lncRNAs GAS5 and MALAT1 Contained in Human Adipose Stem Cell (hASC)-Derived Exosomes Drive the Cell-Free Repair and Regeneration of Wounds In Vivo
Abstract
1. Introduction
2. Results
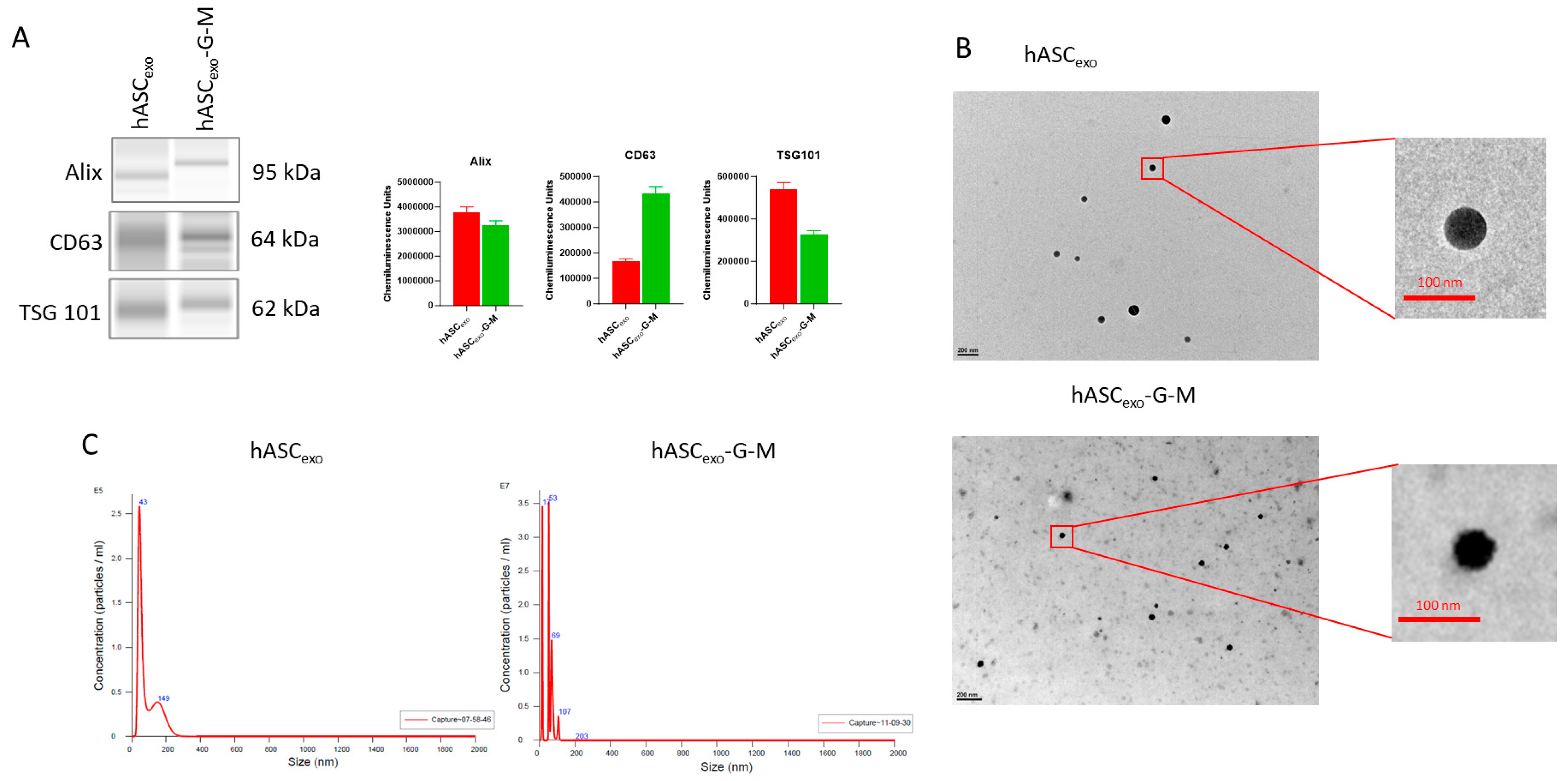
2.1. Automatic Western Blotting and NTA Verify That Isolated sEVs Are Exosomes
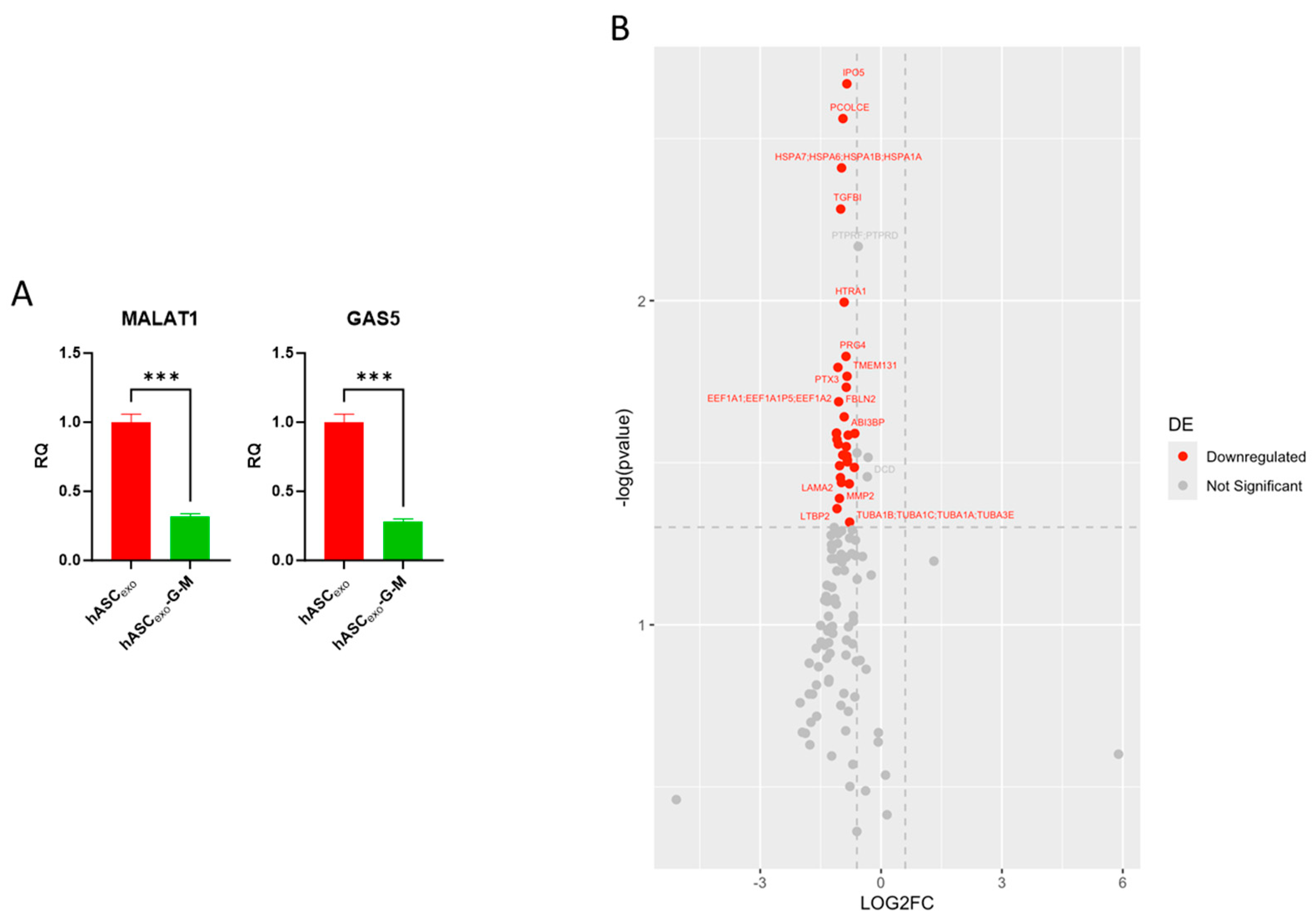
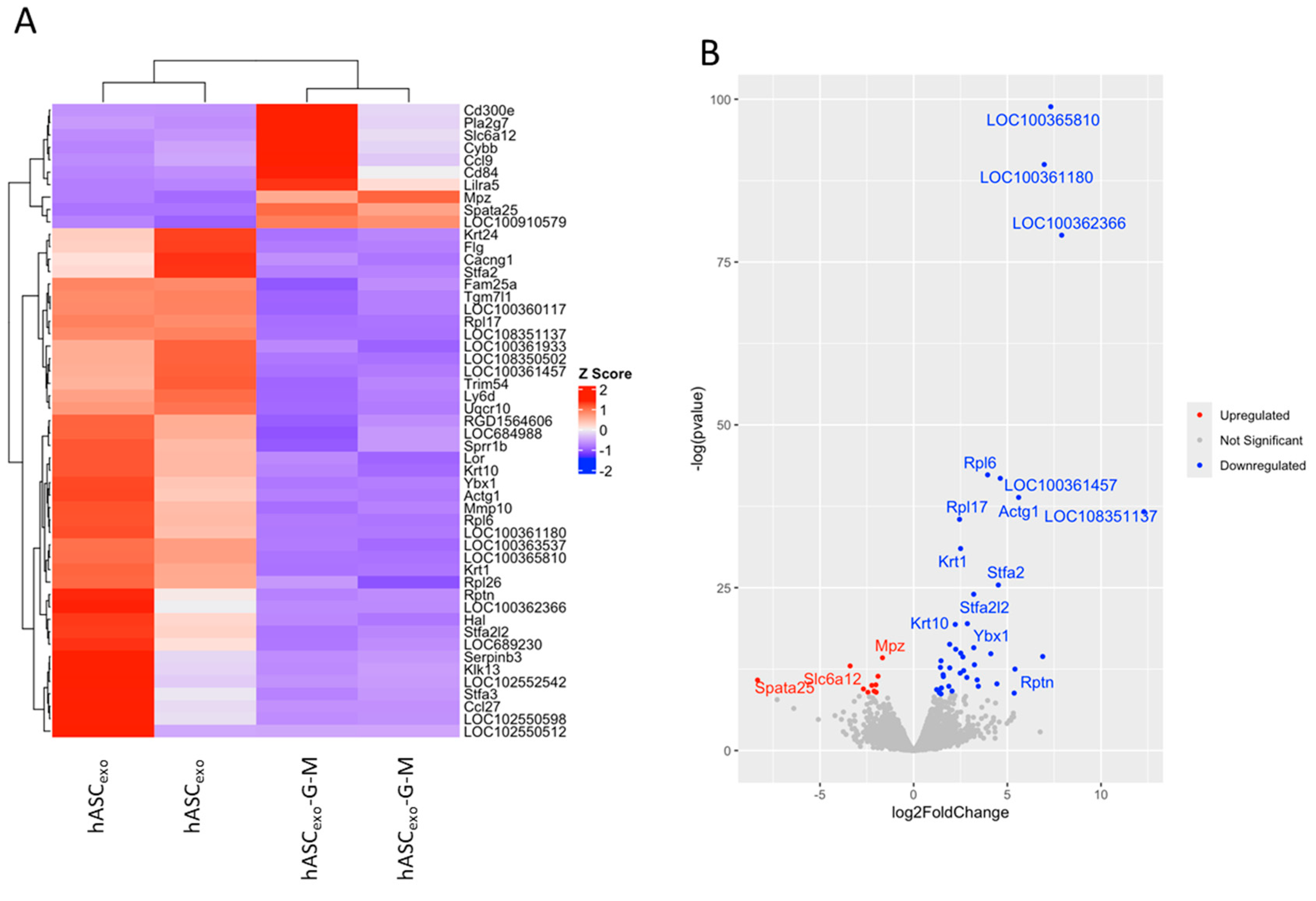
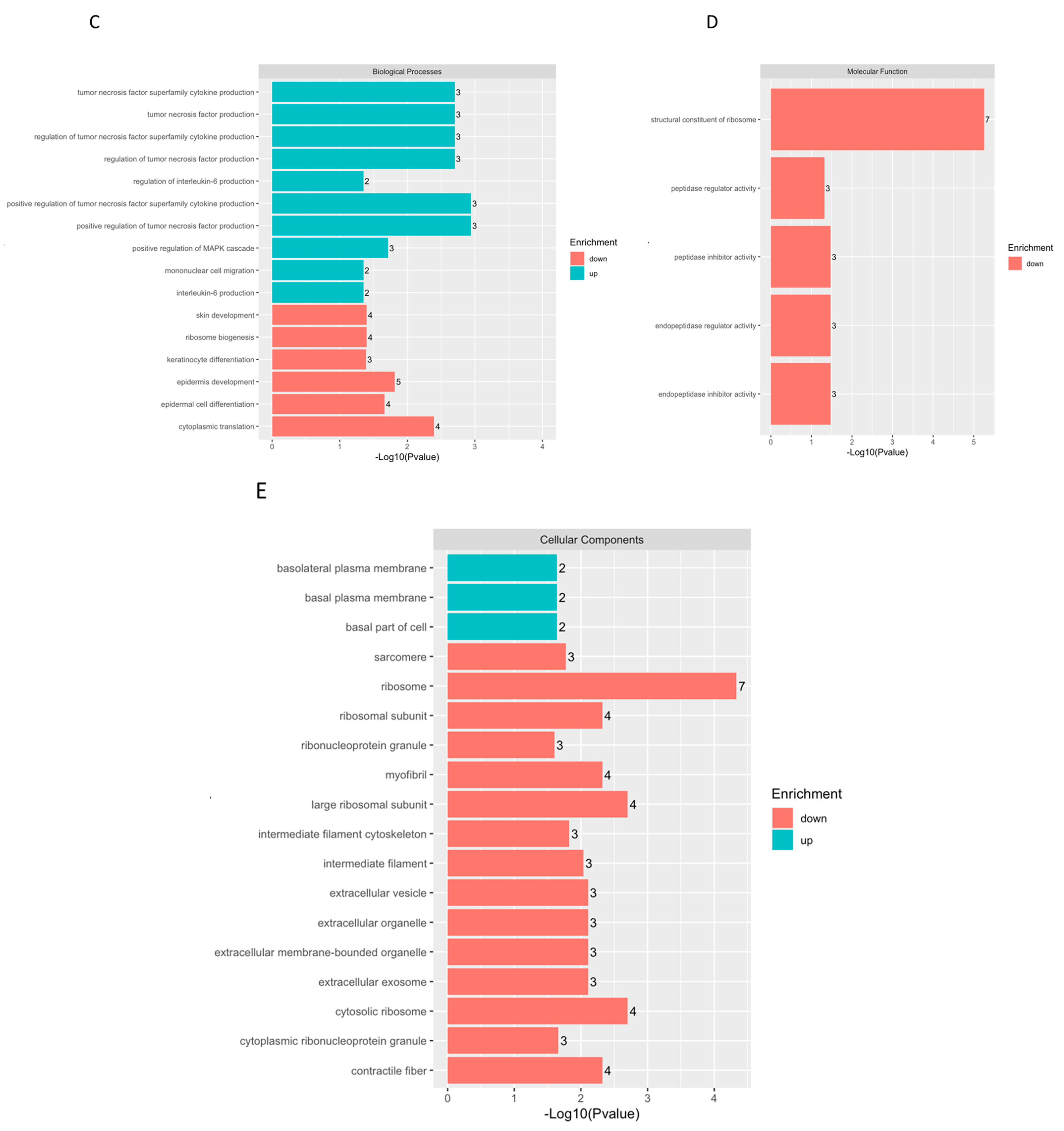
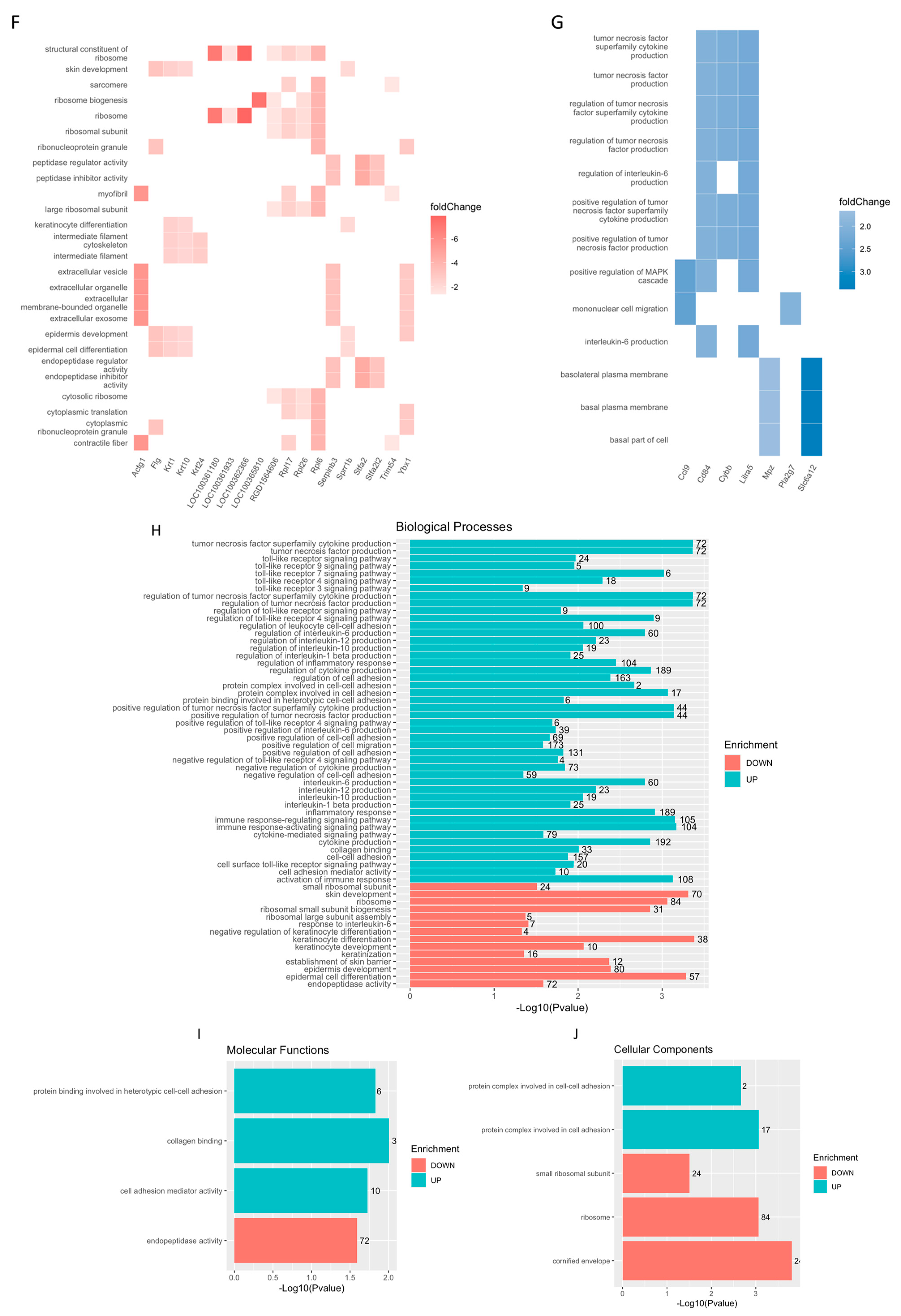
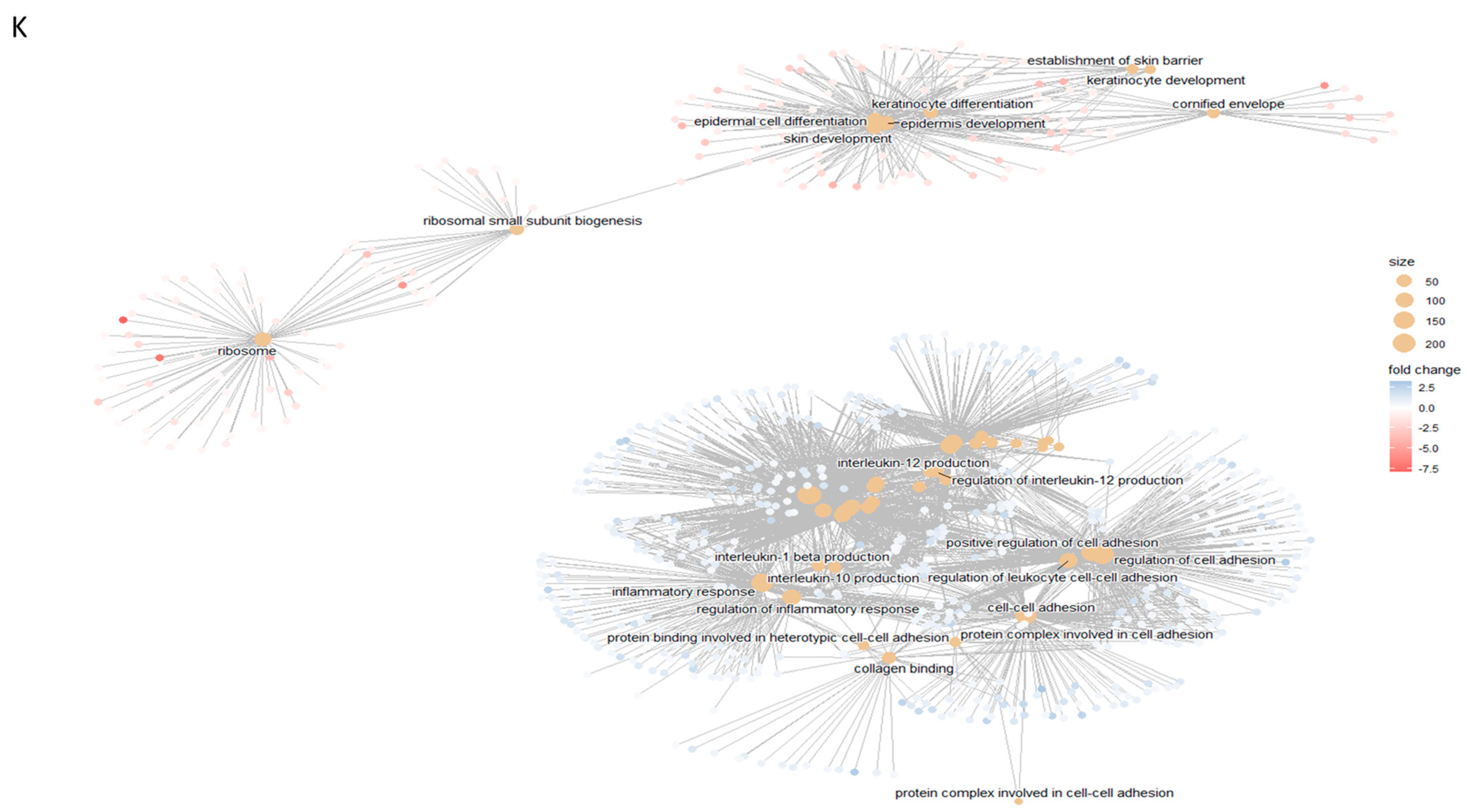
2.2. Biochemical Testing of hASCexo and hASCexo-G-M Demonstrate Differences in Cargos
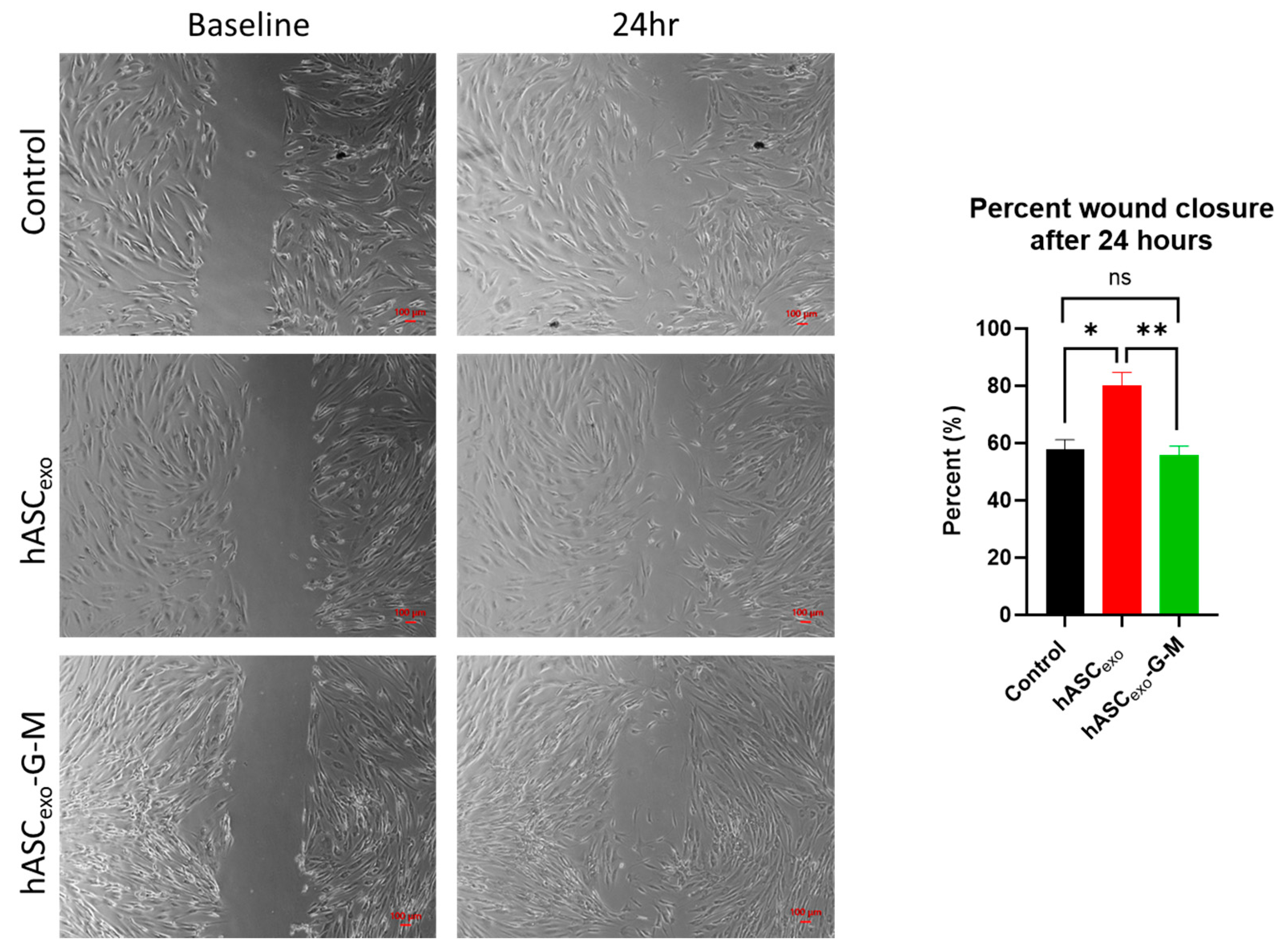
2.3. GAS5 and MALAT1 Improve Wound Healing In Vitro
2.4. The Knockdown of GAS5 and MALAT1 in hASCexo Slows Wound Healing In Vivo
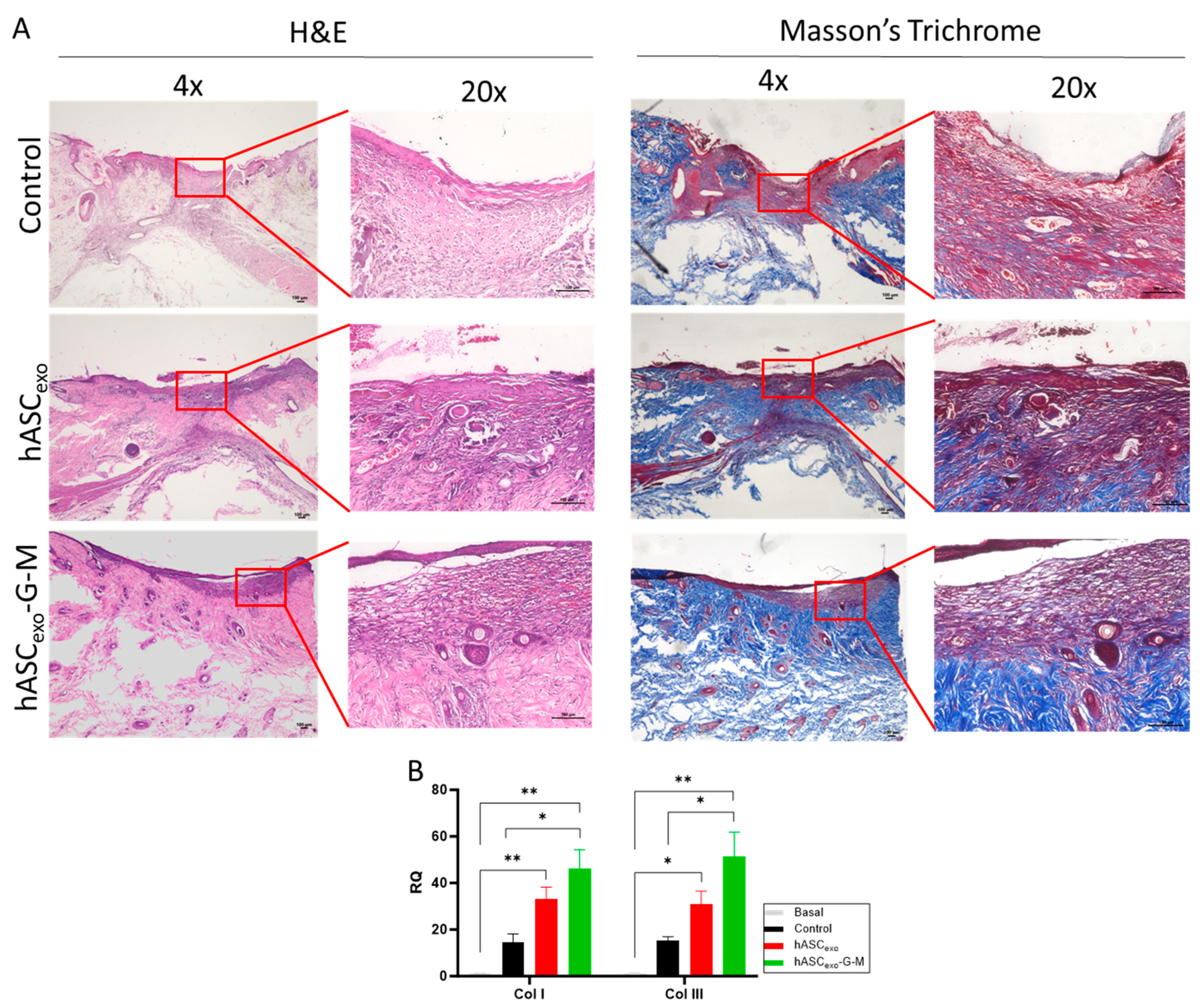
2.5. GAS5 and MALAT1 Knockdown Results in Increased Collagen I and III in the Wound Bed
2.6. Skin, Epidermis, and Keratinocyte Development Is Suppressed in hASCexo-G-M-Treated Wounds While Inflammatory Pathways Are Activated
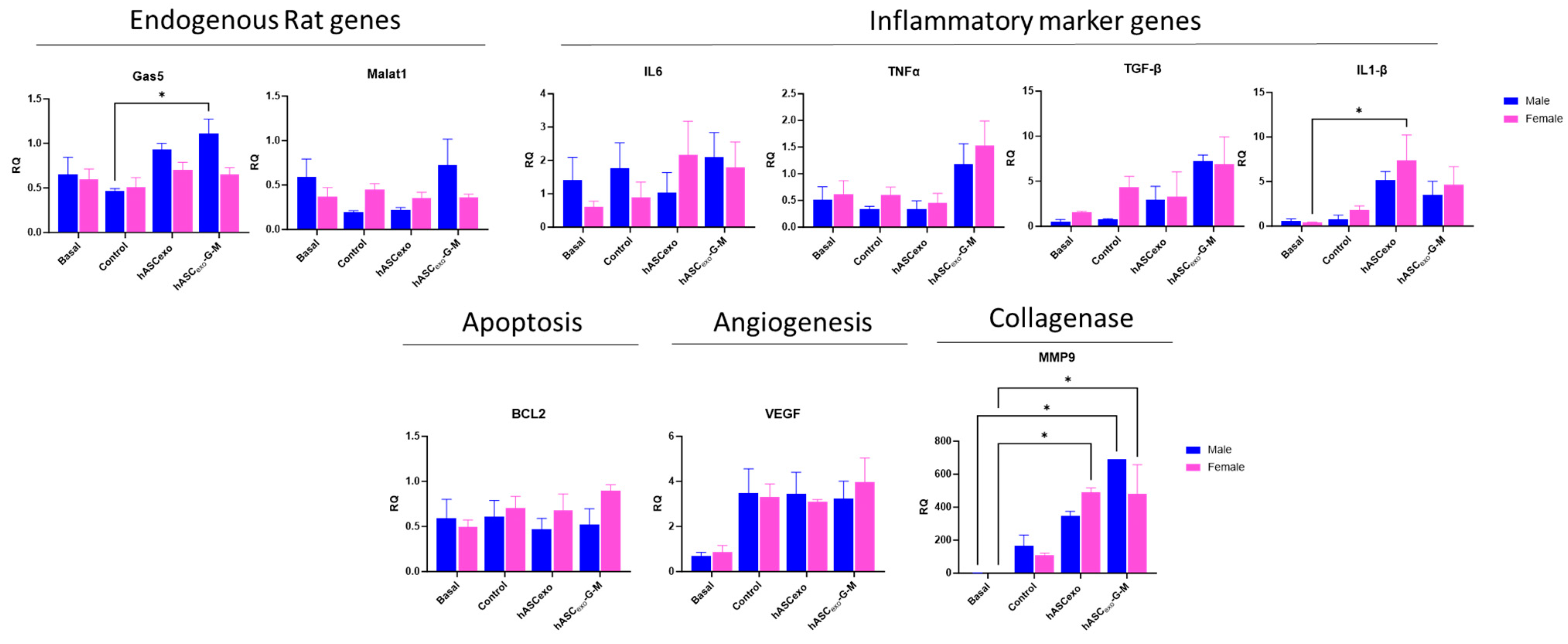
2.7. Treatment of Wounds with hASCexo and hASCexo-G-M Alters Expression of Molecular Markers for Inflammation, Apoptosis, Angiogenesis, and Collagenase Compared to Untreated Control Wounds
2.8. Male and Female Rats Respond Equally to Exosome Treatment
3. Discussion
4. Materials and Methods
4.1. Isolation of Exosomes from hASCs
4.2. Knockdown of GAS5 and MALAT1 in hASCs
4.3. Transmission Electron Microscopy (TEM)
4.4. In Vitro Wound Healing Model
4.5. Proteomic Evaluation of Exosome Cargo
4.6. Quantitative Real-Time PCR
4.7. ProteinSimple Jess Automated Western Blot
4.8. Animals
4.9. Wounding and Exosome Treatment of Rats
4.10. Immunohistocytology
4.11. RNA Sequencing of Wounds
4.12. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Extracellular vesicle |
| hASC | Human adipose stem cell |
| exo | exosome |
| GO | Gene ontology |
| GSEA | Gene set enrichment analysis |
| ORA | Over-representation analysis |
| BP | Biological process |
| MF | Molecular function |
| CC | Cellular component |
| DEG | Differentially expressed gene |
| TEM | Transmission electron microscopy |
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Liu, Z.J.; Xiao, M.; Chen, H.; Goldstein, L.J.; Buerk, D.G.; Nedeau, A.; Thom, S.R.; Velazquez, O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Investig. 2007, 117, 1249–1259. [Google Scholar] [CrossRef]
- Mandrika, I.; Kumar, S.; Zandersone, B.; Eranezhath, S.S.; Petrovska, R.; Liduma, I.; Jezupovs, A.; Pirags, V.; Tracevska, T. Antibacterial and Anti-Inflammatory Potential of Polyherbal Formulation Used in Chronic Wound Healing. Evid. Based Complement. Alternat Med. 2021, 2021, 9991454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, J.Q.; Zheng, Z.; Zhang, J.; Wang, S.Y.; Han, S.C.; Zhou, Q.; Guan, H.; Li, C.; Su, L.L.; et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res. 2016, 365, 85–99. [Google Scholar] [CrossRef]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal 2023, 21, 77. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, Y.J.; Kim, H.; Choi, M.; Lee, E.; Ryoou, B.; Lee, S.G.; Cho, B.S. Adipose Tissue-Derived Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing and Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 10434. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Brandi, J.; Manfredi, M.; Scambi, I.; Schiaffino, L.; Merigo, F.; Turano, E.; Bonetti, B.; Marengo, E.; Cecconi, D.; et al. The Anti-Apoptotic Effect of ASC-Exosomes in an In Vitro ALS Model and Their Proteomic Analysis. Cells 2019, 8, 1087. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Thomas, M.; McLaughlin, K.; Oguljahan, B.; Henderson, J.; Yang, Q.; Chen, Y.E.; Liu, D. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1alpha pathway. J. Mol. Cell Cardiol. 2022, 162, 10–19. [Google Scholar] [CrossRef]
- Tevlin, R.; des Jardins-Park, H.; Huber, J.; DiIorio, S.E.; Longaker, M.T.; Wan, D.C. Musculoskeletal tissue engineering: Adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials 2022, 286, 121544. [Google Scholar] [CrossRef]
- Patel, R.S.; Carter, G.; El Bassit, G.; Patel, A.A.; Cooper, D.R.; Murr, M.; Patel, N.A. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: Role of protein kinase C delta (PKCdelta) in adipose stem cell niche. Stem Cell Investig. 2016, 3, 2. [Google Scholar] [CrossRef]
- Patel, N.A.; Moss, L.D.; Lee, J.Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018, 15, 204. [Google Scholar] [CrossRef]
- Patel, R.S.; Impreso, S.; Lui, A.; Vidyarthi, G.; Albear, P.; Patel, N.A. Long Noncoding RNA GAS5 Contained in Exosomes Derived from Human Adipose Stem Cells Promotes Repair and Modulates Inflammation in a Chronic Dermal Wound Healing Model. Biology 2022, 11, 426. [Google Scholar] [CrossRef]
- Cooper, D.R.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef] [PubMed]
- El Bassit, G.; Patel, R.S.; Carter, G.; Shibu, V.; Patel, A.A.; Song, S.; Murr, M.; Cooper, D.R.; Bickford, P.C.; Patel, N.A. MALAT1 in Human Adipose Stem Cells Modulates Survival and Alternative Splicing of PKCdeltaII in HT22 Cells. Endocrinology 2017, 158, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Zhang, C.; Li, B.; Deng, H.; Chen, R.; Li, G. Human Keratinocyte-Derived Exosomal MALAT1 Promotes Diabetic Wound Healing by Upregulating MFGE8 via microRNA-1914-3p. Int. J. Nanomed. 2023, 18, 949–970. [Google Scholar] [CrossRef]
- Carter, G.; Miladinovic, B.; Patel, A.A.; Deland, L.; Mastorides, S.; Patel, N.A. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015, 4, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Parag, S.; Patel, R.; Lui, A.; Murr, M.; Cai, J.; Patel, N.A. Stabilization of lncRNA GAS5 by a Small Molecule and Its Implications in Diabetic Adipocytes. Cell Chem. Biol. 2019, 26, 319–330.e6. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Z.; Lei, X.; Gan, R. Long non-coding RNA GAS5 in human cancer. Oncol. Lett. 2020, 20, 2587–2594. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Wang, Q.; Xu, Z.; Jiang, J.; Gao, Y.; Gao, M.; Kang, J.; Wu, M.; Xiong, J.; et al. Long non-coding RNA GAS5 controls human embryonic stem cell self-renewal by maintaining NODAL signalling. Nat. Commun. 2016, 7, 13287. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, L.; Liu, X.; Zhou, L.; Wang, W.; Han, Z.; Sui, H.; Tang, Y.; Wang, Y.; Liu, N.; et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br. J. Cancer 2014, 111, 736–748. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Gao, Y.; Wang, Y.W.; Zhang, G.Q.; Pan, S.H.; Ji, L.; Kong, R.; Wang, G.; Jia, Y.H.; et al. Long Noncoding RNA MALAT1 Promotes Aggressive Pancreatic Cancer Proliferation and Metastasis via the Stimulation of Autophagy. Mol. Cancer Ther. 2016, 15, 2232–2243. [Google Scholar] [CrossRef]
- Latorre, E.; Carelli, S.; Raimondi, I.; D’Agostino, V.; Castiglioni, I.; Zucal, C.; Moro, G.; Luciani, A.; Ghilardi, G.; Monti, E.; et al. The Ribonucleic Complex HuR-MALAT1 Represses CD133 Expression and Suppresses Epithelial-Mesenchymal Transition in Breast Cancer. Cancer Res. 2016, 76, 2626–2636. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Z.; Wu, T.; Huang, Y.; Cheng, Z.; Li, X.; Sun, T.; Xie, X.; Zhou, Y.; Du, Z. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016, 7, e2123. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Wu, N.; Cheng, C.J.; Zhong, J.J.; He, J.C.; Zhang, Z.S.; Wang, Z.G.; Sun, X.C.; Liu, H. Essential role of MALAT1 in reducing traumatic brain injury. Neural Regen. Res. 2022, 17, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, J.; Han, Z.; Chen, Z.; Zhang, Q. Silencing of lncRNA MALAT1 Prevents Inflammatory Injury after Lung Transplant Ischemia-Reperfusion by Downregulation of IL-8 via p300. Mol. Ther. Nucleic Acids 2019, 18, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Stone, S.S.; Liu, N.; Gong, K.; Ren, C.; Sun, K.; Zhang, C.; Shao, G. The Role of the lncRNA MALAT1 in Neuroprotection against Hypoxic/Ischemic Injury. Biomolecules 2022, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, C.H.; Sun, Y.J. Overexpression of lncRNA GAS5 attenuates cardiac fibrosis through regulating PTEN/MMP-2 signal pathway in mice. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4414–4418. [Google Scholar]
- Yu, F.; Zheng, J.; Mao, Y.; Dong, P.; Lu, Z.; Li, G.; Guo, C.; Liu, Z.; Fan, X. Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA. J. Biol. Chem. 2015, 290, 28286–28298. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, H.; Niu, Y.; Huang, J.; Zhang, X.; Liu, X.; Zhang, Y.; Liu, S.; Fu, H.; Yu, C. Long noncoding RNA-GAS5 retards renal fibrosis through repressing miR-21 activity. Exp. Mol. Pathol. 2020, 116, 104518. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The lncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Cai, X.; Liu, Y.; Yang, W.; Xia, Y.; Yang, C.; Yang, S.; Liu, X. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J. Orthop. Res. 2016, 34, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.B.; Brito, B.L.; Dutrieux, R.P.; Lassance, F.P.; Hage, J.J. MMP-2 Assessment as an Indicator of Wound Healing: A Feasibility Study. Adv. Skin. Wound Care 2006, 19, 324–327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma. 2012, 2, 18–28. [Google Scholar]
- GenÇ, S.; Yenİ, Y.; ÇİÇEk, B.; Hacımüftüoğlu, A. Wound Healing of Quinic Acid in Human Dermal Fibroblasts by Regulating Expression of FN1 and COL1A1 Gene. Türk Doğa Fen Dergisi 2022, 11, 63–69. [Google Scholar] [CrossRef]
- Jones, B.; Patel, R.; Wang, B.; Evans-Nguyen, T.; Patel, N.A. Lyophilized Small Extracellular Vesicles (sEVs) Derived from Human Adipose Stem Cells Maintain Efficacy to Promote Healing in Neuronal Injuries. Biomedicines 2025, 13, 275. [Google Scholar] [CrossRef]


| Primer | Sense | AntiSense |
|---|---|---|
| Rat Malat1 | GGTTACCAGCCCAAACCTCA | GCATCAAGGTGAGGGGTGAA |
| Rat Gas5 | CTGTGATGGGACATCTGGTGG | TCCCATTTTCTGGCTTCCCAT |
| Rat IL1-β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC |
| Rat MMP9 | AGGCGCCGTGGTCCCCACTTACTT | GCAGGGTTTGCCGTCTCCGTTGCC |
| Rat TGF-β | GCAACAACGCAATCTSTGAC | CCTGTATTCCGTCTCCTT |
| Rat BCL2 | ATCGCTCTGTGTGGATGACTGAGTAC | AGAGACAGCCAGGAGAAATCAAAC |
| Rat VEGF | CCAGGACTACCCCGATGAGATAG | CTGGCTTTGGTGAGGTTTGATC |
| Rat IL6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| Rat TNFα | AGCACAGAAAGCATGATCCGAG | CCTGGTATGAAGTGGCAAATCG |
| Rat Col1 | AGGGAACAACTGATGGTGCTACTG | GGACTGCTGTGCCAAAATAAGAGA |
| Rat Col3 | AGGGAACAACTGATGCTGCTACTG | GGACTGCTGTGCCAAAATAAGAGA |
| Rat GAPDH | GGCAAGTTCAATGGCACAGT | TGGTGAAGACGCCAGTAGACTC |
| Human GAS5 | CTTCTGGGCTCAAGTGATCCT | TGTGCCATGAGACTCCATCAG |
| Human MALAT1 | CTTCCCTAGGGGATTTCAGG | GCCCACAGGAACAAGTCCTA |
| Human GAPDH | GATCATCAGCAATGCCTCCT | TGTGGTCATGAGTCCTTCCA |
| Antibody | Source | Cat # |
|---|---|---|
| Anti-TSG101 | Abcam (Cambridge, United Kingdom) | ab125011 |
| Anti-Hu CD63 | Millipore Sigma (Burlington, MA, USA) | CBL553 |
| Alix | Cell Signaling Technology (Danvers, MA, USA) | 92880 |
| Secondary HRP for rabbit | BioRad (Hercules, CA, USA) | 1706515 |
| Secondary HRP for mouse | Invitrogen (Waltham, MA, USA) | 62-6520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause-Hauch, M.; Patel, R.S.; Wang, B.; Osborne, B.; Jones, B.; Albear, P.; Patel, N.A. lncRNAs GAS5 and MALAT1 Contained in Human Adipose Stem Cell (hASC)-Derived Exosomes Drive the Cell-Free Repair and Regeneration of Wounds In Vivo. Int. J. Mol. Sci. 2025, 26, 3479. https://doi.org/10.3390/ijms26083479
Krause-Hauch M, Patel RS, Wang B, Osborne B, Jones B, Albear P, Patel NA. lncRNAs GAS5 and MALAT1 Contained in Human Adipose Stem Cell (hASC)-Derived Exosomes Drive the Cell-Free Repair and Regeneration of Wounds In Vivo. International Journal of Molecular Sciences. 2025; 26(8):3479. https://doi.org/10.3390/ijms26083479
Chicago/Turabian StyleKrause-Hauch, Meredith, Rekha S. Patel, Bangmei Wang, Brenna Osborne, Brianna Jones, Paul Albear, and Niketa A. Patel. 2025. "lncRNAs GAS5 and MALAT1 Contained in Human Adipose Stem Cell (hASC)-Derived Exosomes Drive the Cell-Free Repair and Regeneration of Wounds In Vivo" International Journal of Molecular Sciences 26, no. 8: 3479. https://doi.org/10.3390/ijms26083479
APA StyleKrause-Hauch, M., Patel, R. S., Wang, B., Osborne, B., Jones, B., Albear, P., & Patel, N. A. (2025). lncRNAs GAS5 and MALAT1 Contained in Human Adipose Stem Cell (hASC)-Derived Exosomes Drive the Cell-Free Repair and Regeneration of Wounds In Vivo. International Journal of Molecular Sciences, 26(8), 3479. https://doi.org/10.3390/ijms26083479






