Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes
Abstract
1. Introduction
2. Results
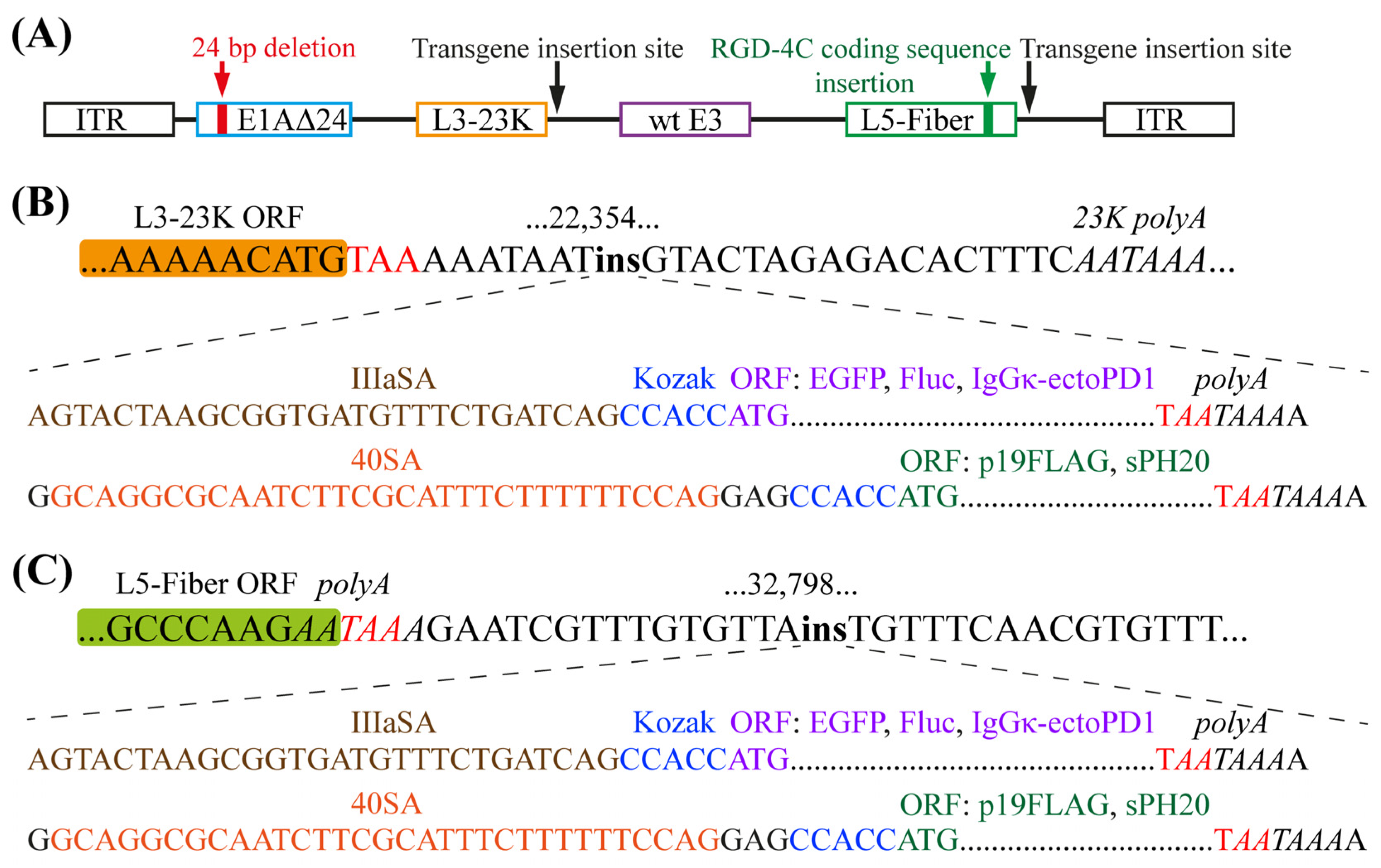
2.1. Generation of Transgene-Armed Oncolytic Adenoviruses
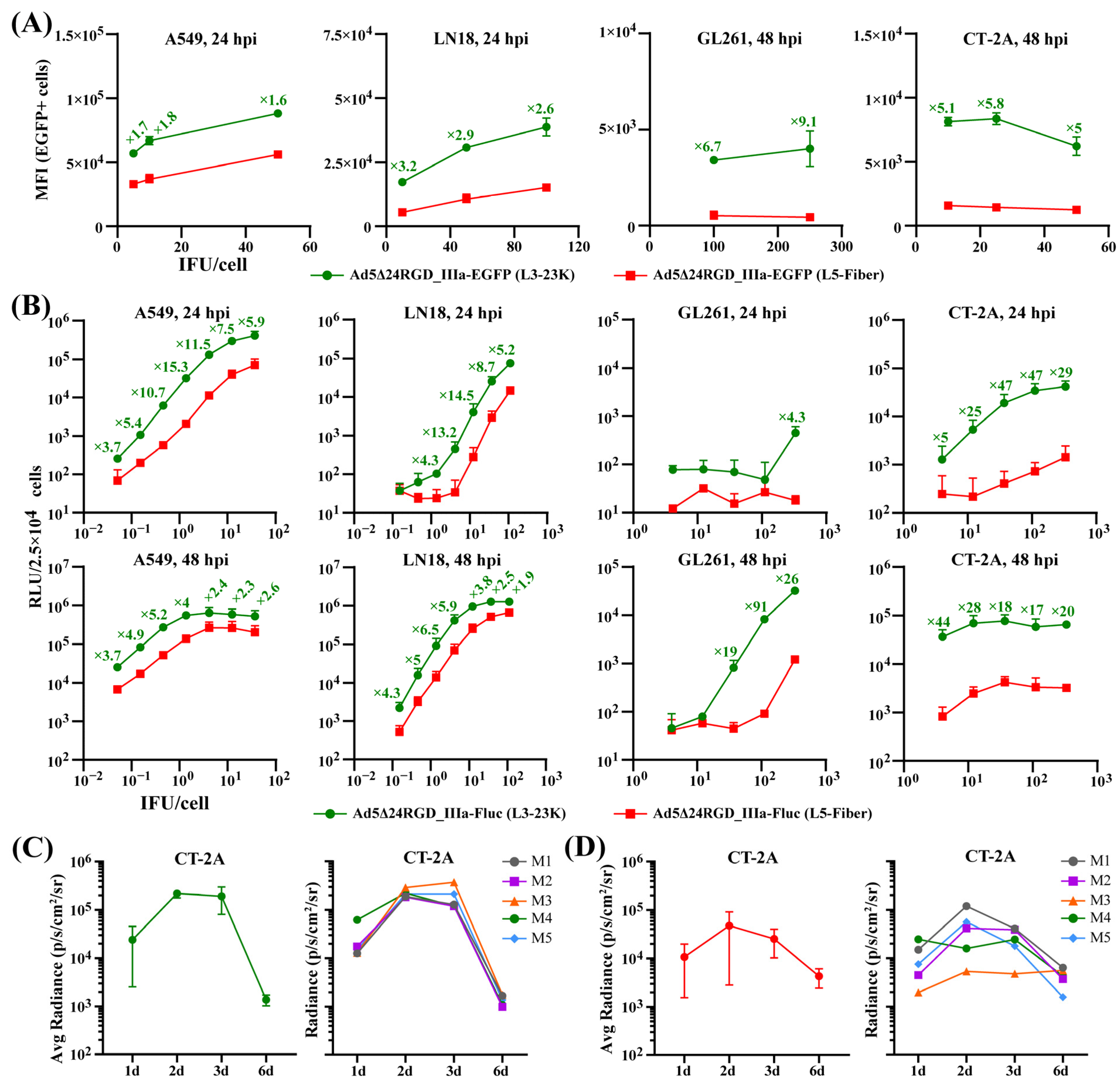
2.2. The Insertion of Transgenes Downstream of the L3-23K Region Ensures Their Production at Considerably Higher Levels
2.3. The Insertion of Transgenes Downstream of the L3-23K or L5-Fiber Region Differentially and Unpredictably Affects the Oncolytic Potency of Ad5Δ24RGD
2.4. Characterization of Ad5Δ24RGD Expressing the Wild-Type Murine PD-1, Human PD-1 or the Human Mutant High-Affinity PD-1 Ectodomain
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Construction of Oncolytic Adenoviruses
4.3. Adenovirus Particle Purification
4.4. Adenovirus Titration
4.5. Resazurin/Alamar Blue Cell Viability Assay
4.6. Total Virus Yield Assay
4.7. Plaque Assay
4.8. Flow Cytometry
4.9. In Vitro Bioluminescence Assay
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Hyaluronidase Activity Assay
4.12. Western Blot Analysis
4.13. In Vivo Bioluminescence Imaging (IVIS)
4.14. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OAd | Oncolytic adenovirus |
| SA | Splicing acceptor |
| MLP | Major late promoter |
| GC3% | Percentage of codons with G/C at the third base position |
| EGFP | Enhanced green fluorescent protein |
| BiTE | Bispecific T-cell engager |
| RGD | Arginine-glycine-aspartic acid motif |
| RLU | Relative light units |
| IFU | Infectious units |
| PD-1 | Programmed cell death receptor-1 |
| FAP | Fibroblast activation protein |
| PH20 | Hyaluronidase posterior head 20 |
References
- Lathwal, A.; Kumar, R.; Raghava, G.P.S. OvirusTdb: A database of oncolytic viruses for the advancement of therapeutics in cancer. Virology 2020, 548, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, P.; Teodoro, J.G. A Renaissance for Oncolytic Adenoviruses? Viruses 2023, 15, 358. [Google Scholar] [CrossRef] [PubMed]
- Nettelbeck, D.M. Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer. J. Mol. Med. 2008, 86, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Chekhonin, V.P. Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res. 2018, 257, 40–51. [Google Scholar] [CrossRef]
- Yoon, A.-R.; Hong, J.; Kim, S.W.; Yun, C.-O. Redirecting adenovirus tropism by genetic, chemical, and mechanical modification of the adenovirus surface for cancer gene therapy. Expert Opin. Drug Deliv. 2016, 13, 843–858. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Chekhonin, V.P. A compendium of adenovirus genetic modifications for enhanced replication, oncolysis, and tumor immunosurveillance in cancer therapy. Gene 2018, 679, 11–18. [Google Scholar] [CrossRef]
- Gros, A.; Guedan, S. Adenovirus Release from the Infected Cell as a Key Factor for Adenovirus Oncolysis. Open Gene Ther. J. 2010, 3, 24–30. [Google Scholar] [CrossRef]
- Gryciuk, A.; Rogalska, M.; Baran, J.; Kuryk, L.; Staniszewska, M. Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment. Cancers 2023, 15, 1947. [Google Scholar] [CrossRef]
- Lu, S.-C.; Barry, M.A. Locked and loaded: Engineering and arming oncolytic adenoviruses to enhance anti-tumor immune responses. Expert Opin. Biol. Ther. 2022, 22, 1359–1378. [Google Scholar] [CrossRef]
- Biegert, G.W.G.; Rosewell Shaw, A.; Suzuki, M. Current development in adenoviral vectors for cancer immunotherapy. Mol. Ther.-Oncolytics 2021, 23, 571–581. [Google Scholar] [CrossRef]
- Goradel, N.H.; Negahdari, B.; Ghorghanlu, S.; Jahangiri, S.; Arashkia, A. Strategies for enhancing intratumoral spread of oncolytic adenoviruses. Pharmacol. Ther. 2020, 213, 107586. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Prevec, L.; Graham, F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 1993, 67, 5911–5921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hallden, G.; Hill, R.; Anand, A.; Liu, T.-C.; Francis, J.; Brooks, G.; Lemoine, N.; Kirn, D. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat. Biotechnol. 2003, 21, 1328–1335. [Google Scholar] [CrossRef]
- Bortolanza, S.; Bunuales, M.; Alzuguren, P.; Lamas, O.; Aldabe, R.; Prieto, J.; Hernandez-Alcoceba, R. Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters. Cancer Gene Ther. 2009, 16, 703–712. [Google Scholar] [CrossRef]
- Farrera-Sal, M.; de Sostoa, J.; Nuñez-Manchón, E.; Moreno, R.; Fillat, C.; Bazan-Peregrino, M.; Alemany, R. Arming Oncolytic Adenoviruses: Effect of Insertion Site and Splice Acceptor on Transgene Expression and Viral Fitness. Int. J. Mol. Sci. 2020, 21, 5158. [Google Scholar] [CrossRef]
- Robinson, M.; Ge, Y.; Ko, D.; Yendluri, S.; Laflamme, G.; Hawkins, L.; Jooss, K. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008, 15, 9–17. [Google Scholar] [CrossRef]
- Fuerer, C.; Iggo, R. 5-Fluorocytosine increases the toxicity of Wnt-targeting replicating adenoviruses that express cytosine deaminase as a late gene. Gene Ther. 2004, 11, 142–151. [Google Scholar] [CrossRef]
- García-Castro, J.; Martínez-Palacio, J.; Lillo, R.; García-Sánchez, F.; Alemany, R.; Madero, L.; Bueren, J.A.; Ramírez, M. Tumor cells as cellular vehicles to deliver gene therapies to metastatic tumors. Cancer Gene Ther. 2005, 12, 341–349. [Google Scholar] [CrossRef]
- Núñez-Manchón, E.; Farrera-Sal, M.; Otero-Mateo, M.; Castellano, G.; Moreno, R.; Medel, D.; Alemany, R.; Villanueva, E.; Fillat, C. Transgene codon usage drives viral fitness and therapeutic efficacy in oncolytic adenoviruses. NAR Cancer 2021, 3, zcab015. [Google Scholar] [CrossRef]
- Hia, F.; Yang, S.F.; Shichino, Y.; Yoshinaga, M.; Murakawa, Y.; Vandenbon, A.; Fukao, A.; Fujiwara, T.; Landthaler, M.; Natsume, T.; et al. Codon bias confers stability to human mRNAs. EMBO Rep. 2019, 20, e48220. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chernysheva, A.A.; Cherepanov, S.A.; Yusubalieva, G.M.; Ruzsics, Z.; Lipatova, A.V.; Chekhonin, V.P. Superior infectivity of the fiber chimeric oncolytic adenoviruses Ad5/35 and Ad5/3 over Ad5-delta-24-RGD in primary glioma cultures. Mol. Ther.-Oncolytics 2022, 24, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Hermiston, T.; Johnson, L.; Brooks, G.; Sampson-Johannes, A.; Williams, A.; Hawkins, L.; Kirn, D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000, 6, 1134–1139. [Google Scholar] [CrossRef]
- Dmitriev, I.; Krasnykh, V.; Miller, C.R.; Wang, M.; Kashentseva, E.; Mikheeva, G.; Belousova, N.; Curiel, D.T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998, 72, 9706–9713. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Fuchs, N.; Zhang, L.; Calvo-Barreiro, L.; Kuncewicz, K.; Gabr, M. Inhibitors of Immune Checkpoints: Small Molecule- and Peptide-Based Approaches. J. Pers. Med. 2024, 14, 68. [Google Scholar] [CrossRef]
- Maute, R.L.; Gordon, S.R.; Mayer, A.T.; McCracken, M.N.; Natarajan, A.; Ring, N.G.; Kimura, R.; Tsai, J.M.; Manglik, A.; Kruse, A.C.; et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc. Natl. Acad. Sci. USA 2015, 112, E6506–E6514. [Google Scholar] [CrossRef]
- Westergren Jakobsson, A.; Segerman, B.; Wallerman, O.; Bergström Lind, S.; Zhao, H.; Rubin, C.-J.; Pettersson, U.; Akusjärvi, G. The Human Adenovirus 2 Transcriptome: An Amazing Complexity of Alternatively Spliced mRNAs. J. Virol. 2021, 95, e01869-20. [Google Scholar] [CrossRef] [PubMed]
- Donovan-Banfield, I.; Turnell, A.S.; Hiscox, J.A.; Leppard, K.N.; Matthews, D.A. Deep splicing plasticity of the human adenovirus type 5 transcriptome drives virus evolution. Commun. Biol. 2020, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Steinbock, R.T.; Lauman, R.; Charman, M.; Hayer, K.E.; Kumar, N.; Halko, E.; Lum, K.K.; Wei, M.; Wilson, A.C.; et al. Novel viral splicing events and open reading frames revealed by long-read direct RNA sequencing of adenovirus transcripts. PLoS Pathog. 2022, 18, e1010797. [Google Scholar] [CrossRef] [PubMed]
- Alemán, M.V.; Bertzbach, L.D.; Speiseder, T.; Ip, W.H.; González, R.A.; Dobner, T. Global Transcriptome Analyses of Cellular and Viral mRNAs during HAdV-C5 Infection Highlight New Aspects of Viral mRNA Biogenesis and Cytoplasmic Viral mRNA Accumulations. Viruses 2022, 14, 2428. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; Butowski, N.A. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neuro-Oncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef]
- Letchuman, V.; Ampie, L.; Shah, A.H.; Brown, D.A.; Heiss, J.D.; Chittiboina, P. Syngeneic murine glioblastoma models: Reactionary immune changes and immunotherapy intervention outcomes. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef]
- Puigdelloses, M.; Garcia-Moure, M.; Labiano, S.; Laspidea, V.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; De la Nava, D.; Ausejo, I.; et al. CD137 and PD-L1 targeting with immunovirotherapy induces a potent and durable antitumor immune response in glioblastoma models. J. Immunother. Cancer 2021, 9, e002644. [Google Scholar] [CrossRef]
- Vera, B.; Martínez-Vélez, N.; Xipell, E.; Acanda de la Rocha, A.; Patiño-García, A.; Saez-Castresana, J.; Gonzalez-Huarriz, M.; Cascallo, M.; Alemany, R.; Alonso, M.M. Characterization of the Antiglioma Effect of the Oncolytic Adenovirus VCN-01. PLoS ONE 2016, 11, e0147211. [Google Scholar] [CrossRef]
- Rivera-Molina, Y.; Jiang, H.; Fueyo, J.; Nguyen, T.; Shin, D.H.; Youssef, G.; Fan, X.; Gumin, J.; Alonso, M.M.; Phadnis, S.; et al. GITRL-armed Delta-24-RGD oncolytic adenovirus prolongs survival and induces anti-glioma immune memory. Neuro-Oncol. Adv. 2019, 1, vdz009. [Google Scholar] [CrossRef]
- Martínez-Vélez, N.; Garcia-Moure, M.; Marigil, M.; González-Huarriz, M.; Puigdelloses, M.; Gallego Pérez-Larraya, J.; Zalacaín, M.; Marrodán, L.; Varela-Guruceaga, M.; Laspidea, V.; et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat. Commun. 2019, 10, 2235. [Google Scholar] [CrossRef]
- Young, A.-M.; Archibald, K.M.; Tookman, L.A.; Pool, A.; Dudek, K.; Jones, C.; Williams, S.L.; Pirlo, K.J.; Willis, A.E.; Lockley, M.; et al. Failure of translation of human adenovirus mRNA in murine cancer cells can be partially overcome by L4-100K expression in vitro and in vivo. Mol. Ther. 2012, 20, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Garcia-Carbonero, R.; Laquente, B.; Álvarez, R.; Mato-Berciano, A.; Gimenez-Alejandre, M.; Morgado, S.; Rodríguez-García, A.; Maliandi, M.V.; Riesco, M.C.; et al. VCN-01 disrupts pancreatic cancer stroma and exerts antitumor effects. J. Immunother. Cancer 2021, 9, e003254. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quintanilla, J.; He, D.; Wakimoto, H.; Alemany, R.; Shah, K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol. Ther. 2015, 23, 108–118. [Google Scholar] [CrossRef]
- Kiyokawa, J.; Kawamura, Y.; Ghouse, S.M.; Acar, S.; Barçın, E.; Martínez-Quintanilla, J.; Martuza, R.L.; Alemany, R.; Rabkin, S.D.; Shah, K.; et al. Modification of Extracellular Matrix Enhances Oncolytic Adenovirus Immunotherapy in Glioblastoma. Clin. Cancer Res. 2021, 27, 889–902. [Google Scholar] [CrossRef]
- Martínez-Vélez, N.; Xipell, E.; Vera, B.; Acanda de la Rocha, A.; Zalacain, M.; Marrodán, L.; Gonzalez-Huarriz, M.; Toledo, G.; Cascallo, M.; Alemany, R.; et al. The Oncolytic Adenovirus VCN-01 as Therapeutic Approach Against Pediatric Osteosarcoma. Clin. Cancer Res. 2016, 22, 2217–2225. [Google Scholar] [CrossRef]
- Guedan, S.; Rojas, J.J.; Gros, A.; Mercade, E.; Cascallo, M.; Alemany, R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010, 18, 1275–1283. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Giménez-Alejandre, M.; Rojas, J.J.; Moreno, R.; Bazan-Peregrino, M.; Cascalló, M.; Alemany, R. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015, 21, 1406–1418. [Google Scholar] [CrossRef]
- Pascual-Pasto, G.; Bazan-Peregrino, M.; Olaciregui, N.G.; Restrepo-Perdomo, C.A.; Mato-Berciano, A.; Ottaviani, D.; Weber, K.; Correa, G.; Paco, S.; Vila-Ubach, M.; et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 2019, 11, eaat9321. [Google Scholar] [CrossRef]
- Scholthof, H.B. The Tombusvirus-encoded P19: From irrelevance to elegance. Nat. Rev. Microbiol. 2006, 4, 405–411. [Google Scholar] [CrossRef]
- Danielson, D.C.; Pezacki, J.P. Studying the RNA silencing pathway with the p19 protein. FEBS Lett. 2013, 587, 1198–1205. [Google Scholar] [CrossRef]
- Rauschhuber, C.; Mueck-Haeusl, M.; Zhang, W.; Nettelbeck, D.M.; Ehrhardt, A. RNAi suppressor P19 can be broadly exploited for enhanced adenovirus replication and microRNA knockdown experiments. Sci. Rep. 2013, 3, 1363. [Google Scholar] [CrossRef] [PubMed]
- Doerner, J.; Sallard, E.; Zhang, W.; Solanki, M.; Liu, J.; Ehrke-Schulz, E.; Zirngibl, H.; Lieber, A.; Ehrhardt, A. Novel Group C Oncolytic Adenoviruses Carrying a miRNA Inhibitor Demonstrate Enhanced Oncolytic Activity In Vitro and In Vivo. Mol. Cancer Ther. 2022, 21, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Zhao, Z.; Arooj, S.; Fu, Y.; Liao, G. Soluble PD-1: Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front. Immunol. 2020, 11, 587460. [Google Scholar] [CrossRef]
- Moss, M.J.; Chamness, L.M.; Clark, P.L. The Effects of Codon Usage on Protein Structure and Folding. Annu. Rev. Biophys. 2024, 53, 87–108. [Google Scholar] [CrossRef]
- Paremskaia, A.I.; Kogan, A.A.; Murashkina, A.; Naumova, D.A.; Satish, A.; Abramov, I.S.; Feoktistova, S.G.; Mityaeva, O.N.; Deviatkin, A.A.; Volchkov, P.Y. Codon-optimization in gene therapy: Promises, prospects and challenges. Front. Bioeng. Biotechnol. 2024, 12, 1371596. [Google Scholar] [CrossRef]
- Komar, A.A. A Code Within a Code: How Codons Fine-Tune Protein Folding in the Cell. Biochemistry 2021, 86, 976–991. [Google Scholar] [CrossRef]
- Love, A.M.; Nair, N.U. Specific codons control cellular resources and fitness. Sci. Adv. 2024, 10, eadk3485. [Google Scholar] [CrossRef]
- Magiera-Mularz, K.; Kocik, J.; Musielak, B.; Plewka, J.; Sala, D.; Machula, M.; Grudnik, P.; Hajduk, M.; Czepiel, M.; Siedlar, M.; et al. Human and mouse PD-L1: Similar molecular structure, but different druggability profiles. iScience 2021, 24, 101960. [Google Scholar] [CrossRef]
- Bartee, M.Y.; Dunlap, K.M.; Bartee, E. Tumor-Localized Secretion of Soluble PD1 Enhances Oncolytic Virotherapy. Cancer Res. 2017, 77, 2952–2963. [Google Scholar] [CrossRef]
- Shin, S.-P.; Seo, H.-H.; Shin, J.-H.; Park, H.-B.; Lim, D.-P.; Eom, H.-S.; Bae, Y.-S.; Kim, I.-H.; Choi, K.; Lee, S.-J. Adenovirus expressing both thymidine kinase and soluble PD1 enhances antitumor immunity by strengthening CD8 T-cell response. Mol. Ther. 2013, 21, 688–695. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wei, M.; Mou, T.; Shi, T.; Ma, Y.; Cai, X.; Li, Y.; Dong, J.; Wei, J. Recombinant Adenovirus Expressing a Soluble Fusion Protein PD-1/CD137L Subverts the Suppression of CD8+ T Cells in HCC. Mol. Ther. 2019, 27, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chekhonin, V.P. A new insight into aggregation of oncolytic adenovirus Ad5-delta-24-RGD during CsCl gradient ultracentrifugation. Sci. Rep. 2021, 11, 16088. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, F.; Hagedorn, C.; Kreppel, F. Combined Genetic and Chemical Capsid Modifications of Adenovirus-Based Gene Transfer Vectors for Shielding and Targeting. J. Vis. Exp. 2018, 140, 58480. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Vasiukova, A.A.; Abramova, O.V.; Lipatova, A.V.; Yusubalieva, G.M.; Chekhonin, V.P. Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes. Int. J. Mol. Sci. 2025, 26, 3700. https://doi.org/10.3390/ijms26083700
Stepanenko AA, Sosnovtseva AO, Valikhov MP, Vasiukova AA, Abramova OV, Lipatova AV, Yusubalieva GM, Chekhonin VP. Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes. International Journal of Molecular Sciences. 2025; 26(8):3700. https://doi.org/10.3390/ijms26083700
Chicago/Turabian StyleStepanenko, Aleksei A., Anastasiia O. Sosnovtseva, Marat P. Valikhov, Anastasiia A. Vasiukova, Olga V. Abramova, Anastasiia V. Lipatova, Gaukhar M. Yusubalieva, and Vladimir P. Chekhonin. 2025. "Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes" International Journal of Molecular Sciences 26, no. 8: 3700. https://doi.org/10.3390/ijms26083700
APA StyleStepanenko, A. A., Sosnovtseva, A. O., Valikhov, M. P., Vasiukova, A. A., Abramova, O. V., Lipatova, A. V., Yusubalieva, G. M., & Chekhonin, V. P. (2025). Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes. International Journal of Molecular Sciences, 26(8), 3700. https://doi.org/10.3390/ijms26083700




