Efforts to Downsize Base Editors for Clinical Applications
Abstract
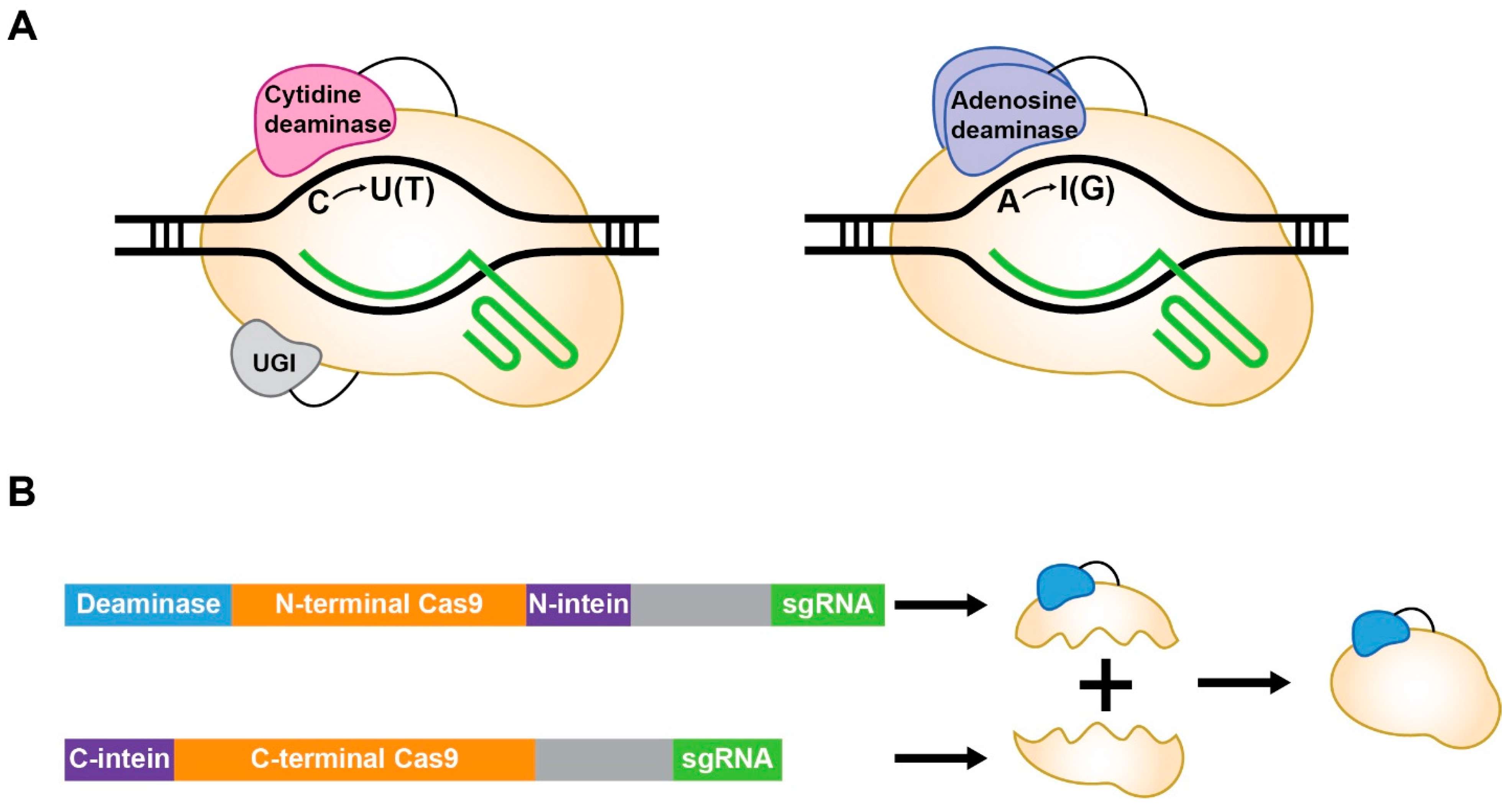
1. Introduction
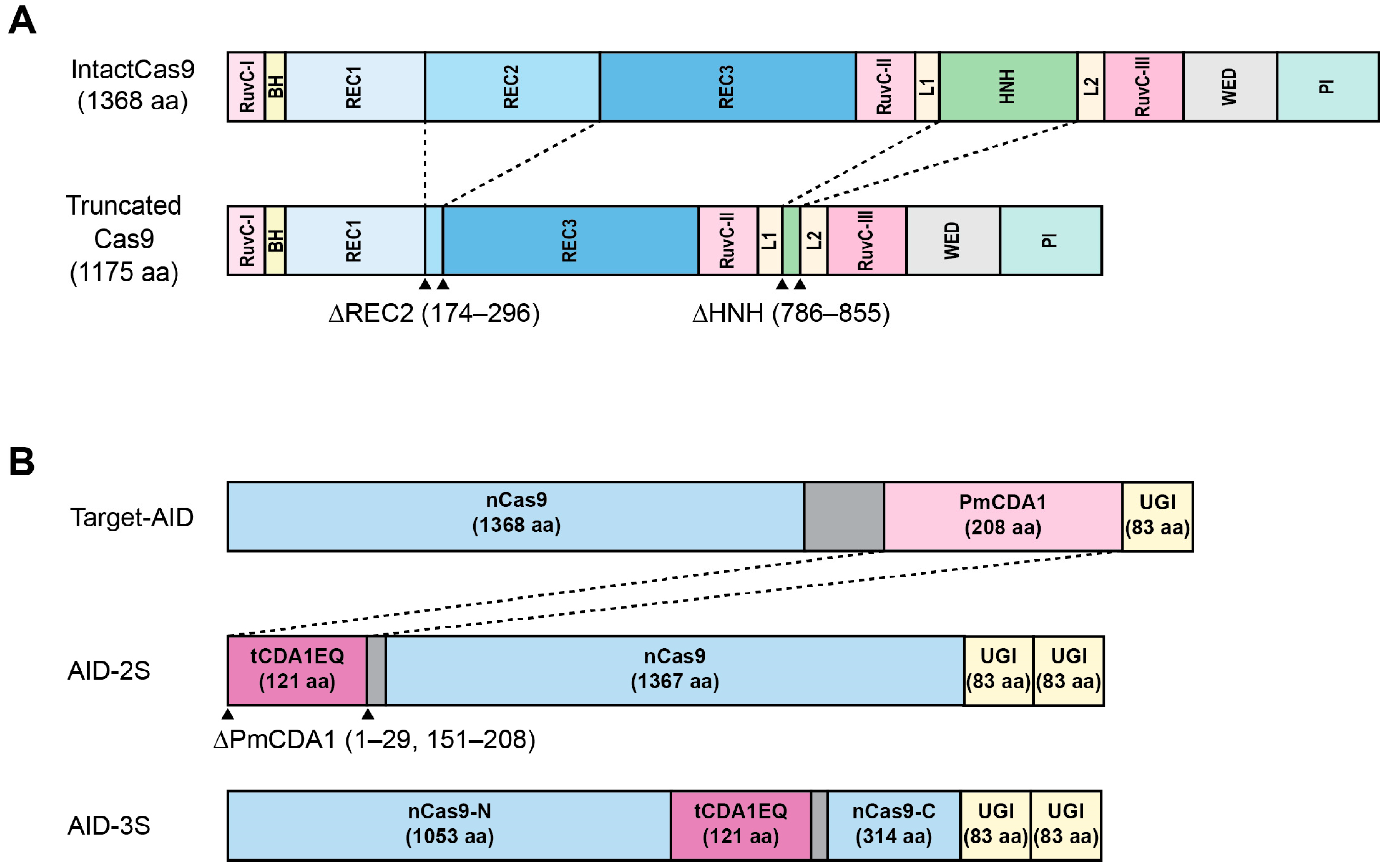
2. Truncation of the BE Components
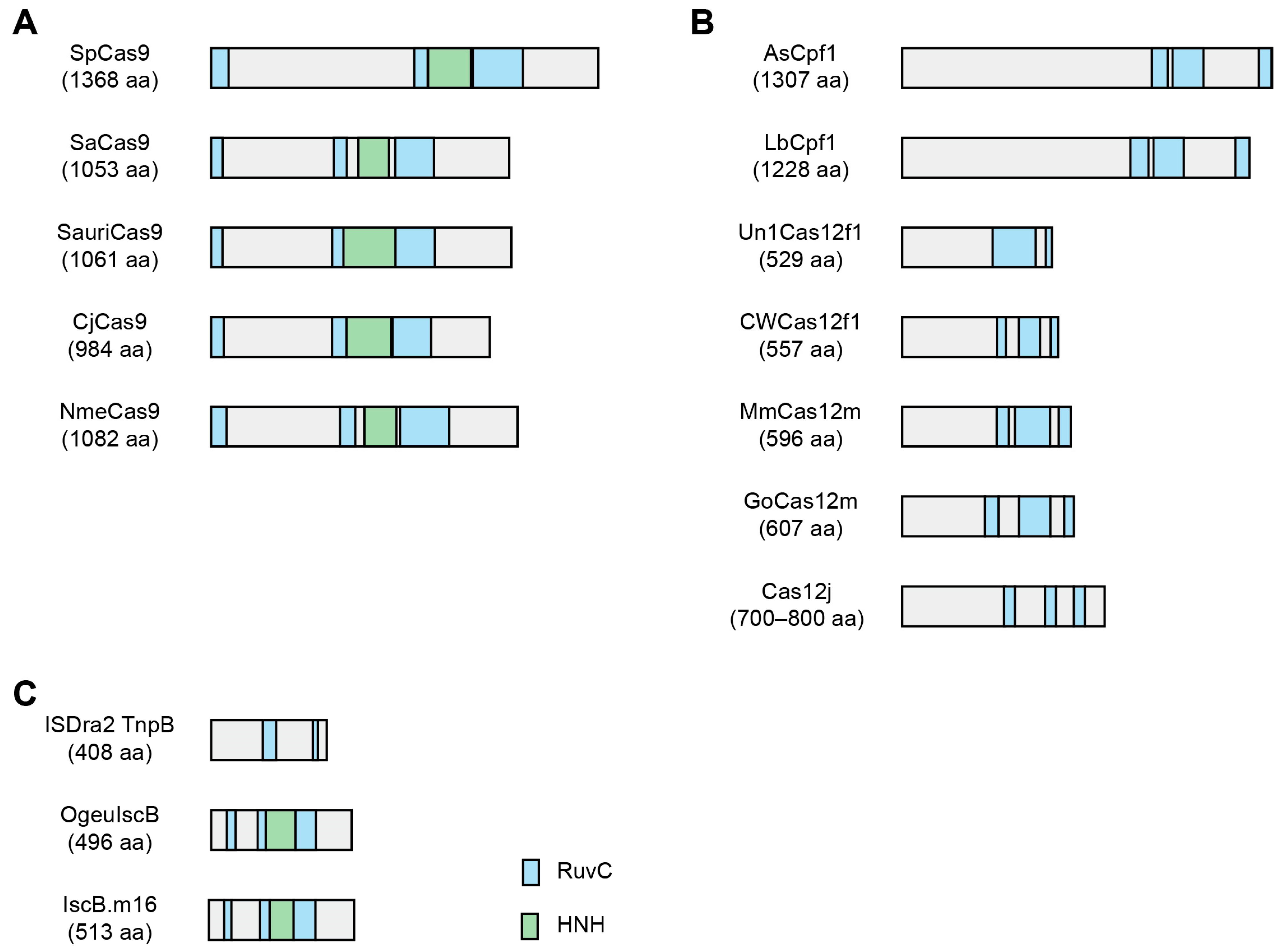
3. Adoption of Small Cas9 Orthologs
4. Employment of Cas12f (Also Known as Cas14) Orthologs
5. Harnessing the Ancestors of Cas Proteins
6. Employment of Small Deaminases
7. Adoption of the Type I-F CRISPR-Associated Complex for Antiviral Defense (Cascade)
8. Efforts to Overcome Other Limitations of BEs
8.1. Off-Target Effects
8.2. Bystander Mutations
8.3. Limited Editing Scopes
9. Conclusions
Funding
Conflicts of Interest
References
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.D.; Richardson, C.D.; Corn, J.E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 2019, 21, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, E.; Botla, S.; Persson, J.; Schmierer, B.; Taipale, J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018, 24, 927–930. [Google Scholar] [CrossRef]
- Ihry, R.J.; Worringer, K.A.; Salick, M.R.; Frias, E.; Ho, D.; Theriault, K.; Kommineni, S.; Chen, J.; Sondey, M.; Ye, C.; et al. p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018, 24, 939–946. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, H.; Chen, S.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Efficient base editing with expanded targeting scope using an engineered Spy-mac Cas9 variant. Cell Discov. 2019, 5, 58. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]
- Gehrke, J.M.; Cervantes, O.; Clement, M.K.; Wu, Y.; Zeng, J.; Bauer, D.E.; Pinello, L.; Joung, J.K. An APOBEC3A-Cas9 base editor with minimized bystander and off-target activities. Nat. Biotechnol. 2018, 36, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Epstein, B.E.; Schaffer, D.V. Genome Engineering Using Adeno-associated Virus: Basic and Clinical Research Applications. Mol. Ther. 2016, 24, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.E.; Schaffer, D.V. Combining Engineered Nucleases with Adeno-associated Viral Vectors for Therapeutic Gene Editing. Adv. Exp. Med. Biol. 2017, 1016, 29–42. [Google Scholar]
- Kuzmin, D.A.; Shutova, M.V.; Johnston, N.R.; Smith, O.P.; Fedorin, V.V.; Kukushkin, Y.S.; van der Loo, J.C.M.; Johnstone, E.C. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Discov. 2021, 20, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Pena, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef]
- Dong, J.Y.; Fan, P.D.; Frizzell, R.A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996, 7, 2101–2112. [Google Scholar] [CrossRef]
- Levy, J.M.; Yeh, W.H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.T.; Kim, H.S.; Kim, D.E.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Mohr, F.; McMillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 2021, 74, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Shams, A.; Higgins, S.A.; Fellmann, C.; Laughlin, T.G.; Oakes, B.L.; Lew, R.; Kim, S.; Lukarska, M.; Arnold, M.; Staahl, B.T.; et al. Comprehensive deletion landscape of CRISPR-Cas9 identifies minimal RNA-guided DNA-binding modules. Nat. Commun. 2021, 12, 5664. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, D.; Niu, W.; Zhao, D.; Li, J.; Liu, Z.; Gao, X.; Han, Y.; Lai, L.; Li, Z. A new compact adenine base editor generated through deletion of HNH and REC2 domain of SpCas9. BMC Biol. 2023, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Sola-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.J.; Liquori, A.J.; et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef]
- Yan, D.; Ren, B.; Liu, L.; Yan, F.; Li, S.; Wang, G.; Sun, W.; Zhou, X.; Zhou, H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant 2021, 14, 722–731. [Google Scholar] [CrossRef]
- Li, A.; Mitsunobu, H.; Yoshioka, S.; Suzuki, T.; Kondo, A.; Nishida, K. Cytosine base editing systems with minimized off-target effect and molecular size. Nat. Commun. 2022, 13, 4531. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Expanding the genome-targeting scope and the site selectivity of high-precision base editors. Nat. Commun. 2020, 11, 629. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Song, B.; Bae, S. Genome editing using CRISPR, CAST, and Fanzor systems. Mol. Cells 2024, 47, 100086. [Google Scholar] [CrossRef]
- Chylinski, K.; Le Rhun, A.; Charpentier, E. The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol. 2013, 10, 726–737. [Google Scholar] [CrossRef]
- Chylinski, K.; Makarova, K.S.; Charpentier, E.; Koonin, E.V. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res. 2014, 42, 6091–6105. [Google Scholar] [CrossRef]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Xie, H.; Tang, L.; He, X.; Liu, X.; Zhou, C.; Liu, J.; Ge, X.; Li, J.; Liu, C.; Zhao, J.; et al. SaCas9 Requires 5′-NNGRRT-3′ PAM for Sufficient Cleavage and Possesses Higher Cleavage Activity than SpCas9 or FnCpf1 in Human Cells. Biotechnol. J. 2018, 13, e1700561. [Google Scholar] [CrossRef]
- Yang, Z.X.; Fu, Y.W.; Zhao, J.J.; Zhang, F.; Li, S.A.; Zhao, M.; Wen, W.; Zhang, L.; Cheng, T.; Zhang, J.P.; et al. Superior Fidelity and Distinct Editing Outcomes of SaCas9 Compared with SpCas9 in Genome Editing. Genom. Proteom. Bioinform. 2023, 21, 1206–1220. [Google Scholar] [CrossRef]
- Kim, Y.B.; Komor, A.C.; Levy, J.M.; Packer, M.S.; Zhao, K.T.; Liu, D.R. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 2017, 35, 371–376. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Wang, L.; Yin, S.; Zhu, B.; Xie, L.; Duan, Q.; Hu, H.; Zheng, R.; Wei, Y.; et al. Increasing targeting scope of adenosine base editors in mouse and rat embryos through fusion of TadA deaminase with Cas9 variants. Protein Cell 2018, 9, 814–819. [Google Scholar] [CrossRef]
- Huang, T.P.; Zhao, K.T.; Miller, S.M.; Gaudelli, N.M.; Oakes, B.L.; Fellmann, C.; Savage, D.F.; Liu, D.R. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat. Biotechnol. 2019, 37, 626–631. [Google Scholar] [CrossRef]
- Grunewald, J.; Zhou, R.; Lareau, C.A.; Garcia, S.P.; Iyer, S.; Miller, B.R.; Langner, L.M.; Hsu, J.Y.; Aryee, M.J.; Joung, J.K. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat. Biotechnol. 2020, 38, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Tran, M.T.; Mohd Khalid, M.K.N.; Wang, Q.; Walker, J.K.R.; Lidgerwood, G.E.; Dilworth, K.L.; Lisowski, L.; Pebay, A.; Hewitt, A.W. Engineering domain-inlaid SaCas9 adenine base editors with reduced RNA off-targets and increased on-target DNA editing. Nat. Commun. 2020, 11, 4871. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, S.; Zhang, C.; Gao, N.; Li, M.; Wang, D.; Wang, D.; Liu, D.; Liu, H.; Ong, S.G.; et al. A compact Cas9 ortholog from Staphylococcus Auricularis (SauriCas9) expands the DNA targeting scope. PLoS Biol. 2020, 18, e3000686. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Wang, X.; Witte, I.P.; Huang, T.P.; Levy, J.M.; Raguram, A.; Banskota, S.; Seidah, N.G.; Musunuru, K.; Liu, D.R. Efficient in vivo base editing via single adeno-associated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng. 2022, 6, 1272–1283. [Google Scholar] [CrossRef]
- Kim, E.; Koo, T.; Park, S.W.; Kim, D.; Kim, K.; Cho, H.Y.; Song, D.W.; Lee, K.J.; Jung, M.H.; Kim, S.; et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017, 8, 14500. [Google Scholar] [CrossRef]
- Fonfara, I.; Le Rhun, A.; Chylinski, K.; Makarova, K.S.; Lecrivain, A.L.; Bzdrenga, J.; Koonin, E.V.; Charpentier, E. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 2014, 42, 2577–2590. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Wang, B.; Xia, Y.; Zheng, Y.; Du, L.; Xu, D.; Xing, D.; DePinho, R.A.; Lu, Z. Programmable base editing of mutated TERT promoter inhibits brain tumour growth. Nat. Cell Biol. 2020, 22, 282–288. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, Y.; Gootenberg, J.S.; Hirano, H.; Ran, F.A.; Nakane, T.; Ishitani, R.; Zhang, F.; Nishimasu, H.; Nureki, O. Crystal Structure of the Minimal Cas9 from Campylobacter jejuni Reveals the Molecular Diversity in the CRISPR-Cas9 Systems. Mol. Cell 2017, 65, 1109–1121.e3. [Google Scholar] [CrossRef]
- Nakagawa, R.; Ishiguro, S.; Okazaki, S.; Mori, H.; Tanaka, M.; Aburatani, H.; Yachie, N.; Nishimasu, H.; Nureki, O. Engineered Campylobacter jejuni Cas9 variant with enhanced activity and broader targeting range. Commun. Biol. 2022, 5, 211. [Google Scholar] [CrossRef]
- Kweon, J.; Jang, A.H.; Kwon, E.; Kim, U.; Shin, H.R.; See, J.; Jang, G.; Lee, C.; Koo, T.; Kim, S.; et al. Targeted dual base editing with Campylobacter jejuni Cas9 by single AAV-mediated delivery. Exp. Mol. Med. 2023, 55, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Amrani, N.; Gao, X.D.; Liu, P.; Edraki, A.; Mir, A.; Ibraheim, R.; Gupta, A.; Sasaki, K.E.; Wu, T.; Donohoue, P.D.; et al. NmeCas9 is an intrinsically high-fidelity genome-editing platform. Genome Biol. 2018, 19, 214. [Google Scholar] [CrossRef]
- Mir, A.; Edraki, A.; Lee, J.; Sontheimer, E.J. Type II-C CRISPR-Cas9 Biology, Mechanism, and Application. ACS Chem. Biol. 2018, 13, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Cradick, T.J.; Bao, G. The Neisseria meningitidis CRISPR-Cas9 System Enables Specific Genome Editing in Mammalian Cells. Mol. Ther. 2016, 24, 645–654. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Jia, Y.; Shan, H.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Efficient and high-fidelity base editor with expanded PAM compatibility for cytidine dinucleotide. Sci. China Life Sci. 2021, 64, 1355–1367. [Google Scholar] [CrossRef]
- Thuronyi, B.W.; Koblan, L.W.; Levy, J.M.; Yeh, W.H.; Zheng, C.; Newby, G.A.; Wilson, C.; Bhaumik, M.; Shubina-Oleinik, O.; Holt, J.R.; et al. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat. Biotechnol. 2019, 37, 1070–1079. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wang, Y.; Yang, B.; Wei, J.; Wu, J.; Wang, R.; Huang, X.; Chen, J.; Yang, L. Efficient base editing in methylated regions with a human APOBEC3A-Cas9 fusion. Nat. Biotechnol. 2018, 36, 946–949. [Google Scholar] [CrossRef]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.L.; Gao, C. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef]
- Zhang, H.; Bamidele, N.; Liu, P.; Ojelabi, O.; Gao, X.D.; Rodriguez, T.; Cheng, H.; Kelly, K.; Watts, J.K.; Xie, J.; et al. Adenine Base Editing In Vivo with a Single Adeno-Associated Virus Vector. GEN Biotechnol. 2022, 1, 285–299. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Xie, W.; Yu, H.; Lai, L.; Li, Z. Versatile and efficient genome editing with Neisseria cinerea Cas9. Commun. Biol. 2022, 5, 1296. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, H.; Chen, S.; Chen, M.; Zhang, Q.; Lai, L.; Li, Z. Improved base editor for efficient editing in GC contexts in rabbits with an optimized AID-Cas9 fusion. FASEB J. 2019, 33, 9210–9219. [Google Scholar] [CrossRef]
- Badon, I.W.; Oh, Y.; Kim, H.J.; Lee, S.H. Recent application of CRISPR-Cas12 and OMEGA system for genome editing. Mol. Ther. 2024, 32, 32–43. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Karvelis, T.; Bigelyte, G.; Young, J.K.; Hou, Z.; Zedaveinyte, R.; Budre, K.; Paulraj, S.; Djukanovic, V.; Gasior, S.; Silanskas, A.; et al. PAM recognition by miniature CRISPR-Cas12f nucleases triggers programmable double-stranded DNA target cleavage. Nucleic Acids Res. 2020, 48, 5016–5023. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, J.M.; Moon, S.B.; Chin, H.J.; Park, S.; Lim, Y.; Kim, D.; Koo, T.; Ko, J.-H.; Kim, Y.-S. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol. 2021, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chemparathy, A.; Zeng, L.; Kempton, H.R.; Shang, S.; Nakamura, M.; Qi, L.S. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol. Cell 2021, 81, 4333–4345.e4. [Google Scholar] [CrossRef]
- Chavez, A.; Tuttle, M.; Pruitt, B.W.; Ewen-Campen, B.; Chari, R.; Ter-Ovanesyan, D.; Haque, S.J.; Cecchi, R.J.; Kowal, E.J.K.; Buchthal, J.; et al. Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, L.; Yuan, B.; Zhang, C.; Cao, J.; Chen, J.; Qiu, J.; Tai, Y.; Chen, J.; Qiu, Z.; et al. TadA reprogramming to generate potent miniature base editors with high precision. Nat. Commun. 2023, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, Y.; Yan, R.; Liu, Y.; Zuo, E.; Gu, C.; Han, L.; Wei, Y.; Hu, X.; Zeng, R.; et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 2019, 571, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, C.; Yu, W.; Wang, Y.; He, S.; Yang, B.; Xiong, Y.C.; Wei, J.; Li, J.; Liang, J.; et al. Cas12a Base Editors Induce Efficient and Specific Editing with Low DNA Damage Response. Cell Rep. 2020, 31, 107723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, B.; Cao, J.; Chen, J.; Chen, J.; Qiu, J.; Zhao, X.M.; Wang, X.; Qiu, Z.; Cheng, T.L. Docking sites inside Cas9 for adenine base editing diversification and RNA off-target elimination. Nat. Commun. 2020, 11, 5827. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jeong, Y.K.; Hur, J.K.; Kim, J.S.; Bae, S. Adenine base editors catalyze cytosine conversions in human cells. Nat. Biotechnol. 2019, 37, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Lee, S.; Hwang, G.H.; Hong, S.A.; Park, S.E.; Kim, J.S.; Woo, J.S.; Bae, S. Adenine base editor engineering reduces editing of bystander cytosines. Nat. Biotechnol. 2021, 39, 1426–1433. [Google Scholar] [CrossRef]
- Hu, Y.; Han, L.; Mo, Q.; Du, Z.; Jiang, W.; Wu, X.; Zheng, J.; Xiao, X.; Sun, Y.; Ma, H. Engineering miniature CRISPR-Cas Un1Cas12f1 for efficient base editing. Mol. Ther. Nucleic Acids 2024, 35, 102201. [Google Scholar] [CrossRef]
- Choli, T.; Henning, P.; Wittmann-Liebold, B.; Reinhardt, R. Isolation, characterization and microsequence analysis of a small basic methylated DNA-binding protein from the Archaebacterium, Sulfolobus solfataricus. Biochmica Biophys. Acta 1988, 950, 193–203. [Google Scholar] [CrossRef]
- Agback, P.; Baumann, H.; Knapp, S.; Ladenstein, R.; Härd, T. Architecture of nonspecific protein-DNA interactions in the Sso7d-DNA complex. Nat. Struct. Biol. 1998, 5, 579–584. [Google Scholar] [CrossRef]
- Kalichuk, V.; Behar, G.; Renodon-Corniere, A.; Danovski, G.; Obal, G.; Barbet, J.; Mouratou, B.; Pecorari, F. The archaeal “7 kDa DNA-binding” proteins: Extended characterization of an old gifted family. Sci. Rep. 2016, 6, 37274. [Google Scholar] [CrossRef]
- Kim, D.Y.; Chung, Y.; Lee, Y.; Jeong, D.; Park, K.-H.; Chin, H.J.; Lee, J.M.; Park, S.; Ko, S.; Ko, J.H.; et al. Hypercompact adenine base editors based on transposase B guided by engineered RNA. Nat. Chem. Biol. 2022, 18, 1005–1013. [Google Scholar] [CrossRef]
- Wu, W.Y.; Mohanraju, P.; Liao, C.; Adiego-Perez, B.; Creutzburg, S.C.A.; Makarova, K.S.; Keessen, K.; Lindeboom, T.A.; Khan, T.S.; Prinsen, S.; et al. The miniature CRISPR-Cas12m effector binds DNA to block transcription. Mol. Cell 2022, 82, 4487–4502. [Google Scholar] [CrossRef]
- Omura, S.N.; Nakagawa, R.; Sudfeld, C.; Villegas Warren, R.; Wu, W.Y.; Hirano, H.; Laffeber, C.; Kusakizako, T.; Kise, Y.; Lebbink, J.H.G.; et al. Mechanistic and evolutionary insights into a type V-M CRISPR-Cas effector enzyme. Nat. Struct. Mol. Biol. 2023, 30, 1172–1182. [Google Scholar] [CrossRef]
- Bigelyte, G.; Duchovska, B.; Zedaveinyte, R.; Sasnauskas, G.; Sinkunas, T.; Dalgediene, I.; Tamulaitiene, G.; Silanskas, A.; Kazlauskas, D.; Valancauskas, L.; et al. Innate programmable DNA binding by CRISPR-Cas12m effectors enable efficient base editing. Nucleic Acids Res. 2024, 52, 3234–3248. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Sousa, A.A.; Walton, R.T.; Tak, Y.E.; Hsu, J.Y.; Clement, K.; Welch, M.M.; Horng, J.E.; Malagon-Lopez, J.; Scarfo, I.; et al. Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 2019, 37, 276–282. [Google Scholar] [CrossRef]
- Pausch, P.; Al-Shayeb, B.; Bisom-Rapp, E.; Tsuchida, C.A.; Li, Z.; Cress, B.F.; Knott, G.J.; Jacobsen, S.E.; Banfield, J.F.; Doudna, J.A. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science 2020, 369, 333–337. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, T.; Liu, J.; Yang, Y.; Wang, Z.; Wang, Y.; Wang, T.; Li, M.; Li, M.; Lu, D.; et al. A highly specific CRISPR-Cas12j nuclease enables allele-specific genome editing. Sci. Adv. 2023, 9, eabo6405. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Makarova, K.S.; Koonin, E.V. ISC, a Novel Group of Bacterial and Archaeal DNA Transposons That Encode Cas9 Homologs. J. Bacteriol. 2015, 198, 797–807. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef]
- Altae-Tran, H.; Kannan, S.; Demircioglu, F.E.; Oshiro, R.; Nety, S.P.; McKay, L.J.; Dlakić, M.; Inskeep, W.P.; Makarova, K.S.; Macrae, R.K.; et al. The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. Science 2021, 374, 57–65. [Google Scholar] [CrossRef]
- Han, D.; Xiao, Q.; Wang, Y.; Zhang, H.; Dong, X.; Li, G.; Kong, X.; Wang, S.; Song, J.; Zhang, W.; et al. Development of miniature base editors using engineered IscB nickase. Nat. Methods 2023, 20, 1029–1036. [Google Scholar] [CrossRef]
- Han, L.; Hu, Y.; Mo, Q.; Yang, H.; Gu, F.; Bai, F.; Sun, Y.; Ma, H. Engineering miniature IscB nickase for robust base editing with broad targeting range. Nat. Chem. Biol. 2024, 20, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, X.; Liu, Y.; Liu, N.; Li, Y.; Luo, J.; Ma, Q.; Wu, D.; Li, J.; Xu, C.; et al. Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase. Nat. Biotechnol. 2023, 41, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, G.; Han, D.; Wang, H.; Yao, M.; Ma, T.; Zhou, J.; Zhang, Y.; Zhang, X.; He, B.; et al. Engineered IscB-omegaRNA system with expanded target range for base editing. Nat. Chem. Biol. 2024, 21, 100–108. [Google Scholar] [CrossRef]
- Karvelis, T.; Druteika, G.; Bigelyte, G.; Budre, K.; Zedaveinyte, R.; Silanskas, A.; Kazlauskas, D.; Venclovas, C.; Siksnys, V. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 2021, 599, 692–696. [Google Scholar] [CrossRef]
- Karmakar, S.; Panda, D.; Panda, S.; Dash, M.; Saha, R.; Das, P.; Avinash, S.P.; Shih, J.; Yang, Y.; Nayak, A.K.; et al. A miniature alternative to Cas9 and Cas12: Transposon-associated TnpB mediates targeted genome editing in plants. Plant Biotechnol. J. 2024, 22, 2950–2953. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Q.; Fei, H.; He, Z.; Xu, H.; Li, Y.; Qu, K.; Han, P.; Gao, Q.; Li, B.; et al. Discovery of deaminase functions by structure-based protein clustering. Cell 2023, 186, 3182–3195.e14. [Google Scholar] [CrossRef]
- Doman, J.L.; Raguram, A.; Newby, G.A.; Liu, D.R. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat. Biotechnol. 2020, 38, 620–628. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Yu, H.; Yang, L.; Wang, Z.; Yi, W.; Liu, Y.; Xiao, W.; Xiang, H.; Xie, Z.; et al. Accelerated discovery and miniaturization of novel single-stranded cytidine deaminases. Nucleic Acids Res. 2024, 52, 11188–11202. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.J.; Jung, S.J.; Yang, J.; Choi, D.E.; Kim, V.N. Functional viromic screens uncover regulatory RNA elements. Cell 2023, 186, 3291–3306.e21. [Google Scholar] [CrossRef]
- Guo, J.; Gong, L.; Yu, H.; Li, M.; An, Q.; Liu, Z.; Fan, S.; Yang, C.; Zhao, D.; Han, J.; et al. Engineered minimal type I CRISPR-Cas system for transcriptional activation and base editing in human cells. Nat. Commun. 2024, 15, 7277. [Google Scholar] [CrossRef] [PubMed]
- Pausch, P.; Muller-Esparza, H.; Gleditzsch, D.; Altegoer, F.; Randau, L.; Bange, G. Structural Variation of Type I-F CRISPR RNA Guided DNA Surveillance. Mol. Cell 2017, 67, 622–632.e4. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, S.; Brenzinger, S.; Gleditzsch, D.; Plagens, A.; Klingl, A.; Thormann, K.; Randau, L. Interference activity of a minimal Type I CRISPR-Cas system from Shewanella putrefaciens. Nucleic Acids Res. 2015, 43, 8913–8923. [Google Scholar] [CrossRef]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F.; et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011, 9, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Luo, M.; Hayes, R.P.; Kim, J.; Ng, S.; Ding, F.; Liao, M.; Ke, A. Structure Basis for Directional R-loop Formation and Substrate Handover Mechanisms in Type I CRISPR-Cas System. Cell 2017, 170, 48–60.e11. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ni, D.; Nam, K.H.; Majumdar, S.; McLean, J.; Stahlberg, H.; Terns, M.P.; Ke, A. Allosteric control of type I-A CRISPR-Cas3 complexes and establishment as effective nucleic acid detection and human genome editing tools. Mol. Cell 2022, 82, 2754–2768.e5. [Google Scholar] [CrossRef]
- Szczelkun, M.D.; Tikhomirova, M.S.; Sinkunas, T.; Gasiunas, G.; Karvelis, T.; Pschera, P.; Siksnys, V.; Seidel, R. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc. Natl. Acad. Sci. USA 2014, 111, 9798–9803. [Google Scholar] [CrossRef]
- Grunewald, J.; Zhou, R.; Iyer, S.; Lareau, C.A.; Garcia, S.P.; Aryee, M.J.; Joung, J.K. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat. Biotechnol. 2019, 37, 1041–1048. [Google Scholar] [CrossRef]
- Grunewald, J.; Zhou, R.; Garcia, S.P.; Iyer, S.; Lareau, C.A.; Aryee, M.J.; Joung, J.K. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 2019, 569, 433–437. [Google Scholar] [CrossRef]
- Rees, H.A.; Wilson, C.; Doman, J.L.; Liu, D.R. Analysis and minimization of cellular RNA editing by DNA adenine base editors. Sci. Adv. 2019, 5, eaax5717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, W.; Huang, S.; Wu, S.; Li, L.; Zhou, J.; Cao, Y.; Huang, X.; Qiao, Y. Structure-guided engineering of adenine base editor with minimized RNA off-targeting activity. Nat. Commun. 2021, 12, 2287. [Google Scholar] [CrossRef]
- Voss, S.; Klewer, L.; Wu, Y.W. Chemically induced dimerization: Reversible and spatiotemporal control of protein function in cells. Curr. Opin. Chem. Biol. 2015, 28, 194–201. [Google Scholar] [CrossRef]
- Berrios, K.N.; Evitt, N.H.; DeWeerd, R.A.; Ren, D.; Luo, M.; Barka, A.; Wang, T.; Bartman, C.R.; Lan, Y.; Green, A.M.; et al. Controllable genome editing with split-engineered base editors. Nat. Chem. Biol. 2021, 17, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Liu, N.; Tang, W.; Xie, L.; Qin, F.; Zhou, L.; Tao, R.; Wang, Y.; Hu, Y.; Jiao, Y.; et al. A split cytosine deaminase architecture enables robust inducible base editing. FASEB J. 2021, 35, e22045. [Google Scholar] [CrossRef]
- Zeng, H.; Yuan, Q.; Peng, F.; Ma, D.; Lingineni, A.; Chee, K.; Gilberd, P.; Osikpa, E.C.; Sun, Z.; Gao, X. A split and inducible adenine base editor for precise in vivo base editing. Nat. Commun. 2023, 14, 5573. [Google Scholar] [CrossRef] [PubMed]
- Kawano, F.; Suzuki, H.; Furuya, A.; Sato, M. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun. 2015, 6, 6256. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Q.; Cheng, Y.; Wang, X.; Deng, Z.; Zhou, F.; Sun, Y. Design and Engineering of Light-Induced Base Editors Facilitating Genome Editing with Enhanced Fidelity. Adv. Sci. 2024, 11, e2305311. [Google Scholar] [CrossRef]
- Zou, Q.; Lu, Y.; Qing, B.; Li, N.; Zhou, T.; Pan, J.; Zhang, X.; Zhang, X.; Chen, Y.; Sun, S.K. Photoactivatable base editors for spatiotemporally controlled genome editing in vivo. Biomaterials 2023, 302, 122328. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, C.; Huang, S.; Dang, L.; Wei, Y.; He, J.; Zhou, Y.; Mao, S.; Tao, W.; Zhang, Y.; et al. A Cas-embedding strategy for minimizing off-target effects of DNA base editors. Nat. Commun. 2020, 11, 6073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, L.; Liu, N.; Yao, S. BE-PIGS: A base-editing tool with deaminases inlaid into Cas9 PI domain significantly expanded the editing scope. Signal Transduct Target Ther. 2019, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Bamidele, N.; Zhang, H.; Dong, X.; Cheng, H.; Gaston, N.; Feinzig, H.; Cao, H.; Kelly, K.; Watts, J.K.; Xie, J.; et al. Domain-inlaid Nme2Cas9 adenine base editors with improved activity and targeting scope. Nat. Commun. 2024, 15, 1458. [Google Scholar] [CrossRef]
- Hu, L.; Han, J.; Wang, H.D.; Cheng, Z.H.; Lv, C.C.; Liu, D.F.; Yu, H.Q. A universal and wide-range cytosine base editor via domain-inlaid and fidelity-optimized CRISPR-FrCas9. Nat. Commun. 2025, 16, 1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, W.; Zhang, H.; Gao, R.; Qiu, H.; Wei, J.; Zhou, L.; Lei, Y.N.; Wu, X.; Li, X.; et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat. Cell Biol. 2021, 23, 552–563. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Z.; Yang, G.; Huang, S.; Li, G.; Feng, S.; Liu, Y.; Li, J.; Yu, W.; Zhang, Y.; et al. Efficient generation of mouse models of human diseases via ABE- and BE-mediated base editing. Nat. Commun. 2018, 9, 2338. [Google Scholar] [CrossRef]
- Lee, H.K.; Willi, M.; Miller, S.M.; Kim, S.; Liu, C.; Liu, D.R.; Hennighausen, L. Targeting fidelity of adenine and cytosine base editors in mouse embryos. Nat. Commun. 2018, 9, 4804. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, B.; Ru, G.; Meng, H.; Yan, Y.; Hong, M.; Zhang, D.; Luan, C.; Zhang, S.; Wu, H.; et al. Re-engineering the adenine deaminase TadA-8e for efficient and specific CRISPR-based cytosine base editing. Nat. Biotechnol. 2022, 41, 663–672. [Google Scholar] [CrossRef]
- Neugebauer, M.E.; Hsu, A.; Arbab, M.; Krasnow, N.A.; McElroy, A.N.; Pandey, S.; Doman, J.L.; Huang, T.P.; Raguram, A.; Banskota, S.; et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity. Nat. Biotechnol. 2022, 41, 673–685. [Google Scholar] [CrossRef]
- Lam, D.K.; Feliciano, P.R.; Arif, A.; Bohnuud, T.; Fernandez, T.P.; Gehrke, J.M.; Grayson, P.; Lee, K.D.; Ortega, M.A.; Sawyer, C.; et al. Improved cytosine base editors generated from TadA variants. Nat. Biotechnol. 2023, 41, 686–697. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, B.; Cao, J.; Song, L.; Chen, J.; Qiu, J.; Qiu, Z.; Zhao, X.M.; Chen, J.; Cheng, T.L. TadA orthologs enable both cytosine and adenine editing of base editors. Nat. Commun. 2023, 14, 414. [Google Scholar] [CrossRef]
- Li, G.; Dong, X.; Luo, J.; Yuan, T.; Li, T.; Zhao, G.; Zhang, H.; Zhou, J.; Zeng, Z.; Cui, S.; et al. Engineering TadA ortholog-derived cytosine base editor without motif preference and adenosine activity limitation. Nat. Commun. 2024, 15, 8090. [Google Scholar] [CrossRef]
- Pallaseni, A.; Peets, E.M.; Koeppel, J.; Weller, J.; Vanderstichele, T.; Ho, U.L.; Crepaldi, L.; van Leeuwen, J.; Allen, F.; Parts, L. Predicting base editing outcomes using position-specific sequence determinants. Nucleic Acids Res. 2022, 50, 3551–3564. [Google Scholar] [CrossRef]
- Xiong, X.; Li, Z.; Liang, J.; Liu, K.; Li, C.; Li, J.F. A cytosine base editor toolkit with varying activity windows and target scopes for versatile gene manipulation in plants. Nucleic Acids Res. 2022, 50, 3565–3580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Shan, H.; Jia, Y.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Precise base editing with CC context-specificity using engineered human APOBEC3G-nCas9 fusions. BMC Biol. 2020, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Zuo, E.; Sun, Y.; Yuan, T.; He, B.; Zhou, C.; Ying, W.; Liu, J.; Wei, W.; Zeng, R.; Li, Y.; et al. A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects. Nat. Methods 2020, 17, 600–604. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Xue, N.; Hong, M.; Zhang, X.; Zhang, D.; Yang, J.; Bai, S.; Huang, Y.; Meng, H.; et al. Engineering a precise adenine base editor with minimal bystander editing. Nat. Chem. Biol. 2023, 19, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Engineering of high-precision base editors for site-specific single nucleotide replacement. Nat. Commun. 2019, 10, 439. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Shan, H.; Zhang, Q.; Chen, M.; Lai, L.; Li, Z. Efficient and precise base editing in rabbits using human APOBEC3A-nCas9 fusions. Cell Discov. 2019, 5, 31. [Google Scholar] [CrossRef]
- Zhao, N.; Zhou, J.; Tao, T.; Wang, Q.; Tang, J.; Li, D.; Gou, S.; Guan, Z.; Olajide, J.S.; Lin, J.; et al. Evolved cytidine and adenine base editors with high precision and minimized off-target activity by a continuous directed evolution system in mammalian cells. Nat. Commun. 2024, 15, 8140. [Google Scholar] [CrossRef]
- Lee, S.; Ding, N.; Sun, Y.; Yuan, T.; Li, J.; Yuan, Q.; Liu, L.; Yang, J.; Wang, Q.; Kolomeisky, A.B.; et al. Single C-to-T substitution using engineered APOBEC3G-nCas9 base editors with minimum genome- and transcriptome-wide off-target effects. Sci. Adv. 2020, 6, eaba1773. [Google Scholar] [CrossRef]
- Lian, M.; Chen, F.; Huang, X.; Zhao, X.; Gou, S.; Li, N.; Jin, Q.; Shi, H.; Liang, Y.; Xie, J.; et al. Improving the Cpf1-mediated base editing system by combining dCas9/dead sgRNA with human APOBEC3A variants. J. Genet. Genom. 2021, 48, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lian, M.; Ma, B.; Gou, S.; Luo, X.; Yang, K.; Shi, H.; Xie, J.; Ge, W.; Ouyang, Z.; et al. Multiplexed base editing through Cas12a variant-mediated cytosine and adenine base editors. Commun. Biol. 2022, 5, 1163. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grunewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Koblan, L.W.; Arbab, M.; Shen, M.W.; Hussmann, J.A.; Anzalone, A.V.; Doman, J.L.; Newby, G.A.; Yang, D.; Mok, B.; Replogle, J.M.; et al. Efficient C*G-to-G*C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nat. Biotechnol. 2021, 39, 1414–1425. [Google Scholar] [CrossRef]
- Yuan, T.; Yan, N.; Fei, T.; Zheng, J.; Meng, J.; Li, N.; Liu, J.; Zhang, H.; Xie, L.; Ying, W.; et al. Optimization of C-to-G base editors with sequence context preference predictable by machine learning methods. Nat. Commun. 2021, 12, 4902. [Google Scholar] [CrossRef]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nat. Commun. 2021, 12, 1384. [Google Scholar] [CrossRef]
- Chen, L.; Hong, M.; Luan, C.; Gao, H.; Ru, G.; Guo, X.; Zhang, D.; Zhang, S.; Li, C.; Wu, J.; et al. Adenine transversion editors enable precise, efficient A*T-to-C*G base editing in mammalian cells and embryos. Nat. Biotechnol. 2023, 42, 638–650. [Google Scholar] [CrossRef]
- Tong, H.; Liu, N.; Wei, Y.; Zhou, Y.; Li, Y.; Wu, D.; Jin, M.; Cui, S.; Li, H.; Li, G.; et al. Programmable deaminase-free base editors for G-to-Y conversion by engineered glycosylase. Natl. Sci. Rev. 2023, 10, nwad143. [Google Scholar] [CrossRef]
- Tong, H.; Wang, H.; Wang, X.; Liu, N.; Li, G.; Wu, D.; Li, Y.; Jin, M.; Li, H.; Wei, Y.; et al. Development of deaminase-free T-to-S base editor and C-to-G base editor by engineered human uracil DNA glycosylase. Nat. Commun. 2024, 15, 4897. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, B.; Chen, L.; Xie, L.; Yu, W.; Wang, Y.; Li, L.; Yin, S.; Yang, L.; Hu, H.; et al. Dual base editor catalyzes both cytosine and adenine base conversions in human cells. Nat. Biotechnol. 2020, 38, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.C.; Ishiguro, S.; Mori, H.; Tanaka, M.; Tatsuno, K.; Ueda, H.; Yamamoto, S.; Seki, M.; Masuyama, N.; Nishida, K.; et al. Base editors for simultaneous introduction of C-to-T and A-to-G mutations. Nat. Biotechnol. 2020, 38, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, X.; Wang, X.; Gou, S.; Liang, Y.; Chen, F.; Li, N.; Ouyang, Z.; Zhang, Q.; Ge, W.; et al. ACBE, a new base editor for simultaneous C-to-T and A-to-G substitutions in mammalian systems. BMC Biol. 2020, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Shelake, R.M.; Pramanik, D.; Kim, J.-Y. Improved Dual Base Editor Systems (iACBEs) for Simultaneous Conversion of Adenine and Cytosine in the Bacterium Escherichia coli. mBio 2023, 14, e0229622. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Meng, X.; Chen, S.; Zong, Y.; Lu, C.; Qiu, J.L.; Chen, Y.H.; Li, J.; Gao, C. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 2020, 38, 875–882. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, J.; Zhang, Q.; Wang, X.; Gou, S.; Lin, L.; Chen, T.; Ge, W.; Zhuang, Z.; Lian, M.; et al. AGBE: A dual deaminase-mediated base editor by fusing CGBE with ABE for creating a saturated mutant population with multiple editing patterns. Nucleic Acids Res. 2022, 50, 5384–5399. [Google Scholar] [CrossRef]
- Gammage, P.A.; Moraes, C.T.; Minczuk, M. Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. 2018, 34, 101–110. [Google Scholar] [CrossRef]
- Mok, Y.G.; Lee, J.M.; Chung, E.; Lee, J.; Lim, K.; Cho, S.I.; Kim, J.S. Base editing in human cells with monomeric DddA-TALE fusion deaminases. Nat. Commun. 2022, 13, 4038. [Google Scholar] [CrossRef]
- Cho, S.I.; Lee, S.; Mok, Y.G.; Lim, K.; Lee, J.; Lee, J.M.; Chung, E.; Kim, J.S. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 2022, 185, 1764–1776.e12. [Google Scholar] [CrossRef]
- Guo, J.; Yu, W.; Li, M.; Chen, H.; Liu, J.; Xue, X.; Lin, J.; Huang, S.; Shu, W.; Huang, X.; et al. A DddA ortholog-based and transactivator-assisted nuclear and mitochondrial cytosine base editors with expanded target compatibility. Mol. Cell 2023, 83, 1710–1724.e7. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]

| Name of BE | Strategy for Downsizing | Approximate Size (Excluding the Promoter) | Ref. |
|---|---|---|---|
| sABE | Dual deletion of REC2 and HNH | ~4.15 kb | [27] |
| SaAID-2S | Truncation of PmCDA1 and adoption of SaCas9 | ~3.9 kb | [30] |
| SaAID-3S | ~3.9 kb | ||
| SaBE3 | Adoption of SaCas9 | ~4.2 kb | [39] |
| SaBE3-KKH | ~4.2 kb | ||
| SaKKH-ABE7.10 | ~4.3 kb | [40] | |
| SaKKH-ABE8e | ~3.9 kb | [15] | |
| miniABEmax (V82G)-nSaCas9 | ~3.9 kb | [43] | |
| miniABEmax | Removal of wtTadA | ~4.8 kb | [42] |
| SauriBE4max | Adoption of SauriCas9 | ~4.5 kb | [44] |
| SauriABEmax | ~4.6 kb | ||
| SauriABE8e | ~3.9 kb | [45] | |
| cjABE8e | Adoption of CjCas9 | ~3.6 kb | [45] |
| cjCBEmax | ~4.3 kb | [51] | |
| nNme2-CBE | Adoption of Nme2Cas9 | ~4.6 kb | [55] |
| Nme2-ABE8e | ~3.9 kb | [45,59] | |
| nNc-CBE | Adoption of NcCas9 | ~3.9 kb | [60] |
| nNc-ABE | ~3.9 kb | ||
| dCasMINI-ABE | Adoption of Un1Cas12f1 | ~3.0 kb | [67] |
| miniABE | ~2.3 kb | [69] | |
| miniCBE | ~2.5 kb | ||
| UminiABE | ~2.6 kb | [75] | |
| STUminiABE | ~2.8 kb | ||
| STUminiCBE | ~2.4 kb | ||
| TaRGET-ABE-C3.0 | Adoption of CWCas9 | ~3.0 kb | [79] |
| TaRGET-ABE-C3.1 | ~3.0 kb | ||
| dCas12m-CBE1 | Adoption of MmdCas12m | ~3.1 kb | [80] |
| GoABE | Adoption of GoCas12m | ~2.8 kb | [85] |
| miABE | Adoption of enIscB | ~2.8 kb | [90] |
| miCBE | ~2.8 kb | ||
| IminiCBE | Adoption of OgeuIscB | ~2.4 kb | [91] |
| IminiABE | ~2.6 kb | ||
| IminiCGBE | ~2.4 kb | ||
| IminiAYBE | ~3.5 kb | ||
| SIminiBE (CBE) | ~2.6 kb | ||
| SIminiBE (ABE) | ~2.8 kb | ||
| enOgeuIscB-CBE | ~2.4 kb | [93] | |
| enOgeuIscB-ABE | ~2.6 kb | ||
| dTnpB-ABE8e-N | Adoption of ISDra2 TnpB | ~2.0 kb | [95] |
| dTnpB-ABE8e-C | ~2.0 kb | ||
| mini-Sdd6 | Adoption of Sdd6 | ~4.0 kb | [99] |
| SflSdd-CBE | Adoption of SflSdd | ~4.5 kb | [101] |
| 5CABE | Adoption of type I CRISPR | ~3.4 kb | [103] |
| 5NABE | ~3.4 kb | ||
| 7CABE | ~3.4 kb | ||
| 7NABE | ~3.4 kb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B. Efforts to Downsize Base Editors for Clinical Applications. Int. J. Mol. Sci. 2025, 26, 2357. https://doi.org/10.3390/ijms26052357
Song B. Efforts to Downsize Base Editors for Clinical Applications. International Journal of Molecular Sciences. 2025; 26(5):2357. https://doi.org/10.3390/ijms26052357
Chicago/Turabian StyleSong, Beomjong. 2025. "Efforts to Downsize Base Editors for Clinical Applications" International Journal of Molecular Sciences 26, no. 5: 2357. https://doi.org/10.3390/ijms26052357
APA StyleSong, B. (2025). Efforts to Downsize Base Editors for Clinical Applications. International Journal of Molecular Sciences, 26(5), 2357. https://doi.org/10.3390/ijms26052357





