Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review
Abstract
1. Introduction
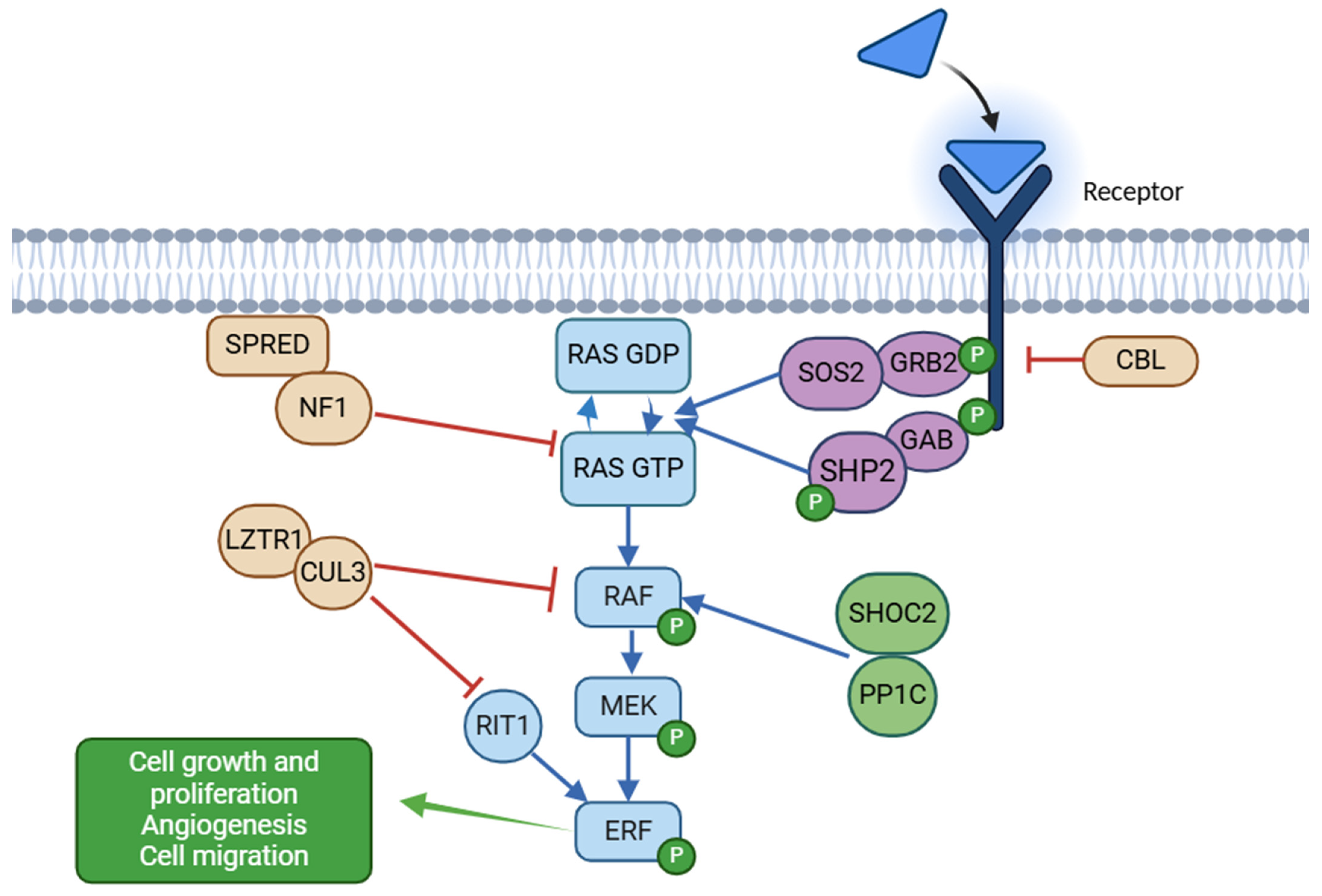
1.1. Biology of the RAS/MAPK Cascade
1.2. Genetic Causes of RASopathies and Genotype–Phenotype Correlations
1.3. Objectives of the Study
2. Materials and Methods
2.1. Study Design and Data Collection of the Retrospective Analysis of Our Cohort
2.2. Molecular Methods of the Retrospective Analysis of Our Cohort
2.3. Clinical Data Collection and Phenotypic Assessment of the Retrospective Analysis of Our Cohort
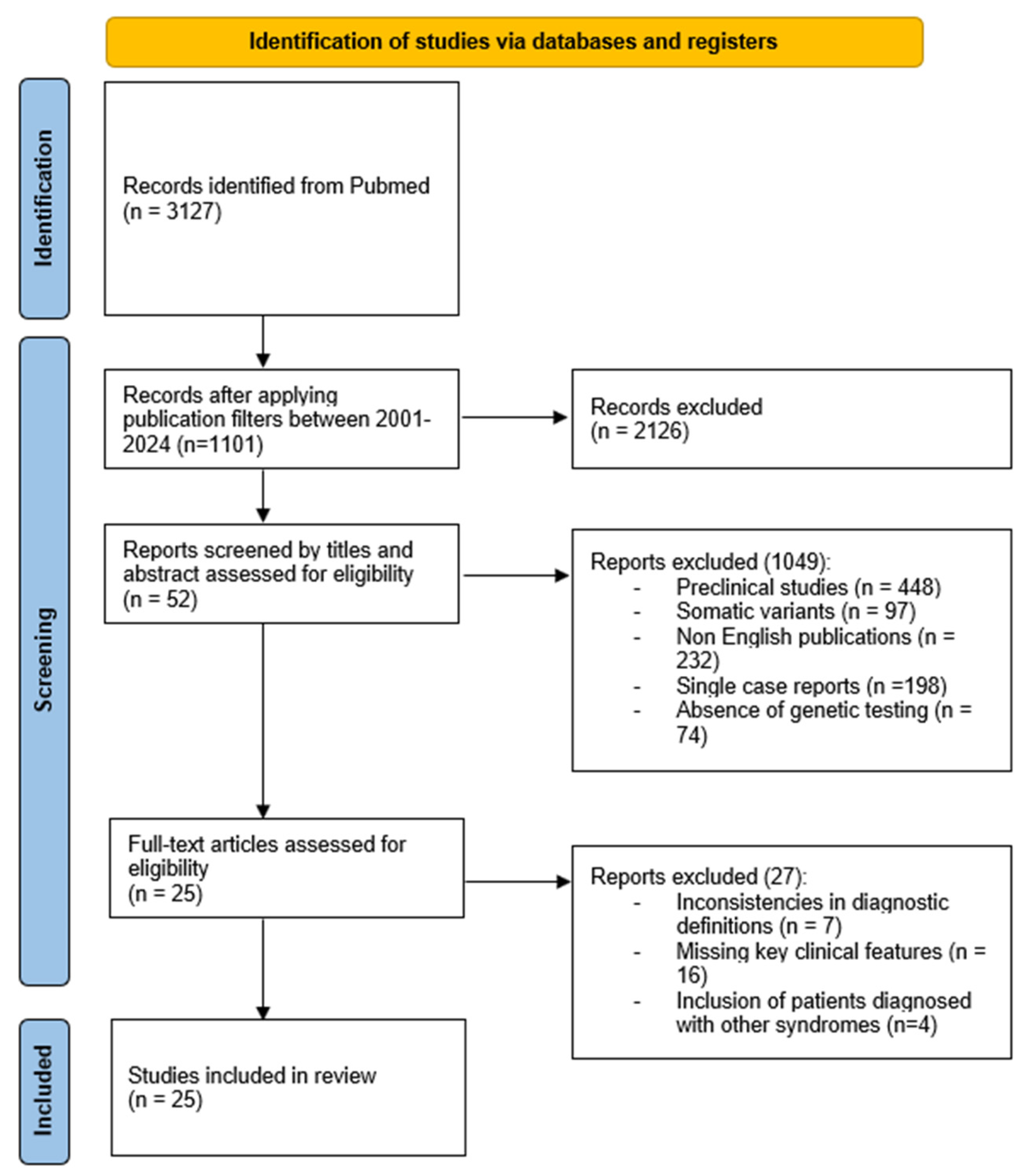
2.4. Methods of the Systematic Review
2.5. Statistical Analysis
3. Results
3.1. Genetic Findings
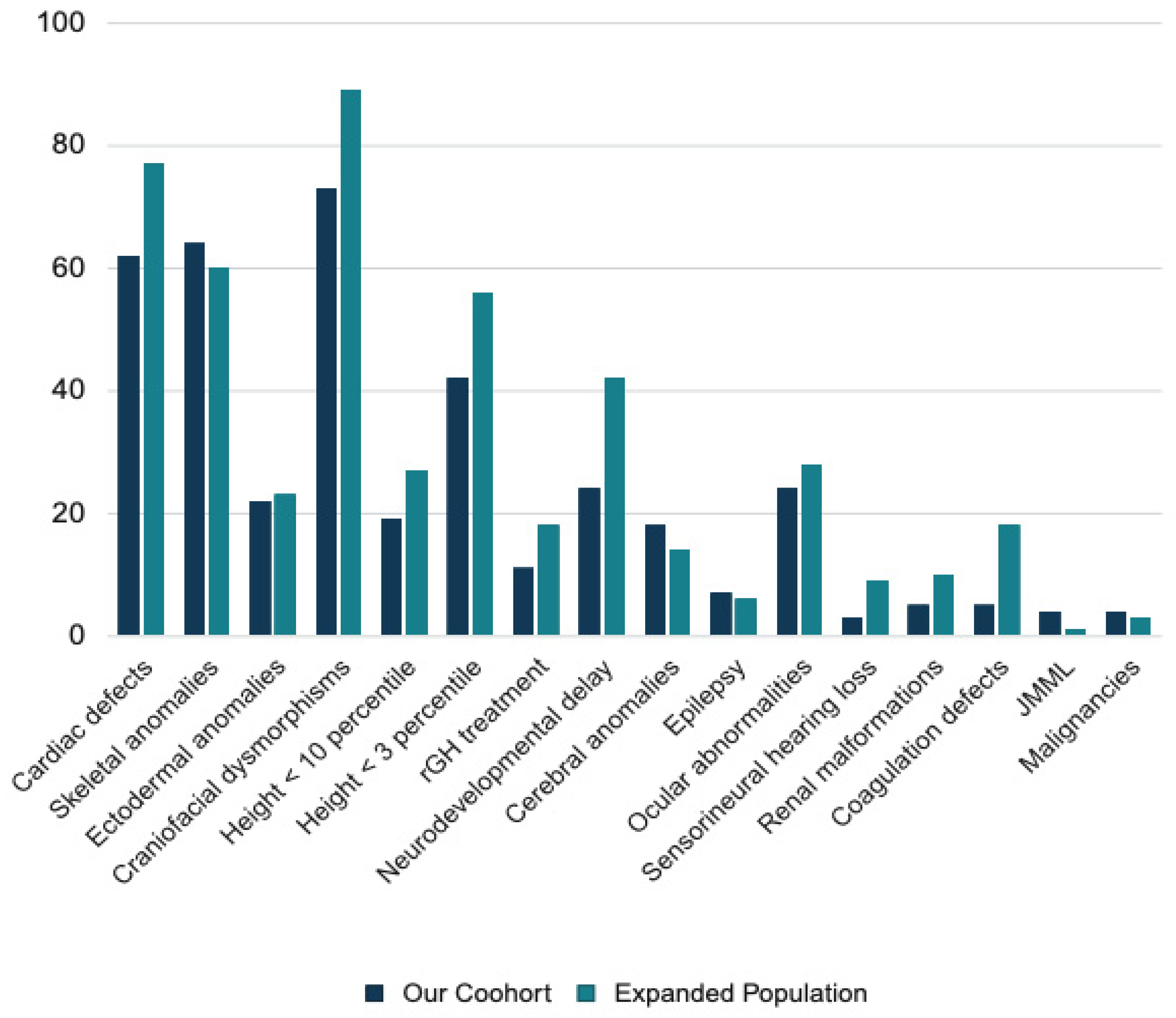
3.2. Clinical Findings in NS
3.3. Clinical Finding in Noonan Syndrome with Multiple Lentigines
3.4. Clinical Findings in Cardiofaciocutaneous Syndrome
3.5. Clinical Findings in Legius Syndrome
3.6. Clinical Findings in Noonan-like Syndrome with Loose Anagen Hair
3.7. Clinical Findings in Noonan Syndrome-like Disorder With or Without Juvenile Myelomonocytic Leukemia
4. Discussion
4.1. Genes Involved in Noonan Syndrome
4.2. Cardiac Phenotype in Noonan Syndrome
4.3. Growth in Noonan Syndrome
4.4. Cancer Risk in Noonan Syndrome
4.5. Neurodevelopmental Phenotype in Noonan Syndrome
4.6. Other Clinical Features of Noonan Syndrome
4.7. Molecular Correlations in Noonan Syndrome
4.8. Other RASopathies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noonan, J.A. Hypertelorism with Turner Phenotype: A New Syndrome with Associated Congenital Heart Disease. Am. J. Dis. Child. 1968, 116, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.A.; Allanson, J.E.; Dahlgren, J.; Gelb, B.D.; Hall, B.; Pierpont, M.E.; Roberts, A.E.; Robinson, W.; Takemoto, C.M.; Noonan, J.A. Noonan Syndrome: Clinical Features, Diagnosis, and Management Guidelines. Pediatrics 2010, 126, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Allanson, J.E.; Roberts, A.E. Noonan Syndrome. In Cassidy and Allanson’s Management of Genetic Syndromes; Wiley: Hoboken, NJ, USA, 2021; pp. 651–669. [Google Scholar]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Paccoud, R.; Branka, S.; Edouard, T.; Yart, A. The RASopathy family: Consequences of germline activation of the RAS/MAPK pathway. Endocr. Rev. 2018, 39, 676–700. [Google Scholar] [CrossRef]
- Rauen, K.A. The RASopathies. Annu. Rev. Genom. Hum. Genet. 2013, 14, 355–369. [Google Scholar] [CrossRef]
- Atay, O.; Skotheim, J.M. Spatial and temporal signal processing and decision making by MAPK pathways. J. Cell Biol. 2017, 216, 317–330. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Dentici, M.L.; Niceta, M.; Lepri, F.R.; Mancini, C.; Priolo, M.; Bonnard, A.A.; Cappelletti, C.; Leoni, C.; Ciolfi, A.; Pizzi, S.; et al. Loss-of-function variants in ERF are associated with a Noonan syndrome-like phenotype with or without craniosynostosis. Eur. J. Hum. Genet. 2024, 32, 954–963. [Google Scholar] [CrossRef]
- Motta, M.; Pannone, L.; Pantaleoni, F.; Bocchinfuso, G.; Radio, F.C.; Cecchetti, S.; Ciolfi, A.; Di Rocco, M.; Elting, M.W.; Brilstra, E.H.; et al. Enhanced MAPK1 Function Causes a Neurodevelopmental Disorder within the RASopathy Clinical Spectrum. Am. J. Hum. Genet. 2020, 107, 499–513. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Jahromi, B.R.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.-R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef]
- Nauth, T.; Bazgir, F.; Voß, H.; Brandenstein, L.I.; Mosaddeghzadeh, N.; Rickassel, V.; Deden, S.; Gorzelanny, C.; Schlüter, H.; Ahmadian, M.R.; et al. Cutaneous manifestations in Costello syndrome: HRAS p.Gly12Ser affects RIN1-mediated integrin trafficking in immortalized epidermal keratinocytes. Hum. Mol. Genet. 2023, 32, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Lepri, F.; De Luca, A.; Stella, L.; Rossi, C.; Baldassarre, G.; Pantaleoni, F.; Cordeddu, V.; Williams, B.J.; Dentici, M.L.; Caputo, V.; et al. SOS1 mutations in Noonan syndrome: Molecular spectrum, structural insights on pathogenic effects, and genotype-phenotype correlations. Hum. Mutat. 2011, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Cordeddu, V.; Yin, J.C.; Gunnarsson, C.; Virtanen, C.; Drunat, S.; Lepri, F.; De Luca, A.; Rossi, C.; Ciolfi, A.; Pugh, T.J.; et al. Activating Mutations Affecting the Dbl Homology Domain of SOS2 Cause Noonan Syndrome. Hum. Mutat. 2015, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Bouchikhi, E.I.; Belhassan, K.; Moufid, F.Z.; Houssaini, I.M.; Bouguenouch, L.; Samri, I.; Atmani, S.; Ouldim, K. Noonan syndrome-causing genes: Molecular update and an assessment of the mutation rate. Int. J. Pediatr. Adolesc. Med. 2016, 3, 133–142. [Google Scholar] [CrossRef]
- Brand, K.; Kentsch, H.; Glashoff, C.; Rosenberger, G. RASopathy-associated CBL germline mutations cause aberrant ubiquitylation and trafficking of EGFR. Hum. Mutat. 2014, 35, 1372–1381. [Google Scholar] [CrossRef]
- Martinelli, S.; De Luca, A.; Stellacci, E.; Rossi, C.; Checquolo, S.; Lepri, F.; Caputo, V.; Silvano, M.; Buscherini, F.; Consoli, F.; et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a noonan syndrome-like phenotype. Am. J. Hum. Genet. 2010, 87, 250–257. [Google Scholar] [CrossRef]
- Darouich, S.; Chakroun, A.S.; Bellamine, H.; Khamassi, I. A severe clinicopathologic phenotype of RAF1 Ser257Leu neomutation in a preterm infant without cardiac anomaly. Am. J. Med. Genet. A 2023, 191, 630–633. [Google Scholar] [CrossRef]
- Tajan, M.; de Rocca Serra, A.; Valet, P.; Edouard, T.; Yart, A. SHP2 sails from physiology to pathology. Eur. J. Med. Genet. 2015, 58, 509–525. [Google Scholar] [CrossRef]
- Motta, M.; Fidan, M.; Bellacchio, E.; Pantaleoni, F.; Schneider-Heieck, K.; Coppola, S.; Borck, G.; Salviati, L.; Zenker, M.; Cirstea, I.C.; et al. Dominant Noonan syndrome-causing LZTR1 mutations specifically affect the Kelch domain substrate-recognition surface and enhance RAS-MAPK signaling. Hum. Mol. Genet. 2019, 28, 1007–1022. [Google Scholar] [CrossRef]
- Yamamoto, G.L.; Aguena, M.; Gos, M.; Hung, C.; Pilch, J.; Fahiminiya, S.; Abramowicz, A.; Cristian, I.; Buscarilli, M.; Naslavsky, M.S.; et al. Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome. J. Med. Genet. 2015, 52, 413–421. [Google Scholar] [CrossRef]
- Bertola, D.R.; Yamamoto, G.L.; Almeida, T.F.; Buscarilli, M.; Jorge, A.A.L.; Malaquias, A.C.; Kim, C.A.; Takahashi, V.N.V.; Passos-Bueno, M.R.; Pereira, A.C. Further evidence of the importance of RIT1 in Noonan syndrome. Am. J. Med. Genet. Part A 2014, 164, 2952–2957. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, M.; Niihori, T.; Mizuno, S.; Okamoto, N.; Hayashi, S.; Watanabe, A.; Yokozawa, M.; Suzumura, H.; Nakahara, A.; Nakano, Y.; et al. Spectrum of mutations and genotype–phenotype analysis in Noonan syndrome patients with RIT1 mutations. Hum. Genet. 2016, 135, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Shimizu, A.; Torii, C.; Kosaki, R.; Takahashi, T.; Saya, H.; Kosaki, K. Multiple café au lait spots in familial patients with MAP2K2 mutation. Am. J. Med. Genet. A 2014, 164A, 392–396. [Google Scholar] [CrossRef]
- Zakharova, V.; Raykina, E.; Mersiyanova, I.; Deordieva, E.; Pershin, D.; Vedmedskia, V.; Rodina, Y.; Kuzmenko, N.; Maschan, M.; Shcherbina, A. Cancer-causing MAP2K1 mutation in a mosaic patient with cardio-facio-cutaneous syndrome and immunodeficiency. Hum. Mutat. 2022, 43, 1852–1855. [Google Scholar] [CrossRef]
- Pierpont, E.I.; Semrud-Clikeman, M.; Pierpont, M.E. Variability in clinical and neuropsychological features of individuals with MAP2K1 mutations. Am. J. Med. Genet. A 2017, 173, 452–459. [Google Scholar] [CrossRef]
- Chinton, J.; Huckstadt, V.; Moresco, A.; Gravina, L.P.; Obregon, M.G. Clinical and molecular characterization of children with Noonan syndrome and other RASopathies in Argentina. Arch. Argent. Pediatr. 2019, 117, 330–336. [Google Scholar] [CrossRef]
- Nava, C.; Hanna, N.; Michot, C.; Pereira, S.; Pouvreau, N.; Niihori, T.; Aoki, Y.; Matsubara, Y.; Arveiler, B.; Lacombe, D.; et al. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: Genotype-phenotype relationships and overlap with Costello syndrome. J. Med. Genet. 2007, 44, 763–771. [Google Scholar] [CrossRef]
- Sarkozy, A.; Carta, C.; Moretti, S.; Zampino, G.; Digilio, M.C.; Pantaleoni, F.; Scioletti, A.P.; Esposito, G.; Cordeddu, V.; Lepri, F.; et al. Germline BRAF mutations in noonan, LEOPARD, and cardiofaciocutaneous Syndromes: Molecular diversity and associated phenotypic spectrum. Hum. Mutat. 2009, 30, 695–702. [Google Scholar] [CrossRef]
- Rauen, K.A.; Banerjee, A.; Bishop, W.R.; Lauchle, J.O.; Mccormick, F.; Mcmahon, M.; Melese, T.; Munster, P.N.; Nadaf, S.; Packer, R.J.; et al. Costello and cardio-facio-cutaneous syndromes: Moving toward clinical trials in RASopathies. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157, 136–146. [Google Scholar] [CrossRef]
- Jongmans, M.C.J.; Hoogerbrugge, P.M.; Hilkens, L.; Flucke, U.; van der Burgt, I.; Noordam, K.; Ruiterkamp-Versteeg, M.; Yntema, H.G.; Nillesen, W.M.; Ligtenberg, M.J.L.; et al. Noonan syndrome, the SOS1 gene and embryonal rhabdomyosarcoma. Genes Chromosom. Cancer 2010, 49, 635–641. [Google Scholar] [CrossRef]
- Yin, J.C.; Platt, M.J.; Tian, X.; Wu, X.; Backx, P.H.; Simpson, J.A.; Araki, T.; Neel, B.G. Cellular interplay via cytokine hierarchy causes pathological cardiac hypertrophy in RAF1-mutant Noonan syndrome. Nat. Commun. 2017, 8, 15518. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, R.J.; Anderson, R.C.; Blaw, M. Multiple Lentigenes Syndrome: Complex Comprising Multiple Lentigenes, Electrocardiographic Conduction Abnormalities, Ocular Hypertelorism, Pulmonary Stenosis, Abnormalities of Genitalia, Retardation of Growth, Sensorineural Deafness, and Autosomal Dominan. Am. J. Dis. Child. 1969, 117, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, A.; Conti, E.; Digilio, M.C.; Marino, B.; Morini, E.; Pacileo, G.; Wilson, M.; Calabrò, R.; Pizzuti, A.; Dallapiccola, B. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J. Med. Genet. 2004, 41, e68. [Google Scholar] [CrossRef]
- Johnston, J.J.; van der Smagt, J.J.; Rosenfeld, J.A.; Pagnamenta, A.T.; Alswaid, A.; Baker, E.H.; Blair, E.; Borck, G.; Brinkmann, J.; Craigen, W.; et al. Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genet. Med. 2018, 20, 1175–1185. [Google Scholar] [CrossRef]
- Chinton, J.; Huckstadt, V.; Mucciolo, M.; Lepri, F.; Novelli, A.; Gravina, L.P.; Obregon, M.G. Providing more evidence on LZTR1 variants in Noonan syndrome patients. Am. J. Med. Genet. A 2020, 182, 409–414. [Google Scholar] [CrossRef]
- Tartaglia, M.; Kalidas, K.; Shaw, A.; Song, X.; Musat, D.L.; van der Burgt, I.; Brunner, H.G.; Bertola, D.R.; Crosby, A.; Ion, A.; et al. PTPN11 mutations in noonan syndrome: Molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet. 2002, 70, 1555–1563. [Google Scholar] [CrossRef]
- Roberts, A.E.; Araki, T.; Swanson, K.D.; Montgomery, K.T.; Schiripo, T.A.; Joshi, V.A.; Li, L.; Yassin, Y.; Tamburino, A.M.; Neel, B.G.; et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat. Genet. 2007, 39, 70–74. [Google Scholar] [CrossRef]
- Razzaque, M.A.; Nishizawa, T.; Komoike, Y.; Yagi, H.; Furutani, M.; Amo, R.; Kamisago, M.; Momma, K.; Katayama, H.; Nakagawa, M.; et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat. Genet. 2007, 39, 1013–1017. [Google Scholar] [CrossRef]
- Tartaglia, M.; Aoki, Y.; Gelb, B.D. The molecular genetics of RASopathies: An update on novel disease genes and new disorders. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190C, 425–439. [Google Scholar] [CrossRef]
- Motta, M.; Fasano, G.; Gredy, S.; Brinkmann, J.; Bonnard, A.A.; Simsek-Kiper, P.O.; Gulec, E.Y.; Essaddam, L.; Utine, G.E.; Prandi, I.G.; et al. SPRED2 loss-of-function causes a recessive Noonan syndrome-like phenotype. Am. J. Hum. Genet. 2021, 108, 2112–2129. [Google Scholar] [CrossRef]
- Kruszka, P.; Porras, A.R.; Addissie, Y.A.; Moresco, A.; Medrano, S.; Mok, G.T.K.; Leung, G.K.C.; Tekendo-Ngongang, C.; Uwineza, A.; Thong, M.K.; et al. Noonan syndrome in diverse populations. Am. J. Med. Genet. Part A 2017, 173, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Gazzin, A.; Fornari, F.; Niceta, M.; Leoni, C.; Dentici, M.L.; Carli, D.; Villar, A.M.; Calcagni, G.; Banaudi, E.; Massuras, S.; et al. Defining the variant-phenotype correlation in patients affected by Noonan syndrome with the RAF1:c.770C>T p.(Ser257Leu) variant. Eur. J. Hum. Genet. 2024, 32, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Tekendo-Ngongang, C.; Agenbag, G.; Bope, C.D.; Esterhuizen, A.I.; Wonkam, A. Noonan syndrome in South Africa: Clinical and molecular profiles. Front. Genet. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Bertola, D.R.; Castro, M.A.A.; Yamamoto, G.L.; Honjo, R.S.; Ceroni, J.R.; Buscarilli, M.M.; Freitas, A.B.; Malaquias, A.C.; Pereira, A.C.; Jorge, A.A.L.; et al. Phenotype–genotype analysis of 242 individuals with RASopathies: 18-year experience of a tertiary center in Brazil. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 896–911. [Google Scholar] [CrossRef]
- Athota, J.P.; Bhat, M.; Nampoothiri, S.; Gowrishankar, K.; Narayanachar, S.G.; Puttamallesh, V.; Farooque, M.O.; Shetty, S. Molecular and clinical studies in 107 Noonan syndrome affected individuals with PTPN11 mutations. J. Hum. Genet. 2018, 83, 87–93. [Google Scholar] [CrossRef]
- Li, X.; Yao, R.; Tan, X.; Li, N.; Ding, Y.; Li, J.; Chang, G.; Chen, Y.; Ma, L.; Wang, J.; et al. Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients. Clin. Genet. 2019, 96, 290–299. [Google Scholar] [CrossRef]
- Jongmans, M.; Sistermans, E.A.; Rikken, A.; Nillesen, W.M.; Tamminga, R.; Patton, M.; Maier, E.M.; Tartaglia, M.; Noordam, K.; van der Burgt, I. Genotypic and phenotypic characterization of Noonan syndrome: New data and review of the literature. Am. J. Med. Genet. A 2005, 134A, 165–170. [Google Scholar] [CrossRef]
- Shoji, Y.; Ida, S.; Niihori, T.; Aoki, Y.; Okamoto, N.; Etani, Y.; Kawai, M. Genotype-phenotype correlation analysis in Japanese patients with Noonan syndrome. Endocr. J. 2019, 66, 983–994. [Google Scholar] [CrossRef]
- Zenker, M.; Buheitel, G.; Rauch, R.; Koenig, R.; Bosse, K.; Kress, W.; Tietze, H.-U.; Doerr, H.-G.; Hofbeck, M.; Singer, H.; et al. Genotype-phenotype correlations in Noonan syndrome. J. Pediatr. 2004, 144, 368–374. [Google Scholar] [CrossRef]
- Kouz, K.; Lissewski, C.; Spranger, S.; Mitter, D.; Riess, A.; Lopez-Gonzalez, V.; Lüttgen, S.; Aydin, H.; Von Deimling, F.; Evers, C.; et al. Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genet. Med. 2016, 18, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Kehl, H.G.; Majewski, F.; Meinecke, P.; Schweiger, S.; Gillessen-Kaesbach, G.; Wieczorek, D.; Hinkel, G.K.; Tinschert, S.; Hoeltzenbein, M.; et al. Spectrum of mutations in PTPN11 and genotype-phenotype correlation in 96 patients with Noonan syndrome and five patients with cardio-facio-cutaneous syndrome. Eur. J. Hum. Genet. 2003, 11, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.M.; Kim, J.M.; Kim, G.H.; Yoo, H.W. PTPN11, SOS1; KRAS; RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome. J. Hum. Genet. 2008, 53, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.; Papadopoulou, A.; Kosma, K.; Papadimitriou, A.; Papaevangelou, V.; Kanaka-Gantenbein, C.; Bountouvi, E.; Kitsiou-Tzeli, S. Molecular and clinical profile of patients referred as Noonan or Noonan-like syndrome in Greece: A cohort of 86 patients. Eur. J. Pediatr. 2022, 181, 3691–3700. [Google Scholar] [CrossRef]
- Sarkozy, A.; Conti, E.; Seripa, D.; Digilio, M.C.; Grifone, N.; Tandoi, C.; Fazio, V.M.; Di Ciommo, V.; Marino, B.; Pizzuti, A.; et al. Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. J. Med. Genet. 2003, 40, 704–708. [Google Scholar] [CrossRef]
- Bertola, D.R.; Pereira, A.C.; Albano, L.M.J.; De Oliveira, P.S.L.; Kim, C.A.; Krieger, J.E. PTPN11 gene analysis in 74 Brazilian patients with Noonan syndrome or Noonan-like phenotype. Genet. Test. 2006, 10, 186–191. [Google Scholar] [CrossRef]
- Essawi, M.L.; Ismail, M.F.; Afifi, H.H.; Kobesiy, M.M.; El Kotoury, A.; Barakat, M.M. Mutational analysis of the PTPN11 gene in Egyptian patients with Noonan syndrome. J. Formos. Med. Assoc. 2013, 112, 707–712. [Google Scholar] [CrossRef][Green Version]
- Lallar, M.; Bijarnia-Mahay, S.; Verma, I.C.; Mandal, K.; Puri, R.D. Mutation and Phenotypic Spectrum of Patients with RASopathies. Indian Pediatr. 2021, 58, 30–33. [Google Scholar] [CrossRef]
- Şimşek-Kiper, P.; Alanay, Y.; Gülhan, B.; Lissewski, C.; Türkyilmaz, D.; Alehan, D.; Çetin, M.; Utine, G.E.; Zenker, M.; Boduroǧlu, K. Clinical and molecular analysis of RASopathies in a group of Turkish patients. Clin. Genet. 2013, 83, 181–186. [Google Scholar] [CrossRef]
- Louati, R.; Abdelmoula, N.B.; Trabelsi, I.; Abid, D.; Lissewski, C.; Kharrat, N.; Kamoun, S.; Zenker, M.; Rebai, T. Clinical and molecular findings of tunisian patients with rasopathies. Mol. Syndromol. 2014, 5, 212–217. [Google Scholar] [CrossRef]
- van Trier, D.C.; Rinne, T.; Noordam, K.; Draaisma, J.M.; van der Burgt, I. Variable phenotypic expression in a large Noonan syndrome family segregating a novel SOS1 mutation. Am. J. Med. Genet. A 2017, 173, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Kaisaki, P.J.; Bennett, F.; Burkitt-Wright, E.; Martin, H.C.; Ferla, M.P.; Taylor, J.M.; Gompertz, L.; Lahiri, N.; Tatton-Brown, K.; et al. Delineation of dominant and recessive forms of LZTR1-associated Noonan syndrome. Clin. Genet. 2019, 95, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Tafazoli, A.; Eshraghi, P.; Pantaleoni, F.; Vakili, R.; Moghaddassian, M.; Ghahraman, M.; Muto, V.; Paolacci, S.; Golyan, F.F.; Abbaszadegan, M.R. Novel mutations and their genotype-phenotype correlations in patients with Noonan syndrome, using next-generation sequencing. Adv. Med. Sci. 2018, 63, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, J.M.; Jin, H.Y.; Kim, G.H.; Choi, J.H.; Yoo, H.W. Spectrum of mutations in Noonan syndrome and their correlation with phenotypes. J. Pediatr. 2011, 159, 1029–1035. [Google Scholar] [CrossRef]
- Schubbert, S.; Zenker, M.; Rowe, S.L.; Böll, S.; Klein, C.; Bollag, G.; van der Burgt, I.; Musante, L.; Kalscheuer, V.; Wehner, L.E.; et al. Germline KRAS mutations cause Noonan syndrome. Nat. Genet. 2006, 38, 331–336. [Google Scholar] [CrossRef]
- Pandit, B.; Sarkozy, A.; Pennacchio, L.A.; Carta, C.; Oishi, K.; Martinelli, S.; Pogna, E.A.; Schackwitz, W.; Ustaszewska, A.; Landstrom, A.; et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 2007, 39, 1007–1012. [Google Scholar] [CrossRef]
- Umeki, I.; Niihori, T.; Abe, T.; Kanno, S.-I.; Okamoto, N.; Mizuno, S.; Kurosawa, K.; Nagasaki, K.; Yoshida, M.; Ohashi, H.; et al. Delineation of LZTR1 mutation-positive patients with Noonan syndrome and identification of LZTR1 binding to RAF1-PPP1CB complexes. Hum. Genet. 2019, 138, 21–35. [Google Scholar] [CrossRef]
- Aoki, Y.; Niihori, T.; Banjo, T.; Okamoto, N.; Mizuno, S.; Kurosawa, K.; Ogata, T.; Takada, F.; Yano, M.; Ando, T.; et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am. J. Hum. Genet. 2013, 93, 173–180. [Google Scholar] [CrossRef]
- Sarkozy, A.; Digilio, M.C.; Marino, B.; Mingarelli, R.; Tartaglia, M.; Dallapiccola, B. Noonan’s syndrome and related disorders: Clinical-molecular update and guidelines. Ital. J. Pediatr. 2006, 32, 145–155. [Google Scholar]
- Romano, A.A.; Blethen, S.L.; Dana, K.; Noto, R.A. Growth hormone treatment in Noonan syndrome: The National Cooperative Growth Study experience. J. Pediatr. 1996, 128, S18–S21. [Google Scholar] [CrossRef]
- Romano, A.A.; Dana, K.; Bakker, B.; Davis, D.A.; Hunold, J.J.; Jacobs, J.; Lippe, B. Growth response, near-adult height, and patterns of growth and puberty in patients with Noonan syndrome treated with growth hormone. J. Clin. Endocrinol. Metab. 2009, 94, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Serra-Nédélec, D.R.A.; Edouard, T.; Tréguer, K.; Tajan, M.; Araki, T.; Dance, M.; Mus, M.; Montagner, A.; Tauber, M.; Salles, J.P.; et al. Noonan syndrome-causing SHP2 mutants inhibit insulinlike growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc. Natl. Acad. Sci. USA 2012, 109, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Binder, G. Response to growth hormone in short children with noonan syndrome: Correlation to genotype. Horm. Res. 2009, 72, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.V.; Souza, S.A.L.; Arnhold, I.J.P.; Mendonca, B.B.; Jorge, A.A.L. PTPN11 (Protein Tyrosine Phosphatase, Nonreceptor Type 11) Mutations and Response to Growth Hormone Therapy in Children with Noonan Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5156–5160. [Google Scholar] [CrossRef]
- Binder, G.; Neuer, K.; Ranke, M.B.; Wittekindt, N.E. PTPN11 mutations are associated with mild growth hormone resistance in individuals with noonan syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5377–5381. [Google Scholar] [CrossRef]
- Noonan, J.A.; Kappelgaard, A.M. The efficacy and safety of growth hormone therapy in children with noonan syndrome: A review of the evidence. Horm. Res. Paediatr. 2015, 83, 157–166. [Google Scholar] [CrossRef]
- Castinetti, F.; Reynaud, R.; Brue, T. Syndrome de Noonan et hormone de croissance. Ann. Endocrinol. 2008, 69, S2–S5. [Google Scholar] [CrossRef]
- Raaijmakers, R.; Noordam, C.; Karagiannis, G.; Gregory, J.W.; Hertel, N.T.; Sipilä, I.; Otten, B.J. Response to growth hormone treatment and final height in Noonan syndrome in a large cohort of patients in the KIGS database. J. Pediatr. Endocrinol. Metab. 2008, 21, 267–273. [Google Scholar] [CrossRef]
- MacFarlane, C.E.; Brown, D.C.; Johnston, L.B.; Patton, M.A.; Dunger, D.B.; Savage, M.O.; McKenna, W.J.; Kelnar, C.J.H. Growth Hormone Therapy and Growth in Children with Noonan’s Syndrome: Results of 3 Years’ Follow-Up*. J. Clin. Endocrinol. Metab. 2001, 86, 1953–1956. [Google Scholar] [CrossRef][Green Version]
- Lee, P.A.; Ross, J.; Germak, J.A.; Gut, R. Effect of 4 years of growth hormone therapy in children with Noonan syndrome in the American Norditropin Studies: Web-Enabled Research (ANSWER) Program® registry. Int. J. Pediatr. Endocrinol. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Shaw, A.C.; Kalidas, K.; Crosby, A.H.; Jeffery, S.; Patton, M.A. The natural history of Noonan syndrome: A long-term follow-up study. Arch. Dis. Child. 2007, 92, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Schubbert, S.; Shannon, K.; Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 2007, 7, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kratz, C.P.; Franke, L.; Peters, H.; Kohlschmidt, N.; Kazmierczak, B.; Finckh, U.; Bier, A.; Eichhorn, B.; Blank, C.; Kraus, C.; et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br. J. Cancer 2015, 112, 1392–1397. [Google Scholar] [CrossRef]
- Kratz, C.P.; Niemeyer, C.M.; Castleberry, R.P.; Cetin, M.; Bergsträsser, E.; Emanuel, P.D.; Hasle, H.; Kardos, G.; Klein, C.; Kojima, S.; et al. The mutational spectrum of PTPN11 in juvenile myelomonocytic leukemia and Noonan syndrome/myeloproliferative disease. Blood 2005, 106, 2183–2185. [Google Scholar] [CrossRef]
- Choong, K.; Freedman, M.H.; Chitayat, D.; Kelly, E.N.; Taylor, G.; Zipursky, A. Juvenile myelomonocytic leukemia and Noonan syndrome. J. Pediatr. Hematol. Oncol. 1999, 21, 523–527. [Google Scholar] [CrossRef]
- Perrino, M.R.; Das, A.; Scollon, S.R.; Mitchell, S.G.; Greer, M.-L.C.; Yohe, M.E.; Hansford, J.R.; Kalish, J.M.; Schultz, K.A.P.; MacFarland, S.P.; et al. Update on Pediatric Cancer Surveillance Recommendations for Patients with Neurofibromatosis Type 1, Noonan Syndrome, CBL Syndrome, Costello Syndrome, and Related RASopathies. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2024, 30, 4834–4843. [Google Scholar] [CrossRef]
- Villani, A.; Greer, M.L.C.; Kalish, J.M.; Nakagawara, A.; Nathanson, K.L.; Pajtler, K.W.; Pfister, S.M.; Walsh, M.F.; Wasserman, J.D.; Zelley, K.; et al. Recommendations for cancer surveillance in individuals with RASopathies and other rare genetic conditions with increased cancer risk. Clin. Cancer Res. 2017, 23, e83–e90. [Google Scholar] [CrossRef]
- Mcwilliams, G.D.; Santacruz, K.; Hart, B.; Clericuzio, C. Occurrence of DNET and other brain tumors in Noonan syndrome warrants caution with growth hormone therapy. Am. J. Med. Genet. Part A 2016, 170, 195–201. [Google Scholar] [CrossRef]
- Khan, S.; McDowell, H.; Upadhyaya, M.; Fryer, A. Vaginal rhabdomyosarcoma in a patient with Noonan syndrome. J. Med. Genet. 1995, 32, 743–745. [Google Scholar] [CrossRef]
- Shatara, M.; Schieffer, K.M.; Melas, M.; Varga, E.A.; Thomas, D.; Bucknor, B.A.; Costello, H.M.; Wheeler, G.; Kelly, B.J.; Miller, K.E.; et al. Molecular characterization of gliomas and glioneuronal tumors amid Noonan syndrome: Cancer predisposition examined. Front. Oncol. 2024, 14, 1453309. [Google Scholar] [CrossRef]
- Russ, S.A.; Larson, K.; Halfon, N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012, 129, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Trevathan, E.; Yeargin-Allsopp, M. Prevalence of epilepsy and epileptic seizures in 10-year-old children: Results from the Metropolitan Atlanta Developmental Disabilities Study. Epilepsia 1995, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Wellesley, D.G.; Barisic, I.; Addor, M.-C.; Bergman, J.E.H.; Braz, P.; Cavero-Carbonell, C.; Draper, E.S.; Gatt, M.; Haeusler, M.; et al. Epidemiology of congenital cerebral anomalies in Europe: A multicentre, population-based EUROCAT study. Arch. Dis. Child. 2019, 104, 1181–1187. [Google Scholar] [CrossRef]
- Kim, I.-J.; Drahushuk, K.M.; Kim, W.-Y.; Gonsiorek, E.A.; Lein, P.; Andres, D.A.; Higgins, D. Extracellular signal-regulated kinases regulate dendritic growth in rat sympathetic neurons. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 3304–3312. [Google Scholar] [CrossRef]
- Davico, C.; D’Alessandro, R.; Borgogno, M.; Campagna, F.; Torta, F.; Ricci, F.; Amianto, F.; Vittorini, R.; Carli, D.; Mussa, A.; et al. Epilepsy in a cohort of children with Noonan syndrome and related disorders. Eur. J. Pediatr. 2022, 181, 2919–2926. [Google Scholar] [CrossRef]
- Sharland, M.; Burch, M.; McKenna, W.M.; Paton, M.A. A clinical study of Noonan syndrome. Arch. Dis. Child. 1992, 67, 178–183. [Google Scholar] [CrossRef]
- Duenas, D.A.; Preissig, S.; Summitt, R.L.; Wilroy, R.S.; Lemmi, H.; Dews, J.E. Neurologic manifestations of the noonan syndrome. South Med. J. 1973, 66, 193–196. [Google Scholar] [CrossRef]
- Adviento, B.; Corbin, I.L.; Widjaja, F.; Desachy, G.; Enrique, N.; Rosser, T.; Risi, S.; Marco, E.J.; Hendren, R.L.; Bearden, C.E.; et al. Autism traits in the RASopathies. J. Med. Genet. 2014, 51, 10–20. [Google Scholar] [CrossRef]
- Chen, B.; Bronson, R.T.; Klaman, L.D.; Hampton, T.G.; Wang, J.F.; Green, P.J.; Magnuson, T.; Douglas, P.S.; Morgan, J.P.; Neel, B.G. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 2000, 24, 296–299. [Google Scholar] [CrossRef]
- Tartaglia, M.; Pennacchio, L.A.; Zhao, C.; Yadav, K.K.; Fodale, V.; Sarkozy, A.; Pandit, B.; Oishi, K.; Martinelli, S.; Schackwitz, W.; et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat. Genet. 2007, 39, 75–79. [Google Scholar] [CrossRef]
- Kobayashi, T.; Aoki, Y.; Niihori, T.; Cavé, H.; Verloes, A.; Okamoto, N.; Kawame, H.; Fujiwara, I.; Takada, F.; Ohata, T.; et al. Molecular and clinical analysis of RAF1 in Noonan syndrome and related disorders: Dephosphorylation of serine 259 as the essential mechanism for mutant activation. Hum. Mutat. 2010, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, A.; Xie, J.; Liu, Y.F.; Poplawski, A.B.; Gomes, A.R.; Madanecki, P.; Fu, C.; Crowley, M.R.; Crossman, D.K.; Armstrong, L.; et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat. Genet. 2014, 46, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kuske, M.; Westphal, D.; Wehner, R.; Schmitz, M.; Beissert, S.; Praetorius, C.; Meier, F. Immunomodulatory effects of BRAF and MEK inhibitors: Implications for Melanoma therapy. Pharmacol. Res. 2018, 136, 151–159. [Google Scholar] [CrossRef]
- Gorlin, R.J.; Anderson, R.C.; Moller, J.H. The leopard (multiple lentigines) syndrome revisited. Laryngoscope 1971, 81, 1674–1681. [Google Scholar] [CrossRef]
- Coppin, B.D.; Temple, I.K. Multiple lentigines syndrome (LEOPARD syndrome or progressive cardiomyopathic lentiginosis). J. Med. Genet. 1997, 34, 582–586. [Google Scholar] [CrossRef]
- Leoni, C.; Onesimo, R.; Giorgio, V.; Diamanti, A.; Giorgio, D.; Martini, L.; Rossodivita, A.; Tartaglia, M.; Zampino, G. Understanding Growth Failure in Costello Syndrome: Increased Resting Energy Expenditure. J. Pediatr. 2016, 170, 322–324. [Google Scholar] [CrossRef]
- Gripp, K.W.; Aldinger, K.A.; Bennett, J.T.; Baker, L.; Tusi, J.; Powell-Hamilton, N.; Stabley, D.; Sol-Church, K.; Timms, A.E.; Dobyns, W.B. A novel rasopathy caused by recurrent de novo missense mutations in PPP1CB closely resembles Noonan syndrome with loose anagen hair. Am. J. Med. Genet. Part A 2016, 170, 2237–2247. [Google Scholar] [CrossRef]
- Martinelli, S.; Stellacci, E.; Pannone, L.; D’Agostino, D.; Consoli, F.; Lissewski, C.; Silvano, M.; Cencelli, G.; Lepri, F.; Maitz, S.; et al. Molecular Diversity and Associated Phenotypic Spectrum of Germline CBL Mutations. Hum. Mutat. 2015, 36, 787–796. [Google Scholar] [CrossRef]
| Title | DOI | Reference | Number of Cases |
|---|---|---|---|
| Phenotype–genotype analysis of 242 individuals with RASopathies: 18-year experience of a tertiary center in Brazil | DOI: 10.1002/ajmg.c.31851 | [46] | 181 |
| This cohort | 116 | ||
| Molecular and clinical studies in 107 Noonan syndrome affected individuals with PTPN11 variants | DOI: 10.1186/s12881-020-0986-5 | [47] | 107 |
| Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients | DOI: 10.1111/cge.13588 | [48] | 102 |
| Genotypic and Phenotypic Characterization of Noonan Syndrome: New Data and Review of the Literature | DOI: 10.1002/ajmg.a.30598 | [49] | 56 |
| PTPN11 Variants in Noonan Syndrome: Molecular Spectrum, Genotype–Phenotype Correlation, and Phenotypic Heterogeneity | DOI: 10.1086/340847 | [37] | 54 |
| Spectrum of variants and genotype–phenotype analysis in Noonan syndrome patients with RIT1 variants | DOI: 10.1007/s00439-015-1627-5 | [23] | 48 |
| Genotype–phenotype correlation analysis in Japanese patients with Noonan syndrome | DOI: 10.1507/endocrj.EJ18-0564 | [50] | 39 |
| Genotype–phenotype correlations in Noonan syndrome | DOI: 10.1016/j.jpeds.2003.11.032 | [51] | 34 |
| Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation | DOI: 10.1038/gim.2016.32 | [52] | 33 |
| Spectrum of variants in PTPN11 and genotype–phenotype correlation in 96 patients with Noonan syndrome and five patients with cardio-facio-cutaneous syndrome | DOI: 10.1038/sj.ejhg.5200935 | [53] | 32 |
| PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype–phenotype correlation in Korean patients with Noonan syndrome | DOI: 10.1007/s10038-008-0343-6 | [54] | 30 |
| Molecular and clinical profile of patients referred as Noonan or Noonan-like syndrome in Greece: a cohort of 86 patients | DOI: 10.1007/s00431-022-04574-w | [55] | 28 |
| Correlation between PTPN11 gene variants and congenital heart defects in Noonan and LEOPARD syndromes | DOI: 10.1136/jmg.40.9.704 | [56] | 23 |
| PTPN11 Gene Analysis in 74 Brazilian Patients with Noonan Syndrome or Noonan-like Phenotype | DOI: 10.1089/gte.2006.10.186 | [57] | 21 |
| Mutational analysis of the PTPN11 gene in Egyptian patients with Noonan syndrome | DOI: 10.1016/j.jfma.2012.06.002 | [58] | 21 |
| Mutation and Phenotypic Spectrum of Patients With RASopathies | PMID: 33452774 | [59] | 19 |
| Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome | DOI: 10.1136/jmedgenet-2015-103018 | [21] | 18 |
| Clinical and molecular analysis of RASopathies in a group of Turkish patients | DOI: 10.1111/j.1399-0004.2012.01875.x | [60] | 15 |
| Providing more evidence on LZTR1 variants in Noonan syndrome patients | DOI: 10.1002/ajmg.a.61445 | [36] | 14 |
| Clinical and Molecular Findings of Tunisian Patients with RASopathies | DOI: 10.1159/000362898 | [61] | 11 |
| Variable phenotypic expression in a large Noonan syndrome family segregating a novel SOS1 mutation | DOI: 10.1002/ajmg.a.38466 | [62] | 10 |
| Delineation of dominant and recessive forms of LZTR1-associated Noonan syndrome | DOI: 10.1111/cge.13533 | [63] | 9 |
| Novel variants and their genotype–phenotype correlations in patients with Noonan syndrome, using next-generation sequencing | DOI: 10.1016/j.advms.2017.07.001 | [64] | 8 |
| Activating Variants Affecting the Dbl Homology Domain of SOS2 Cause Noonan Syndrome | DOI: 10.1002/humu.22834 | [14] | 7 |
| Noonan Syndrome in South Africa: Clinical and Molecular Profiles | DOI: 10.3389/fgene.2019.00333 | [44] | 3 |
| PTPN11 (n = 79) | SOS1 (n = 12) | RAF1 (n = 10) | LZTR1 (n = 9) | BRAF (n = 2) | KRAS (n = 2) | RIT1 (n = 1) | SOS2 (n = 1) | Total (n = 116) | |
|---|---|---|---|---|---|---|---|---|---|
| Cardiac features | |||||||||
| Cardiopathy | |||||||||
| Combination | 28 (35.4%) | 7 (58.3%) | 4 (40.0%) | - | - | - | - | - | 39 (33.6%) |
| PVS | 37 (46.8%) | 8 (66.7%) | 4 (40.0%) | 3 (33.3%) | 2 (100%) | 1 (50.0%) | 1 (100%) | - | 56 (48.3%) |
| ASD | 23 (19.0%) | 1 (8.3%) | - | - | - | - | - | - | 24 (20.7%) |
| VSD | 1 (1.3%) | 4 (33.3%) | - | - | - | - | - | 1 (100%) | 6 (5.2%) |
| HCM | 7 (8.9%) | 2 (16.7%) | 8 (80.0%) | - | - | - | - | - | 17 (14.7%) |
| AS | 4 (5.1%) | - | 1 (10.0%) | - | - | - | - | - | 5 (4.3%) |
| AVD | 3 (3.8%) | 1 (8.3%) | - | - | - | - | - | - | 4 (3.4%) |
| MVD | 6 (7.6%) | 2 (16.7%) | 2 (20.0%) | - | - | - | - | - | 10 (8.6%) |
| TVD | 1 (1.3%) | - | 1 (10.0%) | - | - | - | - | - | 2 (1.6%) |
| TOF | 1 (1.3%) | - | - | - | - | - | - | - | 1 (0.9%) |
| AVC | 2 (2.5%) | - | - | - | - | - | - | - | 2 (1.7%) |
| PVRA | 1 (1.3%) | - | - | - | - | - | - | - | 1 (0.9%) |
| PDA | - | 3 (25.0%) | - | - | - | - | - | - | 3 (2.6%) |
| None | 23 (29.1%) | 1 (8.3%) | 2 (20.0%) | 6 (66.7%) | - | 1 (50.0%) | - | - | 33 (28.4%) |
| Severity of cardiac phenotype | |||||||||
| Mild cardiopathy | 11 (13.9%) | 7 (58.3%) | 2 (20.0%) | 3 (33.3%) | 1 (50.0%) | 1 (50.0%) | - | 1 (100%) | 26 (22.4%) |
| Cardiopathy requiring chronic drug therapy | 4 (5.1%) | 1 (8.3%) | 4 (40.0%) | - | - | - | - | - | 9 (7.8%) |
| Cardiopathy requiring surgery | 28 (35.4%) | 4 (33.3%) | 1 (10.0%) | 1 (11.1%) | 1 (50.0%) | 1 (50.0%) | 1 (100%) | - | 37 (31.9%) |
| Severe cardiopathy with death | - | - | 1 (10.0%) | - | - | - | - | - | 1 (0.9%) |
| Arrhythmia | 2 (2.5%) | - | 2 (20.0%) | - | - | - | - | - | 4 (3.4%) |
| Orthopedic features | |||||||||
| Skeletal anomalies | 33 (41.8%) | 7 (58.3%) | 2 (20.0%) | 3 (33.3%) | 1 (50.0%) | 2 (100%) | 1 (100%) | 1 (100%) | 50 (43.1%) |
| Severe scoliosis | 4 (5.1%) | 1 (8.3%) | - | - | - | - | - | - | 5 (4.3%) |
| Severe pectus excavatus | - | 1 (8.3%) | - | - | - | - | - | - | 1 (0.9%) |
| Ectodermal features | |||||||||
| Ectodermal anomalies | 8 (10.1%) | 3 (25.0%) | 4 (40.0%) | 1 (11.1%) | 1 (50.0%) | 1 (50.0%) | - | 1 (100%) | 19 (16.4%) |
| Severe craniofacial dysmorphisms | 45 (56.9%) | 11 (91.7%) | 9 (90.0%) | 5 (55.5%) | 1 (50.0%) | 2 (100%) | - | 1 (100%) | 74 (63.8%) |
| Mild craniofacial dysmorphisms | 7 (8.9%) | - | - | 3 (33.3%) | 1 (50.0%) | - | 1 (100%) | - | 12 (10.3%) |
| Prenatal features | |||||||||
| Medically assisted reproduction | 5 (6.3%) | 1 (8.3%) | - | 1 (11.1%) | - | 1 (50.0%) | U | - | 8 (6.9%) |
| Intrauterine growth restriction | 7 (8.9%) | - | 1 (10.0%) | - | - | - | U | - | 8 (6.9%) |
| Polyhydramnios | 20 (25.3%) | 6 (50.0%) | 5 (50.0%) | 1 (11.1%) | 1 (50.0%) | - | U | 1 (100%) | 34 (29.3%) |
| Oligohydramnios | 2 (2.5%) | - | - | - | - | - | U | - | 2 (1.7%) |
| Pathological second-trimester ultrasound | 30 (37.9%) | 7 (58.3%) | 5 (50.0%) | 1 (11.1%) | 2 (100.0%) | - | U | 1 (100%) | 46 (39.6%) |
| Increased nuchal translucency | 8 (10.1%) | 6 (50.0%) | - | 1 (11.1%) | - | - | U | - | 15 (12.9%) |
| Positive triple screen | 9 (11.4%) | 2 (16.7%) | - | - | - | - | U | - | 11 (9.5%) |
| Twin pregnancy | 1 (1.3%) | - | - | 1 (11.1%) | - | - | U | - | 2 (1.7%) |
| Premature birth | 12 (15.2%) | 7 (58.3%) | 5 (50.0%) | 1 (11.1%) | 1 (50.0%) | 2 (100%) | U | - | 28 (24.1%) |
| Growth | |||||||||
| Height < 10° | 19 (24.1%) | 1 (8.3%) | - | 2 (22.2%) | - | - | - | - | 22 (19.0%) |
| Height < 3° | 36 (45.6%) | 4 (33.3%) | 4 (40.0%) | 2 (22.2%) | 2 (100.0%) | 2 (100%) | - | - | 50 (43.1%) |
| rGH treatment | 13 (16.5%) | - | - | - | - | - | - | - | 13 (11.2%) |
| Neurological features | |||||||||
| Severe neurodevelopmental delay | 16 (20.3%) | 5 (41.7%) | 2 (20.0%) | 2 (22.2%) | 2 (50.0%) | - | - | 1 (100%) | 28 (24.1%) |
| Cerebral anomalies at MRI | 13 (16.5%) | 2 (16.7%) | 3 (30.0%) | - | 1 (50.0%) | - | 1 (100%) | - | 20 (17.2%) |
| Epilepsy | 3 (3.8%) | 1 (8.3%) | 1 (10.0%) | - | 1 (50.0%) | 1 (50.0%) | - | - | 7 (6.0%) |
| Ocular abnormalities | 18 (22.8%) | 6 (50.0%) | 2 (20.0%) | 1 (11.1%) | 1 (50.0%) | 1 (50.0%) | - | - | 29 (25.0%) |
| Sensorineural hearing loss | 2 (2.5%) | - | 1 (10.0%) | - | - | - | - | - | 3 (2.6%) |
| Urological features | |||||||||
| Cryptorchidism | 26/39 (66.7%) | 6/8 (75.0%) | 1/4 (25.0%) | 2/7 (28.6%) | 0/2 (0.0%) | 0/0 (0.0%) | 1/1 (100%) | 1/1 (100%) | 37/62 (59.7%) |
| Renal malformations | 4 (5.1%) | 1 (8.3%) | 2 (20.0%) | - | - | - | - | - | 7 (6.0%) |
| Hematological features | |||||||||
| Coagulation defects | 2 (2.5%) | 2 (16.7%) | 1 (10.0%) | - | - | 1 (50.0%) | - | - | 6 (5.2%) |
| JMML | 4 (5.1%) | - | - | - | - | - | - | - | 4 (3.5%) |
| Other malignancies | 4 (5.1%) | 1 (8.3%) | - | - | - | - | - | - | 5 (4.3%) |
| Sex | Gene | Coding Variant NM_002834.5 | Protein Variant | Inherited | Diagnosis | Age at Diagnosis | Treatment | Other Relevant Clinical Features | Death |
|---|---|---|---|---|---|---|---|---|---|
| M | PTPN11 | c.417G>C | p.(Glu139Asp) | No | Myelodysplasia | 9 months | No | PS, ASD, typical craniofacial dysmorphisms | No |
| M | PTPN11 | c.561G>A | p.(Asp61Asn) | No | JMML | At birth | No | Dorsal mast cell tumor, AVC, vWF deficiency, cryptorchidism, short stature, mild neurodevelopmental delay | No |
| M | PTPN11 | c.797G>C | p.(Glu139Asp) | No | JMML | 3 months | No | Cerebral palsy, ASD, aortic ectasia, cerebral hamartomas | No |
| F | PTPN11 | c.845T>G | p.(Phe285Ile) | No | JMML | At birth | Corticosteroids | HCM, PS, severe lymphatic dysplasia, typical craniofacial dysmorphisms | Yes at 21 months |
| F | CBL | c.1222T>C | p.(Trp408Arg) | Yes (M) | JMML | 25 months | Corticosteroids during flares | Recurrent vasculitis, mild craniofacial dysmorphisms, mild neurodevelopmental delay | No |
| M | PTPN11 | c.1851C>A | p.(Pro491His) | No | DNET | 72 months | No | VSD, ectopic atrial tachycardia, focal epilepsy | No |
| M | PTPN11 | c.241G>T | p.(Ala72Ser) | No | Glioma | 22 months | Surgical exeresis, trametinib, dabrafenib | ASD, mild craniofacial dysmorphisms, mild neurodevelopmental delay | No |
| M | PTPN11 | c.794G>A | p.(Arg265Gln) | Yes (M) | Testicular juvenile granulosa cell tumor | 12 months | Orchidectomy | Mild craniofacial dysmorphisms | No |
| F | SOS1 | c.797c>A | p.(Thr266Lys) | No | Embryonal pleomorphic rhabdomyosarcoma | 24 months | Chemotherapy † | ASD, AVC, typical craniofacial dysmorphisms | No |
| PTPN11 (n = 675) | SOS1 (n = 87) | RAF1 (n = 42) | LZTR1 (n = 59) | BRAF (n = 9) | KRAS (n = 30) | RIT1 (n = 102) | SOS2 (n = 18) | Total (n = 1022) | ANOVA p Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac defects | 517/665 (77.7%) | 57/84 (67.8%) | 40/42 (95.2%) * | 32/59 (54.2%) | 8/9 (88.9%) | 26/29 (89.6%) | 88/102 (86.3%) | 10/18 (55.5%) | 778/1021 (76.2%) | <0.0001 |
| Skeletal anomalies | 377/555 (67.9%) * | 38/79 (48.1%) | 17/41 (41.5%) | 32/59 (54.2%) | 4/7 (57.1%) | 11/27 (40.7%) | 45/89 (50.6%) | 9/18 (50.0%) | 533/875 (60.9%) | <0.0001 |
| Ectodermal anomalies | 41/294 (13.9%) | 20/48 (41.7%) * | 7/30 (23.3%) | 8/43 (18.6%) | 2/6 (33.3%) | 4/10 (40%) | 28/71 (39.4%) | 6/8 (75.0%) * | 116/510 (22.7%) | <0.0001 |
| Craniofacial dysmorphisms | 507/581 (87.6%) | 75/80 (93.7%) | 35/36 (97.2%) | 57/59 (96.6%) | 7/7 (100%) | 22/25 (88.0%) | 46/55 (83.6%) | 11/11 (100%) | 760/854 (89.0%) | 0.0422 |
| Height < 10° | 93/251 (37.1%) * | 7/40 (17.5%) | 1/23 (4.3%) | 6/36 (16.7%) | 0/6 (0%) | 0/10 (0%) | 4/36 (11.1%) | 2/13 (15.4%) | 113/415 (27.2%) | <0.0001 |
| Height < 3° | 376/591 (63.6%) * | 18/80 (22.5%) | 26/41 (63.4%) | 9/49 (18.4%) | 5/7 (71.4%) | 20/27 (74.1%) * | 34/65 (52.3%) | 4/15 (26.7%) | 492/875 (56.2%) | <0.0001 |
| rGH treatment | 39/162 (24.1%) | 2/30 (6.7%) | 3/23 (13.0%) | 1/22 (4.5%) | 1/4 (25.0%) | 0/9 (0%) | 0/9 (0%) | 0/10 (0%) | 46/269 (17.1%) | 0.0281 |
| Neurodevelopmental delay | 194/499 (38.9%) | 32/73 (43.8%) | 13/33 (39.4%) | 23/50 (46.0%) | 5/7 (71.4%) | 18/25 (72.0%) * | 26/49 (53.1%) | 7/16 (43.7%) | 318/752 (42.3%) | 0.0241 |
| Cerebral anomalies at MRI | 17/190 (8.9%) | 4/27 (14.8%) | 4/22 (18.2%) | 2/29 (6.9%) | 2/7 (28.6%) | 2/12 (16.7%) | 10/10 (100%) * | 0/9 (0%) | 41/306 (13.4%) | <0.0001 |
| Epilepsy | 12/207 (5.7%) | 2/33 (6.0%) | 2/21 (9.5%) | 0/26 (0%) | 3/7 (42.9%) * | 1/12 (8.3%) | 2/45 (4.4%) | 0/14 (0%) | 22/376 (5.8%) | 0.0007 |
| Ocular abnormalities | 31/154 (20.1%) | 16/40 (40.0%) | 3/18 (16.7%) | 7/46 (15.2%) | 1/2 (50.0%) | 5/12 (41.7%) | 29/41 (70.7%) * | 2/8 (25.0%) | 94/321 (29.3%) | <0.0001 |
| Sensorineural hearing loss | 21/199 (10.5%) | 4/57 (7.0%) | 3/41 (7.3%) | 1/19 (5.3%) | 0/6 (0%) | 2/18 (11.1%) | 3/43 (6.9%) | 0/8 (0%) | 34/391 (8.7%) | 0.9347 |
| Cryptorchidism | 185/311 (59.5%) | 23/48 (47.9%) | 5/23 (16.1%) | 5/17 (29.4%) | 1/4 (25.0%) | 1/15 (6.7%) | 25/40 (62.5%) | 7/8 (87.5%) | 252/466 (54.1%) | <0.0001 |
| Renal malformations | 22/266 (8.2%) | 7/50 (14.0%) | 5/31 (16.1%) | 2/51 (3.9%) | 0/5 (0%) | 2/20 (10%) | 10/44 (22.7%) | 2/17 (11.8%) | 50/484 (10.3%) | 0.0936 |
| Coagulation defects | 66/352 (18.7%) | 14/52 (26.9%) | 7/36 (19.4%) | 5/53 (9.4%) | 0/5 (0%) | 3/22 (13.6%) | 12/51 (23.5%) | 6/17 (35.3%) | 113/588 (19.2%) | 0.0919 |
| JMML | 6/376 (1.6%) | 0/56 (0%) | 0/36 (0%) | 0/39 (0%) | 0/9 (0%) | 0/24 (0%) | 0/22 (0%) | 0/17 (0%) | 6/579 (1.0%) | 0.4077 |
| Malignancies | 17/398 (4.3%) | 5/65 (7.7%) | 2/42 (4.8%) | 2/53 (3.8%) | 0/9 (0%) | 1/30 (3.3%) | 5/54 (9.3%) | 0/9 (0%) | 32/660 (4.8%) | 0.6791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, G.; Gazzin, A.; Carli, D.; Massuras, S.; Cardaropoli, S.; Luca, M.; Defilippi, B.; Tartaglia, M.; Ferrero, G.B.; Mussa, A. Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review. Int. J. Mol. Sci. 2025, 26, 3515. https://doi.org/10.3390/ijms26083515
Reynolds G, Gazzin A, Carli D, Massuras S, Cardaropoli S, Luca M, Defilippi B, Tartaglia M, Ferrero GB, Mussa A. Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review. International Journal of Molecular Sciences. 2025; 26(8):3515. https://doi.org/10.3390/ijms26083515
Chicago/Turabian StyleReynolds, Giuseppe, Andrea Gazzin, Diana Carli, Stefania Massuras, Simona Cardaropoli, Maria Luca, Beatrice Defilippi, Marco Tartaglia, Giovanni Battista Ferrero, and Alessandro Mussa. 2025. "Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review" International Journal of Molecular Sciences 26, no. 8: 3515. https://doi.org/10.3390/ijms26083515
APA StyleReynolds, G., Gazzin, A., Carli, D., Massuras, S., Cardaropoli, S., Luca, M., Defilippi, B., Tartaglia, M., Ferrero, G. B., & Mussa, A. (2025). Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review. International Journal of Molecular Sciences, 26(8), 3515. https://doi.org/10.3390/ijms26083515







