 ± 0 b | Mg2+ | % dw | 0.39 ± 0.02 a | 0.38 ± 0.01 a | Ni3 | mg kg−1 | 0.93 ± 0.07 a | 1.09 ± 0.07 a |
| S | % dw | 0.24 ± 0.01 b | 0.21 ± 0.01 a | ||||||||
| NO3− | % dw | 0.34 ± 0.43 a | 0.84 ± 0.25 a | ||||||||
| PO43− | % dw | 1.62 ± 0.15 a | 2.23 ± 0.61 a | ||||||||
| SO42− | % dw | 1.48 ± 0.45 a | 2.27 ± 0.09 a | ||||||||
| Amino Acids (ng g−1) * | Phytohormones (ng g−1) * | ||||
|---|---|---|---|---|---|
| FW | RW | FW | RW | ||
| Ala | 32,882.50 ± 79.43 b | 10,915.80 ± 1318.94 a | ACC | 2563.85 ± 344.29 b | 1664.95 ± 78.98 a |
| Pro | 10,994.40 ± 869.72 a | 6403.35 ± 2027.23 a | tZ | 709.377 ± 126.13 a | 1150.7 ± 43.23 b |
| Tyr | 2450.23 ± 1010.53 a | 2597.47 ± 237.78 a | SA | 127.66 ± 17.08 a | 327.55 ± 44.28 b |
| GABA | 1411.76 ± 332.792 a | 816.86 ± 108.39 a | GA4 | 95.64 ± 12.62 b | 20.59 ± 4.56 a |
| Ser | 1119.24 ± 73.54 b | 607.38 ± 182.69 a | JA | 4.95 ± 0.60 a | 5.29 ± 1.13 a |
| Cys–Gly | 878.17 ± 287.304 a | 693.27 ± 394.28 a | ABA | 0.08 ± 0.05 a | 2.54 ± 0.25 b |
| Leu | 95.79 ± 35.23 a | 654.13 ± 575.74 a | Zr | 1.35 ± 1.62 a | 0 ± 0 a |
| Arg | 422.40 ± 60.68 a | 321.83 ± 39.09 a | IPA | 0.14 ± 0.01 a | 0.15 ± 0.01 a |
| Asn | 242.63 ± 63.50 a | 267.72 ± 108.00 a | GA3 | 0 ± 0 a | 0.14 ± 0.03 b |
| Glu | 251.53 ± 95.95 a | 136.36 ± 30.72 a | |||
| Val | 150.80 ± 63.34 a | 150.05 ± 25.31 a | |||
| Met | 128.61 ± 18.507 a | 79.15 ± 25.21 a | |||
| Asp | 112.07 ± 3.15 a | 50.38 ± 40.17 a | |||
| Gly | 89.47 ± 5.46 b | 39.59 ± 17.34 a | |||
| Phe | 38.80 ± 25.097 a | 38.17 ± 27.72 a | |||
| Citrulline | 46.40 ± 25.423 a | 27.35 ± 4.46 a | |||
| Thr | 25.22 ± 4.51 a | 22.29 ± 5.00 a | |||
| His | 19.45 ± 8.87 a | 12.17 ± 3.45 a | |||
| Units | FW | RW | |
|---|---|---|---|
| CG | m3 | 20.26 ± 1.79 b | 18.18 ± 2.60 a |
| Yield | kg pl−1 | 68.49 ± 12.48 a | 58.18 ± 13.53 a |
| FN | 809.77 ± 134.09 a | 720.88 ± 167.71 a | |
| W | g | 93.53 ± 16.13 b | 80.70 ± 5.68 a |
| D | mm | 60.04 ± 4.44 b | 56.47 ± 1.97 a |
| PT | mm | 3.02 ± 0.40 b | 1.57 ± 0.23 a |
| JC | mL | 42.93 ± 4.88 b | 38.67 ± 5.77 a |
| SSC | °Brix | 13.77 ± 0.48 b | 11.68 ± 0.29 a |
| TA | % | 0.99 ± 0.08 a | 1.41 ± 0.11 b |
| MI | 14.04 ± 1.32 b | 8.32 ± 0.72 a |
| B | Br− | Ala | ACC | tZ | SA | GA4 | ABA | GA3 | GT | W | D | PT | JC | SSC | TA | MI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | 0.9268 | 0.8850 | −0.8955 | −0.9195 | 0.9321 | 0.9601 * | −0.9368 | 0.9355 | 0.9582 * | −0.8183 | −0.9854 * | −0.9993 ** | −0.9476 | −0.9979 ** | −0.8573 | 0.9392 | −0.9217 |
| B | 0.9893 * | −0.9907 ** | −0.9320 | 0.9687 * | 0.9464 | −0.9811 * | 0.9713 * | 0.9366 | −0.9680 * | −0.9688 * | −0.9120 | −0.9957 ** | −0.9218 | −0.8941 | 0.8660 | −0.9100 | |
| Br− | −0.9997 ** | −0.8702 | 0.9231 | 0.9455 | −0.9856 * | 0.9789 * | 0.9365 | −0.9918 ** | −0.9286 | −0.8673 | −0.9872 * | −0.8718 | −0.9304 | 0.8667 | −0.9251 | ||
| Ala | 0.8742 | −0.9261 | −0.9529 * | 0.9893 * | −0.9832 * | −0.9444 | 0.9884 * | 0.9355 | 0.8786 | 0.9904 ** | 0.8823 | 0.9348 | −0.8777 | 0.9325 | |||
| ACC | −0.9927 ** | −0.8369 | 0.8660 | −0.8470 | −0.8241 | 0.8259 | 0.9655 * | 0.9111 | 0.9161 | 0.9372 | 0.6987 | −0.7477 | 0.7606 | ||||
| tZ | 0.8825 | −0.9157 | 0.8991 | 0.8702 | −0.8870 | −0.9793 * | −0.9211 | −0.9554 * | −0.9427 | −0.7712 | 0.7930 | −0.8178 | |||||
| SA | −0.9869 * | 0.9915 ** | 0.9996 ** | −0.9019 | −0.9484 | −0.9535 * | −0.9723 * | −0.9416 | −0.9671 * | 0.9808 * | −0.9917 ** | ||||||
| GA4 | −0.9990 ** | −0.9822 * | 0.9588 * | 0.9524 * | 0.9247 | 0.9934 ** | 0.9208 | 0.9618 * | −0.9378 | 0.9723 * | |||||||
| ABA | 0.9880 * | −0.9504 * | −0.9444 | −0.9242 | −0.9876 * | −0.9171 | −0.9720 * | 0.9503 * | −0.9817 * | ||||||||
| GA3 | −0.8911 | −0.9417 | −0.9524 * | −0.9651 * | −0.9386 | −0.9684 * | 0.9860 * | −0.9938 ** | |||||||||
| GT | 0.8773 | 0.7965 | 0.9589 * | 0.8031 | 0.9097 | −0.8088 | 0.8860 | ||||||||||
| W | 0.9792 * | 0.9745 * | 0.9875 * | 0.8467 | −0.8948 | 0.8999 | |||||||||||
| D | 0.9351 | 0.9975 ** | 0.8464 | −0.9388 | 0.9152 | ||||||||||||
| PT | 0.9388 | 0.9241 | −0.9087 | 0.9433 | |||||||||||||
| JC | 0.8249 | −0.9152 | 0.8954 | ||||||||||||||
| SSC | −0.9535 * | 0.9877 * | |||||||||||||||
| TA | −0.9869 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auñón-Calles, D.; Pinciroli, M.; Nicolás, E.; Gil-Izquierdo, A.; Gabaldón, J.A.; Sánchez-Iglesias, M.P.; Carbonell-Barrachina, A.A.; Ferreres, F.; García, C.J.; Romero-Trigueros, C. Agronomic and Metabolic Responses of Citrus clementina to Long-Term Irrigation with Saline Reclaimed Water as Abiotic Factor. Int. J. Mol. Sci. 2025, 26, 3450. https://doi.org/10.3390/ijms26073450
Auñón-Calles D, Pinciroli M, Nicolás E, Gil-Izquierdo A, Gabaldón JA, Sánchez-Iglesias MP, Carbonell-Barrachina AA, Ferreres F, García CJ, Romero-Trigueros C. Agronomic and Metabolic Responses of Citrus clementina to Long-Term Irrigation with Saline Reclaimed Water as Abiotic Factor. International Journal of Molecular Sciences. 2025; 26(7):3450. https://doi.org/10.3390/ijms26073450
Chicago/Turabian StyleAuñón-Calles, David, María Pinciroli, Emilio Nicolás, Angel Gil-Izquierdo, José Antonio Gabaldón, María Puerto Sánchez-Iglesias, Angel Antonio Carbonell-Barrachina, Federico Ferreres, Carlos J. García, and Cristina Romero-Trigueros. 2025. "Agronomic and Metabolic Responses of Citrus clementina to Long-Term Irrigation with Saline Reclaimed Water as Abiotic Factor" International Journal of Molecular Sciences 26, no. 7: 3450. https://doi.org/10.3390/ijms26073450
APA StyleAuñón-Calles, D., Pinciroli, M., Nicolás, E., Gil-Izquierdo, A., Gabaldón, J. A., Sánchez-Iglesias, M. P., Carbonell-Barrachina, A. A., Ferreres, F., García, C. J., & Romero-Trigueros, C. (2025). Agronomic and Metabolic Responses of Citrus clementina to Long-Term Irrigation with Saline Reclaimed Water as Abiotic Factor. International Journal of Molecular Sciences, 26(7), 3450. https://doi.org/10.3390/ijms26073450












