Advancements in Crop PUFAs Biosynthesis and Genetic Engineering: A Systematic and Mixed Review System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic and Mixed with Bibliometric Reviews: Key Methods in Evidence Synthesis
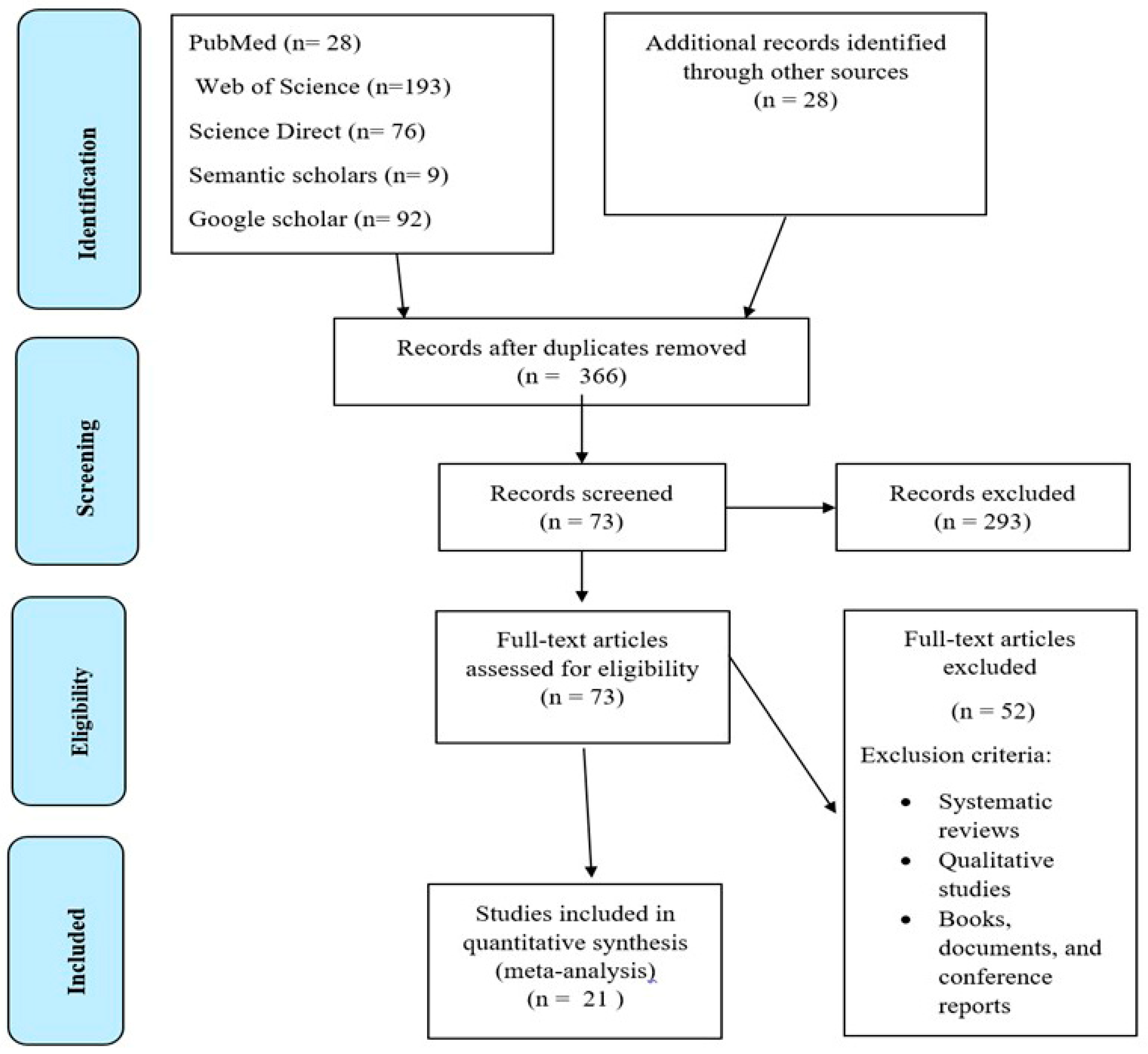
2.2. Review Question and Searches Strategy
2.3. Data Sources
2.4. Data Extraction and Software Application
2.5. Term Map
3. Results and Discussion
3.1. Evidence Synthesis
3.2. Citation Counts
3.3. Core Journals
3.4. Time of Publication, Authors, and Countries/Territories
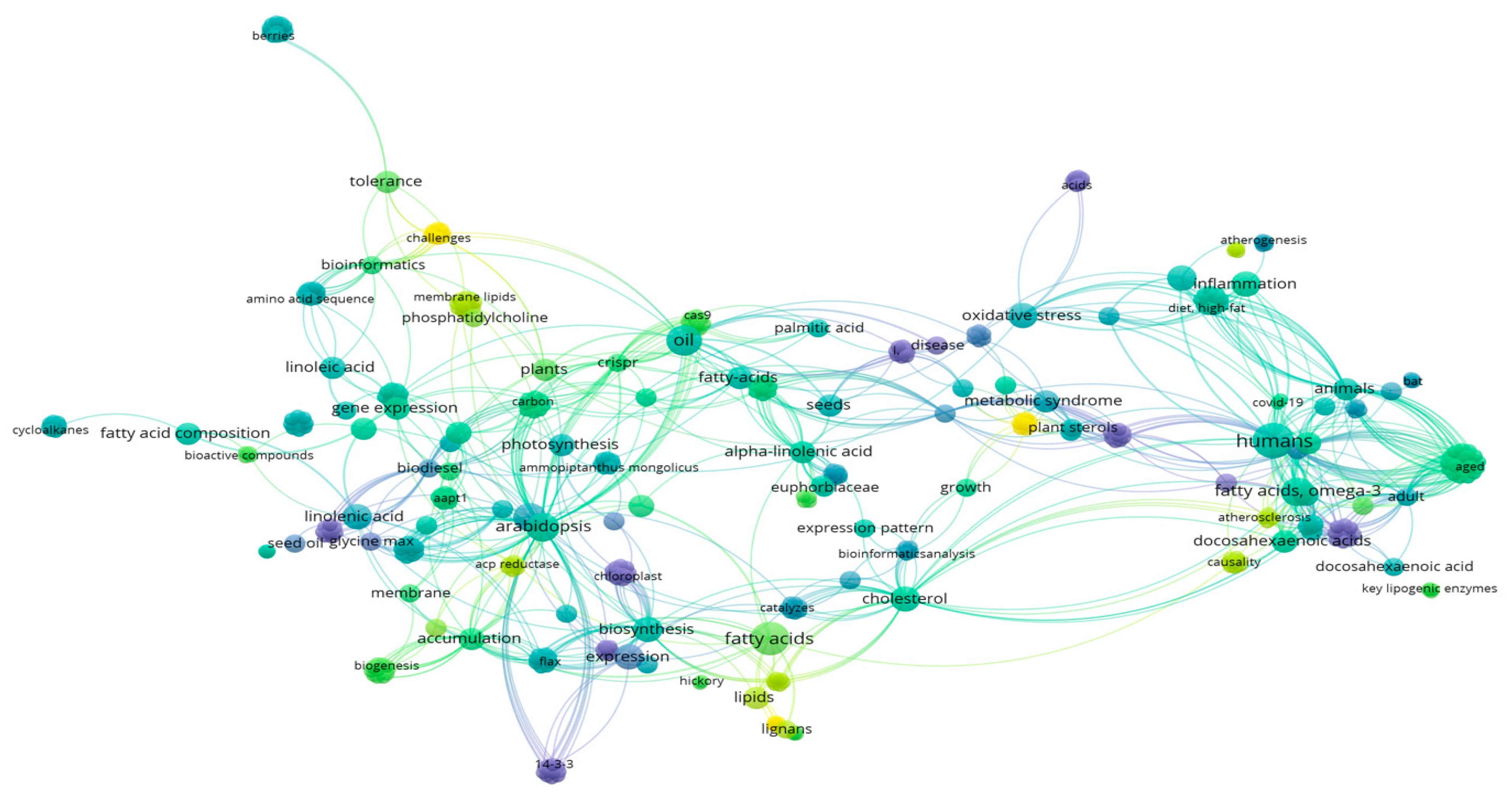
3.5. Term Map
3.6. Appraising the Evidence of 21 Original Research Articles and Summary
3.7. The Broad Spectrum of PUFAs: ALA and LA Oil Crop Biosynthesis and Progression (2014–2024)
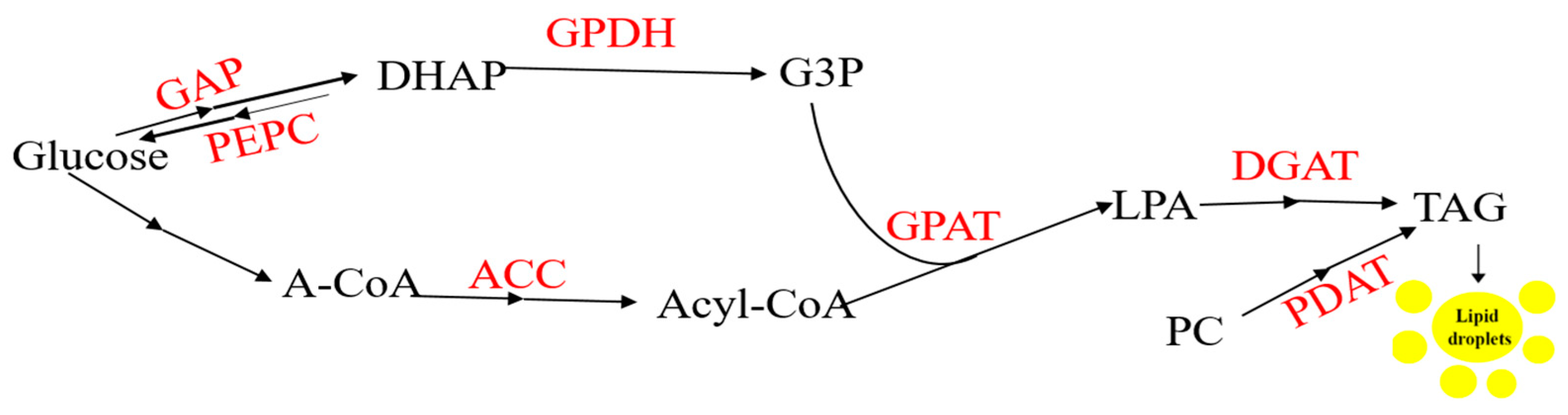
3.8. Profiling Naturally Oil Rich Crops and Schematic Representation of Lipid Biosynthetic Pathway
3.9. Study Limitations
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awasthi, S.; Kaushik, N.; Plaha, N.S.; Kaur, V.; Kumar, A. Exploring lipid health indices and protein quality in ninety Indian linseed varieties by comprehensive analysis of fatty acid composition, lignan content, and amino acid composition. Ind. Crops Prod. 2024, 212, 118366. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, K.; Xi, J.; Xu, Y.; Si, X.; Pei, D.; Lyu, S.; Xia, G.; Wang, J.; et al. Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development. Food Chem. 2022, 370, 130975. [Google Scholar] [CrossRef] [PubMed]
- Neale, E.P.; Tapsell, L.C. Perspective: The Evidence-Based Framework in Nutrition and Dietetics: Implementation, Challenges, and Future Directions. Adv. Nutr. 2019, 10, 1–8. [Google Scholar] [PubMed]
- Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Foubert, I.; Pottel, H.; Duprez, F.; Goethals, L.; Debruyne, P.R. Omega-3 fatty acids: Physiology, biological sources and potential applications in supportive cancer care. Phytochem. Rev. 2014, 13, 223–244. [Google Scholar] [CrossRef]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; San Pablo, G. Alpha-linolenic and linoleic fatty acids in the vegan diet: Do they require dietary reference intake/adequate intake special consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef]
- Segui-Simarro, J.M.; Moreno, J.; Fernández, M.; Mir, R. Doubled haploid technology. Methods Mol. Bsiology 2021, 2287, 50. [Google Scholar]
- Wu, G.-Z.; Xue, H.-W. Arabidopsis β-ketoacyl-[acyl carrier protein] synthase I is crucial for fatty acid synthesis and plays a role in chloroplast division and embryo development. Plant Cell 2010, 22, 3726–3744. [Google Scholar]
- Lee, K.-R.; Chen, G.Q.; Kim, H.U. Current progress towards the metabolic engineering of plant seed oil for hydroxy fatty acids production. Plant Cell Rep. 2015, 34, 603–615. [Google Scholar]
- Wang, M.L.; Khera, P.; Pandey, M.K.; Wang, H.; Qiao, L.; Feng, S.; Tonnis, B.; Barkley, N.A.; Pinnow, D.; Holbrook, C.C. Genetic mapping of QTLs controlling fatty acids provided insights into the genetic control of fatty acid synthesis pathway in peanut (Arachis hypogaea L.). PLoS ONE 2015, 10, e0119454. [Google Scholar]
- Dvorianinova, E.M.; Zinovieva, O.L.; Pushkova, E.N.; Zhernova, D.A.; Rozhmina, T.A.; Povkhova, L.V.; Novakovskiy, R.O.; Sigova, E.A.; Turba, A.A.; Borkhert, E.V. Key FAD2, FAD3, and SAD genes involved in the fatty acid synthesis in flax identified based on genomic and transcriptomic data. Int. J. Mol. Sci. 2023, 24, 14885. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [PubMed]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [PubMed]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 gene in plants: Occurrence, regulation, and role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar]
- Hijawi, T. Characterizing of Oil Quality and Fatty Acid Profiles of Old Olive Trees in Palestine. J. Oleo Sci. 2021, 70, 1585–1606. [Google Scholar]
- Jarvis, B.; Romsdahl, T.; McGinn, M.; Nazarenus, T.; Cahoon, E.; Chapman, K.; Sedbrook, J. CRISPR/Cas9-Induced fad2 and rod1 Mutations Stacked With fae1 Confer High Oleic Acid Seed Oil in Pennycress (Thlaspi arvense L.). Front. Plant Sci. 2021, 12, 652319. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Hou, Q.; Zhao, J.; Fang, C.; An, X.; Wan, X. Fatty acid de novo biosynthesis in plastids: Key enzymes and their critical roles for male reproduction and other processes in plants. Plant Physiol. Biochem. 2024, 210, 108654. [Google Scholar]
- Grimberg, Å.; Carlsson, A.; Marttila, S.; Bhalerao, R.; Hofvander, P. Transcriptional transitions in Nicotiana benthamiana leaves upon induction of oil synthesis by WRINKLED1 homologs from diverse species and tissues. BMC Plant Biol. 2015, 15, 192. [Google Scholar] [CrossRef]
- Li, L.; Liang, C.; Zhang, W.; Zhang, X.; Yu, H.; Liu, X.; Bi, Q.; Wang, L. 3-ketoacyl-CoA synthase 7 from Xanthoceras sorbifolium seeds is a crucial regulatory enzyme for nervonic acid biosynthesis. Plant Sci. 2024, 347, 112184. [Google Scholar]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Physiological Effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA)—A Review. Food Rev. Int. 2006, 22, 291–307. [Google Scholar]
- Yang, Y.; Kong, Q.; Lim, A.R.; Lu, S.; Zhao, H.; Guo, L.; Yuan, L.; Ma, W. Transcriptional regulation of oil biosynthesis in seed plants: Current understanding, applications, and perspectives. Plant Commun. 2022, 3, 100328. [Google Scholar] [PubMed]
- Huang, X.; Zhou, Y.; Shi, X.; Wen, J.; Sun, Y.; Chen, S.; Hu, T.; Li, R.; Wang, J.; Jia, X. PfbZIP85 transcription factor mediates ω-3 fatty acid-enriched oil biosynthesis by Down-regulating PfLPAT1B gene expression in plant tissues. Int. J. Mol. Sci. 2024, 25, 4375. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M. Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 2019, 256, 279–290. [Google Scholar]
- Marzi, G.; Balzano, M.; Caputo, A.; Pellegrini, M.M. Guidelines for Bibliometric-Systematic Literature Reviews: 10 steps to combine analysis, synthesis and theory development. Int. J. Manag. Rev. 2025, 27, 81–103. [Google Scholar]
- Linnenluecke, M.K.; Marrone, M.; Singh, A.K. Conducting systematic literature reviews and bibliometric analyses. Aust. J. Manag. 2020, 45, 175–194. [Google Scholar]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar]
- El Masri, O.Y.; Lui, E.M. Behavior and design of steel delta girders for flexure and shear. J. Struct. Eng. 2021, 147, 04021141. [Google Scholar]
- Samson, D.; Schoelles, K.M. Chapter 2: Medical Tests Guidance (2) Developing the Topic and Structuring Systematic Reviews of Medical Tests: Utility of PICOTS, Analytic Frameworks, Decision Trees, and Other Frameworks. J. Gen. Intern. Med. 2012, 27, 11–19. [Google Scholar] [CrossRef]
- Konda, A.R.; Nazarenus, T.J.; Nguyen, H.; Yang, J.; Gelli, M.; Swenson, S.; Shipp, J.M.; Schmidt, M.A.; Cahoon, R.E.; Ciftci, O.N. Metabolic engineering of soybean seeds for enhanced vitamin E tocochromanol content and effects on oil antioxidant properties in polyunsaturated fatty acid-rich germplasm. Metab. Eng. 2020, 57, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Li, L.; Cai, G.; Ye, J.; Liu, M.; Wang, S.; Li, Z. Molecular cloning and function analysis of FAD2 gene in Idesia polycarpa. Phytochemistry 2019, 168, 112114. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Z.; Shang, X.; Peng, Y.; Gao, L. Overexpression of PvFAD3 Gene from Plukenetia volubilis Promotes the Biosynthesis of α-Linolenic Acid in Transgenic Tobacco Seeds. Genes 2022, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-D.; Pan, B.-Z.; Fu, Q.; Niu, L.; Chen, M.-S.; Xu, Z.-F. De novo transcriptome assembly of the eight major organs of Sacha Inchi (Plukenetia volubilis) and the identification of genes involved in α-linolenic acid metabolism. BMC Genom. 2018, 19, 380. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.; de Castro, M. Fatty acids, triterpenes and cycloalkanes in ficus seed oils. Plant Physiol. Biochem. 2019, 135, 127–131. [Google Scholar] [CrossRef]
- Kodahl, N.; Sorensen, M. Sacha Inchi (Plukenetia volubilis L.) Is an Underutilized Crop with a Great Potential. Agronomy 2021, 11, 1066. [Google Scholar] [CrossRef]
- Gotor, A.; Berger, M.; Labalette, F.; Centis, S.; Daydé, J.; Calmon, A. Oleic conversion effect on the tocopherol and phytosterol contents in sunflower oil. Phyton 2014, 83, 319–324. [Google Scholar]
- Liu, L.; Wang, D.; Hua, J.; Kong, X.; Wang, X.; Wang, J.; Si, A.; Zhao, F.; Liu, W.; Yu, Y.; et al. Genetic and Morpho-Physiological Differences among Transgenic and No-Transgenic Cotton Cultivars. Plants 2023, 12, 3437. [Google Scholar] [CrossRef]
- Hernández, M.; Sicardo, M.; Arjona, P.; Martínez-Rivas, J. Specialized Functions of Olive FAD2 Gene Family Members Related to Fruit Development and the Abiotic Stress Response. Plant Cell Physiol. 2020, 61, 427–441. [Google Scholar] [CrossRef]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W.; Wang, M. Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 143, 375–387. [Google Scholar] [CrossRef]
- Xie, D.; Dai, Z.; Yang, Z.; Tang, Q.; Deng, C.; Xu, Y.; Wang, J.; Chen, J.; Zhao, D.; Zhang, S.; et al. Combined genome-wide association analysis and transcriptome sequencing to identify candidate genes for flax seed fatty acid metabolism. Plant Sci. 2019, 286, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, L.; Wang, Y.; Li, Y.; Zhang, X.; Xue, F.; Nie, X.; Zhu, Q.; Sun, J. GhFAD2-3 is required for anther development in Gossypium hirsutum. BMC Plant Biol. 2019, 19, 393. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Yadava, P.; Sharma, S.; Gupta, N.C.; Bansal, R.; Yadav, R.; Kalia, S.; Kumar, A. Food and nutraceutical functions of sesame oil: An underutilized crop for nutritional and health benefits. Food Chem. 2022, 389, 132990. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, J.; Zhang, X.; Wang, S.; Fu, D.; Chen, M. Yacon (Smallanthus sonchifolius) tuber: A novel and promising feedstock for enhanced high-value docosahexaenoic acid production by Schizochytrium sp. Ind. Crops Prod. 2022, 188, 115597. [Google Scholar] [CrossRef]
- Huang, Z.; Yuan, Y.; Tan, Z.; Zheng, J.; Zhang, W.; Huang, S.; Wang, Y.; Chen, M.; Zhang, L.; Li, H. Metabolomics in combination with network pharmacology reveals the potential anti-neuroinflammatory mechanism of essential oils from four Curcuma species. Ind. Crops Prod. 2023, 195, 116411. [Google Scholar] [CrossRef]
- Shams, R.; Azizi, A.; Hamzei, J.; Noroozisharaf, A.; Moghadam, S.; Kordrostami, M. Genetic structure and diversity of Iranian Cannabis populations based on phytochemical, agro-morphological and molecular markers. Ind. Crops Prod. 2020, 158, 112950. [Google Scholar] [CrossRef]
- Alberghini, B.; Zanetti, F.; Corso, M.; Boutet, S.; Lepiniec, L.; Vecchi, A.; Monti, A. Camelina [Camelina sativa (L.) Crantz] seeds as a multi-purpose feedstock for bio-based applications. Ind. Crops Prod. 2022, 182, 114944. [Google Scholar] [CrossRef]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Aggarwal, B.B.; Barreca, D.; Battino, M.; Belwal, T.; Horbanczuk, O.K.; Berindan-Neagoe, I.; Bishayee, A.; Daglia, M.; Devkota, H.P. Dietary natural products and their potential to influence health and disease including animal model studies. Anim. Sci. Pap. Rep. 2018, 36, 345–358. [Google Scholar]
- Suh, M.; Kim, H.; Nakamura, Y. Plant lipids: Trends and beyond. J. Exp. Bot. 2022, 73, 2715–2720. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Steinwand, M.A.; Ronald, P.C. Crop biotechnology and the future of food. Nat. Food 2020, 1, 273–283. [Google Scholar]
- Wallis, J.G.; Bengtsson, J.D.; Browse, J. Molecular approaches reduce saturates and eliminate trans fats in food oils. Front. Plant Sci. 2022, 13, 908608. [Google Scholar]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Issa, A.M.; Mojica, W.A.; Morton, S.C.; Traina, S.B.; Newberry, S.; Hilton, L.G.; Garland, R.H.; MacLean, C.H. The Efficacy of Omega–3 Fatty Acids on Cognitive Function in Aging and Dementia: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2006, 21, 88–96. [Google Scholar]
- Qin, J.; Kurt, E.; LBassi, T.; Sa, L.; Xie, D. Biotechnological production of omega-3 fatty acids: Current status and future perspectives. Front. Microbiol. 2023, 14, 1280296. [Google Scholar]
- Nam, J.-W.; Yeon, J.; Jeong, J.; Cho, E.; Kim, H.B.; Hur, Y.; Lee, K.-R.; Yi, H. Overexpression of acyl-ACP thioesterases, CpFatB4 and CpFatB5, induce distinct gene expression reprogramming in developing seeds of Brassica napus. Int. J. Mol. Sci. 2019, 20, 3334. [Google Scholar] [CrossRef]
- Maheshwari, P.; Kovalchuk, I. Genetic engineering of oilseed crops. Biocatal. Agric. Biotechnol. 2014, 3, 31–37. [Google Scholar]
- Sharma, M.; Samota, M.K. Metabolic engineering of linseed crop for enhancing production yield. In Linseed; Elsevier: Amsterdam, The Netherlands, 2024; pp. 107–117. [Google Scholar]
- Schübel, R.; Jaudszus, A.; Krüger, R.; Roth, A.; Klempt, M.; Barth, S.W. Dietary essential α-linolenic acid and linoleic acid differentially modulate TNFα-induced NFκB activity in FADS2-deficient HEK-293 cells. Int. J. Food Sci. Nutr. 2017, 68, 553–559. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Brainard, J.; Deane, K.H.; Song, F. Creation of a database to assess effects of omega-3, omega-6 and total polyunsaturated fats on health: Methodology for a set of systematic reviews. BMJ Open 2019, 9, e029554. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of refined Buglossoides oil as a novel food ingredient. EFSA J. 2015, 13, 4029. [Google Scholar]
- Britten-Jones, A.C.; Craig, J.P.; Downie, L.E. Omega-3 polyunsaturated fatty acids and corneal nerve health: Current evidence and future directions. Ocul. Surf. 2023, 27, 1–12. [Google Scholar] [PubMed]
- Ursin, V.; Froman, B.; Nava, A.J.; Gonzales, J. U.S. Patent and Trademark Office. U.S. Patent 8,378,186, 2013. [Google Scholar]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Sherratt, S.C.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of omega-3 fatty acids in cardiovascular disease: The debate continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary fat and cancer—Which is good, which is bad, and the body of evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, W. Fatty acids and pregnancy-induced hypertension: A Mendelian randomization study. Lipids Health Dis. 2023, 22, 131. [Google Scholar]
- Park, H.; Graef, G.; Xu, Y.; Tenopir, P.; Clemente, T.E. Stacking of a stearoyl-ACP thioesterase with a dual-silenced palmitoyl-ACP thioesterase and∆ 12 fatty acid desaturase in transgenic soybean. Plant Biotechnol. J. 2014, 12, 1035–1043. [Google Scholar]
- Burdziej, A.; Pączkowski, C.; Destrac-Irvine, A.; Richard, T.; Cluzet, S.; Szakiel, A. Triterpenoid profiles of the leaves of wild and domesticated grapevines. Phytochem. Lett. 2019, 30, 302–308. [Google Scholar]
- Furse, S.; Martel, C.; Yusuf, A.; Shearman, G.C.; Koch, H.; Stevenson, P.C. Sterol composition in plants is specific to pollen, leaf, pollination and pollinator. Phytochemistry 2023, 214, 113800. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.-Y.; Su, P.; Zhao, Y.-J.; Zhang, X.-N.; Dai, Z.-B.; Guo, J.; Tong, Y.-R.; Liu, Y.-J.; Hu, T.-Y.; Yin, Y.; et al. Cloning and functional analysis of two sterol-C24-methyltransferase 1 (SMT1) genes from Paris polyphylla. J. Asian Nat. Prod. Res. 2018, 20, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Jankiewicz, A.; del Bosque-Plata, L.; Tejero, M.E. Combined effect of plant sterols and dietary fiber for the treatment of hypercholesterolemia. Plant Foods Hum. Nutr. 2014, 69, 93–100. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, L.; Zhang, X.; Gao, W. A cytochrome P450 monooxygenase responsible for the C-22 hydroxylation step in the Paris polyphylla steroidal saponin biosynthesis pathway. Phytochemistry 2018, 156, 116–123. [Google Scholar] [CrossRef]
- Kamisah, Y.; Periyah, V.; Lee, K.T.; Noor-Izwan, N.; Nurul-Hamizah, A.; Nurul-Iman, B.S.; Qodriyah, H.M.S. Cardioprotective effect of virgin coconut oil in heated palm oil diet-induced hypertensive rats. Pharm. Biol. 2015, 53, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Carnahan, S.; Brown, L. Coconut products improve signs of diet-induced metabolic syndrome in rats. Plant Foods Hum. Nutr. 2017, 72, 418–424. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K.; Chen, X.; Wang, J.; Shu, G.; Ping, Z.; Zhang, S. Health outcomes associated with phytosterols: An umbrella review of systematic reviews and meta-analyses of randomized controlled trials. Phytomedicine 2023, 122, 155151. [Google Scholar] [CrossRef]
- Huang, A.H. Plant lipid droplets and their associated proteins: Potential for rapid advances. Plant Physiol. 2018, 176, 1894–1918. [Google Scholar] [CrossRef]
- Scholz, P.; Chapman, K.D.; Mullen, R.T.; Ischebeck, T. Finding new friends and revisiting old ones–how plant lipid droplets connect with other subcellular structures. New Phytol. 2022, 236, 833–838. [Google Scholar] [CrossRef]
- Kitaoka, N.; Lu, X.; Yang, B.; Peters, R.J. The application of synthetic biology to elucidation of plant mono-, sesqui-, and diterpenoid metabolism. Mol. Plant 2015, 8, 6–16. [Google Scholar] [CrossRef]
- Liu, F.; Wang, P.; Xiong, X.; Zeng, X.; Zhang, X.; Wu, G. A review of nervonic acid production in plants: Prospects for the genetic engineering of high nervonic acid cultivars plants. Front. Plant Sci. 2021, 12, 626625. [Google Scholar]
- Bai, Y.; Zhu, X.; Guo, X.; Zhang, W.; Zhang, G.; Chen, H.; Zhang, Q. Molecular cloning and functional characterization of GmAAPTs from soybean (Glycine max). Plant Signal. Behav. 2021, 16, 1845048. [Google Scholar] [PubMed]
- Sun, M.; Zhang, J.; Wang, N.; Wei, X.; Fang, H.; Ding, X.; Wang, X. Genome-Wide Identification, Phylogenetic and Expression Pattern Analysis of Fatty Acid Desaturase Genes in Castor (Ricinus communis L.). Trop. Plant Biol. 2024, 17, 52–64. [Google Scholar]
- Matzner, M.; Launhardt, L.; Barth, O.; Humbeck, K.; Goss, R.; Heilmann, I. Inter-Organellar Effects of Defective ER-Localized Linolenic Acid Formation on Thylakoid Lipid Composition, Non-Photochemical Quenching of Chlorophyll Fluorescence and Xanthophyll Cycle Activity in the Arabidopsis fad3 Mutant. Plant Cell Physiol. 2023, 65, pcad141. [Google Scholar]
- Tran, H.T.D.; Le, N.T.; Khuat, V.L.U.; Nguyen, T.T.H. Identification and functional characterization of a soybean (Glycine max) thioesterase that acts on intermediates of fatty acid biosynthesis. Plants 2019, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Kong, Q.; Mantyla, J.J.; Yang, Y.; Ohlrogge, J.B.; Benning, C. 14-3-3 protein mediates plant seed oil biosynthesis through interaction with AtWRI1. Plant J. 2016, 88, 228–235. [Google Scholar]
- Tang, Y.; Huang, J.; Ji, H.; Pan, L.; Hu, C.; Qiu, X.; Qiao, L. Identification of AhFatB genes through genome-wide analysis and knockout of AhFatB reduces the content of saturated fatty acids in peanut (Arichis hypogaea L.). Plant Sci. 2022, 319, 111247. [Google Scholar]
- Kobayashi, K.; Fujii, S.; Sato, M.; Toyooka, K.; Wada, H. Specific role of phosphatidylglycerol and functional overlaps with other thylakoid lipids in Arabidopsis chloroplast biogenesis. Plant Cell Rep. 2015, 34, 631–642. [Google Scholar] [PubMed]
- Zimmer, B.; Angioni, C.; Osthues, T.; Toewe, A.; Thomas, D.; Pierre, S.C.; Sisignano, M. The oxidized linoleic acid metabolite 12, 13-DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 669–678. [Google Scholar]
- Zheng, J.; Yang, J.; Yang, X.; Cao, Z.; Cai, S.; Wang, B.; Xu, F. Transcriptome and miRNA sequencing analyses reveal the regulatory mechanism of α-linolenic acid biosynthesis in Paeonia rockii. Food Res. Int. 2022, 155, 111094. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, W.; Han, X.; Hu, J.; Yin, L.; Lv, Z. Integrated analysis of fatty acid, sterol and tocopherol components of seed oils obtained from four varieties of industrial and environmental protection crops. Ind. Crops Prod. 2020, 154, 112655. [Google Scholar]
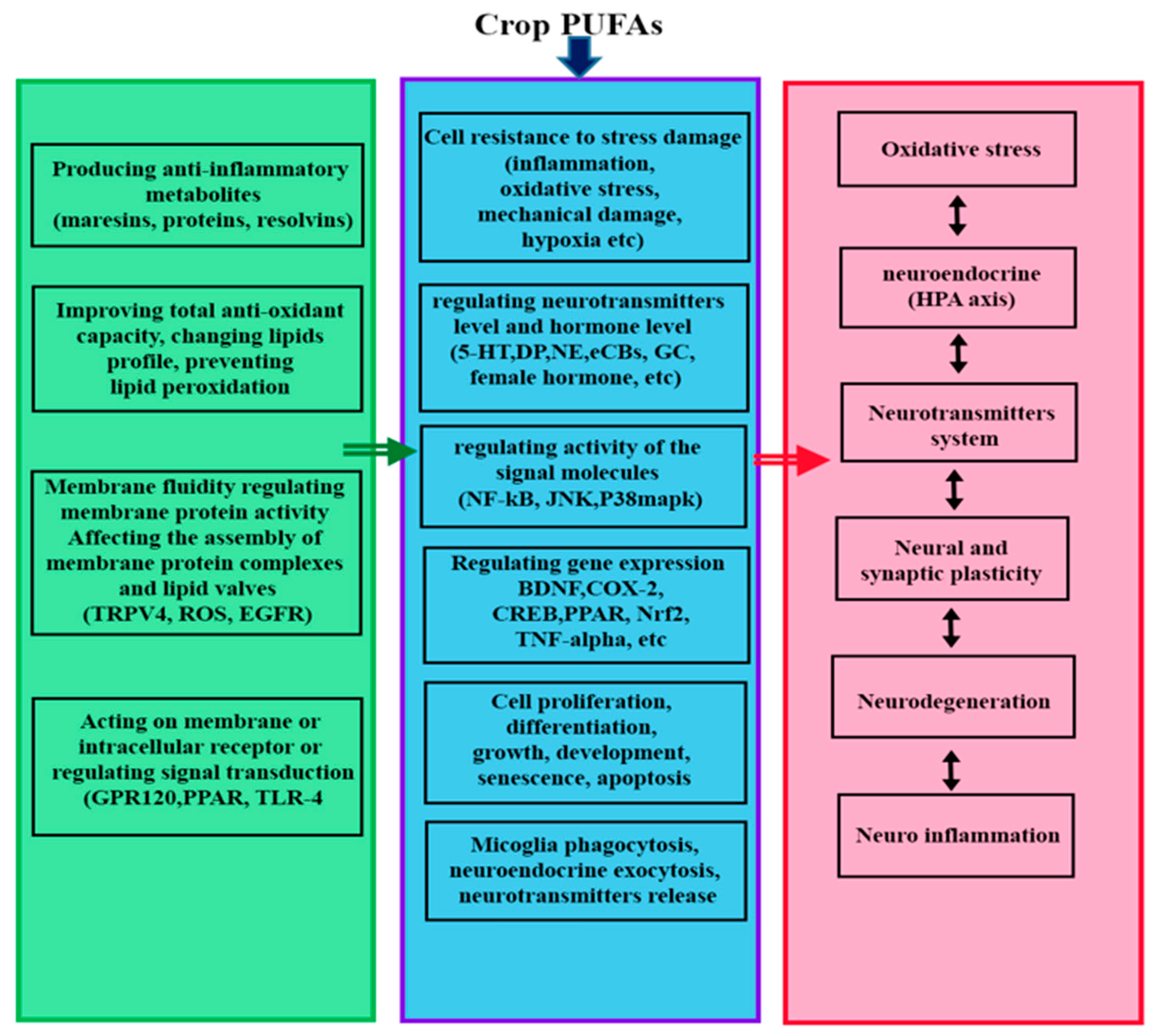
- Gao, H.; Yan, P.; Zhang, S.; Nie, S.; Huang, F.; Han, H.; Liu, L. Chronic alpha-linolenic acid treatment alleviates age-associated neuropathology: Roles of PERK/eIF2α signaling pathway. Brain Behav. Immun. 2016, 57, 314–325. [Google Scholar]
- García-Viñuales, S.; Sciacca, M.F.; Lanza, V.; Santoro, A.M.; Grasso, G.; Tundo, G.R.; Milardi, D. The interplay between lipid and Aβ amyloid homeostasis in Alzheimer’s Disease: Risk factors and therapeutic opportunities. Chem. Phys. Lipids 2021, 236, 105072. [Google Scholar] [PubMed]
- Reemst, K.; Lopizzo, N.; Abbink, M.R.; Engelenburg, H.J.; Cattaneo, A.; Korosi, A. Molecular underpinnings of programming by early-life stress and the protective effects of early dietary ω6/ω3 ratio, basally and in response to LPS: Integrated mRNA-miRNAs approach. Brain Behav. Immun. 2024, 117, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.B.; Dátilo, M.N.; Sant'Ana, M.R.; Nogueira, G.A.d.S.; Marin, R.M.; Nakandakari, S.C.B.R.; de Moura, L.P.; da Silva, A.S.R.; Ropelle, E.R.; Pauli, J.R.; et al. The early impact of diets enriched with saturated and unsaturated fatty acids on intestinal inflammation and tight junctions. J. Nutr. Biochem. 2023, 119, 109410. [Google Scholar]
- Moura-Assis, A.; Afonso, M.S.; de Oliveira, V.; Morari, J.; Dos Santos, G.A.; Koike, M.; Cintra, D.E.C. Flaxseed oil rich in omega-3 protects aorta against inflammation and endoplasmic reticulum stress partially mediated by GPR120 receptor in obese, diabetic and dyslipidemic mice models. J. Nutr. Biochem. 2018, 53, 9–19. [Google Scholar]
- Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Let food be thy medicine and medicine be thy food: A bibliometric analysis of the most cited papers focusing on nutraceuticals and functional foods. Food Chem. 2018, 269, 455–465. [Google Scholar]
- Fan, R.; Toney, A.M.; Jang, Y.; Ro, S.H.; Chung, S. Maternal n-3 PUFA supplementation promotes fetal brown adipose tissue development through epigenetic modifications in C57BL/6 mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1488–1497. [Google Scholar] [CrossRef]
- Al-Khudairy, L.; Hartley, L.; Clar, C.; Flowers, N.; Hooper, L.; Rees, K. Omega 6 fatty acids for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2015, 11, CD011094. [Google Scholar] [CrossRef]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front. Oncol. 2013, 3, 224. [Google Scholar]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Huerta-Yepez, S. Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sci. 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Deane, K.H. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD011094. [Google Scholar] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3,-6 and-9 fatty acids in animals. Anim. Biotechnol. 2022, 33, 1678–1690. [Google Scholar] [PubMed]

| Authors/Year | Titles | Genes/Genotypes/Varieties Used for Study) | Measurements | Findings/Conclusions |
|---|---|---|---|---|
| [31], USA/China | Metabolic engineering of soybean seeds for enhanced vitamin E tocochromanol content and effects on oil antioxidant properties in polyunsaturated fatty acid-rich germplasm | Soybean line (SDA-1/535-9); γTMT gene, HGGT (Barley) gene | Oil contents of transgenic soybean seeds oi, transgenic HGGT barley gene | Soybean line crossed—γTMT showed oil enriched in SDA, ALA, GLA; the progeny of HGGT expressed had ≥6 fold increased free radicals scavenging activity in cell metabolic activity |
| [32], China | Molecular cloning and function analysis of FAD2 gene in Idesia polycarpa | FADS2 gene, Indesia polycarpa fruit tree | Lipid accumulation, linoleic accumulation rates and final linoleic content | IpFAD2 gene could encode a bio-functional omega-6 fatty acid desaturase; IpFAD2 has significant contribution in linoleic synthesis |
| [33], China | Overexpression of PvFAD3 gene from Plukenetia volubilis promotes the biosynthesis of linolenic acid in transgenic tobacco seeds | Gene PvFAD3, Nicotiana benthamiana (for transformation and test material) | Expression of PvFAD3 gene in different tissues of P. volubilis. | The transgenic seed showed a significant increase in-ALA content, a dramatic decrease in LA content; PvFAD3 gene of was confirmed as a key enzyme gene for ALA synthesis |
| [34], China | De novo transcriptome assembly of the eight major organs of Sacha Inchi (Plukenetia volubilis) and the identification of genes involved in α-linolenic acid metabolism | Major organs of Sacha: roots, stems, shoot apexes, mature leaves, male flowers, female flowers, fruits, and seeds | Expression of genes related to the ALA metabolism based on the de novo assembly and annotation transcriptome | Sacha Inchi accumulates high level of ALA in seeds by strong expression of biosynthesis-related genes, the upregulation of FAD3 and FAD7 is consistent with high level of ALA in seeds of Sacha Inchi |
| [19], Sweden | Transcriptional transitions in Nicotiana benthamiana leaves upon induction of oil synthesis by WRI1 homologs from diverse species and tissues | WRI1 (A. thaliana/potato/poplar/oat) were expressed in Nicotiana benthamiana | Oil synthesis by WRI1 | Transcripts representing fatty acid degradation were upregulated indicating that fatty acids might be degraded to feed the increased need to channel carbons into fatty acid synthesis creating a futile cycle. WRI1 may exert on global gene expression during seed and embryo development |
| [35], USA/Philippines | Fatty acids, triterpenes, and cycloalkanes in ficus seed oils | Ficus spp: F. nota, F. septica, F. ulmifolia) | Level of FAs studied in various plant parts | ALA is the most prominent FA in the seed oils followed by LA, with these two fatty acid comprising about 75% of the fatty acids in the oils |
| [36], Denmark | Sacha Inchi (Plukenetia volubilis L.) is an underutilized crop with a great potential | P. volubilis | FA contents studied | The seed contains 35.2–50.8% ALA and 33.4–41.0% LA |
| [37], France | Oleic conversion effect on the tocopherol and phytosterol contents in sunflower oil | Hundreds of hybrids and parental lines | FA contents studied | The results indicated that sunflower oil is rich in α-tocopherol and β-sitosterol |
| [38], China | Genetic and morpho-physiological differences among transgenic and no-transgenic cotton cultivars | G. hirsutum genotypes namely: GhKAR, GhHAD, and GhENR, and cultivars (10H1014, 10H1041, 10H1007 and 2074) | Expression patterns of genotypes and cultivars were studied | Oil contents of GhKAR and GhENR overexpression lines increased 1.05~1.08 folds; these results indicated that GhHAD, GhENR, and GhKAR were involved in both seed oil synthesis |
| [39], Spain | Specialized functions of olive FAD2 gene family members related to fruit development and the abiotic stress response | Three cDNA sequences: OepFAD2-3, OepFAD2-4 OepFAD2-5 all encoding three microsomal FAD2 gene from olive (Olea europaea cv. Picual) | Three cDNA genes expression and lipid analysis | OeFAD2-5, together with OeFAD2-2 contributes mostly to the linoleic acid present in the mesocarp and, therefore, in the olive oil |
| [40], China | Constitutive expression of chloroplast G3P acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis | AmGPAT, Arabidopsis | Characterize the physiological function of AmGPAT from A. mongolicus | Transgenic lines of AmGPAT in Arabidopsis increased the levels of cis-unsaturated fatty acids: ALA |
| [41], China | Combined genome-wide association analysis and transcriptome sequencing to identify candidate genes for flax seed fatty acid metabolism | Flax seeds (224 samples) | Based on GWAS and RNA-seq methods to identify candidate genes for fatty acid metabolism in flax seeds | Among 10 candidate genes screened, 2 most genes were significantly correlated with 5 fatty acid contents in seeds of the high oil variety: (Shuangya4vs.NEW), and both of these genes encode acyl-lipid omega-3 desaturase |
| [42], China | GhFAD2–3 is required for another development in Gossypium hirsutisms | Cotton GhFAD2 gene family | Molecular characterization of GhFAD2 gene | The ratio of monounsaturated to polyunsaturated fatty acid was 5.43 in fad2–3 anther, which was much higher than that of the WT (only 0.39) |
| [17],United States | CRISPR/Cas9-induced fad2 and rod1 mutations stacked with fae1 confer high oleic acid seed oil in pennycress (Thlaspi arvense L.) | Genes FAD2 and ROD1 | Knockout mutation | fad2, fae1 and rod1 fae1 double mutants produced 90% and 60% oleic acid in seed oil, respectively, with PUFAs in fad2 fae1 as well as fad2 single mutants reduced to less than 5% |
| [43], India | Food and nutraceutical functions of sesame oil: an underutilized crop for nutritional and health benefits | Sesame crop | Sesame crop demonstration | Potential candidate to maintain the diversity of food oils |
| [44], China | Yacon (Smallanthus sonchifolius) tuber: a novel and promising feedstock for enhanced high-value docosahexaenoic acid production by Schizochytrium sp. | Yacon tuber hydrolysate (YTH) | Demonstration | YTH is a novel potential substrate for Schizochytrium sp. ATCC 20888 to produce DHA |
| [45], China/Switzerland | Metabolomics in combination with network pharmacology reveals the potential anti-neuro inflammatory mechanism of essential oils from four Curcuma species | Four Curcuma species: Curcuma longa L. (CL), Curcuma kwangsiensis S.G. Lee and C.F. Liang (CK), Curcuma zedoaria (Christm.) Roscoe (CZ) and Curcuma aromatica Salisb. (CA) | Demonstration | 11 (e.g., ar-turmerone, curzerenone, nootkatone, curlone, β-Elemene, curzerene) metabolites mainly associated with arachidonic acid metabolism, linoleic acid metabolism, and aminoacyl-tRNA biosynthesis were identified |
| [46], Iran | Genetic structure and diversity of Iranian Cannabis populations based on phytochemical, agro-morphological and molecular markers | Hemp (Cannabis sativa), 10 local cultivated landraces | Diversity based seed oil fatty and biochemical traits | The studied populations showed that Kermanshah had the highest proportion of linoleic acid (54.03%), Hamedan; α-linolenic acid (21.47%), Alborz; oleic acid (16.75%), Kohkylouih-and-Boyerahmad; palmitic acid (6.82%), and Khuzestan; stearic acid (3.23%) |
| [1], India | Exploring lipid health indices and protein quality in ninety Indian linseed varieties by comprehensive analysis of fatty acid composition, lignan content, and amino acid composition | 90 linseed varieties | Diversity based seed oil fatty and biochemical traits | The analysis FA composition revealed 5 major FAs as ALA, LA, oleic acid (OA), palmitic acid (PA), and stearic acid (SA) ranging from 1.68% to 59.19%, 5.61–62.44%, 18.14–45.54%, 5.69–9.5%, and 3.94–10.29%, respectively |
| [2], China | Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development | Hickory nuts | Lipid omics profiling and analysis | 544 kinds of lipids were identified in mature hickory nuts: TAG, DAG, and other related lipids had high relative content with abundance of unsaturated fatty acids, such as oleic acid, linoleic acid and linolenic acid, localized mainly at sn-2 lipid position. |
| [47], Italy/France | Camelina [Camelina sativa (L.) Crantz] seeds as a multi-purpose feedstock for bio-based applications | Six camelina cultivars (Cypress, Midas, 789-02, Pearl, Omega, and WUR) | Profiling Camelina seeds for FA contents and related lipids | Pearl and 789-02 were identified as the most suitable for specific bio-based applications because of the increased omega-3 to omega-6 ratio of the oil. Pearl represent a starting point for future research targeting the increase/decrease of specific fatty acids |
| Source | Unit (g) | 18:2n-6 | 18:3n-3 | References |
|---|---|---|---|---|
| Canola | 100 g | 18.64 | 9.137 | [6,54] |
| Flaxseed/linseed | 100 g | 14.25 | 53.37 | |
| Soybean | 100 g | 50.42 | 6.789 | |
| Walnut | 100 g | 52.90 | 10.40 | |
| Sunflower | 100 g | 3.61 | 0.192 | |
| High oleic safflower | 100 g | 12.72 | 0.096 | |
| Almonds, raw | 100 g | 12.30 | 0.003 | |
| Amaranth | 100 g | 2.736 | 0.042 | |
| Avocados | 100 g | 1.674 | 0.111 | |
| Brazil nuts | 100 g | 23.859 | 0.018 | |
| Cashews, raw | 100 g | 7.782 | 0.062 | |
| Chia seeds | 100 g | 5.840 | 17.80 | |
| Hempseed | 100 g | 1.340 | 8.864 | |
| Millet, cooked | 100 g | 0.480 | 0.028 | |
| Oat bran, cooked | 100 g | 0.324 | 0.015 | |
| Pistachio, raw | 100 g | 13.10 | 0.210 | |
| Poppy seeds | 100 g | 28.30 | 0.273 | |
| Quinoa | 100 g | 2.977 | 0.260 | |
| Rye | 100 g | 0.659 | 0.108 | |
| Sesame seeds | 100 g | 21.375 | 0.376 | |
| Aracauriaceae oil | 100 g | 36.9 | 11 | |
| Boraginase oil | 100 g | 22 | 21.6 | |
| Echium oil | 100 g | 19.5 | 28.1 | |
| Blackcurrant oil | 100 g | 40–50 | 12–15 | |
| Evening promise | 100 g | 70–77 | 0.1–1 | |
| Primulaceae | 100 g | 33.1 | 20.8 | |
| Rapeseed oil | 100 g | 21.6 | 7.3–11.1 | [5] |
| Corn oil | 100 g | 59.0 | 1.16 | |
| Mustard seed oil | 100 g | 15.0 | 6.0 |
| Genus | FA Modified | Target Enzyme Engineered | Sources of Genes | Molecular Technique Applied | Reference |
|---|---|---|---|---|---|
| B. napus | Palmitic acid | Acyl-ACP thioesterase | Cuphea | OE | [57] |
| Lauric acid | Acyl-ACP thioesterase | California Bay | OE | ||
| Caprylic acid | Acyl-ACP thioesterase | Cuphea | OE | ||
| Stearic acid | Acyl-ACP thioesterase Laurate-specific Lysophosphatidic acid acyl-transferase | Mangosteen Coconut | OE | ||
| Oleic acid | Oleoyl-Δ-12-desaturase | B. napus | DR | ||
| GLA(18:3ω-6) | Oleoyl-Δ-6-desaturase and Oleoyl-Δ-12-desaturase | Mortierellaapina | OE | ||
| Saturated fatty acids | Palmitoyl-ACP-desaturase | Doxanthaunguis cati | DR | ||
| Oil rich in ricinoleic acid | Fatty acid hydroxylase | Castor | OE | ||
| B.juncea | Oleic acid | Oleoyl-Δ-12-desaturase | Brassica | DR | |
| Cotton seed | Stearic acid | Stearoyl-ACP-Δ-9-desaturase | Cotton seed | DR | [38] |
| Oleic acid | Oleoyl-Δ-12-desaturase | Cotton seed | DR | ||
| Flax | C18 omega-3, stearidonic acid | omega-3 desaturase | Primula | OE | [41] |
| Soybean | Stearic acid | Stearoyl-ACP-Δ-9-desaturaseand oleoyl-Δ-12-desaturase | Soybean | DR | [58] |
| Oleic acid | Oleoyl-Δ-12-desaturase | Soybean | DR | ||
| Eleostearic acid | Conjugase | Momordica | OE | ||
| Δ-5 Eicosenoic acid | β-Ketoacyl-CoAsynthase andacyl-CoAdesaturase | Meadowfoam | OE | ||
| Oleic acid | Δ-12Fatty acid desaturase | Soybean | OE | ||
| Palmitic acid | Palmitoyl-thioesterase | Soybean | DR | ||
| GLA and stearidonic acid | Δ-6Desaturase | Arabidopsis thaliana | OE | ||
| Arachidonic acid | Δ-5Desaturase, Δ-6desaturase, GLELO elongase, Δ-15desaturase | Mortierella alpine and soybean | OE | ||
| Stearidonic acid | Δ-6Desaturase, Δ-15desaturase | Brassica officinalis and A. thaliana | OE | ||
| C20-LCPUFAs | Δ-6Desaturase, Δ-6elongase, Δ-5 desaturase | Marchantiapolymorpha | OE | ||
| Improved oil yield and composition | Fatty acid ω-3 destaurase2, ACP thioesterase, diacylglycerol acyl-transferase, dihydrodipicolinate synthase, high-lysineprotein, truncatedcysteine synthase | Soybean, Yarrowia lipolytica, Corynebacteriumglutamicum, barely | OE | ||
| Increase oil content | Sphingo lipid compensation | Saccharomycescerevisiae | OE | ||
| Increase oil content | Diacylglycerolacyl-transferase2A | Umbelopsisramanniana | OE | ||
| Epoxy fatty acid | Epoxygenase, diacylglycerolacyl transferase | StokesialaevisVernonia galamensis | OE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefa, M.; Zhao, Y.; Zhou, C.; Song, Y.; Zhao, X. Advancements in Crop PUFAs Biosynthesis and Genetic Engineering: A Systematic and Mixed Review System. Int. J. Mol. Sci. 2025, 26, 3462. https://doi.org/10.3390/ijms26083462
Assefa M, Zhao Y, Zhou C, Song Y, Zhao X. Advancements in Crop PUFAs Biosynthesis and Genetic Engineering: A Systematic and Mixed Review System. International Journal of Molecular Sciences. 2025; 26(8):3462. https://doi.org/10.3390/ijms26083462
Chicago/Turabian StyleAssefa, Molalign, Yajie Zhao, Chao Zhou, Yuanda Song, and Xiangyu Zhao. 2025. "Advancements in Crop PUFAs Biosynthesis and Genetic Engineering: A Systematic and Mixed Review System" International Journal of Molecular Sciences 26, no. 8: 3462. https://doi.org/10.3390/ijms26083462
APA StyleAssefa, M., Zhao, Y., Zhou, C., Song, Y., & Zhao, X. (2025). Advancements in Crop PUFAs Biosynthesis and Genetic Engineering: A Systematic and Mixed Review System. International Journal of Molecular Sciences, 26(8), 3462. https://doi.org/10.3390/ijms26083462







