CRISPR-Cas Systems: A Functional Perspective and Innovations
Abstract
:1. Introduction
2. Bacterial Adaptive Immunity: CRISPR-Cas Systems and Their Mechanisms of Action
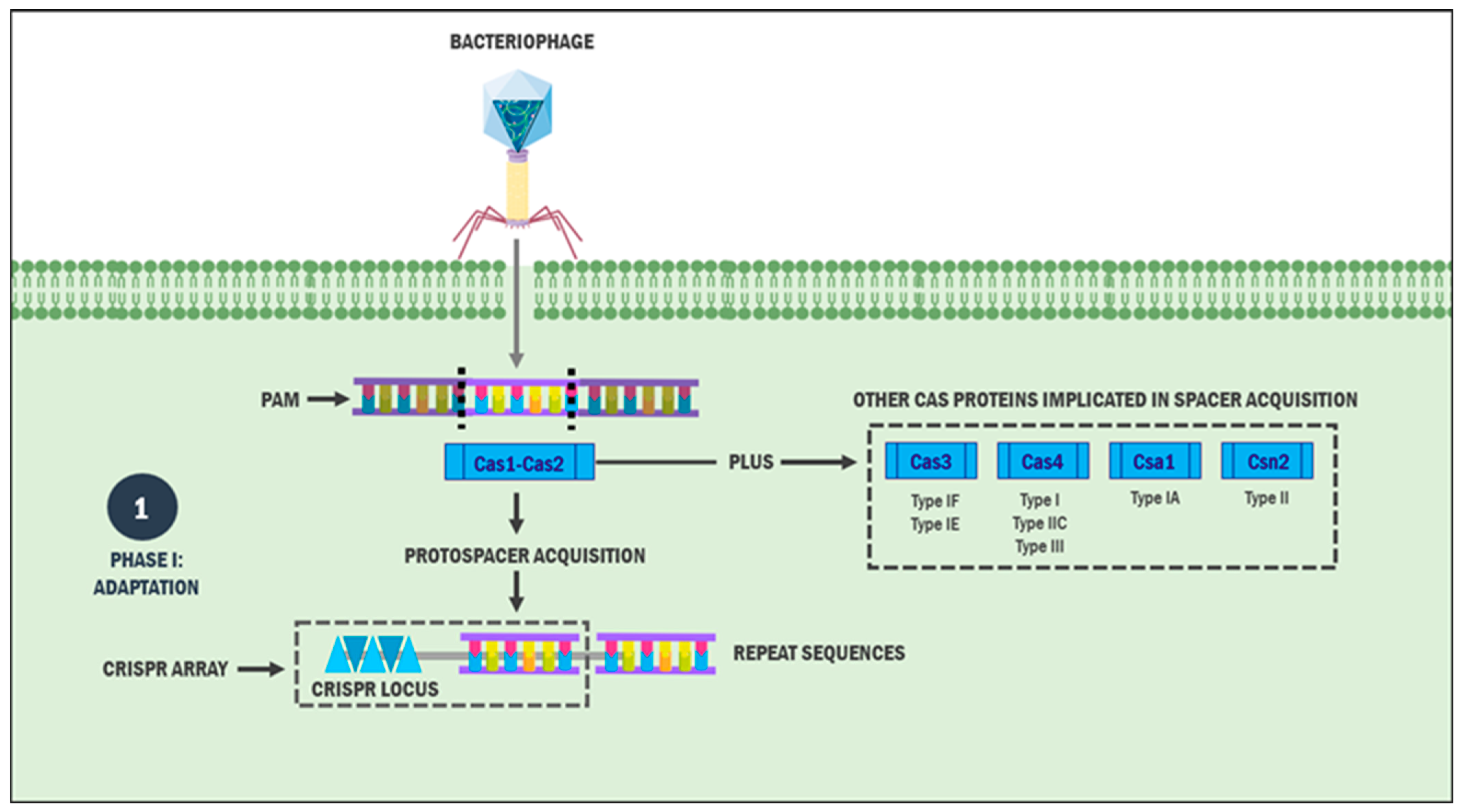
2.1. Phase I: Adaptation
2.2. Phase II: crRNA Biogenesis
2.3. Phase III: Nucleic Acid Interference
3. CRISPR-Cas Delivery Systems
3.1. Non-Viral Delivery Strategies
3.1.1. Microinjections
3.1.2. Electroporation
3.1.3. Hydrodynamic Delivery
3.2. Viral Delivery Strategies
3.2.1. Adenoviral Vectors
3.2.2. Adeno-Associated Viruses
3.2.3. Lentivirus
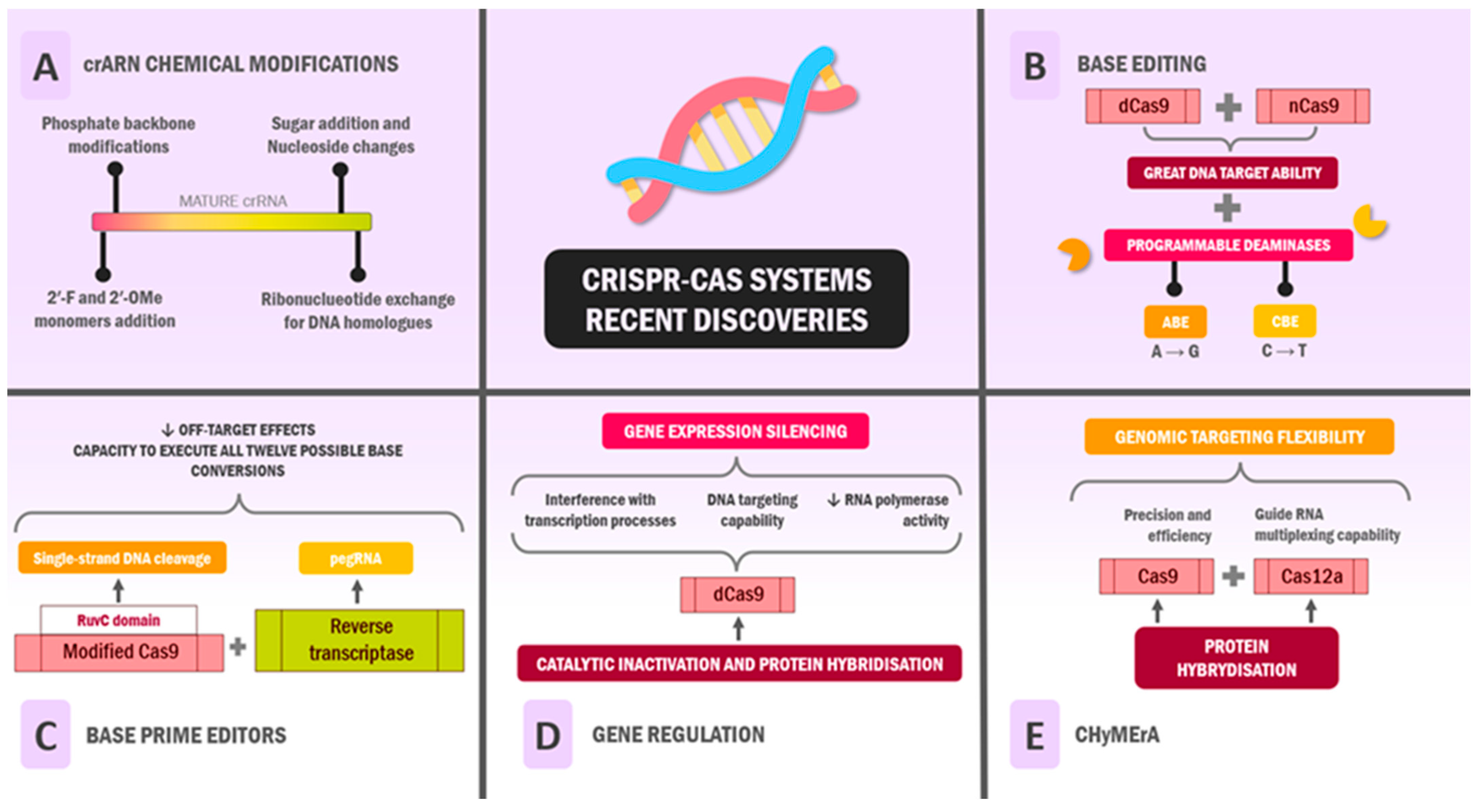
4. Recent Discoveries: Where Are We Now?
4.1. Functional and Chemical Modifications of RNA
4.2. Base Editing
4.3. Base Prime Editors
4.4. Gene Regulation
4.5. CHyMErA
4.6. CRISPR Screening
4.7. Anti-CRISPR Proteins
5. Integrate
5.1. PAM Variability
5.2. Mini CRISPRs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| HIV | Human Immunodeficiency Virus |
| TALEN | Transcription Activator-Like Effector Nuclease |
| ZFN | Zinc Finger Nuclease |
| DNA | Desoxyribonucleic Acid |
| SRSR | Regularly Spaced Short Repetitions |
| RNA | Ribonucleic Acid |
| crRNA | CRISPR RNA |
| NUC Lobe | Nuclease Lobe |
| REC | Recognition Lobe |
| RNP | Ribonucleoprotein Particle |
| tracrRNA | Trans-Activating crRNA |
| CTD | C-terminal Domain |
| PAM | Protospacer Adjacent Motif |
| RRM | RNA Recognition Motif |
| RAMP | Receptor Activity-Modifying Protein |
| RAMPs | Repeat-Associated Mysterious Proteins |
| mRNA | Messenger RNA |
| PAPS | 3′-Phosphoadenosine-5′-phosphosulfate |
| PI Domain | PAM Interacting Domain |
| BH | Bridge Helix |
| dsDNA | Double Strand DNA |
| HEPN Domain | Higher Eukaryotes and Prokaryotes Nucleotide-Binding Domain |
| NTD | N-Terminal Domain |
| RHH | Ribbon-Helix-Helix |
| HTH | Helix-Turn-Helix Domain |
| MGE | Mobile Genome Expression |
| ssDNA | Single-Stranded DNA |
| IHF | Integration-Host Factor |
| ATP | Adenosine Triphosphate |
| ABE | Adenine Base Editor |
| CBE | Cytidine Base Editor |
| pegRNA | Prime Editing Guide RNA |
| CRISPRi | CRISPR Interference |
| CRISPRa | CRISPR Activation |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| TET | Ten-Eleven Translocation |
| ChyMeRa | Cas Hybrid for Multiplexed Editing and Screening Application |
References
- Xu, C.-F.; Chen, G.-J.; Luo, Y.-L.; Zhang, Y.; Zhao, G.; Lu, Z.-D.; Czarna, A.; Gu, Z.; Wang, J. Rational Designs of in Vivo CRISPR-Cas Delivery Systems. Adv. Drug Deliv. Rev. 2021, 168, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The next Generation of CRISPR-Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Goell, J.H.; Hilton, I.B. CRISPR/Cas-Based Epigenome Editing: Advances, Applications, and Clinical Utility. Trends Biotechnol. 2021, 39, 678–691. [Google Scholar] [CrossRef]
- Huang, D.; Miller, M.; Ashok, B.; Jain, S.; Peppas, N.A. CRISPR/Cas Systems to Overcome Challenges in Developing the next Generation of T Cells for Cancer Therapy. Adv. Drug Deliv. Rev. 2020, 158, 17–35. [Google Scholar] [CrossRef]
- Wu, S.-S.; Li, Q.-C.; Yin, C.-Q.; Xue, W.; Song, C.-Q. Advances in CRISPR/Cas-Based Gene Therapy in Human Genetic Diseases. Theranostics 2020, 10, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Babačić, H.; Mehta, A.; Merkel, O.; Schoser, B. CRISPR-Cas Gene-Editing as Plausible Treatment of Neuromuscular and Nucleotide-Repeat-Expansion Diseases: A Systematic Review. PLoS ONE 2019, 14, e0212198. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Dammes, N.; Peer, D. Progress and Challenges towards CRISPR/Cas Clinical Translation. Adv. Drug Deliv. Rev. 2020, 154, 176–186. [Google Scholar] [CrossRef]
- Wilbie, D.; Walther, J.; Mastrobattista, E. Delivery Aspects of CRISPR/Cas for in Vivo Genome Editing. Acc. Chem. Res. 2019, 52, 1555–1564. [Google Scholar] [CrossRef]
- van Dijke, I.; Bosch, L.; Bredenoord, A.L.; Cornel, M.; Repping, S.; Hendriks, S. The Ethics of Clinical Applications of Germline Genome Modification: A Systematic Review of Reasons. Hum. Reprod. 2018, 33, 1777–1796. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Zou, Y.; Yin, T.; Yang, J. Human Embryo Gene Editing: God’s Scalpel or Pandora’s Box? Brief. Funct. Genom. 2020, 19, 154–163. [Google Scholar] [CrossRef]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas Guides the Future of Genetic Engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, Mechanisms and Relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas Immune System: Biology, Mechanisms and Applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Wiedenheft, B. SnapShot: CRISPR-RNA-Guided Adaptive Immune Systems. Cell 2015, 163, 260–260.e1. [Google Scholar] [CrossRef] [PubMed]
- Gleditzsch, D.; Pausch, P.; Müller-Esparza, H.; Özcan, A.; Guo, X.; Bange, G.; Randau, L. PAM Identification by CRISPR-Cas Effector Complexes: Diversified Mechanisms and Structures. RNA Biol. 2019, 16, 504–517. [Google Scholar] [CrossRef]
- Beloglazova, N.; Brown, G.; Zimmerman, M.D.; Proudfoot, M.; Makarova, K.S.; Kudritska, M.; Kochinyan, S.; Wang, S.; Chruszcz, M.; Minor, W.; et al. A Novel Family of Sequence-Specific Endoribonucleases Associated with the Clustered Regularly Interspaced Short Palindromic Repeats. J. Biol. Chem. 2008, 283, 20361–20371. [Google Scholar] [CrossRef]
- Babu, M.; Beloglazova, N.; Flick, R.; Graham, C.; Skarina, T.; Nocek, B.; Gagarinova, A.; Pogoutse, O.; Brown, G.; Binkowski, A.; et al. A Dual Function of the CRISPR-Cas System in Bacterial Antivirus Immunity and DNA Repair. Mol. Microbiol. 2011, 79, 484–502. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Zhou, K.; Jinek, M.; Coyle, S.M.; Ma, W.; Doudna, J.A. Structural Basis for DNase Activity of a Conserved Protein Implicated in CRISPR-Mediated Genome Defense. Structure 2009, 17, 904–912. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Harrington, L.B.; Kranzusch, P.J.; Engelman, A.N.; Doudna, J.A. Foreign DNA Capture during CRISPR-Cas Adaptive Immunity. Nature 2015, 527, 535–538. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Kranzusch, P.J.; Noeske, J.; Wright, A.V.; Davies, C.W.; Doudna, J.A. Cas1-Cas2 Complex Formation Mediates Spacer Acquisition during CRISPR-Cas Adaptive Immunity. Nat. Struct. Mol. Biol. 2014, 21, 528–534. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Almendros, C. Short Motif Sequences Determine the Targets of the Prokaryotic CRISPR Defence System. Microbiology 2009, 155, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Qimron, U. The Escherichia coli CRISPR System Protects from λ Lysogenization, Lysogens, and Prophage Induction. J. Bacteriol. 2010, 192, 6291–6294. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Klumpe, H.E.; Luo, M.L.; Selle, K.; Barrangou, R.; Beisel, C.L. Programmable Removal of Bacterial Strains by Use of Genome-Targeting CRISPR-Cas Systems. mBio 2014, 5, e00928-13. [Google Scholar] [CrossRef] [PubMed]
- Grainy, J.; Garrett, S.; Graveley, B.R.; Terns, M.P. CRISPR Repeat Sequences and Relative Spacing Specify DNA Integration by Pyrococcus furiosus Cas1 and Cas2. Nucleic Acids Res. 2019, 47, 7518–7531. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Lee, A.S.Y.; Engelman, A.; Doudna, J.A. Integrase-Mediated Spacer Acquisition during CRISPR-Cas Adaptive Immunity. Nature 2015, 519, 193–198. [Google Scholar] [CrossRef]
- Arslan, Z.; Hermanns, V.; Wurm, R.; Wagner, R.; Pul, Ü. Detection and Characterization of Spacer Integration Intermediates in Type I-E CRISPR-Cas System. Nucleic Acids Res. 2014, 42, 7884–7893. [Google Scholar] [CrossRef]
- Ivančić-Baće, I.; Cass, S.D.; Wearne, S.J.; Bolt, E.L. Different Genome Stability Proteins Underpin Primed and Naïve Adaptation in E. coli CRISPR-Cas Immunity. Nucleic Acids Res. 2015, 43, 10821–10830. [Google Scholar] [CrossRef]
- Yosef, I.; Goren, M.G.; Qimron, U. Proteins and DNA Elements Essential for the CRISPR Adaptation Process in Escherichia coli. Nucleic Acids Res. 2012, 40, 5569–5576. [Google Scholar] [CrossRef]
- Yoganand, K.N.R.; Sivathanu, R.; Nimkar, S.; Anand, B. Asymmetric Positioning of Cas1-2 Complex and Integration Host Factor Induced DNA Bending Guide the Unidirectional Homing of Protospacer in CRISPR-Cas Type I-E System. Nucleic Acids Res. 2017, 45, 367–381. [Google Scholar] [CrossRef]
- Xue, C.; Sashital, D.G. Mechanisms of Type I-E and I-F CRISPR-Cas Systems in Enterobacteriaceae. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Levy, A.; Goren, M.G.; Yosef, I.; Auster, O.; Manor, M.; Amitai, G.; Edgar, R.; Qimron, U.; Sorek, R. CRISPR Adaptation Biases Explain Preference for Acquisition of Foreign DNA. Nature 2015, 520, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, M.S.; Kowalczykowski, S.C. RecBCD Enzyme and the Repair of Double-Stranded DNA Breaks. Microbiol. Mol. Biol. Rev. 2008, 72, 642–671. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, R.; Xiang, H. Haloarcula Hispanica CRISPR Authenticates PAM of a Target Sequence to Prime Discriminative Adaptation. Nucleic Acids Res. 2014, 42, 7226–7235. [Google Scholar] [CrossRef]
- Plagens, A.; Tjaden, B.; Hagemann, A.; Randau, L.; Hensel, R. Characterization of the CRISPR/Cas Subtype I-A System of the Hyperthermophilic Crenarchaeon Thermoproteus Tenax. J. Bacteriol. 2012, 194, 2491–2500. [Google Scholar] [CrossRef]
- Richter, C.; Gristwood, T.; Clulow, J.S.; Fineran, P.C. In Vivo Protein Interactions and Complex Formation in the Pectobacterium Atrosepticum Subtype I-F CRISPR/Cas System. PLoS ONE 2012, 7, e49549. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Terns, R.M.; Terns, M.P. Cas9 Function and Host Genome Sampling in Type II-A CRISPR–Cas Adaptation. Genes Dev. 2015, 29, 356–361. [Google Scholar] [CrossRef]
- Kim, J.G.; Garrett, S.; Wei, Y.; Graveley, B.R.; Terns, M.P. CRISPR DNA Elements Controlling Site-Specific Spacer Integration and Proper Repeat Length by a Type II CRISPR–Cas System. Nucleic Acids Res. 2019, 47, 8632–8648. [Google Scholar] [CrossRef]
- Arslan, Z.; Wurm, R.; Brener, O.; Ellinger, P.; Nagel-Steger, L.; Oesterhelt, F.; Schmitt, L.; Willbold, D.; Wagner, R.; Gohlke, H.; et al. Double-Strand DNA End-Binding and Sliding of the Toroidal CRISPR-Associated Protein Csn2. Nucleic Acids Res. 2013, 41, 6347–6359. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lee, S.-G.; Eun Lee, K.; Jeon, H.; Robinson, H.; Oh, B.-H. Identification, Structural, and Biochemical Characterization of a Group of Large Csn2 Proteins Involved in CRISPR-Mediated Bacterial Immunity. Proteins 2012, 80, 2573–2582. [Google Scholar] [CrossRef]
- Heler, R.; Samai, P.; Modell, J.W.; Weiner, C.; Goldberg, G.W.; Bikard, D.; Marraffini, L.A. Cas9 Specifies Functional Viral Targets during CRISPR-Cas Adaptation. Nature 2015, 519, 199–202. [Google Scholar] [CrossRef]
- Hooton, S.P.T.; Connerton, I.F. Campylobacter Jejuni Acquire New Host-Derived CRISPR Spacers When in Association with Bacteriophages Harboring a CRISPR-like Cas4 Protein. Front. Microbiol. 2015, 5, 744. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dhingra, Y.; Sashital, D.G. The Cas4-Cas1-Cas2 Complex Mediates Precise Prespacer Processing during CRISPR Adaptation. eLife 2019, 8, e44248. [Google Scholar] [CrossRef] [PubMed]
- Amitai, G.; Sorek, R. CRISPR-Cas Adaptation: Insights into the Mechanism of Action. Nat. Rev. Microbiol. 2016, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lemak, S.; Nocek, B.; Beloglazova, N.; Skarina, T.; Flick, R.; Brown, G.; Joachimiak, A.; Savchenko, A.; Yakunin, A.F. The CRISPR-Associated Cas4 Protein Pcal_0546 from Pyrobaculum Calidifontis Contains a [2Fe-2S] Cluster: Crystal Structure and Nuclease Activity. Nucleic Acids Res. 2014, 42, 11144–11155. [Google Scholar] [CrossRef]
- Zhang, J.; Kasciukovic, T.; White, M.F. The CRISPR Associated Protein Cas4 Is a 5′ to 3′ DNA Exonuclease with an Iron-Sulfur Cluster. PLoS ONE 2012, 7, e47232. [Google Scholar] [CrossRef]
- Silas, S.; Mohr, G.; Sidote, D.J.; Markham, L.M.; Sanchez-Amat, A.; Bhaya, D.; Lambowitz, A.M.; Fire, A.Z. Direct CRISPR Spacer Acquisition from RNA by a Natural Reverse Transcriptase-Cas1 Fusion Protein. Science 2016, 351, aad4234. [Google Scholar] [CrossRef]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An Updated Evolutionary Classification of CRISPR-Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Richter, C.; Dy, R.L.; McKenzie, R.E.; Watson, B.N.J.; Taylor, C.; Chang, J.T.; McNeil, M.B.; Staals, R.H.J.; Fineran, P.C. Priming in the Type I-F CRISPR-Cas System Triggers Strand-Independent Spacer Acquisition, Bi-Directionally from the Primed Protospacer. Nucleic Acids Res. 2014, 42, 8516–8526. [Google Scholar] [CrossRef]
- Fineran, P.C.; Gerritzen, M.J.H.; Suárez-Diez, M.; Künne, T.; Boekhorst, J.; van Hijum, S.A.F.T.; Staals, R.H.J.; Brouns, S.J.J. Degenerate Target Sites Mediate Rapid Primed CRISPR Adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, E1629–E1638. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Pougach, K.; Tikhonov, A.; Wanner, B.L.; Severinov, K.; Semenova, E. Molecular Memory of Prior Infections Activates the CRISPR/Cas Adaptive Bacterial Immunity System. Nat. Commun. 2012, 3, 945. [Google Scholar] [CrossRef]
- Savitskaya, E.; Semenova, E.; Dedkov, V.; Metlitskaya, A.; Severinov, K. High-Throughput Analysis of Type I-E CRISPR/Cas Spacer Acquisition in E. coli. RNA Biol. 2013, 10, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Deecker, S.R.; Ensminger, A.W. Type I-F CRISPR-Cas Distribution and Array Dynamics in Legionella Pneumophila. G3 2020, 10, 1039–1050. [Google Scholar] [CrossRef]
- Bernick, D.L.; Cox, C.L.; Dennis, P.P.; Lowe, T.M. Comparative Genomic and Transcriptional Analyses of CRISPR Systems across the Genus Pyrobaculum. Front. Microbiol. 2012, 3, 251. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR-Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Haurwitz, R.E.; Jinek, M.; Wiedenheft, B.; Zhou, K.; Doudna, J.A. Sequence- and Structure-Specific RNA Processing by a CRISPR Endonuclease. Science 2010, 329, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.R.; Zhao, P.; Olson, S.; Duff, M.O.; Graveley, B.R.; Wells, L.; Terns, R.M.; Terns, M.P. RNA-Guided RNA Cleavage by a CRISPR RNA-Cas Protein Complex. Cell 2009, 139, 945–956. [Google Scholar] [CrossRef]
- Carte, J.; Wang, R.; Li, H.; Terns, R.M.; Terns, M.P. Cas6 Is an Endoribonuclease That Generates Guide RNAs for Invader Defense in Prokaryotes. Genes Dev. 2008, 22, 3489–3496. [Google Scholar] [CrossRef]
- Hatoum-Aslan, A.; Maniv, I.; Marraffini, L.A. Mature Clustered, Regularly Interspaced, Short Palindromic Repeats RNA (crRNA) Length Is Measured by a Ruler Mechanism Anchored at the Precursor Processing Site. Proc. Natl. Acad. Sci. USA 2011, 108, 21218–21222. [Google Scholar] [CrossRef]
- van der Oost, J.; Westra, E.R.; Jackson, R.N.; Wiedenheft, B. Unravelling the Structural and Mechanistic Basis of CRISPR–Cas Systems. Nat. Rev. Microbiol. 2014, 12, 479–492. [Google Scholar] [CrossRef]
- Shao, Y.; Li, H. Recognition and Cleavage of a Non-Structured CRISPR RNA by Its Processing Endoribonuclease Cas6. Structure 2013, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Garside, E.L.; Schellenberg, M.J.; Gesner, E.M.; Bonanno, J.B.; Sauder, J.M.; Burley, S.K.; Almo, S.C.; Mehta, G.; MacMillan, A.M. Cas5d Processes Pre-crRNA and Is a Member of a Larger Family of CRISPR RNA Endonucleases. RNA 2012, 18, 2020–2028. [Google Scholar] [CrossRef]
- Nam, K.H.; Haitjema, C.; Liu, X.; Ding, F.; Wang, H.; DeLisa, M.P.; Ke, A. Cas5d Protein Processes Pre-crRNA and Assembles into a Cascade-like Interference Complex in Subtype I-C/Dvulg CRISPR-Cas System. Structure 2012, 20, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Zoephel, J.; Schermuly, J.; Maticzka, D.; Backofen, R.; Randau, L. Characterization of CRISPR RNA Processing in Clostridium Thermocellum and Methanococcus Maripaludis. Nucleic Acids Res. 2012, 40, 9887–9896. [Google Scholar] [CrossRef]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Barrangou, R. CRISPR-Cas Systems and RNA-Guided Interference. Wiley Interdiscip. Rev. RNA 2013, 4, 267–278. [Google Scholar] [CrossRef]
- Niewoehner, O.; Jinek, M.; Doudna, J.A. Evolution of CRISPR RNA Recognition and Processing by Cas6 Endonucleases. Nucleic Acids Res. 2014, 42, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Redondo, R.; Mayo-Muñoz, D.; Russel, J.; Garrett, R.A.; Randau, L.; Sørensen, S.J.; Shah, S.A. Type IV CRISPR-Cas Systems Are Highly Diverse and Involved in Competition between Plasmids. Nucleic Acids Res. 2020, 48, 2000–2012. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA Maturation by Trans-Encoded Small RNA and Host Factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S.; et al. Structures of Cas9 Endonucleases Reveal RNA-Mediated Conformational Activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Carte, J.; Christopher, R.T.; Smith, J.T.; Olson, S.; Barrangou, R.; Moineau, S.; Glover, C.V.C.; Graveley, B.R.; Terns, R.M.; Terns, M.P. The Three Major Types of CRISPR-Cas Systems Function Independently in CRISPR RNA Biogenesis in Streptococcus Thermophilus. Mol. Microbiol. 2014, 93, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; Bratovič, M.; Le Rhun, A.; Charpentier, E. The CRISPR-Associated DNA-Cleaving Enzyme Cpf1 Also Processes Precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.R. Molecular Mechanisms of RNA Targeting by Cas13-Containing Type VI CRISPR-Cas Systems. J. Mol. Biol. 2019, 431, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR Interference: RNA-Directed Adaptive Immunity in Bacteria and Archaea. Nat. Rev. Genet. 2010, 11, 181–190. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef]
- Leenay, R.T.; Beisel, C.L. Deciphering, Communicating, and Engineering the CRISPR PAM. J. Mol. Biol. 2017, 429, 177–191. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. Self versus Non-Self Discrimination during CRISPR RNA-Directed Immunity. Nature 2010, 463, 568–571. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 Is a Single-Component Programmable RNA-Guided RNA-Targeting CRISPR Effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Jore, M.M.; Lundgren, M.; van Duijn, E.; Bultema, J.B.; Westra, E.R.; Waghmare, S.P.; Wiedenheft, B.; Pul, U.; Wurm, R.; Wagner, R.; et al. Structural Basis for CRISPR RNA-Guided DNA Recognition by Cascade. Nat. Struct. Mol. Biol. 2011, 18, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; van der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Sashital, D.G.; Wiedenheft, B.; Doudna, J.A. Mechanism of Foreign DNA Selection in a Bacterial Adaptive Immune System. Mol. Cell 2012, 46, 606–615. [Google Scholar] [CrossRef]
- Swarts, D.C.; Mosterd, C.; van Passel, M.W.J.; Brouns, S.J.J. CRISPR Interference Directs Strand Specific Spacer Acquisition. PLoS ONE 2012, 7, e35888. [Google Scholar] [CrossRef]
- Hayes, R.P.; Xiao, Y.; Ding, F.; van Erp, P.B.G.; Rajashankar, K.; Bailey, S.; Wiedenheft, B.; Ke, A. Structural Basis for Promiscuous PAM Recognition in Type I-E Cascade from E. coli. Nature 2016, 530, 499–503. [Google Scholar] [CrossRef]
- Semenova, E.; Jore, M.M.; Datsenko, K.A.; Semenova, A.; Westra, E.R.; Wanner, B.; van der Oost, J.; Brouns, S.J.J.; Severinov, K. Interference by Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) RNA Is Governed by a Seed Sequence. Proc. Natl. Acad. Sci. USA 2011, 108, 10098–10103. [Google Scholar] [CrossRef] [PubMed]
- Wiedenheft, B.; van Duijn, E.; Bultema, J.B.; Waghmare, S.P.; Zhou, K.; Barendregt, A.; Westphal, W.; Heck, A.J.R.; Boekema, E.J.; Dickman, M.J.; et al. RNA-Guided Complex from a Bacterial Immune System Enhances Target Recognition through Seed Sequence Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 10092–10097. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Lander, G.C.; Zhou, K.; Jore, M.M.; Brouns, S.J.J.; van der Oost, J.; Doudna, J.A.; Nogales, E. Structures of the RNA-Guided Surveillance Complex from a Bacterial Immune System. Nature 2011, 477, 486–489. [Google Scholar] [CrossRef]
- Westra, E.R.; Nilges, B.; van Erp, P.B.G.; van der Oost, J.; Dame, R.T.; Brouns, S.J.J. Cascade-Mediated Binding and Bending of Negatively Supercoiled DNA. RNA Biol. 2012, 9, 1134–1138. [Google Scholar] [CrossRef]
- Hochstrasser, M.L.; Taylor, D.W.; Bhat, P.; Guegler, C.K.; Sternberg, S.H.; Nogales, E.; Doudna, J.A. CasA Mediates Cas3-Catalyzed Target Degradation during CRISPR RNA-Guided Interference. Proc. Natl. Acad. Sci. USA 2014, 111, 6618–6623. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, M.; Dolan, A.E.; Liao, M.; Ke, A. Structure Basis for RNA-Guided DNA Degradation by Cascade and Cas3. Science 2018, 361, eaat0839. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Luo, M.; Hayes, R.P.; Kim, J.; Ng, S.; Ding, F.; Liao, M.; Ke, A. Structure Basis for Directional R-Loop Formation and Substrate Handover Mechanisms in Type I CRISPR-Cas System. Cell 2017, 170, 48–60.e11. [Google Scholar] [CrossRef] [PubMed]
- Sinkunas, T.; Gasiunas, G.; Waghmare, S.P.; Dickman, M.J.; Barrangou, R.; Horvath, P.; Siksnys, V. In Vitro Reconstitution of Cascade-Mediated CRISPR Immunity in Streptococcus Thermophilus. EMBO J. 2013, 32, 385–394. [Google Scholar] [CrossRef]
- Mulepati, S.; Bailey, S. In Vitro Reconstitution of an Escherichia coli RNA-Guided Immune System Reveals Unidirectional, ATP-Dependent Degradation of DNA Target. J. Biol. Chem. 2013, 288, 22184–22192. [Google Scholar] [CrossRef]
- Pausch, P.; Müller-Esparza, H.; Gleditzsch, D.; Altegoer, F.; Randau, L.; Bange, G. Structural Variation of Type I-F CRISPR RNA Guided DNA Surveillance. Mol. Cell 2017, 67, 622–632.e4. [Google Scholar] [CrossRef]
- Plagens, A.; Richter, H.; Charpentier, E.; Randau, L. DNA and RNA Interference Mechanisms by CRISPR-Cas Surveillance Complexes. FEMS Microbiol. Rev. 2015, 39, 442–463. [Google Scholar] [CrossRef]
- Magadán, A.H.; Dupuis, M.-È.; Villion, M.; Moineau, S. Cleavage of Phage DNA by the Streptococcus Thermophilus CRISPR3-Cas System. PLoS ONE 2012, 7, e40913. [Google Scholar] [CrossRef] [PubMed]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA Ribonucleoprotein Complex Mediates Specific DNA Cleavage for Adaptive Immunity in Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. The Structural Biology of CRISPR-Cas Systems. Curr. Opin. Struct. Biol. 2015, 30, 100–111. [Google Scholar] [CrossRef]
- Anders, C.; Niewoehner, O.; Duerst, A.; Jinek, M. Structural Basis of PAM-Dependent Target DNA Recognition by the Cas9 Endonuclease. Nature 2014, 513, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.H.; LaFrance, B.; Kaplan, M.; Doudna, J.A. Conformational Control of DNA Target Cleavage by CRISPR-Cas9. Nature 2015, 527, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Taylor, D.W.; Chen, J.S.; Kornfeld, J.E.; Zhou, K.; Thompson, A.J.; Nogales, E.; Doudna, J.A. Structures of a CRISPR-Cas9 R-Loop Complex Primed for DNA Cleavage. Science 2016, 351, 867–871. [Google Scholar] [CrossRef]
- Elmore, J.R.; Sheppard, N.F.; Ramia, N.; Deighan, T.; Li, H.; Terns, R.M.; Terns, M.P. Bipartite Recognition of Target RNAs Activates DNA Cleavage by the Type III-B CRISPR–Cas System. Genes Dev. 2016, 30, 447–459. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef]
- Goldberg, G.W.; Jiang, W.; Bikard, D.; Marraffini, L.A. Conditional Tolerance of Temperate Phages via Transcription-Dependent CRISPR-Cas Targeting. Nature 2014, 514, 633–637. [Google Scholar] [CrossRef]
- Deng, L.; Garrett, R.A.; Shah, S.A.; Peng, X.; She, Q. A Novel Interference Mechanism by a Type IIIB CRISPR-Cmr Module in Sulfolobus. Mol. Microbiol. 2013, 87, 1088–1099. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Koonin, E.V. Unification of Cas Protein Families and a Simple Scenario for the Origin and Evolution of CRISPR-Cas Systems. Biol. Direct 2011, 6, 38. [Google Scholar] [CrossRef]
- Zhang, J.; Rouillon, C.; Kerou, M.; Reeks, J.; Brugger, K.; Graham, S.; Reimann, J.; Cannone, G.; Liu, H.; Albers, S.-V.; et al. Structure and Mechanism of the CMR Complex for CRISPR-Mediated Antiviral Immunity. Mol. Cell 2012, 45, 303–313. [Google Scholar] [CrossRef]
- Richter, H.; Randau, L.; Plagens, A. Exploiting CRISPR/Cas: Interference Mechanisms and Applications. Int. J. Mol. Sci. 2013, 14, 14518–14531. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Hatoum-Aslan, A.; Maniv, I.; Samai, P.; Marraffini, L.A. Genetic Characterization of Antiplasmid Immunity through a Type III-A CRISPR-Cas System. J. Bacteriol. 2014, 196, 310–317. [Google Scholar] [CrossRef]
- Staals, R.H.J.; Zhu, Y.; Taylor, D.W.; Kornfeld, J.E.; Sharma, K.; Barendregt, A.; Koehorst, J.J.; Vlot, M.; Neupane, N.; Varossieau, K.; et al. RNA Targeting by the Type III-A CRISPR-Cas Csm Complex of Thermus Thermophilus. Mol. Cell 2014, 56, 518–530. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Ma, J.; Wang, J.; Artamonova, D.; Wang, M.; Liu, L.; Xiang, H.; Severinov, K.; Zhang, X.; Wang, Y. Structure Studies of the CRISPR-Csm Complex Reveal Mechanism of Co-Transcriptional Interference. Cell 2019, 176, 239–253.e16. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Mo, C.Y.; Wang, C.; Eng, E.T.; Marraffini, L.A.; Patel, D.J. Type III-A CRISPR-Cas Csm Complexes: Assembly, Periodic RNA Cleavage, DNase Activity Regulation, and Autoimmunity. Mol. Cell 2019, 73, 264–277.e5. [Google Scholar] [CrossRef]
- Kazlauskiene, M.; Kostiuk, G.; Venclovas, Č.; Tamulaitis, G.; Siksnys, V. A Cyclic Oligonucleotide Signaling Pathway in Type III CRISPR-Cas Systems. Science 2017, 357, 605–609. [Google Scholar] [CrossRef]
- Niewoehner, O.; Garcia-Doval, C.; Rostøl, J.T.; Berk, C.; Schwede, F.; Bigler, L.; Hall, J.; Marraffini, L.A.; Jinek, M. Type III CRISPR-Cas Systems Produce Cyclic Oligoadenylate Second Messengers. Nature 2017, 548, 543–548. [Google Scholar] [CrossRef]
- Rouillon, C.; Athukoralage, J.S.; Graham, S.; Grüschow, S.; White, M.F. Control of Cyclic Oligoadenylate Synthesis in a Type III CRISPR System. eLife 2018, 7, e36734. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Shmakov, S.A.; Yan, W.X.; Cheng, D.R.; Scott, D.A.; Peters, J.E.; Makarova, K.S.; Koonin, E.V. CRISPR-Cas in Mobile Genetic Elements: Counter-Defence and Beyond. Nat. Rev. Microbiol. 2019, 17, 513–525. [Google Scholar] [CrossRef]
- Özcan, A.; Pausch, P.; Linden, A.; Wulf, A.; Schühle, K.; Heider, J.; Urlaub, H.; Heimerl, T.; Bange, G.; Randau, L. Type IV CRISPR RNA Processing and Effector Complex Formation in Aromatoleum Aromaticum. Nat. Microbiol. 2019, 4, 89–96. [Google Scholar] [CrossRef]
- Sinkunas, T.; Gasiunas, G.; Fremaux, C.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas3 Is a Single-Stranded DNA Nuclease and ATP-Dependent Helicase in the CRISPR/Cas Immune System. EMBO J. 2011, 30, 1335–1342. [Google Scholar] [CrossRef]
- Strecker, J.; Jones, S.; Koopal, B.; Schmid-Burgk, J.; Zetsche, B.; Gao, L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. Engineering of CRISPR-Cas12b for Human Genome Editing. Nat. Commun. 2019, 10, 212. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jinek, M. Mechanistic Insights into the Cis- and Trans-Acting Deoxyribonuclease Activities of Cas12a. Mol. Cell 2019, 73, 589–600.e4. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA Destruction by Miniature CRISPR-Cas14 Enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef] [PubMed]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.D.; Tjian, R.; Doudna, J.A. Two Distinct RNase Activities of CRISPR-C2c2 Enable Guide-RNA Processing and RNA Detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A Review of the Challenges and Approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef]
- Demirci, S.; Essawi, K.; Germino-Watnick, P.; Liu, X.; Hakami, W.; Tisdale, J.F. Advances in CRISPR Delivery Methods: Perspectives and Challenges. CRISPR J. 2022, 5, 660–676. [Google Scholar] [CrossRef]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 Systems: Delivery Technologies and Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, H.; Cheng, K. Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic Applications. J. Control. Release 2017, 266, 17–26. [Google Scholar] [CrossRef]
- Szabała, B.M.; Święcicka, M.; Łyżnik, L.A. Microinjection of the CRISPR/Cas9 Editing System through the Germ Pore of a Wheat Microspore Induces Mutations in the Target Ms2 Gene. Mol. Biol. Rep. 2024, 51, 706. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Meng, Q.; Qi, J.; Hu, L.; Huang, J.; Zhang, Y.; Yang, J.; Sun, J. Microinjection-Based CRISPR/Cas9 Mutagenesis in the Decapoda Crustaceans Neocaridina Heteropoda and Eriocheir Sinensis. J. Exp. Biol. 2022, 225, jeb243702. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.; Feng, G.; Liu, M.; Nie, C.; Chen, C.; Wang, J.; Wang, L.; Wan, P.; Liu, C.; Liu, Y.; et al. Electroporation Delivery of Cas9 sgRNA Ribonucleoprotein-Mediated Genome Editing in Sheep IVF Zygotes. Int. J. Mol. Sci. 2024, 25, 9145. [Google Scholar] [CrossRef]
- Xu, H.; Kita, Y.; Bang, U.; Gee, P.; Hotta, A. Optimized Electroporation of CRISPR-Cas9/gRNA Ribonucleoprotein Complex for Selection-Free Homologous Recombination in Human Pluripotent Stem Cells. STAR Protoc. 2021, 2, 100965. [Google Scholar] [CrossRef]
- Niola, F.; Dagnæs-Hansen, F.; Frödin, M. In Vivo Editing of the Adult Mouse Liver Using CRISPR/Cas9 and Hydrodynamic Tail Vein Injection. Methods Mol. Biol. 2019, 1961, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Taghdiri, M.; Mussolino, C. Viral and Non-Viral Systems to Deliver Gene Therapeutics to Clinical Targets. Int. J. Mol. Sci. 2024, 25, 7333. [Google Scholar] [CrossRef]
- Statkute, E.; Wang, E.C.Y.; Stanton, R.J. An Optimized CRISPR/Cas9 Adenovirus Vector (AdZ-CRISPR) for High-Throughput Cloning of sgRNA, Using Enhanced sgRNA and Cas9 Variants. Hum. Gene Ther. 2022, 33, 990–1001. [Google Scholar] [CrossRef]
- Asmamaw Mengstie, M. Viral Vectors for the in Vivo Delivery of CRISPR Components: Advances and Challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Xu, C.L.; Ruan, M.Z.C.; Mahajan, V.B.; Tsang, S.H. Viral Delivery Systems for CRISPR. Viruses 2019, 11, 28. [Google Scholar] [CrossRef]
- Davis, D.J.; McNew, J.F.; Walls, J.N.; Bethune, C.E.; Oswalt, P.S.; Bryda, E.C. CRISPR-Cas9 Genome Editing of Rat Embryos Using Adeno-Associated Virus (AAV) and 2-Cell Embryo Electroporation. J. Vis. Exp. 2024, 205, e66069. [Google Scholar] [CrossRef]
- Zhu, K.; Zhao, R.; Ye, Y.; Xu, G.; Zhang, C. Effect of Lentivirus-Mediated Growth and Differentiation Factor-5 Transfection on Differentiation of Rabbit Nucleus Pulposus Mesenchymal Stem Cells. Eur. J. Med. Res. 2022, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Kantor, B. Lentiviral Vectors for Delivery of Gene-Editing Systems Based on CRISPR/Cas: Current State and Perspectives. Viruses 2021, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene Editing and CRISPR in the Clinic: Current and Future Perspectives. Biosci. Rep. 2020, 40, BSR20200127. [Google Scholar] [CrossRef]
- Raper, A.T.; Stephenson, A.A.; Suo, Z. Sharpening the Scissors: Mechanistic Details of CRISPR/Cas9 Improve Functional Understanding and Inspire Future Research. J. Am. Chem. Soc. 2018, 140, 11142–11152. [Google Scholar] [CrossRef] [PubMed]
- Lennox, K.A.; Behlke, M.A. Chemical Modifications in RNA Interference and CRISPR/Cas Genome Editing Reagents. In RNA Interference and CRISPR Technologies; Sioud, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2115, pp. 23–55. ISBN 978-1-0716-0289-8. [Google Scholar]
- Chen, Q.; Zhang, Y.; Yin, H. Recent Advances in Chemical Modifications of Guide RNA, mRNA and Donor Template for CRISPR-Mediated Genome Editing. Adv. Drug Deliv. Rev. 2021, 168, 246–258. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA Interrogation by the CRISPR RNA-Guided Endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Basila, M.; Kelley, M.L.; Smith, A.v.B. Minimal 2′-O-Methyl Phosphorothioate Linkage Modification Pattern of Synthetic Guide RNAs for Increased Stability and Efficient CRISPR-Cas9 Gene Editing Avoiding Cellular Toxicity. PLoS ONE 2017, 12, e0188593. [Google Scholar] [CrossRef]
- Kartje, Z.J.; Barkau, C.L.; Rohilla, K.J.; Ageely, E.A.; Gagnon, K.T. Chimeric Guides Probe and Enhance Cas9 Biochemical Activity. Biochemistry 2018, 57, 3027–3031. [Google Scholar] [CrossRef]
- Yin, H.; Song, C.-Q.; Suresh, S.; Kwan, S.-Y.; Wu, Q.; Walsh, S.; Ding, J.; Bogorad, R.L.; Zhu, L.J.; Wolfe, S.A.; et al. Partial DNA-Guided Cas9 Enables Genome Editing with Reduced off-Target Activity. Nat. Chem. Biol. 2018, 14, 311–316. [Google Scholar] [CrossRef]
- Wang, L.; Han, H. Strategies for Improving the Genome-Editing Efficiency of Class 2 CRISPR/Cas System. Heliyon 2024, 10, e38588. [Google Scholar] [CrossRef] [PubMed]
- Rozners, E. Chemical Modifications of CRISPR RNAs to Improve Gene-Editing Activity and Specificity. J. Am. Chem. Soc. 2022, 144, 12584–12594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shen, W.; Zhao, Y.; Xu, X.; Liu, X.; Qi, Q.; Huang, S.; Tian, T.; Zhou, X. Strategic Base Modifications Refine RNA Function and Reduce CRISPR-Cas9 off-Targets. Nucleic Acids Res. 2025, 53, gkaf082. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, M.; McMahon, M.A.; Prakash, T.P.; Swayze, E.E.; Bennett, C.F.; Cleveland, D.W. Synthetic CRISPR RNA-Cas9-Guided Genome Editing in Human Cells. Proc. Natl. Acad. Sci. USA 2015, 112, E7110–E7117. [Google Scholar] [CrossRef]
- O’Reilly, D.; Kartje, Z.J.; Ageely, E.A.; Malek-Adamian, E.; Habibian, M.; Schofield, A.; Barkau, C.L.; Rohilla, K.J.; DeRossett, L.B.; Weigle, A.T.; et al. Extensive CRISPR RNA Modification Reveals Chemical Compatibility and Structure-Activity Relationships for Cas9 Biochemical Activity. Nucleic Acids Res. 2019, 47, 546–558. [Google Scholar] [CrossRef]
- Lieberman, J. Tapping the RNA World for Therapeutics. Nat. Struct. Mol. Biol. 2018, 25, 357–364. [Google Scholar] [CrossRef]
- Threlfall, R.N.; Torres, A.G.; Krivenko, A.; Gait, M.J.; Caruthers, M.H. Synthesis and Biological Activity of Phosphonoacetate- and Thiophosphonoacetate-Modified 2′-O-Methyl Oligoribonucleotides. Org. Biomol. Chem. 2012, 10, 746–754. [Google Scholar] [CrossRef]
- Hussen, B.M.; Rasul, M.F.; Abdullah, S.R.; Hidayat, H.J.; Faraj, G.S.H.; Ali, F.A.; Salihi, A.; Baniahmad, A.; Ghafouri-Fard, S.; Rahman, M.; et al. Targeting miRNA by CRISPR/Cas in cancer: Advantages and challenges. Mil. Med. Res. 2023, 10, 32. [Google Scholar] [CrossRef]
- Jiang, T.; Henderson, J.M.; Coote, K.; Cheng, Y.; Valley, H.C.; Zhang, X.-O.; Wang, Q.; Rhym, L.H.; Cao, Y.; Newby, G.A.; et al. Chemical Modifications of Adenine Base Editor mRNA and Guide RNA Expand Its Application Scope. Nat. Commun. 2020, 11, 1979. [Google Scholar] [CrossRef]
- Khan, F.J.; Yuen, G.; Luo, J. Multiplexed CRISPR/Cas9 Gene Knockout with Simple crRNA:tracrRNA Co-Transfection. Cell Biosci. 2019, 9, 41. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base Editing: Precision Chemistry on the Genome and Transcriptome of Living Cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable Base Editing of A•T to G•C in Genomic DNA without DNA Cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Solá-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.-J.; Liquori, A.J.; et al. Directed Evolution of Adenine Base Editors with Increased Activity and Therapeutic Application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef]
- Yu, Y.; Leete, T.C.; Born, D.A.; Young, L.; Barrera, L.A.; Lee, S.-J.; Rees, H.A.; Ciaramella, G.; Gaudelli, N.M. Cytosine Base Editors with Minimized Unguided DNA and RNA Off-Target Events and High on-Target Activity. Nat. Commun. 2020, 11, 2052. [Google Scholar] [CrossRef]
- Liang, M.; Sui, T.; Liu, Z.; Chen, M.; Liu, H.; Shan, H.; Lai, L.; Li, Z. AcrIIA5 Suppresses Base Editors and Reduces Their Off-Target Effects. Cells 2020, 9, 1786. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Marzec, M.; Brąszewska-Zalewska, A.; Hensel, G. Prime Editing: A New Way for Genome Editing. Trends Cell Biol. 2020, 30, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Kim, H.K.; Yu, G.; Park, J.; Min, S.; Lee, S.; Yoon, S.; Kim, H.H. Predicting the Efficiency of Prime Editing Guide RNAs in Human Cells. Nat. Biotechnol. 2021, 39, 198–206. [Google Scholar] [CrossRef]
- Chemello, F.; Chai, A.C.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Atmanli, A.; Mireault, A.A.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Precise Correction of Duchenne Muscular Dystrophy Exon Deletion Mutations by Base and Prime Editing. Sci. Adv. 2021, 7, eabg4910. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef]
- Zhang, J.; Späth, S.S.; Katz, S.G. Genome-Wide CRISPRi/a Screening in an In Vitro Coculture Assay of Human Immune Cells with Tumor Cells. Methods Mol. Biol. 2020, 2097, 231–252. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e17. [Google Scholar] [CrossRef]
- Urbano, A.; Smith, J.; Weeks, R.J.; Chatterjee, A. Gene-Specific Targeting of DNA Methylation in the Mammalian Genome. Cancers 2019, 11, 1515. [Google Scholar] [CrossRef]
- Gonatopoulos-Pournatzis, T.; Aregger, M.; Brown, K.R.; Farhangmehr, S.; Braunschweig, U.; Ward, H.N.; Ha, K.C.H.; Weiss, A.; Billmann, M.; Durbic, T.; et al. Genetic Interaction Mapping and Exon-Resolution Functional Genomics with a Hybrid Cas9-Cas12a Platform. Nat. Biotechnol. 2020, 38, 638–648. [Google Scholar] [CrossRef]
- Pacalin, N.M.; Steinhart, Z.; Shi, Q.; Belk, J.A.; Dorovskyi, D.; Kraft, K.; Parker, K.R.; Shy, B.R.; Marson, A.; Chang, H.Y. Bidirectional Epigenetic Editing Reveals Hierarchies in Gene Regulation. Nat. Biotechnol. 2024, 1, 14. [Google Scholar] [CrossRef]
- Bock, C.; Datlinger, P.; Chardon, F.; Coelho, M.A.; Dong, M.B.; Lawson, K.A.; Lu, T.; Maroc, L.; Norman, T.M.; Song, B.; et al. High-Content CRISPR Screening. Nat. Rev. Methods Primers 2022, 2, 9. [Google Scholar] [CrossRef]
- Le Sage, C.; Lawo, S.; Cross, B.C.S. CRISPR: A Screener’s Guide. SLAS Discov. 2020, 25, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Legut, M.; Daniloski, Z.; Xue, X.; McKenzie, D.; Guo, X.; Wessels, H.-H.; Sanjana, N.E. High-Throughput Screens of PAM-Flexible Cas9 Variants for Gene Knockout and Transcriptional Modulation. Cell Rep. 2020, 30, 2859–2868.e5. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, A.; Amrani, N.; Zhang, Y.; Garcia, B.; Hidalgo-Reyes, Y.; Lee, J.; Edraki, A.; Shah, M.; Sontheimer, E.J.; Maxwell, K.L.; et al. Naturally Occurring Off-Switches for CRISPR-Cas9. Cell 2016, 167, 1829–1838.e9. [Google Scholar] [CrossRef]
- Borges, A.L.; Davidson, A.R.; Bondy-Denomy, J. The Discovery, Mechanisms, and Evolutionary Impact of Anti-CRISPRs. Annu. Rev. Virol. 2017, 4, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, A.; Zhan, Q.; Wang, Y.; Feng, H.; Liu, S.; Gao, G.; Serganov, A.; Gao, P. Diverse Mechanisms of CRISPR-Cas9 Inhibition by Type IIC Anti-CRISPR Proteins. Mol. Cell 2019, 74, 296–309.e7. [Google Scholar] [CrossRef]
- Bondy-Denomy, J.; Garcia, B.; Strum, S.; Du, M.; Rollins, M.F.; Hidalgo-Reyes, Y.; Wiedenheft, B.; Maxwell, K.L.; Davidson, A.R. Multiple Mechanisms for CRISPR-Cas Inhibition by Anti-CRISPR Proteins. Nature 2015, 526, 136–139. [Google Scholar] [CrossRef]
- Dong, D.; Guo, M.; Wang, S.; Zhu, Y.; Wang, S.; Xiong, Z.; Yang, J.; Xu, Z.; Huang, Z. Structural Basis of CRISPR–SpyCas9 Inhibition by an Anti-CRISPR Protein. Nature 2017, 546, 436–439. [Google Scholar] [CrossRef]
- Choudhary, N.; Tandi, D.; Verma, R.K.; Yadav, V.K.; Dhingra, N.; Ghosh, T.; Choudhary, M.; Gaur, R.K.; Abdellatif, M.H.; Gacem, A.; et al. A Comprehensive Appraisal of Mechanism of Anti-CRISPR Proteins: An Advanced Genome Editor to Amend the CRISPR Gene Editing. Front. Plant Sci. 2023, 14, 1164461. [Google Scholar] [CrossRef]
- Meacham, Z.; de Tacca, L.A.; Bondy-Denomy, J.; Rabuka, D.; Schelle, M. Cas9 Degradation in Human Cells Using Phage Anti-CRISPR Proteins. PLoS Biol. 2023, 21, e3002431. [Google Scholar] [CrossRef]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-Encoded CRISPR-Cas Systems Direct RNA-Guided DNA Integration. Nature 2019, 571, 219–225. [Google Scholar] [CrossRef]
- Vo, P.L.H.; Ronda, C.; Klompe, S.E.; Chen, E.E.; Acree, C.; Wang, H.H.; Sternberg, S.H. CRISPR RNA-Guided Integrases for High-Efficiency, Multiplexed Bacterial Genome Engineering. Nat. Biotechnol. 2021, 39, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA Targeting Specificity of RNA-Guided Cas9 Nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 Variants with Broad PAM Compatibility and High DNA Specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 Nuclease with Expanded Targeting Space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Huang, T.P.; Zhao, K.T.; Miller, S.M.; Gaudelli, N.M.; Oakes, B.L.; Fellmann, C.; Savage, D.F.; Liu, D.R. Circularly Permuted and PAM-Modified Cas9 Variants Broaden the Targeting Scope of Base Editors. Nat. Biotechnol. 2019, 37, 626–631. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.; Kim, Y.; Park, J.; Min, S.; Choi, J.W.; Huang, T.P.; Yoon, S.; Liu, D.R.; Kim, H.H. High-Throughput Analysis of the Activities of xCas9, SpCas9-NG and SpCas9 at Matched and Mismatched Target Sequences in Human Cells. Nat. Biomed. Eng. 2020, 4, 111–124. [Google Scholar] [CrossRef]
- Villiger, L.; Joung, J.; Koblan, L.; Weissman, J.; Abudayyeh, O.O.; Gootenberg, J.S. CRISPR technologies for genome, epigenome and transcriptome editing. Nat. Rev. Mol. Cell Biol. 2024, 25, 464–487. [Google Scholar] [CrossRef]
- Xu, X.; Chemparathy, A.; Zeng, L.; Kempton, H.R.; Shang, S.; Nakamura, M.; Qi, L.S. Engineered Miniature CRISPR-Cas System for Mammalian Genome Regulation and Editing. Mol. Cell 2021, 81, 4333–4345.e4. [Google Scholar] [CrossRef] [PubMed]
- Carabias, A.; Fuglsang, A.; Temperini, P.; Pape, T.; Sofos, N.; Stella, S.; Erlendsson, S.; Montoya, G. Structure of the Mini-RNA-Guided Endonuclease CRISPR-Cas12j3. Nat. Commun. 2021, 12, 4476. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR gene therapy: Applications, limitations, and implications for the future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Xuan, Q.; Wang, J.; Nie, Y.; Fang, C.; Liang, W. Research Progress and Application of Miniature CRISPR-Cas12 System in Gene Editing. Int. J. Mol. Sci. 2024, 25, 12686. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, C.; Díaz, M.P.; Duran, P.; Castro, A.; Díaz, A.; Cano, C.; Carbonell-Zabaleta, A.-K.; Solano-Jimenez, D.-S.; Rivera-Porras, D.; Contreras-Velásquez, J.C.; et al. CRISPR-Cas Systems: A Functional Perspective and Innovations. Int. J. Mol. Sci. 2025, 26, 3645. https://doi.org/10.3390/ijms26083645
Navarro C, Díaz MP, Duran P, Castro A, Díaz A, Cano C, Carbonell-Zabaleta A-K, Solano-Jimenez D-S, Rivera-Porras D, Contreras-Velásquez JC, et al. CRISPR-Cas Systems: A Functional Perspective and Innovations. International Journal of Molecular Sciences. 2025; 26(8):3645. https://doi.org/10.3390/ijms26083645
Chicago/Turabian StyleNavarro, Carla, María P. Díaz, Pablo Duran, Ana Castro, Andrea Díaz, Clímaco Cano, Ana-Karina Carbonell-Zabaleta, Donny-Sabrith Solano-Jimenez, Diego Rivera-Porras, Julio César Contreras-Velásquez, and et al. 2025. "CRISPR-Cas Systems: A Functional Perspective and Innovations" International Journal of Molecular Sciences 26, no. 8: 3645. https://doi.org/10.3390/ijms26083645
APA StyleNavarro, C., Díaz, M. P., Duran, P., Castro, A., Díaz, A., Cano, C., Carbonell-Zabaleta, A.-K., Solano-Jimenez, D.-S., Rivera-Porras, D., Contreras-Velásquez, J. C., & Bermúdez, V. (2025). CRISPR-Cas Systems: A Functional Perspective and Innovations. International Journal of Molecular Sciences, 26(8), 3645. https://doi.org/10.3390/ijms26083645





