Valorization of Pomegranate Peel: Mechanisms and Clinical Applications in Irritable Bowel Syndrome Management
Abstract
:1. Introduction
2. Results
2.1. Bioactive Compounds and Target Identification in Pomegranate Peel
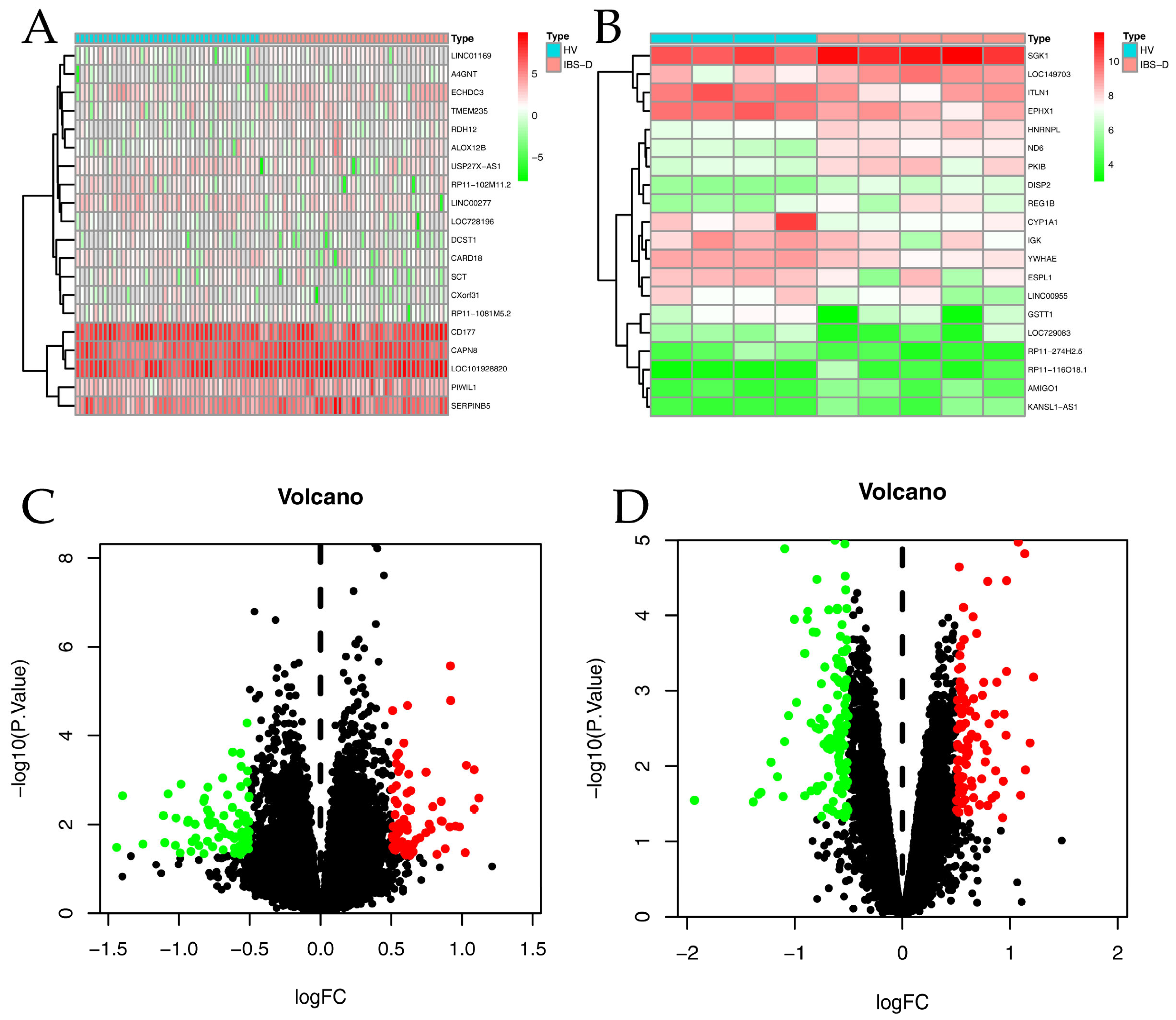
2.2. Analysis of GEO Differentially Expressed Genes Data and Acquisition of Targets for IBS
2.3. Network Construction
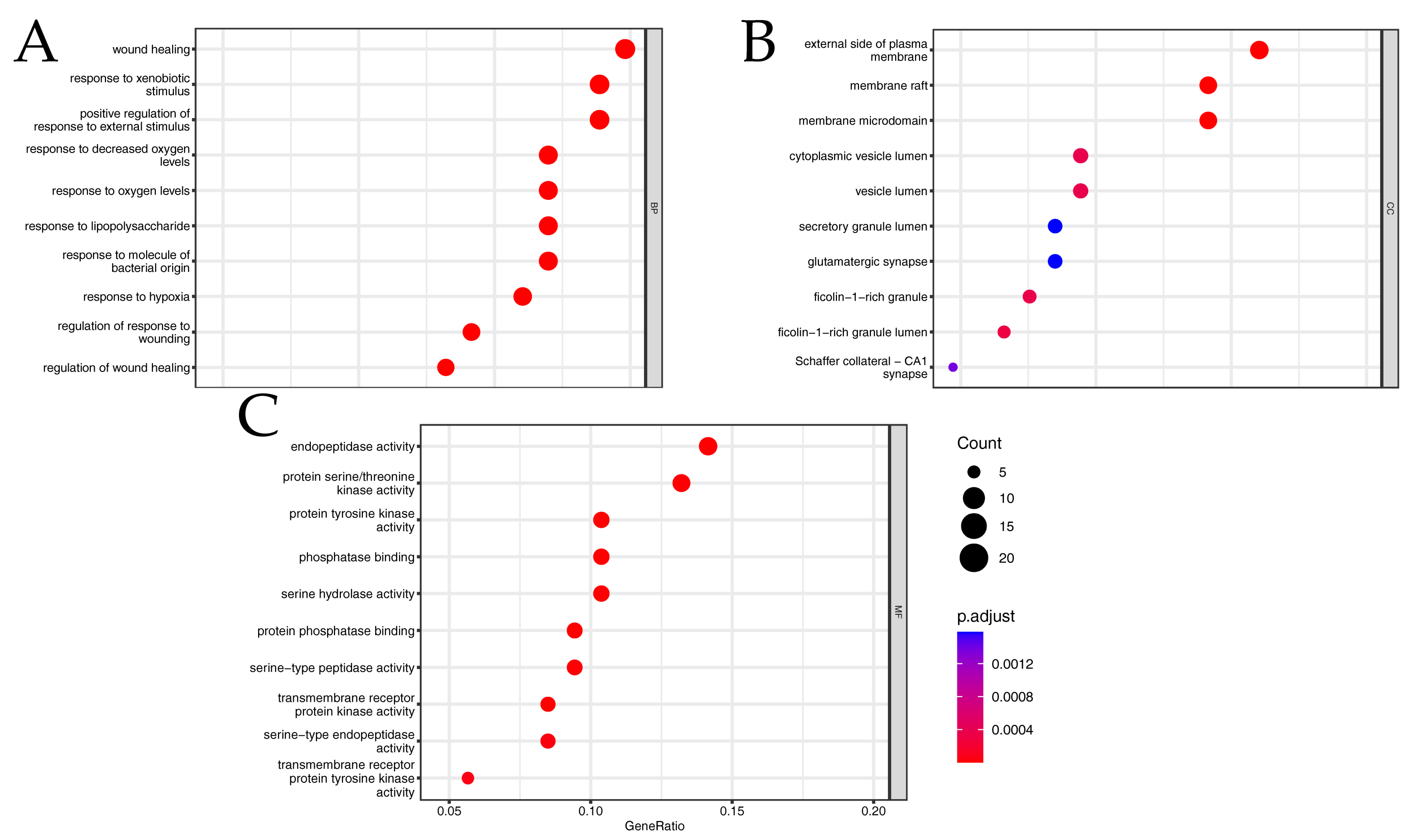
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
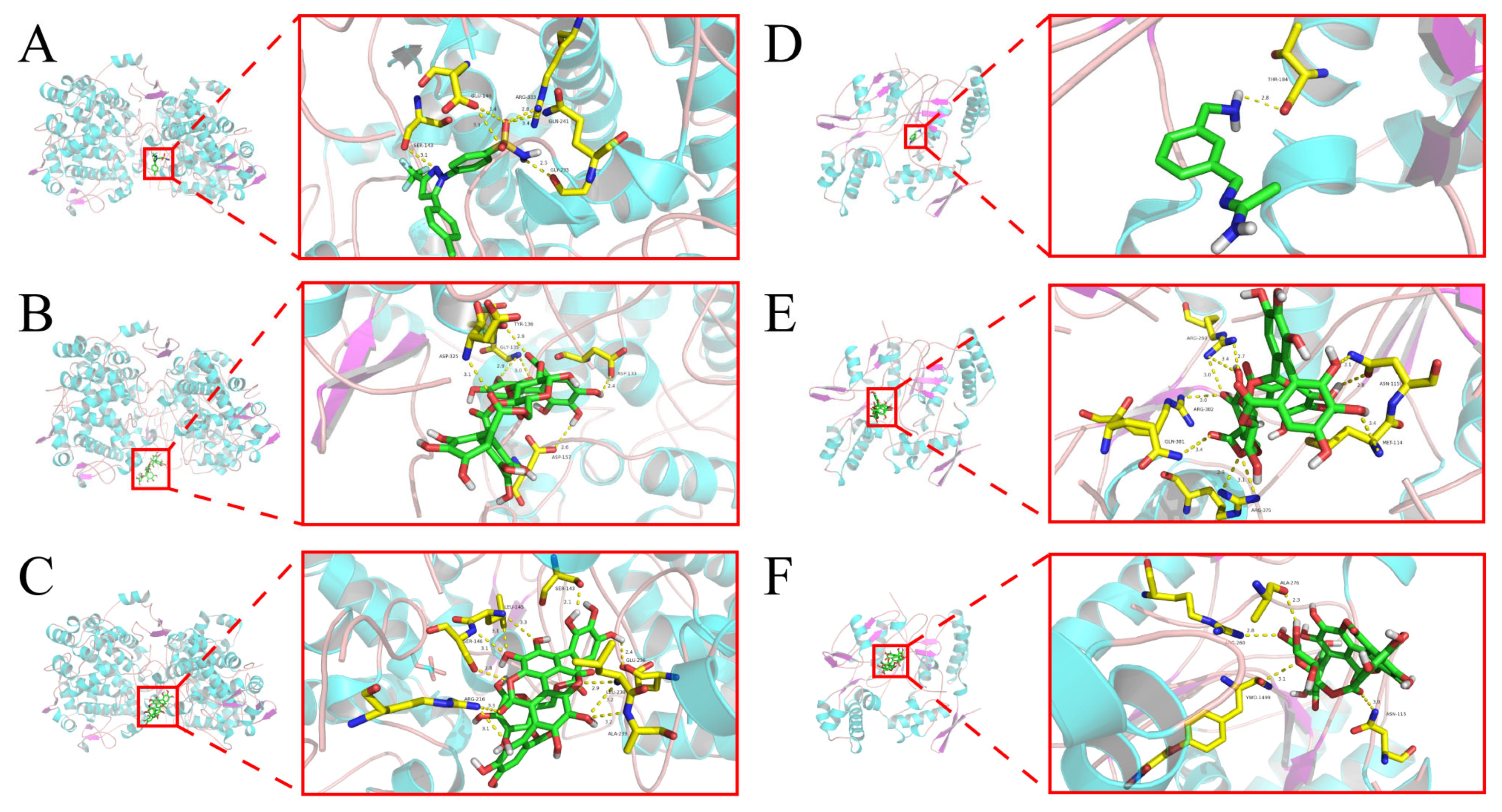
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Collection of the Candidate Compounds of PP
4.2. Screening of Potential Active Compounds
4.3. Prediction of the Relevant Targets of PP Potential Active Compounds
4.4. Acquisition of Targets for IBS
4.5. Network Construction
4.6. Enrichment of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways Analysis
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehdi, A.; Lamiae, B.; Samira, B.; Ramchoun, M.; Abdelouahed, K.; Tamas, F.; Hicham, B. Pomegranate (Punica granatum L.) attenuates neuroinflammation involved in neurodegenerative diseases. Foods 2022, 11, 2570. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Epifano, F.; Palumbo, L.; Collevecchio, C.; Cardellini, F.; Bastianini, M.; Spogli, R.; Fiorito, S. A novel and efficient concentration of pomegranate juice with enhanced antioxidant activity. Food Chem. 2022, 387, 132901. [Google Scholar] [CrossRef] [PubMed]
- El Barnossi, A.; Moussaid, F.; Housseini, A.I. Tangerine, banana and pomegranate peels valorisation for sustainable environment: A review. Biotechnol. Rep. 2021, 29, e00574. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, W.; Chen, Y.; Peng, Y.; Ke, H.; Zhan, L.; Lan, J.; Li, H.; Zhang, Y. Evaluation of greenhouse gas emission and reduction potential of high-food-waste-content municipal solid waste landfills: A case study of a landfill in the east of China. Waste Manag. 2024, 189, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; Wang, W.; Duan, H.; Zhang, H.; He, P.; Lü, F. Emission of odor pollutants and variation in microbial community during the initial decomposition stage of municipal biowaste. Sci. Total Environ. 2023, 861, 160612. [Google Scholar] [CrossRef] [PubMed]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Khatib, M.; Mulinacci, N.; Calosi, L.; Bani, D.; Di Cesare Mannelli, L.; Ghelardini, C. Pomegranate mesocarp against colitis-induced visceral pain in rats: Effects of a decoction and its fractions. Int. J. Mol. Sci. 2020, 21, 4304. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef]
- Yang, S.H.; Tao, G.; Yang, L.; Wu, X.; Liu, J.W.; Dagher, F.; Ou, S.Y.; Song, Y.; Huang, J.Q. Dietary phytochemical and metabolic disease prevention: Focus on plant proteins. Front. Nutr. 2023, 10, 1089487. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, J.S. Irritable bowel syndrome in China: A review on the epidemiology, diagnosis, and management. Chin. Med. J. 2021, 134, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: A review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef] [PubMed]
- The State Commission of Chinese Pharmacopoeia. Pharmacopoeia of People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; pp. 97–98. ISBN 9787521415742. [Google Scholar]
- Li, F.; Qin, C.; Ding, Z. Research progress of pomegranate peel in traditional Chinese and western medicine. Guide China Med. 2022, 20, 69–71. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Usta, C.; Ozdemir, S.; Schiariti, M.; Puddu, P.E. The pharmacological use of ellagic acid-rich pomegranate fruit. Int. J. Food Sci. Nutr. 2013, 64, 907–913. [Google Scholar] [CrossRef]
- Kiyama, R.; Zhu, Y.; Kawaguchi, K.; Iitake, N.; Wada-Kiyama, Y.; Dong, S. Estrogen-responsive genes for environmental studies. Environ. Technol. Innov. 2014, 1, 16–28. [Google Scholar] [CrossRef]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef]
- Jacenik, D.; Cygankiewicz, A.I.; Fichna, J.; Mokrowiecka, A.; Małecka-Panas, E.; Krajewska, W.M. Estrogen signaling deregulation related with local immune response modulation in irritable bowel syndrome. Mol. Cell. Endocrinol. 2018, 471, 89–96. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Gao, J.; Li, J.Y.; Turgeon, D.K.; Owyang, C. Prostaglandin E2, Produced by Mast Cells in Colon Tissues from Patients with Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology 2020, 158, 2195–2207.e6. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, J.; Wu, H.; Li, X.; Wang, F.; Tang, X. Uncovering the Multitarget Therapeutic Mechanism of Tong-Xie-Yao-Fang on Irritable Bowel Syndrome. J. Food Qual. 2024, 2024, 8195739. [Google Scholar] [CrossRef]
- Pantalos, G.; Vaou, N.; Papachristidou, S.; Stavropoulou, E.; Tsigalou, C.; Voidarou, C.; Bezirtzoglou, E. Antioxidant and Anti-Inflammatory Phytochemicals for the Treatment of Inflammatory Bowel Disease: A Systematic Review. Appl. Sci. 2024, 14, 2177. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Zhang, S.; Hao, Z.; Shen, J. Pomegranate Peel Extract Mitigates Diarrhea-Predominant Irritable Bowel Syndromes via MAPK and NF-κB Pathway Modulation in Rats. Nutrients 2024, 16, 3854. [Google Scholar] [CrossRef]
- Sperber, A.D. Epidemiology and Burden of Irritable Bowel Syndrome: An International Perspective. Gastroenterol. Clin. 2021, 50, 489–503. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Akuru, E.A.; Chukwuma, C.I.; Oyeagu, C.E.; Erukainure, O.L.; Mashile, B.; Setlhodi, R.; Mashele, S.S.; Makhafola, T.J.; Unuofin, J.O.; Abifarin, T.O.; et al. Nutritional and phytochemical profile of pomegranate (“Wonderful variety”) peel and its effects on hepatic oxidative stress and metabolic alterations. J. Food Biochem. 2022, 46, e13913. [Google Scholar] [CrossRef]
- Tang, Q.; Han, R.; Xiao, H.; Li, J.; Shen, J.; Luo, Q. Protective effect of tanshinone IIA on the brain and its therapeutic time window in rat models of cerebral ischemia-reperfusion. Exp. Ther. Med. 2014, 8, 1616–1622. [Google Scholar] [CrossRef]
- Zingales, V.; Sirerol-Piquer, M.S.; Fernández-Franzón, M.; Ruiz, M.-J. Role of quercetin on sterigmatocystin-induced oxidative stress-mediated toxicity. Food Chem. Toxicol. 2021, 156, 112498. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Tamir, S.; Song, L.; Ruiz, M.J. NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc. Natl. Acad. Sci. USA 2013, 110, 14676–14681. [Google Scholar] [CrossRef]
- Vinnai, J.R.; Cumming, R.C.; Thompson, G.J.; Timoshenko, A.V. The association between oxidative stress-induced galectins and differentiation of human promyelocytic HL-60 cells. Exp. Cell Res. 2017, 355, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, R.I.; Frigeri, L.G.; Liu, F.T. Activation of rat basophilic leukemia cells by ϵBP, an IgE-binding endogenous lectin. Cell. Immunol. 1994, 156, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.C.G.; Frigeri, L.G.; Liu, F.T. An endogenous lectin, galectin-3 (ϵBP/Mac-2), potentiates IL-1 production by human monocytes. Immunol. Lett. 1994, 42, 113–116. [Google Scholar] [CrossRef]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef]
- Karpe, A.V.; Liu, J.W.; Shah, A.; Koloski, N.; Holtmann, G.; Beale, D.J. Utilising lipid and, arginine and proline metabolism in blood plasma to differentiate the biochemical expression in functional dyspepsia (FD) and irritable bowel syndrome (IBS). Metabolomics 2022, 18, 38. [Google Scholar] [CrossRef]
- Shakya, A.; McKee, N.W.; Dodson, M.; Chapman, E.; Zhang, D.D. Anti-Ferroptotic Effects of Nrf2: Beyond the Antioxidant Response. Mol. Cells 2023, 46, 165–175. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Liu, M.; Lu, J.; Guan, S. Toward improved human health: Nrf2 plays a critical role in regulating ferroptosis. Food Funct. 2021, 12, 9583–9606. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Dodson, M.; Schmidlin, C.J.; Liu, P.; Zhang, D.D. Breakdown of an ironclad defense system: The critical role of NRF2 in mediating ferroptosis. Cell Chem. Biol. 2020, 27, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Choijookhuu, N.; Hino, S.; Oo, P.S.; Batmunkh, B.; Hishikawa, Y. The role of estrogen receptors in intestinal homeostasis and disease. Recept. Clin. Investig. 2016, 3, e1109. [Google Scholar] [CrossRef]
- Baumgartner, C.; Hubacher, T.; Krayer, M.; Gschossmann, J. In vitro spontaneous contractile activity of colonic smooth muscle in naive Lewis rats: Acute effect of gonadal hormones. J. Dig. Dis. 2017, 18, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Sims, S.M.; Jiao, Y.; Preiksaitis, H.G. Regulation of intracellular calcium in human esophageal smooth muscles. Am. J. Physiol.-Cell Physiol. 1997, 273, C1679–C1689. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Ravi, K.; Camilleri, M.; Andrews, C.; Szarka, L.A.; Low, P.A.; Zinsmeister, A.R.; Bharucha, A.E. Effect of neostigmine on gastroduodenal motility in patients with suspected gastrointestinal motility disorders. Neurogastroenterol. Motil. 2015, 27, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.Y.; Sang, X.N.; Hui, H.; Shou, Q.Y.; Fu, H.Y.; Hao, M.; Liu, K.H.; Zhang, Q.Y.; Cao, G.; Qin, L.P. Processing and Polyherbal Formulation of Tetradium ruticarpum (A. Juss.) Hartley: Phytochemistry, Pharmacokinetics, and Toxicity. Front. Pharmacol. 2020, 11, 133. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxidative Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, H.Y. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother. Res. 2001, 15, 206–212. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- de O. Silva, L.; Garrett, R.; Monteiro, M.L.G.; Conte-Junior, C.A.; Torres, A.G. Pomegranate (Punica granatum) peel fractions obtained by supercritical CO2 increase oxidative and colour stability of bluefish (Pomatomus saltatrix) patties treated by UV-C irradiation. Food Chem. 2021, 362, 130159. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, M. Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial identification of antifungal compounds from Punica granatum peel extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Corroto, E.; Marina, M.L.; García, M.C. Extraction and identification by high resolution mass spectrometry of bioactive substances in different extracts obtained from pomegranate peel. J. Chromatogr. A 2019, 1594, 82–92. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić Martinović, L.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Kraljević Pavelić, S. Assessment of the biological activity and phenolic composition of ethanol extracts of pomegranate (Punica granatum L.) peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumaki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Marra, F.; Petrovicova, B.; Canino, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Pomegranate wastes are rich in bioactive compounds with potential benefit on human health. Molecules 2022, 27, 5555. [Google Scholar] [CrossRef]
- González-Hidalgo, I.; Bañón, S.; Ros, J.M. Evaluation of table olive by-product as a source of natural antioxidants. Int. J. Food Sci. Technol. 2012, 47, 674–681. [Google Scholar] [CrossRef]
- Elwej, A.; Grojja, Y.; Ghorbel, I.; Boudawara, O.; Jarraya, R.; Boudawara, T.; Zeghal, N. Barium chloride induces redox status unbalance, upregulates cytokine genes expression and confers hepatotoxicity in rats—Alleviation by pomegranate peel. Environ. Sci. Pollut. Res. 2016, 23, 7559–7571. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Tomás-Barberán, F.A.; García-Villalba, R. Pomegranate fruit and juice (cv. Mollar), rich in ellagitannins and anthocyanins, also provide a significant content of a wide range of proanthocyanidins. J. Agric. Food Chem. 2019, 67, 9160–9167. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, e12803. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Sudakaran, S.V.; Ravichandran, K.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Green synthesis of NiFe nano particles using Punica granatum peel extract for tetracycline removal. J. Clean. Prod. 2019, 210, 767–776. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem DASeeram, N.P. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Kaur, R.; Kaushal, S.; Sharma, P. Antimicrobial and antioxidant potential of pomegranate (Punica granatum L.) peel. Int. J. Chem. Stud. 2018, 6, 3441–3449. [Google Scholar]
- Neuhöfer, H.; Witte, L.; Gorunovic, M.; Czygan, F.C. Alkaloids in the bark of Punica granatum L.(pomegranate) from Yugoslavia. Pharmazie 1993, 48, 389–391. [Google Scholar]
- Keogh, M.F.; O’Donovan, D.G. Biosynthesis of some alkaloids of Punica granatum and Withania somnifera. J. Chem. Soc. C Org. 1970, 13, 1792–1797. [Google Scholar] [CrossRef]
- Ding, W.; Wang, H.; Zhou, Q.; Wu, C.; Gao, X.; Cheng, X.; Tian, L.; Wang, C. Simultaneous determination of polyphenols and triterpenes in pomegranate peel based on high-performance liquid chromatography fingerprint by solvent extraction and ratio blending method in tandem with wavelength switching. Biomed. Chromatogr. 2019, 33, e4690. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.C.A.A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef] [PubMed]



| No. | Name | CAS | Class | No. | Name | CAS | Class |
|---|---|---|---|---|---|---|---|
| PP01 | β-Glucogalin | 13405-60-2 | Polyphenols | PP21 | Granatin B | 77322-54-4 | Polyphenols |
| PP02 | Gallic acid | 149-91-7 | Polyphenols | PP22 | Valoneic acid dilactone | 60202-70-2 | Polyphenols |
| PP03 | Punicalin | 65995-64-4 | Polyphenols | PP23 | Ellagic acid | 476-66-4 | Polyphenols |
| PP04 | 2-O-Galloylpunicalin | 103488-45-5 | Polyphenols | PP24 | Kaempferol-3-O-β-D-glucopyranoside | 480-10-4 | Polyphenols |
| PP05 | Pedunculagin | 7045-42-3 | Polyphenols | PP25 | 3-Glucosylquercetin | 482-35-9 | Polyphenols |
| PP06 | Urolithin D | 131086-98-1 | Polyphenols | PP26 | 5-Hydroxymethylfurfural | 67-47-0 | Furfurals |
| PP07 | Gallocatechin | 970-73-0 | Polyphenols | PP27 | Pelargonidin | 7690-51-9 | Polyphenols |
| PP08 | Punicalagin | 65995-63-3 | Polyphenols | PP28 | 3,3′-Di-O-methylellagic acid 4′-glucoside | 51803-68-0 | Polyphenols |
| PP09 | (−)-Epigallocatechin | 970-74-1 | Polyphenols | PP29 | Apigenin | 520-36-5 | Polyphenols |
| PP10 | Sanguisorbic acid dilactone | 82203-11-0 | Polyphenols | PP30 | 5-hydroxymethylfuran-3-carboxylic acid | 246178-75-6 | Furfurals |
| PP11 | Procyanidin B2 | 15514-06-4 | Polyphenols | PP31 | Cyanidin | 13306-05-3 | Polyphenols |
| PP12 | Tellimagrandin I | 79786-08-6 | Polyphenols | PP32 | Pelletierine | 2858-66-4 | Alkaloids |
| PP13 | Urolithin A | 1143-70-0 | Polyphenols | PP33 | Isopelletierine | 4396-01-4 | Alkaloids |
| PP14 | Granatin A | 161205-11-4 | Polyphenols | PP34 | Pseudopelletierine | 552-70-5 | Alkaloids |
| PP15 | Methyl gallate | 99-24-1 | Polyphenols | PP35 | Oleanic Acid | 508-02-1 | Triterpenoids |
| PP16 | Catechin | 154-23-4 | Polyphenols | PP36 | Kaempferol | 520-18-3 | Polyphenols |
| PP17 | Casuarinin | 79786-01-9 | Polyphenols | PP37 | β-Sitosterol | 83-46-5 | Triterpenoids |
| PP18 | Corilagin | 23094-69-1 | Polyphenols | PP38 | Luteolin | 491-70-3 | Polyphenols |
| PP19 | Castalin | 19086-75-0 | Polyphenols | PP39 | Quercetin | 117-39-5 | Polyphenols |
| PP20 | (−)-Gallocatechin gallate | 4233-96-9 | Polyphenols |
| Compounds | Affinity Energy (kcal/mol) | |
|---|---|---|
| PTGS2 | NOS2 | |
| Pedunculagin | −8.4 | −9.3 |
| Punicalin | −9.2 | −9.4 |
| Celecoxib | −8.4 | / |
| 1400 W | / | −7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, L.; Huang, J.-Q.; Lu, M.-W.; Yang, S.-H. Valorization of Pomegranate Peel: Mechanisms and Clinical Applications in Irritable Bowel Syndrome Management. Int. J. Mol. Sci. 2025, 26, 3530. https://doi.org/10.3390/ijms26083530
Guo Y, Wang L, Huang J-Q, Lu M-W, Yang S-H. Valorization of Pomegranate Peel: Mechanisms and Clinical Applications in Irritable Bowel Syndrome Management. International Journal of Molecular Sciences. 2025; 26(8):3530. https://doi.org/10.3390/ijms26083530
Chicago/Turabian StyleGuo, Yu, Lu Wang, Jun-Qing Huang, Mu-Wen Lu, and Song-Hong Yang. 2025. "Valorization of Pomegranate Peel: Mechanisms and Clinical Applications in Irritable Bowel Syndrome Management" International Journal of Molecular Sciences 26, no. 8: 3530. https://doi.org/10.3390/ijms26083530
APA StyleGuo, Y., Wang, L., Huang, J.-Q., Lu, M.-W., & Yang, S.-H. (2025). Valorization of Pomegranate Peel: Mechanisms and Clinical Applications in Irritable Bowel Syndrome Management. International Journal of Molecular Sciences, 26(8), 3530. https://doi.org/10.3390/ijms26083530






