Circadian Rhythm Dysregulation in Inflammatory Bowel Disease: Mechanisms and Chronotherapeutic Approaches
Abstract
1. Introduction
2. Circadian Rhythms
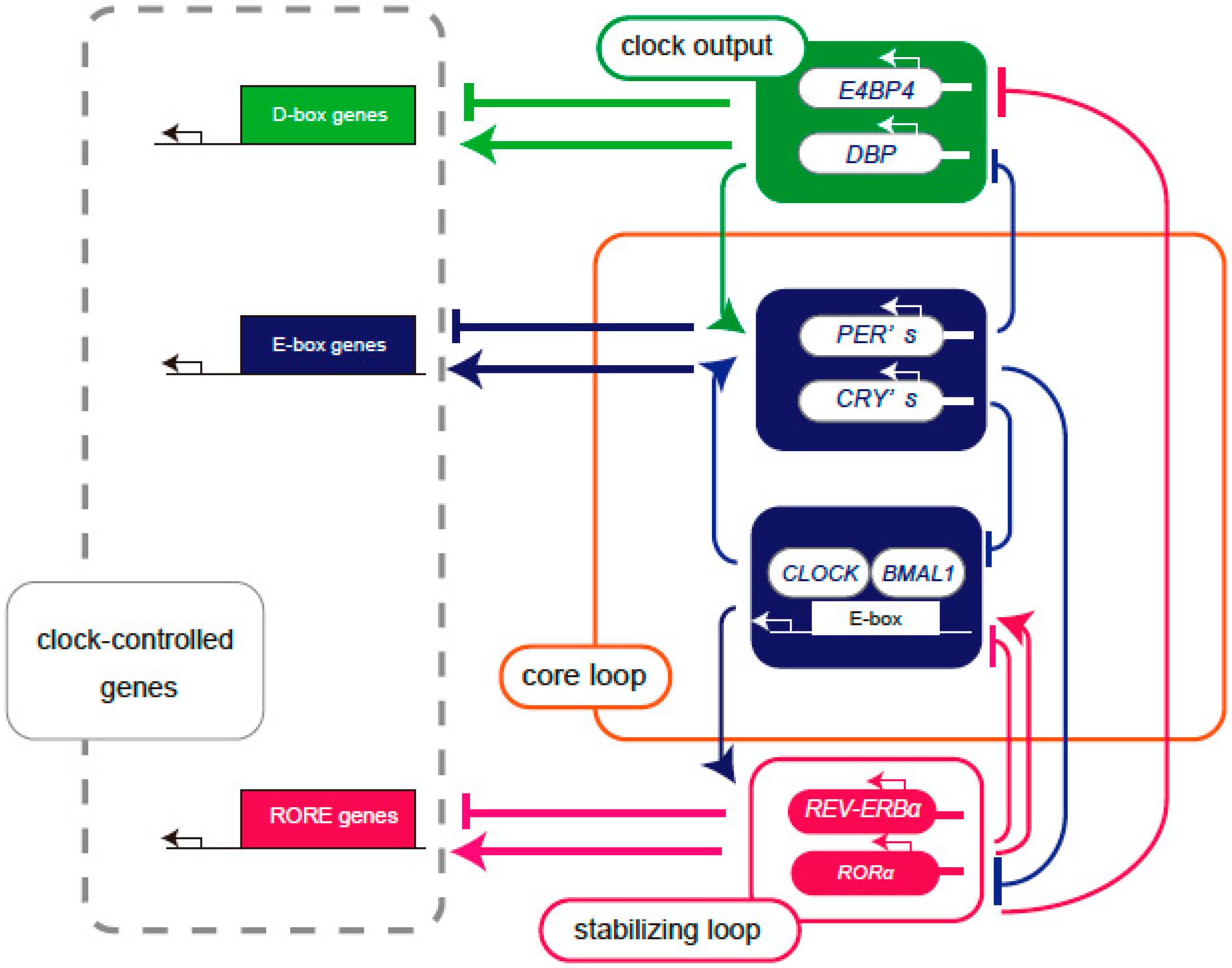
2.1. Molecular Mechanisms Underlying Circadian Rhythms
2.2. Central and Peripheral Clock
2.3. Circadian Rhythms and Immune Function
3. IBD
3.1. Symptoms and Clinical Features
3.2. Diagnosis and Evaluation
3.3. Treatment
3.4. Management
4. The Relationship Between the Intestine and Circadian Rhythm
4.1. Circadian Rhythm Regulation in the Intestinal Tract
4.2. Altered Clock Gene Expression in Patients with IBD
4.3. The Role of E4BP4 in IBD Pathogenesis
5. Treatment Strategies Targeting the Circadian Rhythm
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARNTL | aryl hydrocarbon receptor nuclear translocator like, known as BMAL1 |
| BMAL1 | brain and muscle Arnt-like protein 1 |
| CD | Crohn’s disease |
| CDAI | Crohn’s Disease Activity Index |
| CLOCK | circadian locomotor output cycles kaput, paralogous to NPAS2 |
| CRY | cryptochromes |
| DBP | D-box binding protein |
| DSS | dextran sulfate sodium |
| E4BP4 | E4 promoter-binding protein 4 |
| EIMs | extraintestinal manifestations |
| HBI | Harvey Bradshaw Index |
| HLF | hepatic leukemia factor |
| HPA | hypothalamic-pituitary-adrenal |
| IBD | inflammatory bowel disease |
| IF | intermittent fasting |
| ILC | innate lymphoid cell |
| JAK | Janus kinase |
| NFIL3 | nuclear factor, interleukin 3 regulated protein, known as E4BP4 |
| NR1D1 | nuclear receptor subfamily 1 group D member 1 |
| PEPT1 | peptide cotransporter 1 |
| PER | periods |
| REV-ERBα | reverse erythroblastosis virus-α, known as NR1D1 |
| RRE | ROR/REV-ERB response element |
| ROR | retinoic acid receptor-related orphan receptor |
| SCN | suprachiasmatic nucleus |
| TEF | thyrotroph embryonic factor |
| TLR 9 | Toll-like receptor 9 |
| TRE | time-restricted eating |
| TRF | time-restricted feeding |
| UA | Urolithin A |
| UC | ulcerative colitis |
References
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Giebfried, J.; Lorentz, A. Relationship between the Biological Clock and Inflammatory Bowel Disease. Clocks Sleep 2023, 5, 260–275. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef]
- Liu, X.; Yu, R.; Zhu, L.; Hou, X.; Zou, K. Bidirectional Regulation of Circadian Disturbance and Inflammation in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1741–1751. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Gombert, M. Circadian rhythms in the pathogenesis of gastrointestinal diseases. World J. Gastroenterol. 2018, 24, 4297–4303. [Google Scholar] [CrossRef]
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Lee, C.; Etchegaray, J.P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Ohta, Y.; Nagao, Y.; Tanizawa, Y. The roles of output clock genes in regulating glucose metabolism. J. Diabetes Investig. 2024, 15, 1707. [Google Scholar] [CrossRef] [PubMed]
- Yamajuku, D.; Shibata, Y.; Kitazawa, M.; Katakura, T.; Urata, H.; Kojima, T.; Takayasu, S.; Nakata, O.; Hashimoto, S. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011, 585, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Tousson, E.; Meissl, H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2004, 24, 2983–2988. [Google Scholar] [CrossRef]
- Guo, H.; Brewer, J.M.; Lehman, M.N.; Bittman, E.L. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: Effects of transplanting the pacemaker. J. Neurosci. 2006, 26, 6406–6412. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Segal, E.; Elinav, E. A day in the life of the meta-organism: Diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 2015, 6, 137–142. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef] [PubMed]
- Shimba, A.; Cui, G.; Tani-Ichi, S.; Ogawa, M.; Abe, S.; Okazaki, F.; Kitano, S.; Miyachi, H.; Yamada, H.; Hara, T.; et al. Glucocorticoids Drive Diurnal Oscillations in T Cell Distribution and Responses by Inducing Interleukin-7 Receptor and CXCR4. Immunity 2018, 48, 286–298.e6. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hayano, Y.; Nakai, A.; Furuta, F.; Noda, M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J. Exp. Med. 2016, 213, 2567–2574. [Google Scholar] [CrossRef]
- Silva-Sanchez, A.; Randall, T.D. Fugue G Minor: Getting the Lymph Node Ensemble Together with Circadian Rhythm. Immunity 2017, 46, 6–8. [Google Scholar] [CrossRef]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef]
- Waggoner, S.N. Circadian Rhythms in Immunity. Curr. Allergy Asthma Rep. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Seillet, C.; Rankin, L.C.; Groom, J.R.; Mielke, L.A.; Tellier, J.; Chopin, M.; Huntington, N.D.; Belz, G.T.; Carotta, S. Nfil3 is required for the development of all innate lymphoid cell subsets. J. Exp. Med. 2014, 211, 1733–1740. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Ponder, A.; Long, M.D. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin. Epidemiol. 2013, 5, 237–247. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-L.; Bao, J.-C.; Liao, X.-Y.; Chen, Y.-J.; Wang, L.-W.; Fan, Y.-Y.; Xu, Q.-Y.; Hao, L.-X.; Li, K.-J.; Liang, M.-X.; et al. Trends and projections of inflammatory bowel disease at the global, regional and national levels, 1990–2050: A bayesian age-period-cohort modeling study. BMC Public Health 2023, 23, 2507. [Google Scholar] [CrossRef] [PubMed]
- Calkins, B.M.; Lilienfeld, A.M.; Garland, C.F.; Mendeloff, A.I. Trends in incidence rates of ulcerative colitis and Crohn’s disease. Dig. Dis. Sci. 1984, 29, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.K.; Ding, N.S.; Niewiadomski, O. Management of inflammatory bowel disease. Med. J. Aust. 2018, 209, 318–323. [Google Scholar] [CrossRef]
- D’Amico, F.; Fiorino, G.; Furfaro, F.; Allocca, M.; Roda, G.; Loy, L.; Zilli, A.; Solitano, V.; Peyrin-Biroulet, L.; Danese, S. Patient’s profiling for therapeutic management of inflammatory bowel disease: A tailored approach. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 765–773. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Murakami, M.; Tognini, P.; Liu, Y.; Eckel-Mahan, K.L.; Baldi, P.; Sassone-Corsi, P. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016, 17, 1292–1303. [Google Scholar] [CrossRef]
- Pácha, J.; Sumová, A. Circadian regulation of epithelial functions in the intestine. Acta Physiol. 2013, 208, 11–24. [Google Scholar] [CrossRef]
- Froy, O.; Chapnik, N.; Miskin, R. Mouse intestinal cryptdins exhibit circadian oscillation. FASEB J. 2005, 19, 1920–1922. [Google Scholar] [CrossRef]
- Oh-oka, K.; Kono, H.; Ishimaru, K.; Miyake, K.; Kubota, T.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: Implications in intestinal permeability and susceptibility to colitis. PLoS ONE 2014, 20, e98016. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Terada, T.; Shimakura, J.; Katsura, T.; Inui, K. Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1). Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G395–G402. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, N.R.; Sitren, H.S.; Furuya, S. Circadian rhythmicity in several small intestinal functions is independent of use of the intestine. Am. J. Physiol. 1980, 238, G203–G207. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Palmieri, O.; Corritore, G.; Latiano, T.; Bossa, F.; Scimeca, D.; Biscaglia, G.; Valvano, M.R.; D’Incà, R.; Cucchiara, S.; et al. Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol. Int. 2012, 29, 994–1003. [Google Scholar] [CrossRef]
- Wang, D.; Yin, H.; Wang, X.; Wang, Z.; Han, M.; He, Q.; Chen, J.; Xian, H.; Zhang, B.; Wei, X.; et al. Influence of sleep disruption on inflammatory bowel disease and changes in circadian rhythm genes. Heliyon 2022, 8, e11229. [Google Scholar] [CrossRef]
- Palmieri, O.; Mazzoccoli, G.; Bossa, F.; Maglietta, R.; Palumbo, O.; Ancona, N.; Corritore, G.; Latiano, T.; Martino, G.; Rubino, R.; et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol. Int. 2015, 32, 903–916. [Google Scholar] [CrossRef]
- Labes, S.; Froy, O.; Tabach, Y.; Shamir, R.; Shouval, D.S.; Weintraub, Y. Mucosal Genes Encoding Clock, Inflammation and Their Mutual Regulators Are Disrupted in Pediatric Patients with Active Ulcerative Colitis. Int. J. Mol. Sci. 2024, 25, 1488. [Google Scholar] [CrossRef]
- Weintraub, Y.; Cohen, S.; Chapnik, N.; Ben-Tov, A.; Yerushalmy-Feler, A.; Dotan, I.; Tauman, R.; Froy, O. Clock Gene Disruption Is an Initial Manifestation of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 115–122.e1. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, C.; Wu, R.; Kang, D.; Gao, H.; Lv, H.; Feng, Z.; Shi, Y.; Liu, Z.; Chen, L. Circadian clock component PER2 negatively regulates CD4+ T cell IFN-γ production in ulcerative colitis. Mucosal Immunol. 2024, 17, 1161–1173. [Google Scholar] [CrossRef]
- Mosna, K.; Janega, P.; Sedlak, J.; Babal, P. Complex changes of circadian proteins expression in inflammatory bowel disease. Bratisl. Lek. Listy. 2021, 122, 235–241. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Han, X.; Tian, D.; Yan, W.; Liu, M.; Cao, L. Circadian rhythm disruption-mediated downregulation of Bmal1 exacerbates DSS-induced colitis by impairing intestinal barrier. Front. Immunol. 2024, 15, 1402395. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, D.; Masoumi, S.J.; Mohammad-Kazem Hosseini Asl, S.; Labbe, A.; Razeghian-Jahromi, I.; Fararouei, M.; Lankarani, K.B.; Dara, M. Effects of short-chain fatty acid-butyrate supplementation on expression of circadian-clock genes, sleep quality, and inflammation in patients with active ulcerative colitis: A double-blind randomized controlled trial. Lipids Health Dis. 2024, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Male, V.; Nisoli, I.; Gascoyne, D.M.; Brady, H.J. E4BP4: An unexpected player in the immune response. Trends Immunol. 2012, 33, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, J.; Lu, Q. The role of basic leucine zipper transcription factor E4BP4 in the immune system and immune-mediated diseases. Clin. Immunol. 2017, 180, 5–10. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuoka, K.; Sheikh, S.Z.; Elloumi, H.Z.; Kamada, N.; Hisamatsu, T.; Hansen, J.J.; Doty, K.R.; Pope, S.D.; Smale, S.T.; et al. NFIL3 Is a Regulator of IL-12 p40 in Macrophages and Mucosal Immunity. J. Immunol. 2011, 186, 4649–4655. [Google Scholar] [CrossRef]
- Kajimura, Y.; Taguchi, A.; Nagao, Y.; Yamamoto, K.; Masuda, K.; Shibata, K.; Asaoka, Y.; Furutani-Seiki, M.; Tanizawa, Y.; Ohta, Y. E4BP4 in macrophages induces an anti-inflammatory phenotype that ameliorates the severity of colitis. Commun. Biol. 2024, 7, 527. [Google Scholar] [CrossRef]
- Burgess, H.J.; Bahl, S.; Wilensky, K.; Spence, E.; Jouppi, R.J.; Rizvydeen, M.; Goldstein, C.; Kim, H.M.; Williams, D.A.; Burns, J.W. A 4-week morning light treatment with stable sleep timing for individuals with fibromyalgia: A randomized controlled trial. Pain Med. 2023, 24, 787–795. [Google Scholar] [CrossRef]
- Cohen-Mekelburg, S.; Goldstein, C.A.; Rizvydeen, M.; Fayyaz, Z.; Patel, P.J.; Berinstein, J.A.; Bishu, S.; Cushing-Damm, K.C.; Kim, H.M.; Burgess, H.J. Morning light treatment for inflammatory bowel disease: A clinical trial. BMC Gastroenterol. 2024, 24, 179. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Imai, S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim. Biophys. Acta 2010, 1804, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, L.; Bai, M.; Wang, S.; Ye, X.; Lin, Y.; Luo, X.; Li, Z.; Zhang, L.; Zhu, X.; et al. Time-restricted feeding ameliorates dextran sulfate sodium-induced colitis via reducing intestinal inflammation. Front. Nutr. 2022, 9, 1043783. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Haghdani, S.; Emami, M.H.; Adilipour, H.; Tavakkoli, M.; Tavakkoli, M. Ramadan fasting and inflammatory bowel disease. Indian J. Gastroenterol. 2008, 27, 239–241. [Google Scholar]
- Niu, Y.; Heddes, M.; Altaha, B.; Birkner, M.; Kleigrewe, K.; Meng, C.; Haller, D.; Kiessling, S. Targeting the intestinal circadian clock by meal timing ameliorates gastrointestinal inflammation. Cell Mol. Immunol. 2024, 21, 842–855. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Kajiwara, S.; Orihara, K. Effect of Urolithin A on the Improvement of Circadian Rhythm Dysregulation in Intestinal Barrier Induced by Inflammation. Nutrients 2024, 13, 2263. [Google Scholar] [CrossRef] [PubMed]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Igaki, K.; Nakamura, Y.; Tanaka, M.; Mizuno, S.; Yoshimatsu, Y.; Komoike, Y.; Uga, K.; Shibata, A.; Imaichi, H.; Takayuki, S.; et al. Pharmacological effects of TAK-828F: An orally available RORγt inverse agonist, in mouse colitis model and human blood cells of inflammatory bowel disease. Inflamm. Res. 2019, 68, 493–509. [Google Scholar] [CrossRef]
- Igaki, K.; Nakamura, Y.; Komoike, Y.; Uga, K.; Shibata, A.; Ishimura, Y.; Yamasaki, M.; Tsukimi, Y.; Tsuchimori, N. Pharmacological Evaluation of TAK-828F, a Novel Orally Available RORγt Inverse Agonist, on Murine Colitis Model. Inflammation 2019, 42, 91–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao, Y.; Taguchi, A.; Ohta, Y. Circadian Rhythm Dysregulation in Inflammatory Bowel Disease: Mechanisms and Chronotherapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 3724. https://doi.org/10.3390/ijms26083724
Nagao Y, Taguchi A, Ohta Y. Circadian Rhythm Dysregulation in Inflammatory Bowel Disease: Mechanisms and Chronotherapeutic Approaches. International Journal of Molecular Sciences. 2025; 26(8):3724. https://doi.org/10.3390/ijms26083724
Chicago/Turabian StyleNagao, Yuko, Akihiko Taguchi, and Yasuharu Ohta. 2025. "Circadian Rhythm Dysregulation in Inflammatory Bowel Disease: Mechanisms and Chronotherapeutic Approaches" International Journal of Molecular Sciences 26, no. 8: 3724. https://doi.org/10.3390/ijms26083724
APA StyleNagao, Y., Taguchi, A., & Ohta, Y. (2025). Circadian Rhythm Dysregulation in Inflammatory Bowel Disease: Mechanisms and Chronotherapeutic Approaches. International Journal of Molecular Sciences, 26(8), 3724. https://doi.org/10.3390/ijms26083724




