Using Cancer-Associated Fibroblasts as a Shear-Wave Elastography Imaging Biomarker to Predict Anti-PD-1 Efficacy of Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results
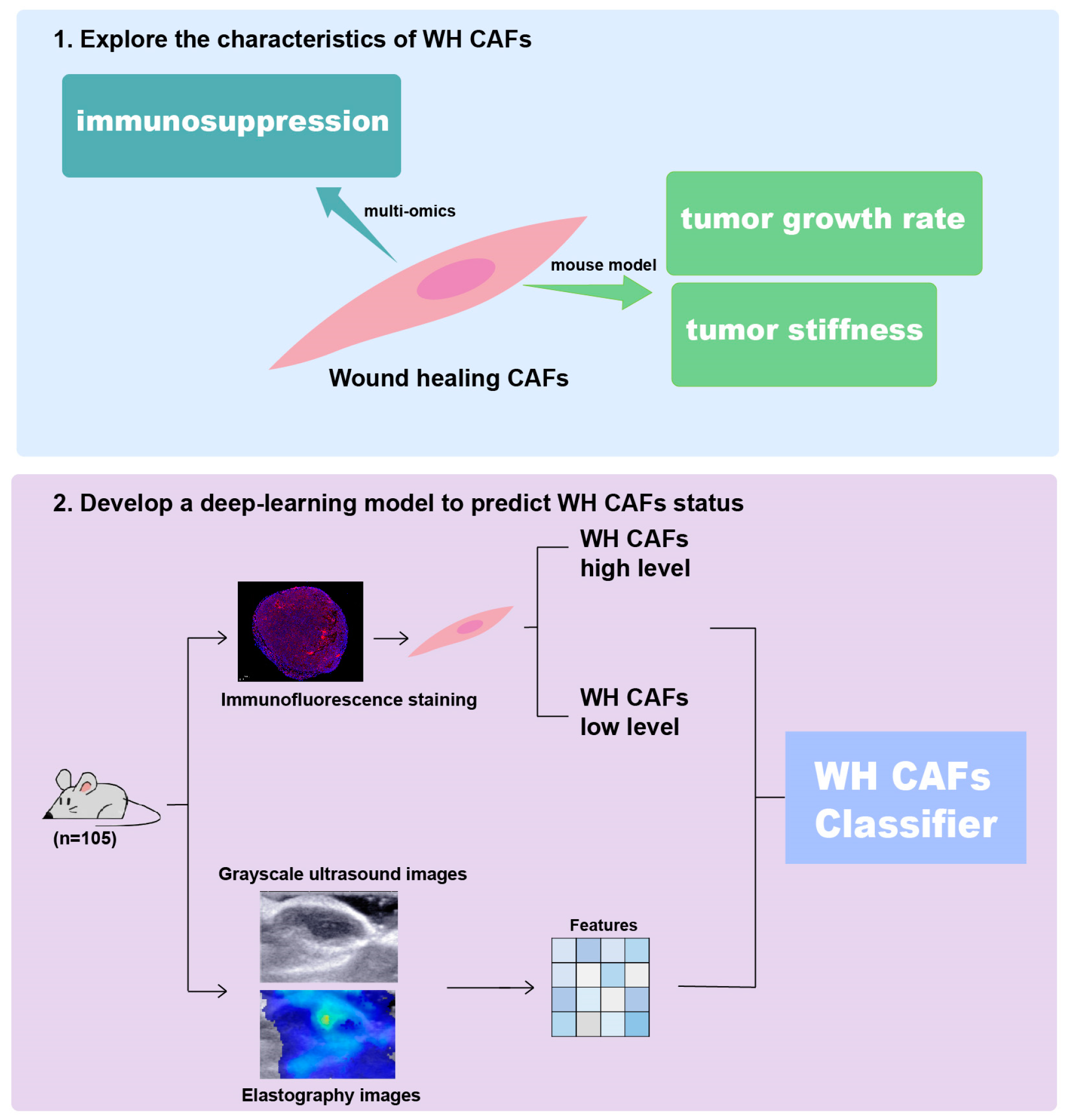
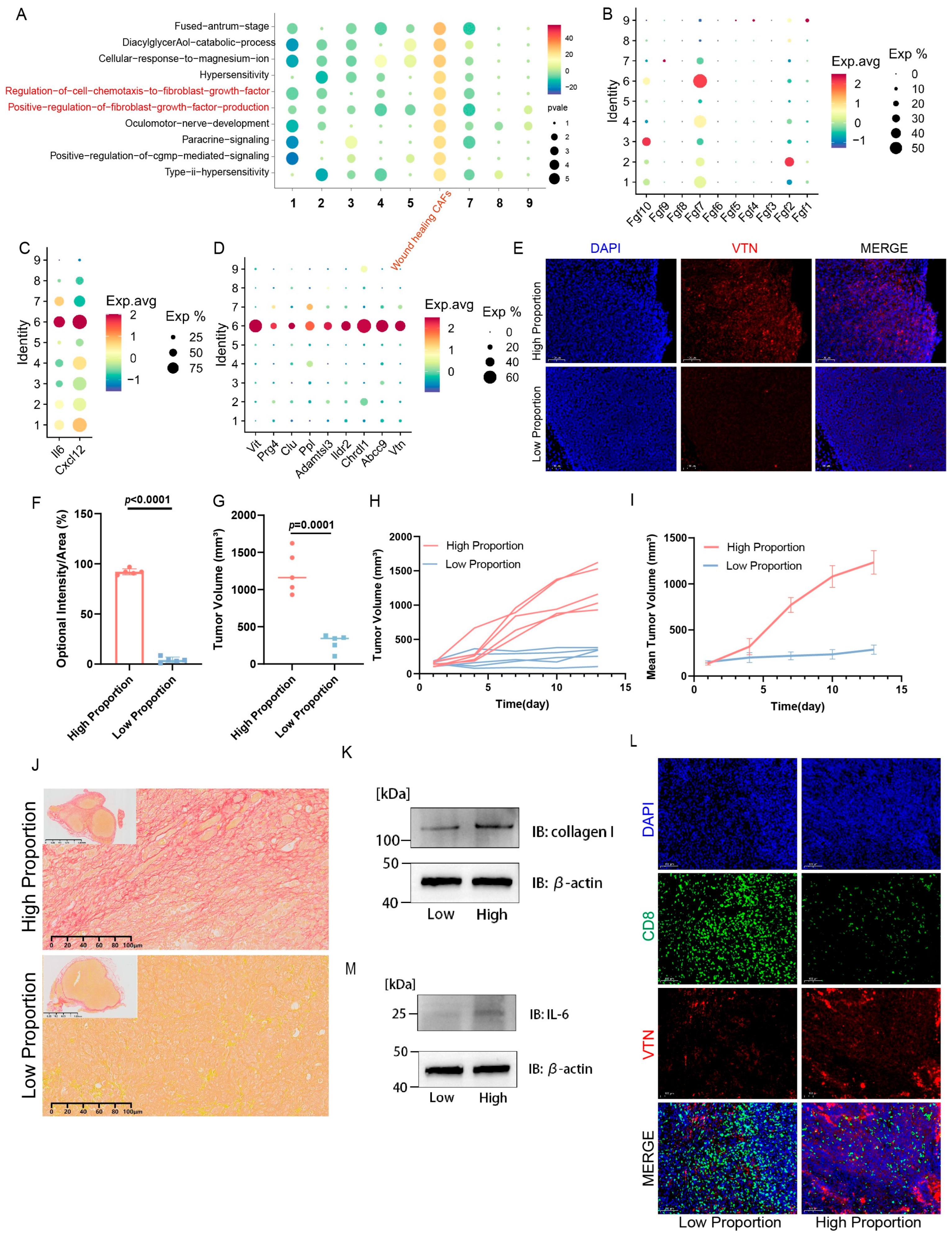
2.1. WH CAFs Are Correlated with Immunosuppression
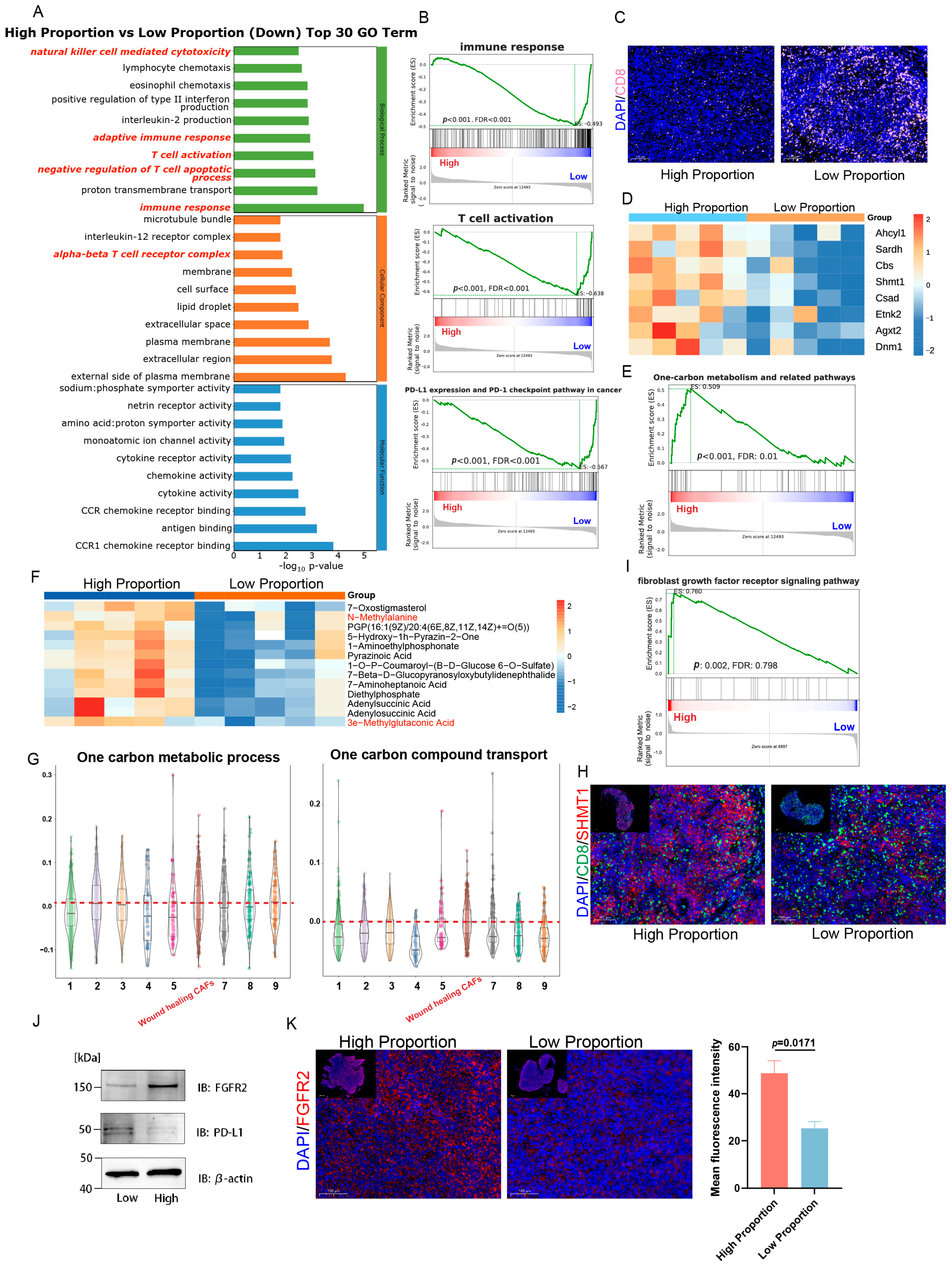
2.2. Multi-Omics Further Confirm That Tumors with a High Proportion of WH CAFs Exhibit Greater Immune Suppression
2.3. Deep Learning Model Predicts WH CAF Level from SWE Imaging
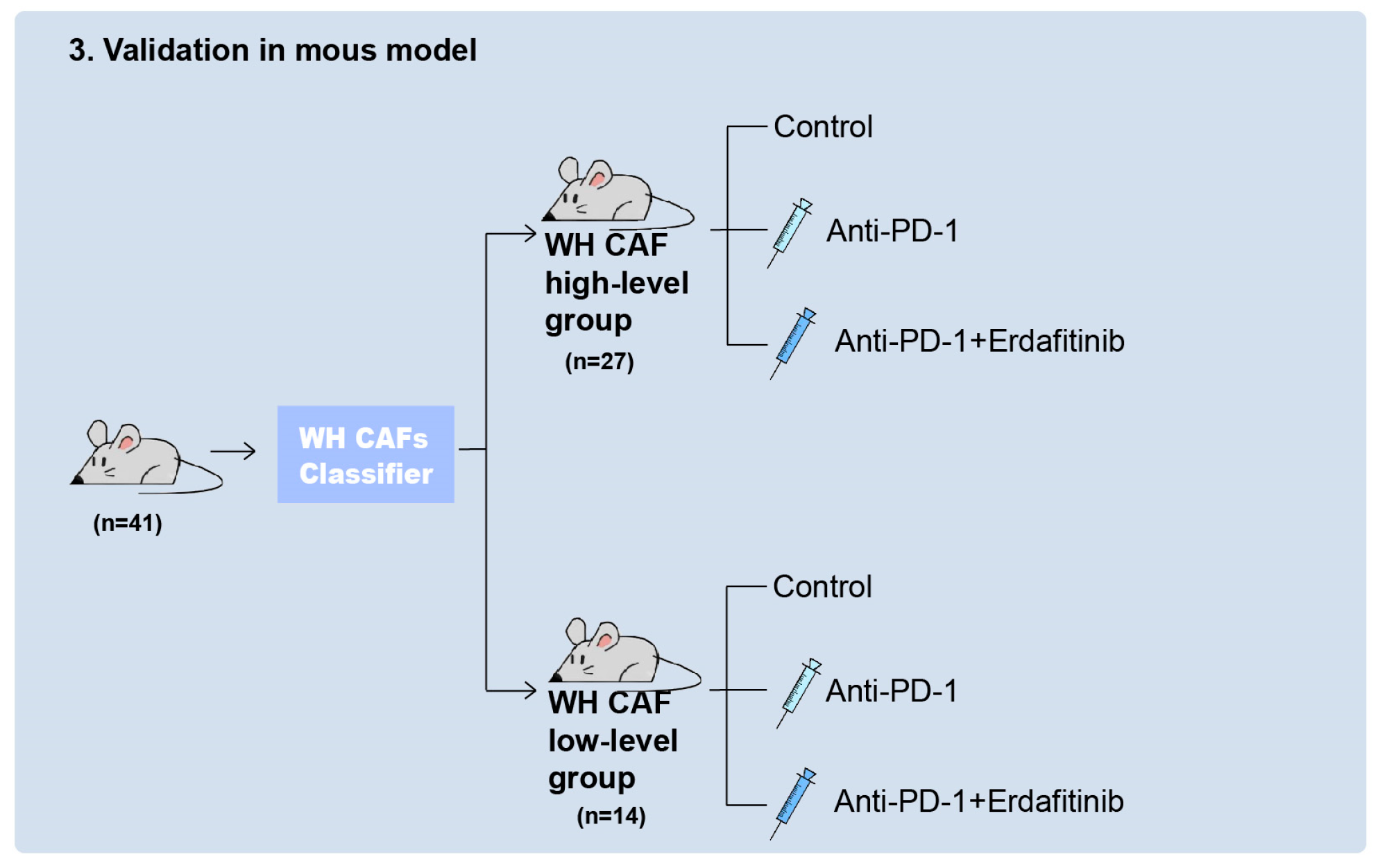
2.4. FGFR Inhibitor Enhances Therapeutic Responses to ICIs in Tumors by Inhibiting WH CAFs
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Mice and Treatments
4.3. Immunofluorescence (IF)
4.4. Immunohistochemistry (IHC)
4.5. Western Blot
4.6. SWE Imaging of Tumors
4.7. Development of the Deep Learning Model
4.8. Experiment Setting of the Deep Learning Model
4.9. Evaluation Metrics for WH CAF Level Classification
4.10. RNA Sequencing and Data Analysis
4.11. 4D-DIA Quantitative Proteomics and Data Analysis
4.12. Metabolomic and Data Analysis
4.13. Single-Cell RNA Sequences Analysis
4.14. Differential Gene Expression and Pathway Enrichment
4.15. GSEA
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | triple-negative breast cancer |
| ICIs | immune checkpoint inhibitors |
| CAFs | cancer-associated fibroblasts |
| WH CAFs | wound-healing CAFs |
| VTN | vitronectin |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death ligand 1 |
| FGFR | fibroblast growth factor receptor |
| TME | tumor microenvironment |
| SWE | shear-wave elastography |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| ECM | extracellular matrix |
| sc-RNA seq | single-cell sequencing |
| IL-6 | interleukin-6 |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| IF | immunofluorescence staining |
| GO | gene ontology |
| GOBP | gene ontology biological process |
| GSEA | gene set enrichment analysis |
| TIL | tumor-infiltrating lymphocytes |
| SHMT | serine hydroxymethyltransferase |
| AUC | area under the curve |
| ROC | receiver operating characteristic curve |
| DEGs | differential expression genes |
References
- Liu, Y.; Hu, Y.; Xue, J.; Li, J.; Yi, J.; Bu, J.; Zhang, Z.; Qiu, P.; Gu, X. Advances in immunotherapy for triple-negative breast cancer. Mol. Cancer 2023, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor-related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Rizvi, H.; Bandlamudi, C.; Sauter, J.L.; Travis, W.D.; Rekhtman, N.; Plodkowski, A.J.; Perez-Johnston, R.; Sawan, P.; Beras, A.; et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann. Oncol. 2020, 31, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Munari, E.; Zamboni, G.; Lunardi, G.; Marchionni, L.; Marconi, M.; Sommaggio, M.; Brunelli, M.; Martignoni, G.; Netto, G.J.; Hoque, M.O.; et al. PD-L1 Expression Heterogeneity in Non–Small Cell Lung Cancer: Defining Criteria for Harmonization between Biopsy Specimens and Whole Sections. J. Thorac. Oncol. 2018, 13, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Mu, W.; Jiang, L.; Shi, Y.; Tunali, I.; Gray, J.E.; Katsoulakis, E.; Tian, J.; Gillies, R.J.; Schabath, M.B. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. J. Immunother. Cancer 2021, 9, e002118. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Stylianopoulos, T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front. Oncol. 2018, 8, 55. [Google Scholar] [CrossRef]
- Ding, J.-H.; Xiao, Y.; Zhao, S.; Xu, Y.; Xiao, Y.-L.; Shao, Z.-M.; Jiang, Y.-Z.; Di, G.-H. Integrated analysis reveals the molecular features of fibrosis in triple-negative breast cancer. Mol. Ther. Oncolytics 2022, 24, 624–635. [Google Scholar] [CrossRef]
- Jenkins, L.; Jungwirth, U.; Avgustinova, A.; Iravani, M.; Mills, A.; Haider, S.; Harper, J.; Isacke, C.M. Cancer-Associated Fibroblasts Suppress CD8+ T-cell Infiltration and Confer Resistance to Immune-Checkpoint Blockade. Cancer Res. 2022, 82, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.; Graves, D.; Korrer, M.; Wang, Y.; Roy, S.; Naveed, A.; Xu, Y.; Luginbuhl, A.; Curry, J.; Gibson, M.; et al. Immunostimulatory Cancer-Associated Fibroblast Subpopulations Can Predict Immunotherapy Response in Head and Neck Cancer. Clin. Cancer Res. 2022, 28, 2094–2109. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, P.; Zhang, X.; Feng, Y.; Li, L.; Lin, H.; Yu, Y. A fibroblast-associated signature predicts prognosis and immunotherapy in esophageal squamous cell cancer. Front. Immunol. 2023, 14, 1199040. [Google Scholar] [CrossRef] [PubMed]
- Voutouri, C.; Mpekris, F.; Panagi, M.; Krolak, C.; Michael, C.; Martin, J.D.; Averkiou, M.A.; Stylianopoulos, T. Ultrasound stiffness and perfusion markers correlate with tumor volume responses to immunotherapy. Acta Biomater. 2023, 167, 121–134. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Wang, K.; Lu, X.; Zhou, H.; Gao, Y.; Zheng, J.; Tong, M.; Wu, C.; Liu, C.; Huang, L.; Jiang, T.; et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: A prospective multicentre study. Gut 2019, 68, 729–741. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, U.; Van Weverwijk, A.; Evans, R.J.; Jenkins, L.; Vicente, D.; Alexander, J.; Gao, Q.; Haider, S.; Iravani, M.; Isacke, C.M. Impairment of a distinct cancer-associated fibroblast population limits tumour growth and metastasis. Nat. Commun. 2021, 12, 3516. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, J.; Qian, L.; Liu, X.; Chang, C.; Tang, S.; Zhang, H.; Zhou, S. Biomimetic nanoparticles drive the mechanism understanding of shear-wave elasticity stiffness in triple negative breast cancers to predict clinical treatment. Bioact. Mater. 2023, 22, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yue, L.; Leng, Q.; Chang, C.; Gan, C.; Ye, T.; Cao, D. Targeting FGFR for cancer therapy. J. Hematol. Oncol. 2024, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Deng, C.-X. Fibroblast Growth Factor Receptor 2 Signaling in Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1163–1171. [Google Scholar] [CrossRef]
- Lei, J.H.; Lee, M.; Miao, K.; Huang, Z.; Yao, Z.; Zhang, A.; Xu, J.; Zhao, M.; Huang, Z.; Zhang, X.; et al. Activation of FGFR2 Signaling Suppresses BRCA1 and Drives Triple-Negative Mammary Tumorigenesis That is Sensitive to Immunotherapy. Adv. Sci. 2021, 8, 2100974. [Google Scholar] [CrossRef]
- Li, P.; Huang, T.; Zou, Q.; Liu, D.; Wang, Y.; Tan, X.; Wei, Y.; Qiu, H. FGFR2 Promotes Expression of PD-L1 in Colorectal Cancer via the JAK/STAT3 Signaling Pathway. J. Immunol. 2019, 202, 3065–3075. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef]
- Felding-Habermann, B.; Cheresh, D.A. Vitronectin and its receptors. Curr. Opin. Cell Biol. 1993, 5, 864–868. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Shang, J.; Zhu, H.; Liao, J.; Hong, X.; Hou, F.F.; Fu, H.; Liu, Y. Macrophage promotes fibroblast activation and kidney fibrosis by assembling a vitronectin-enriched microenvironment. Theranostics 2023, 13, 3897–3913. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Fu, X.; Wang, W.; Liu, H.; McKay, M.J.; Dejkriengkraikul, P.; Nie, Y. Bioinformatic analysis of cancer-associated fibroblast related gene signature as a predictive model in clinical outcomes and immune characteristics of gastric cancer. Ann. Transl. Med. 2022, 10, 698. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.-A.; Guan, L.; Myint, P.K.; Chin, L.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kiweler, N.; Delbrouck, C.; Pozdeev, V.I.; Neises, L.; Soriano-Baguet, L.; Eiden, K.; Xian, F.; Benzarti, M.; Haase, L.; Koncina, E.; et al. Mitochondria preserve an autarkic one-carbon cycle to confer growth-independent cancer cell migration and metastasis. Nat. Commun. 2022, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.; Zhang, J.; Gao, H.; Wang, S.; Li, S.; Wei, P.; Liang, J.; Yu, G.; Wang, X.; et al. Cul4A-DDB1-mediated monoubiquitination of phosphoglycerate dehydrogenase promotes colorectal cancer metastasis via increased S-adenosylmethionine. J. Clin. Investig. 2021, 131, e146187. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Koseki, J.; Taniguchi, M.; Vecchione, A.; Ishii, H. One-carbon metabolism for cancer diagnostic and therapeutic approaches. Cancer Lett. 2020, 470, 141–148. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef]
- Zheng, D.; Wan, C.; Yang, H.; Xu, L.; Dong, Q.; Du, C.; Du, J.; Li, F. Her2-Targeted Multifunctional Nano-Theranostic Platform Mediates Tumor Microenvironment Remodeling and Immune Activation for Breast Cancer Treatment. Int. J. Nanomed. 2020, 15, 10007–10028. [Google Scholar] [CrossRef]
- Qi, Y.-J.; Su, G.-H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.-Z.; Shao, Z.-M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Z.; Yu, Y.; Yuan, Z.; Lin, Y.; Tan, Y.; Duan, X.; Yao, H.; Wang, Y.; Liu, J. Radiomic and clinical data integration using machine learning predict the efficacy of anti-PD-1 antibodies-based combinational treatment in advanced breast cancer: A multicentered study. J. Immunother. Cancer 2023, 11, e006514. [Google Scholar] [CrossRef]
- Li, J.; Sun, B.; Li, Y.; Li, S.; Wang, J.; Zhu, Y.; Lu, H. Correlation analysis between shear-wave elastography and pathological profiles in breast cancer. Breast Cancer Res. Treat. 2023, 197, 269–276. [Google Scholar] [CrossRef]
- Ganau, S.; Andreu, F.J.; Escribano, F.; Martín, A.; Tortajada, L.; Villajos, M.; Baré, M.; Teixidó, M.; Ribé, J.; Sentís, M. Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: Evaluation of maximum and mean elasticity values. Eur. J. Radiol. 2015, 84, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dai, S.; Wang, Q.; Jing, H.; Shao, H.; Zhang, L.; Qin, L.; Qiao, C.; Wang, Z.; Cheng, W. Investigation of correlation between shear wave elastography and lymphangiogenesis in invasive breast cancer and diagnosis of axillary lymph node metastasis. BMC Cancer 2024, 24, 409. [Google Scholar] [CrossRef]
- Xue, Y.; Yao, S.; Li, X.; Zhang, H. Value of shear wave elastography in discriminating malignant and benign breast lesions. Medicine 2017, 96, e7412. [Google Scholar] [CrossRef] [PubMed]
- Tekcan Sanli, D.E.; Yildirim, D.; Kandemirli, S.G.; Sanli, A.N.; Aribal, E. Evaluation of Multiparametric Shear Wave Elastography Indices in Malignant and Benign Breast Lesions. Acad. Radiol. 2022, 29 (Suppl. S1), S50–S61. [Google Scholar] [CrossRef]
- Zheng, S.; Zou, Y.; Tang, Y.; Yang, A.; Liang, J.-Y.; Wu, L.; Tian, W.; Xiao, W.; Xie, X.; Yang, L.; et al. Landscape of cancer-associated fibroblasts identifies the secreted biglycan as a protumor and immunosuppressive factor in triple-negative breast cancer. Oncoimmunology 2022, 11, 2020984. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e10. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Grauel, A.L.; Nguyen, B.; Ruddy, D.; Laszewski, T.; Schwartz, S.; Chang, J.; Chen, J.; Piquet, M.; Pelletier, M.; Yan, Z.; et al. TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat. Commun. 2020, 11, 6315. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Yang, Y.; Le, X.; Tran, H.T.; Elamin, Y.Y.; Yu, X.; Zhang, F.; Poteete, A.; Ren, X.; et al. IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin. Cancer Res. 2023, 29, 1292–1304. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.-F.; Li, Y.; Gupta, V.; Li, C.; et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, X.; Xu, J.; Su, S.M.; Chan, U.I.; Mo, L.; Liu, J.; Zhang, X.; Adhav, R.; Chen, Q.; et al. S100A9-CXCL12 activation in BRCA1-mutant breast cancer promotes an immunosuppressive microenvironment associated with resistance to immunotherapy. Nat. Commun. 2022, 13, 1481. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zuo, X.; Li, G.; Wang, J.; Liu, J.; Wang, X.; Wang, S.; Zhang, W.; Zhang, K.; et al. CXCL12+ tumor-associated endothelial cells promote immune resistance in hepatocellular carcinoma. J. Hepatol. 2025, 82, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.-K.; Berestjuk, I.; Ten Hoeve, J.; et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat. Cancer 2024, 5, 1045–1062. [Google Scholar] [CrossRef]
- Nolte, M.; Margadant, C. Controlling Immunity and Inflammation through Integrin-Dependent Regulation of TGF-β. Trends Cell Biol. 2020, 30, 49–59. [Google Scholar] [CrossRef]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.-L.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef]


| AUC (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Image-level | 86.21 | 80.51 | 81.01 | 80.17 |
| Tumor-level | 85.45 | 80.95 | 90.00 | 72.73 |
| Image-Level | AUC (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Unimodal-g | 82.09 | 66.15 | 98.73 | 43.97 |
| Unimodal-e | 79.12 | 66.67 | 97.47 | 45.69 |
| Bimodal-g and e | 86.21 | 80.51 | 81.01 | 80.17 |
| Tumor-Level | AUC (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Unimodal-g | 83.64 | 71.43 | 100.0 | 45.45 |
| Unimodal-e | 78.18 | 61.90 | 100.0 | 27.27 |
| Bimodal-g and e | 85.45 | 80.95 | 90.00 | 72.73 |
| Training | Validation | Test | |
|---|---|---|---|
| Tumors | 63 | 21 | 21 |
| Bimodal image pairs | 600 | 207 | 195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liang, S.; Zheng, D.; Wang, S.; Zhou, J.; Wang, Z.; Huang, Y.; Chang, C.; Wang, Y.; Guo, Y.; et al. Using Cancer-Associated Fibroblasts as a Shear-Wave Elastography Imaging Biomarker to Predict Anti-PD-1 Efficacy of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 3525. https://doi.org/10.3390/ijms26083525
Zhang Z, Liang S, Zheng D, Wang S, Zhou J, Wang Z, Huang Y, Chang C, Wang Y, Guo Y, et al. Using Cancer-Associated Fibroblasts as a Shear-Wave Elastography Imaging Biomarker to Predict Anti-PD-1 Efficacy of Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2025; 26(8):3525. https://doi.org/10.3390/ijms26083525
Chicago/Turabian StyleZhang, Zhiming, Shuyu Liang, Dongdong Zheng, Shiyu Wang, Jin Zhou, Ziqi Wang, Yunxia Huang, Cai Chang, Yuanyuan Wang, Yi Guo, and et al. 2025. "Using Cancer-Associated Fibroblasts as a Shear-Wave Elastography Imaging Biomarker to Predict Anti-PD-1 Efficacy of Triple-Negative Breast Cancer" International Journal of Molecular Sciences 26, no. 8: 3525. https://doi.org/10.3390/ijms26083525
APA StyleZhang, Z., Liang, S., Zheng, D., Wang, S., Zhou, J., Wang, Z., Huang, Y., Chang, C., Wang, Y., Guo, Y., & Zhou, S. (2025). Using Cancer-Associated Fibroblasts as a Shear-Wave Elastography Imaging Biomarker to Predict Anti-PD-1 Efficacy of Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 26(8), 3525. https://doi.org/10.3390/ijms26083525






