Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia
Abstract
1. Introduction
2. Results
2.1. Bioinformatics Analysis of the rgalT-galU
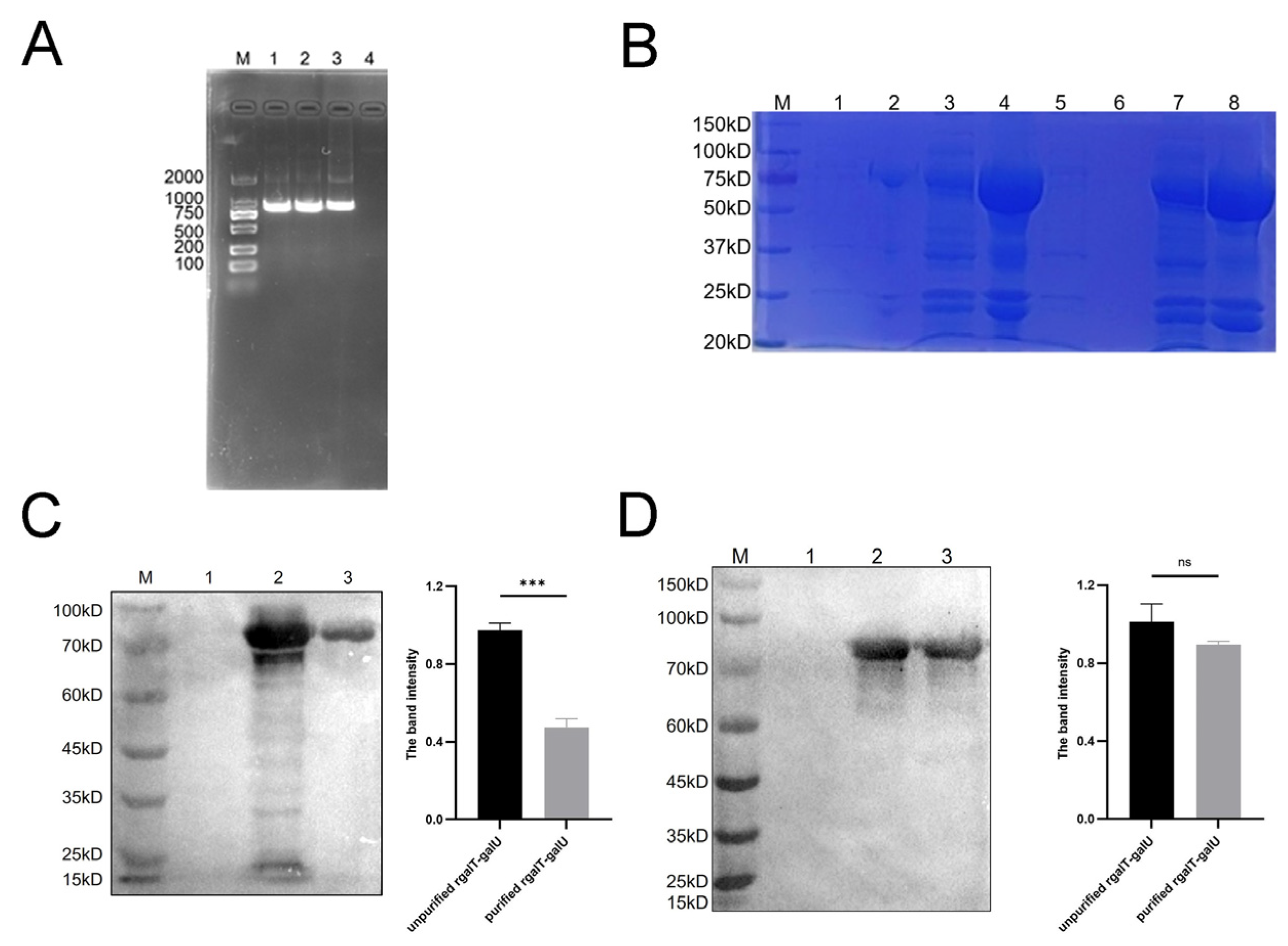
2.2. Identification of rgalT-galU
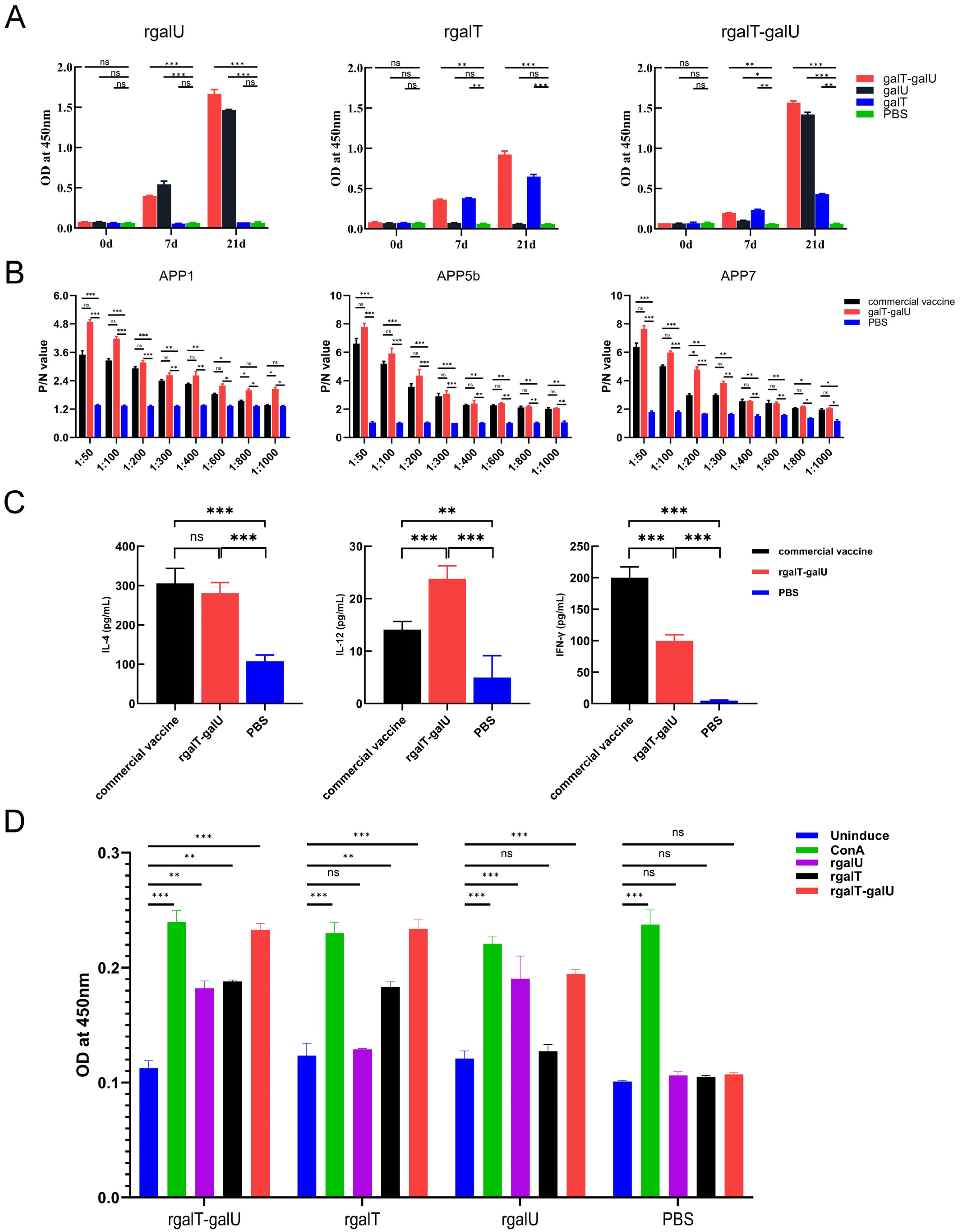
2.3. Immunogenicity of rgalT-galU
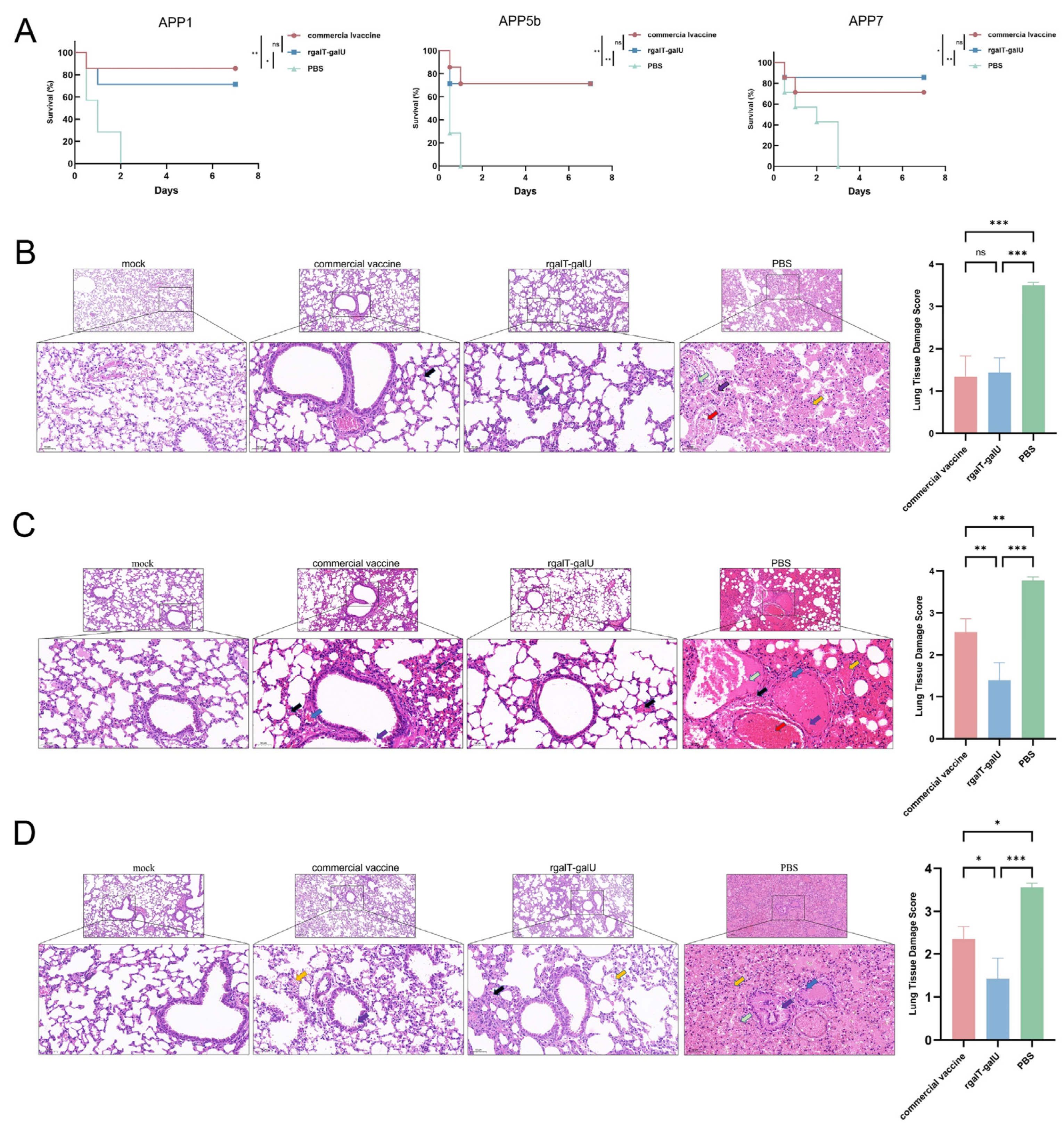
2.4. Protective Efficacy Provided by rgalT-galU
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media, and Growth Condition
4.2. Animals
4.3. Bioinformatics Analysis for rgalT-galU
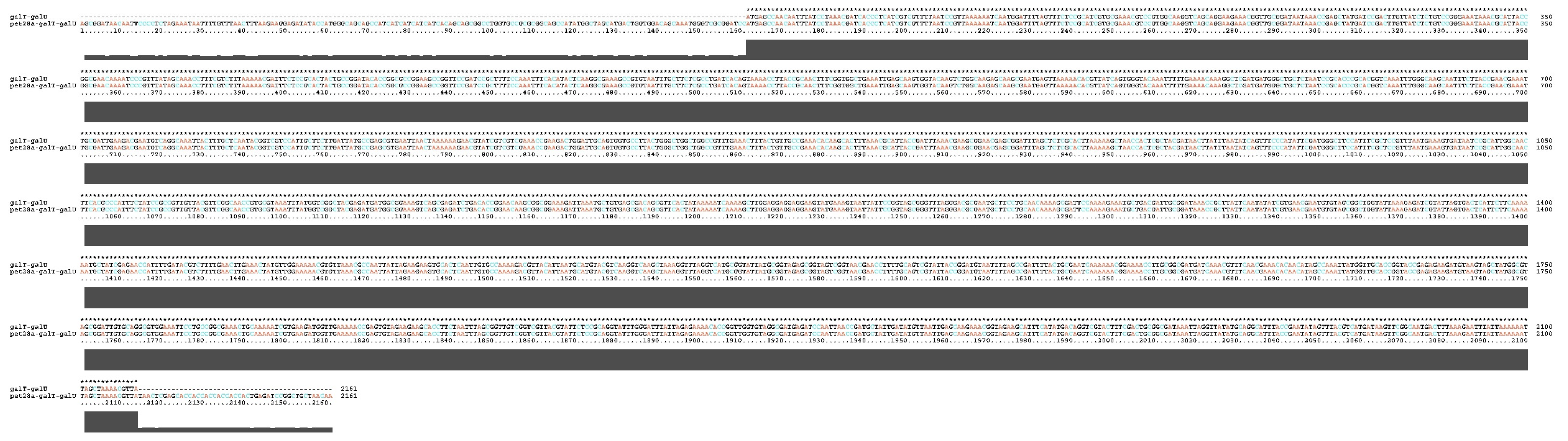
4.4. Construction and Verification of Recombinant Plasmid pET28a-galT-galU
4.5. Expression and Purification of rgalT-galU
4.6. Western Blotting
4.7. Immunization and Challenge
4.8. Indirect Enzyme-Linked Immunosorbent Assay
4.9. Splenocyte Proliferation Assay
4.10. Assessment of Cytokines in Serum
4.11. Histopathological Analysis
4.12. Immunohistochemical Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCP | porcine contagious pleuropneumonia |
| APP | Actinobacillus pleuropneumoniae |
| galT | galactose-1-phosphate uridyltransferase |
| galU | UTP-glucose-1-phosphate uridylyltransferase |
| rgalT-galU | recombinant galT-galU protein |
References
- Sassu, E.L.; Bossé, J.T.; Tobias, T.J.; Gottschalk, M.; Langford, P.R.; Hennig-Pauka, I. Update on Actinobacillus pleuropneumoniae—Knowledge, gaps and challenges. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 72–90. [Google Scholar] [CrossRef]
- Zhu, Z.; Antenucci, F.; Winther-Larsen, H.C.; Skovgaard, K.; Bojesen, A.M. Outer Membrane Vesicles of Actinobacillus pleuropneumoniae Exert Immunomodulatory Effects on Porcine Alveolar Macrophages. Microbiol. Spectr. 2022, 10, e0181922. [Google Scholar] [CrossRef] [PubMed]
- Stringer, O.W.; Li, Y.; Bossé, J.T.; Forrest, M.S.; Hernandez-Garcia, J.; Tucker, A.W.; Nunes, T.; Costa, F.; Mortensen, P.; Velazquez, E.; et al. Rapid Detection of Actinobacillus pleuropneumoniae from Clinical Samples Using Recombinase Polymerase Amplification. Front. Vet. Sci. 2022, 9, 805382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, H.; Yan, C.; Han, W.; Peng, L.; Xu, J.; Chen, X.; Langford, P.R.; Bei, W.; Huang, Q.; et al. The Metabolic Adaptation in Response to Nitrate Is Critical for Actinobacillus pleuropneumoniae Growth and Pathogenicity under the Regulation of NarQ/P. Infect. Immun. 2022, 90, e0023922. [Google Scholar] [CrossRef]
- Aarestrup, F. Sustainable farming: Get pigs off antibiotics. Nature 2012, 486, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, Q.; Wen, X.; Wu, R.; Wen, Y.; Huang, X.; Huang, Y.; Yan, Q.; Han, X.; Ma, X.; et al. A trivalent Apx-fusion protein delivered by E. coli outer membrane vesicles induce protection against Actinobacillus pleuropneumoniae of serotype 1 and 7 challenge in a murine model. PLoS ONE 2018, 13, e0191286. [Google Scholar] [CrossRef]
- Stringer, O.W.; Bossé, J.T.; Lacouture, S.; Gottschalk, M.; Fodor, L.; Angen, Ø.; Velazquez, E.; Penny, P.; Lei, L.; Langford, P.R.; et al. Proposal of Actinobacillus pleuropneumoniae serovar 19, and reformulation of previous multiplex PCRs for capsule-specific typing of all known serovars. Vet. Microbiol. 2021, 255, 109021. [Google Scholar] [CrossRef]
- Guitart-Matas, J.; Gonzalez-Escalona, N.; Maguire, M.; Vilaró, A.; Martinez-Urtaza, J.; Fraile, L.; Migura-Garcia, L. Revealing Genomic Insights of the Unexplored Porcine Pathogen Actinobacillus pleuropneumoniae Using Whole Genome Sequencing. Microbiol. Spectr. 2022, 10, e0118522. [Google Scholar] [CrossRef]
- Chuekwon, K.; Chu, C.Y.; Cheng, L.T. N-terminus of flagellin enhances vaccine efficacy against Actinobacillus pleuropneumoniae. BMC Vet. Res. 2022, 18, 279. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhou, M.; Liu, P.; Zhang, E.; Li, Y.; Lin, J.; Feng, Z.; Yang, Q. Mucosal and systemic immune responses induced by intranasal immunization of recombinant Bacillus subtilis expressing the P97R1, P46 antigens of Mycoplasma hyopneumoniae. Biosci. Rep. 2019, 39, BSR20191126. [Google Scholar] [CrossRef]
- Pham, T.N.; Loupias, P.; Dassonville-Klimpt, A.; Sonnet, P. Drug delivery systems designed to overcome antimicrobial resistance. Med. Res. Rev. 2019, 39, 2343–2396. [Google Scholar] [CrossRef] [PubMed]
- Vilaró, A.; Karstensen, K.T.; Cavaco, L.M.; Angen, Ø.; Solé, E.; Seró, I.; Novell, E.; Enrique-Tarancón, V.; Guitart-Matas, J.; Migura-Garcia, L.; et al. An investigation of the transmission of Actinobacillus pleuropneumoniae within vertically integrated systems using whole genome sequencing. Vet. Microbiol. 2024, 295, 110157. [Google Scholar] [CrossRef] [PubMed]
- Rowney, C. Porcine Pleuropneumonia: Importance of a Broad Vaccine Induced Protection. Available online: https://www.thepigsite.com/articles/porcine-pleuropneumonia-importance-of-a-broad-vaccine-induced-protection?utm_source=chatgpt.com (accessed on 4 April 2025).
- Jensen, K.J.; Hansen, M.S.; Heegaard, P.M.H.; Benn, C.S.; Jungersen, G. The Effect of Inactivated Mycobacterium Paratuberculosis Vaccine on the Response to a Heterologous Bacterial Challenge in Pigs. Front. Immunol. 2019, 10, 1557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, J.; Bao, C.; Zhu, R.; Gu, J.; Sun, C.; Feng, X.; Du, C.; Han, W.; Li, Y.; et al. Recombinant tandem epitope vaccination provides cross protection against Actinobacillus pleuropneumoniae challenge in mice. AMB Express 2020, 10, 123. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, W.; Xiong, R.; Li, H.; Yao, Z.; Zhuo, W.; Zou, G.; Huang, Q.; Zhou, R. A combinatorial vaccine containing inactivated bacterin and subunits provides protection against Actinobacillus pleuropneumoniae infection in mice and pigs. Front. Vet. Sci. 2022, 9, 902497. [Google Scholar] [CrossRef]
- Loera-Muro, A.; Angulo, C. New trends in innovative vaccine development against Actinobacillus pleuropneumoniae. Vet. Microbiol. 2018, 217, 66–75. [Google Scholar] [CrossRef]
- Lopez-Bermudez, J.; Quintanar-Guerrero, D.; Puente, H.L.; Perez, J.T.; Güemez, F.S.; Carrasco, A.C.; Elvira, S.M. Oral immunization against porcine pleuropneumonia using the cubic phase of monoolein and purified toxins of Actinobacillus pleuropneumoniae. Vaccine 2014, 32, 6805–6811. [Google Scholar] [CrossRef]
- Hodson, R. Vaccines. Nature 2019, 575, S43. [Google Scholar] [CrossRef]
- Qin, W.; Wang, L.; Zhai, R.; Ma, Q.; Liu, J.; Bao, C.; Sun, D.; Zhang, H.; Sun, C.; Feng, X.; et al. Apa2H1, the first head domain of Apa2 trimeric autotransporter adhesin, activates mouse bone marrow-derived dendritic cells and immunization with Apa2H1 protects against Actinobacillus pleuropneumoniae infection. Mol. Immunol. 2017, 81, 108–117. [Google Scholar] [CrossRef]
- van Overbeke, I.; Chiers, K.; Donne, E.; Ducatelle, R.; Haesebrouck, F. Effect of endobronchial challenge with Actinobacillus pleuropneumoniae serotype 10 of pigs vaccinated with bacterins consisting of A. pleuropneumoniae serotype 10 grown under NAD-rich and NAD-restricted conditions. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 289–293. [Google Scholar] [CrossRef]
- Goethe, R.; Gonzáles, O.F.; Lindner, T.; Gerlach, G.F. A novel strategy for protective Actinobacillus pleuropneumoniae subunit vaccines: Detergent extraction of cultures induced by iron restriction. Vaccine 2000, 19, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Wen, X.; Huang, X.; Wen, Y.; Wu, R.; Yan, Q.; Huang, Y.; Ma, X.; Zhao, Q.; et al. Identification of Actinobacillus pleuropneumoniae Genes Preferentially Expressed During Infection Using In Vivo-Induced Antigen Technology (IVIAT). J. Microbiol. Biotechnol. 2015, 25, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, S.; Zhu, Z.; Yang, Y.; Wen, X.; Chang, Y.F.; Huang, X.; Wu, R.; Wen, Y.; Yan, Q.; et al. Immunoprotective Efficacy of Six In vivo-Induced Antigens against Actinobacillus pleuropneumoniae as Potential Vaccine Candidates in Murine Model. Front. Microbiol. 2016, 7, 1623. [Google Scholar] [CrossRef]
- Bonofiglio, L.; García, E.; Mollerach, M. Biochemical characterization of the pneumococcal glucose 1-phosphate uridylyltransferase (GalU) essential for capsule biosynthesis. Curr. Microbiol. 2005, 51, 217–221. [Google Scholar] [CrossRef]
- Chai, Y.; Beauregard, P.B.; Vlamakis, H.; Losick, R.; Kolter, R. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio 2012, 3, e00184-12. [Google Scholar] [CrossRef]
- Kong, L.; Huang, Y.; Zeng, X.; Ye, C.; Wu, Z.; Guo, Y.; Pan, D. Effects of galactosyltransferase on EPS biosynthesis and freeze-drying resistance of Lactobacillus acidophilus NCFM. Food Chem. 2022, 5, 100145. [Google Scholar]
- Mollerach, M.; García, E. The galU gene of Streptococcus pneumoniae that codes for a UDP-glucose pyrophosphorylase is highly polymorphic and suitable for molecular typing and phylogenetic studies. Gene 2000, 260, 77–86. [Google Scholar] [CrossRef]
- Mortensen, P.; Toft, N.; Kiss, I.; Palya, V.; Smits, H.; Tenk, M. Comparative Efficacy in Challenge Dose Models of a Toxin Expressing Whole-Cell Vaccine against Eight Serovars of Actinobacillus pleuropneumoniae in Pigs. Animals 2022, 12, 3244. [Google Scholar] [CrossRef]
- Hathroubi, S.; Loera-Muro, A.; Guerrero-Barrera, A.L.; Tremblay, Y.D.N.; Jacques, M. Actinobacillus pleuropneumoniae biofilms: Role in pathogenicity and potential impact for vaccination development. Anim. Health Res. Rev. 2018, 19, 17–30. [Google Scholar] [CrossRef]
- Caboni, M.; Pedron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef]
- Rollins, S.M.; Peppercorn, A.; Hang, L.; Hillman, J.D.; Calderwood, S.B.; Handfield, M.; Ryan, E.T. In vivo induced antigen technology (IVIAT). Cell. Microbiol. 2005, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Díaz, J.; Fumanal, M.; Viguera, E.; Moriñigo, M.; Balebona, M. Use of in vivo induced technology to identify antigens expressed by Photobacterium damselae subsp. piscicida during infection of Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2017, 64, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Pu, W.; Pan, X.; Li, P.; Bai, Q.; Liang, S.; Li, C.; Yu, Y.; Yao, H.; et al. A multi-epitope subunit vaccine providing broad cross-protection against diverse serotypes of Streptococcus suis. NPJ Vaccines 2024, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- İncir, İ.; Kaplan, Ö. Escherichia coli as a versatile cell factory: Advances and challenges in recombinant protein production. Protein Expr. Purif. 2024, 219, 106463. [Google Scholar] [CrossRef]
- Rajacharya, G.H.; Sharma, A.; Yazdani, S.S. Proteomics and metabolic burden analysis to understand the impact of recombinant protein production in E. coli. Sci. Rep. 2024, 14, 12271. [Google Scholar] [CrossRef]
- Alijani, M.; Saffar, B.; Yosefi Darani, H.; Mahzounieh, M.; Fasihi-Ramandi, M.; Shakshi-Niaei, M.; Soltani, S.; Ghaemi, A.; Shirian, S. Immunological evaluation of a novel multi-antigenic DNA vaccine encoding SAG1, SAG3, MIC4, GRA5, GRA7, AMA1and BAG1 against Toxoplasma gondii in BALB/c mice. Exp. Parasitol. 2023, 244, 108409. [Google Scholar] [CrossRef]
- Watts, T.H. Stepping up Th1 immunity to control phagosomal bacteria. Trends Immunol. 2021, 42, 461–463. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Nauseef, W.M. IFN-γ targets macrophage-mediated immune responses toward Staphylococcus aureus. J. Leukoc. Biol. 2017, 101, 751–758. [Google Scholar] [CrossRef]
- Sun, Y.; Long, J.; Chen, W.; Sun, Y.; Zhou, L.; Zhang, L.; Zeng, H.; Yuan, D. Alisol B 23-acetate, a new promoter for cholesterol efflux from dendritic cells, alleviates dyslipidemia and inflammation in advanced atherosclerotic mice. Int. Immunopharmacol. 2021, 99, 107956. [Google Scholar] [CrossRef]
- Cui, A.-H.; Zhao, J.; Liu, S.-X.; Hao, Y.-S. Associations of IL-4, IL-6, and IL-12 levels in peripheral blood with lung function, cellular immune function, and quality of life in children with moderate-to-severe asthma. Medicine 2017, 96, e6265. [Google Scholar] [CrossRef]
- Kisuya, J.; Chemtai, A.; Raballah, E.; Keter, A.; Ouma, C. The diagnostic accuracy of Th1 (IFN-γ, TNF-α, and IL-2) and Th2 (IL-4, IL-6 and IL-10) cytokines response in AFB microscopy smear negative PTB-HIV co-infected patients. Sci. Rep. 2019, 9, 2966. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Sun, H.; Wang, S.; Guo, X.; Ding, H.; Yang, Y.; Shan, Y.; Du, A. Ginseng Stem-and-Leaf Saponin (GSLS)-Enhanced Protective Immune Responses Induced by Toxoplasma gondii Heat Shocked Protein 70 (HSP70) Against Toxoplasmosis in Mice. J. Parasitol. 2017, 103, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, A.; Takahashi, K.; Harada, H.; Kawashima, S.; Oikawa, H.; Fukushima, H.; Hayakawa, Y.; Koizumi, J.; Inoue, N.; Koshizuka, T. Evaluation of the protective effect of the intranasal vaccines adjuvanted with bacterium-like particles against intestinal infection. Vaccine 2024, 42, 125975. [Google Scholar] [CrossRef]
- Whelan, A.O.; Flick-Smith, H.C.; Walker, N.J.; Abraham, A.; Levitz, S.M.; Ostroff, G.R.; Oyston, P.C.F. A glucan-particle based tularemia subunit vaccine induces T-cell immunity and affords partial protection in an inhalation rat infection model. PLoS ONE 2024, 19, e0294998. [Google Scholar] [CrossRef]
- Quan, K.; Zhu, Z.; Cao, S.; Zhang, F.; Miao, C.; Wen, X.; Huang, X.; Wen, Y.; Wu, R.; Yan, Q. Escherichia coli-Derived Outer Membrane Vesicles Deliver Galactose-1-Phosphate Uridyltransferase and Yield Partial Protection against Actinobacillus pleuropneumoniae in Mice. J. Microbiol. Biotechnol. 2018, 28, 2095–2105. [Google Scholar] [CrossRef]
- Kim, J.-M.; Park, S.-M.; Kim, J.-A.; Park, J.-A.; Yi, M.-H.; Kim, N.-S.; Bae, J.-L.; Park, S.G.; Jang, Y.-S.; Yang, M.-S. Functional pentameric formation via coexpression of the Escherichia coli heat-labile enterotoxin B subunit and its fusion protein subunit with a Neutralizing Epitope of ApxIIA Exotoxin improves the mucosal immunogenicity and protection against challenge by Actinobacillus pleuropneumoniae. Clin. Vaccine Immunol. 2011, 18, 2168–2177. [Google Scholar]
- Baxter, R.; Keshavan, P.; Welsch, J.A.; Han, L.; Smolenov, I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum. Vaccin. Immunother. 2016, 12, 1300–1310. [Google Scholar] [CrossRef]
- Haddadi, A.; Chaffey, A.; Ng, S.H.; Yalamati, D.; Wilson, H.L. Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice. Vaccines 2020, 8, 569. [Google Scholar] [CrossRef]
- Kornuta, C.A.; Langellotti, C.A.; Bidart, J.E.; Soria, I.; Quattrocchi, V.; Gammella, M.; Cheuquepán Valenzuela, F.; Mignaqui, A.C.; Ferraris, S.; Charleston, B.; et al. A plasmid encoding the extracellular domain of CD40 ligand and Montanide™ GEL01 as adjuvants enhance the immunogenicity and the protection induced by a DNA vaccine against BoHV-1. Vaccine 2021, 39, 1007–1017. [Google Scholar] [CrossRef]
- Tabynov, K.; Sansyzbay, A.; Tulemissova, Z.; Tabynov, K.; Dhakal, S.; Samoltyrova, A.; Renukaradhya, G.J.; Mambetaliyev, M. Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets. Vet. Microbiol. 2016, 192, 81–89. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Q.; Quan, K.; Zhu, Z.; Yang, Y.; Wen, X.; Chang, Y.-F.; Huang, X.; Wu, R.; Wen, Y. Galactose-1-phosphate uridyltransferase (GalT), an in vivo-induced antigen of Actinobacillus pleuropneumoniae serovar 5b strain L20, provided immunoprotection against serovar 1 strain MS71. PLoS ONE 2018, 13, e0198207. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shang, H.; Niu, X.; Huang, J.; Miao, Y.; Sha, Z.; Qin, L.; Huang, H.; Peng, D.; Zhu, R. Establishment and evaluation of an indirect ELISA for detection of antibodies to goat Klebsiella pneumonia. BMC Vet. Res. 2021, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Kolb, J.G.M. Animal models of pulmonary fibrosis: How far from effective reality? Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L151. [Google Scholar]


| Primer Name | Primer Sequence (5′–3′) | Size | Enzyme | Fragment Length |
|---|---|---|---|---|
| galT-U-F | cactataaaaatcaaaagcttGGAGGAGGAGGAAGT ATGAAAGTAATTATTCCGGTAGC | 59 bp | Hind III | 888 bp |
| galT-U-R | gtggtggtggtggtgctcgagTTATAACGTTTTAGCT AATTTTTTA | 46 bp | Xho I |
| Group | Boost Immunization Dose (0 Day) | Secondary Immunization Dose (14 Day) |
|---|---|---|
| Commercial vaccine | 2.5 μL (12.5 g) | 3.3 μL (16.5 g) |
| galT-galU | 100 μg/200 μL | 120 μg/200 μL |
| galT | 100 μg/200 μL | 120 μg/200 μL |
| galU | 100 μg/200 μL | 120 μg/200 μL |
| PBS | 200 μL | 200 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Deng, Y.; Liu, J.; Wen, X.; Cao, Y.-Q.; Mu, Y.; Sun, M.; Miao, C.; Peng, Z.; Lu, K.; et al. Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia. Int. J. Mol. Sci. 2025, 26, 3634. https://doi.org/10.3390/ijms26083634
Chen J-Y, Deng Y, Liu J, Wen X, Cao Y-Q, Mu Y, Sun M, Miao C, Peng Z, Lu K, et al. Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia. International Journal of Molecular Sciences. 2025; 26(8):3634. https://doi.org/10.3390/ijms26083634
Chicago/Turabian StyleChen, Jia-Yong, Yi Deng, Jiale Liu, Xin Wen, Yu-Qin Cao, Yu Mu, Mengke Sun, Chang Miao, Zhiling Peng, Kun Lu, and et al. 2025. "Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia" International Journal of Molecular Sciences 26, no. 8: 3634. https://doi.org/10.3390/ijms26083634
APA StyleChen, J.-Y., Deng, Y., Liu, J., Wen, X., Cao, Y.-Q., Mu, Y., Sun, M., Miao, C., Peng, Z., Lu, K., Wang, Y.-L., Chen, X., Pang, S., Wang, D., Zhou, J., Li, M., Wen, Y., Wu, R., Zhao, S., ... Zhao, Q. (2025). Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia. International Journal of Molecular Sciences, 26(8), 3634. https://doi.org/10.3390/ijms26083634






