Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice
Abstract
:1. Introduction
2. Results
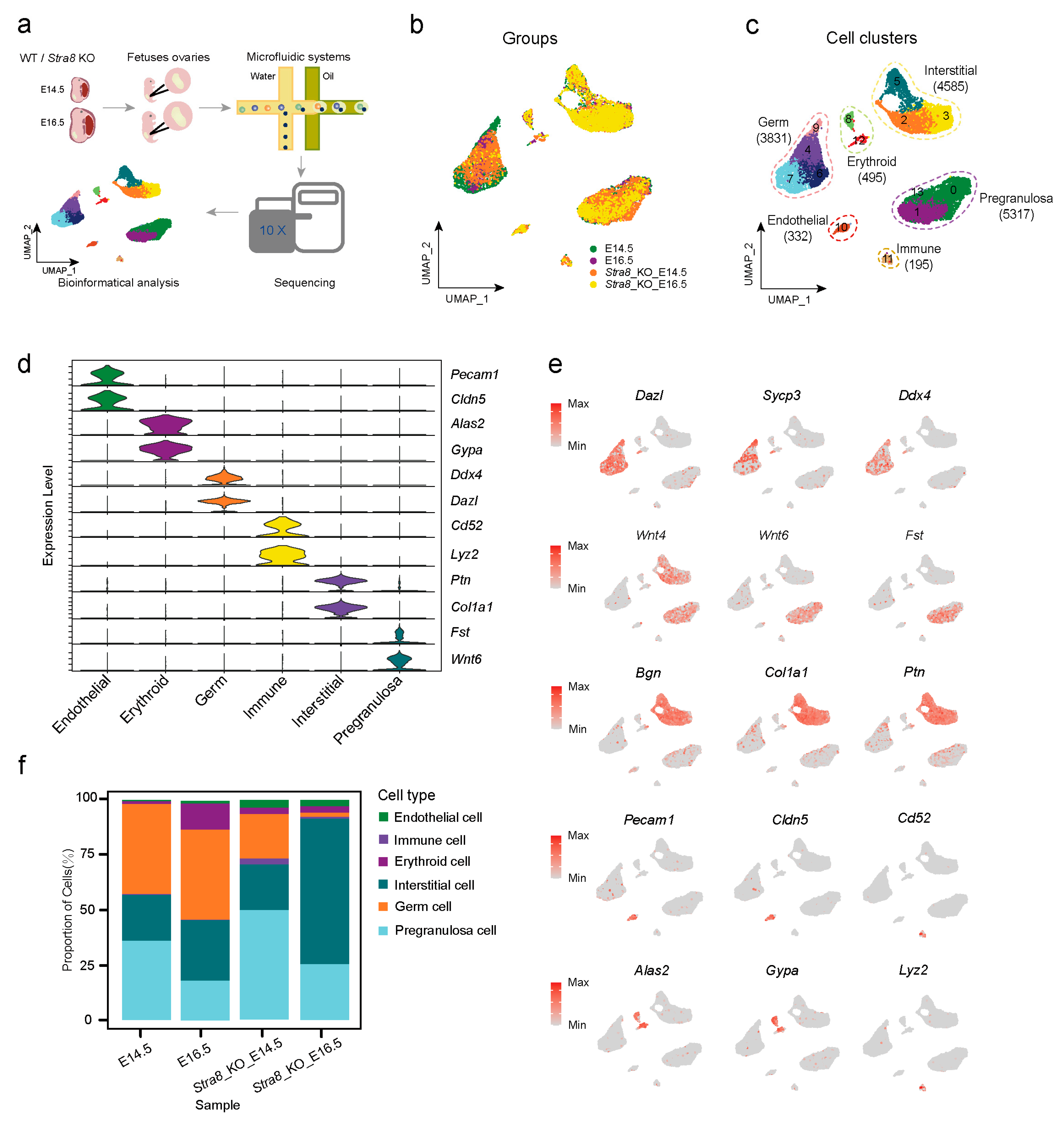
2.1. Construction of Single-Cell Transcriptional Atlases of Ovaries in WT and Stra8-Deficient Mice
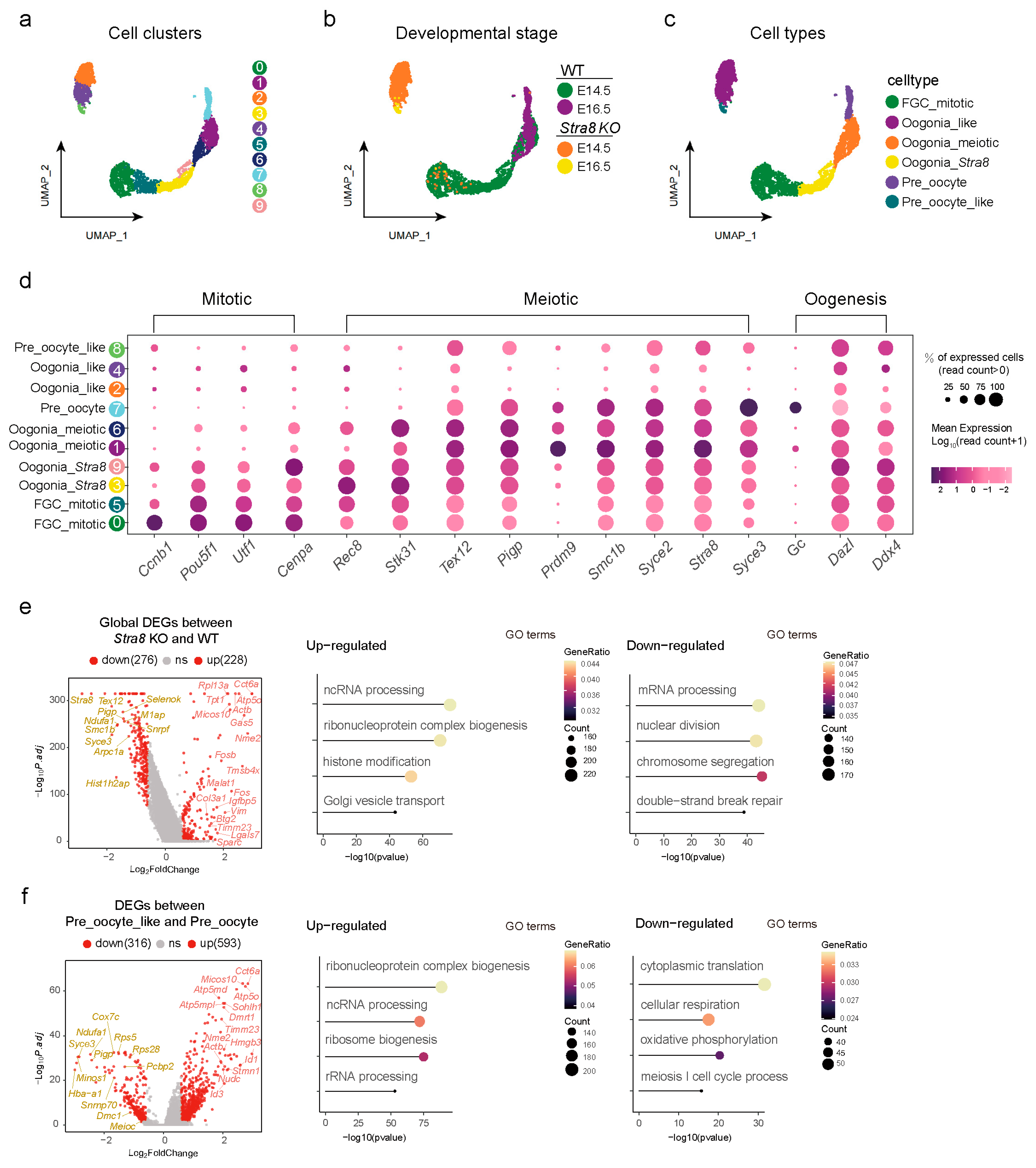
2.2. Analysis of Differentially Expressed Genes (DEGs) Among Germ Cell Subsets
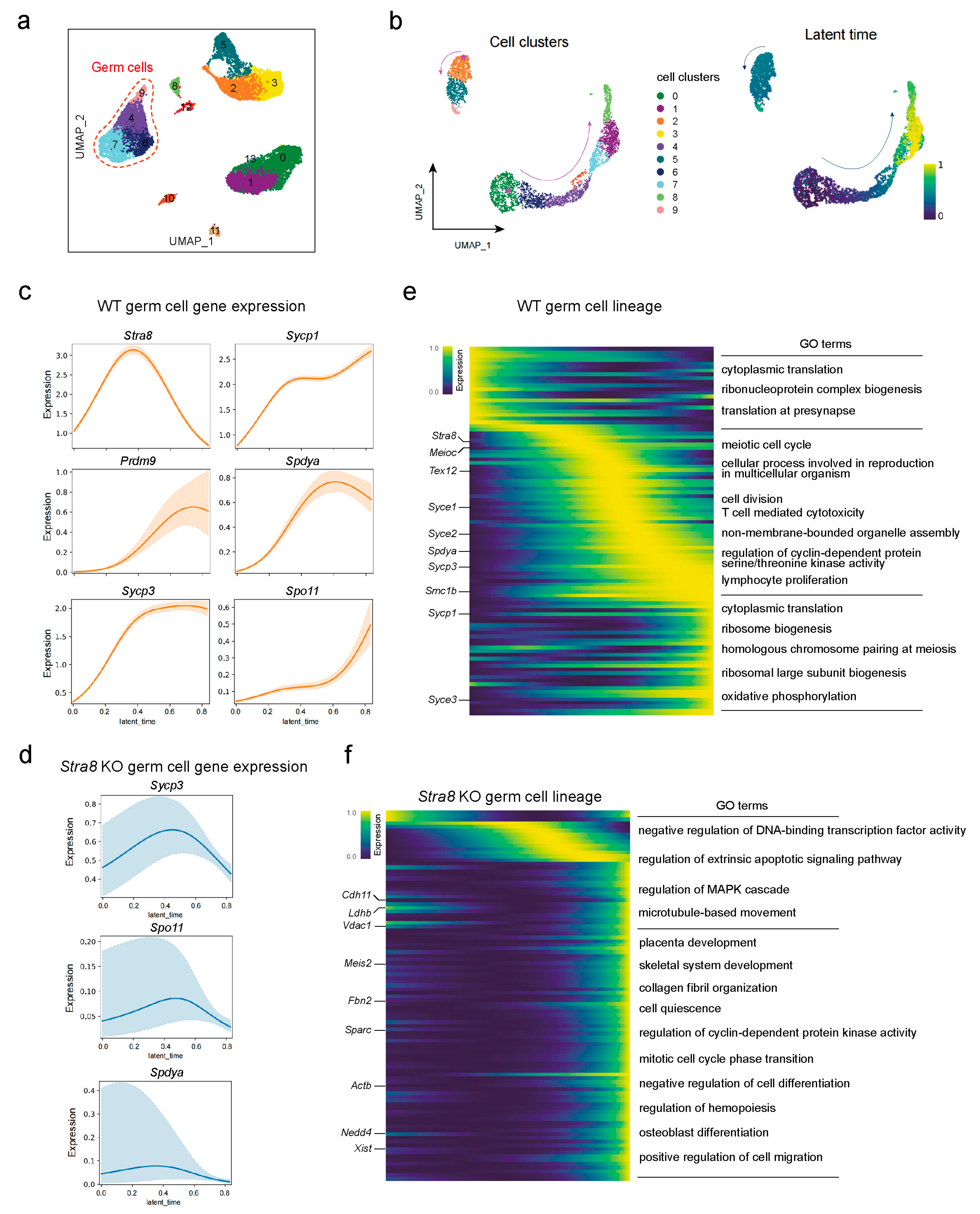
2.3. Differential Analysis of Germ Cell Fate Trajectories Between WT and Stra8-Deficient Mice
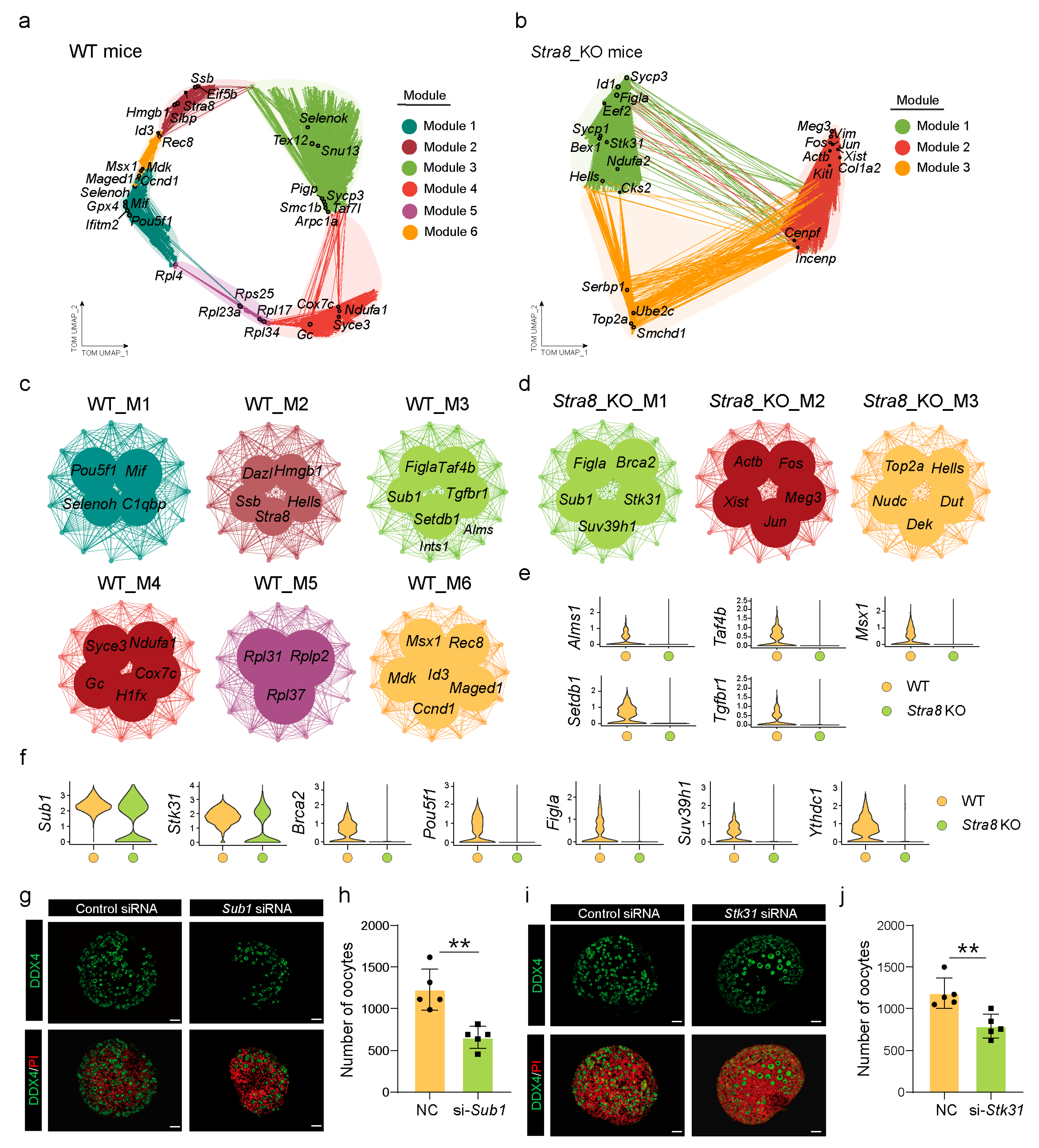
2.4. Construction of Gene Co-Expression Networks to Identify Key Genes Involved in Follicle Formation
2.5. Decreased Mdk–Sdc1 Ligand–Receptor Signaling Affected Primordial Follicle Formation in Stra8-Deficient Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. In Vitro Isolation of Genital Ridges
4.3. Mouse Genotyping
4.4. Preparation of Single-Cell Suspensions
4.5. Single-Cell Library Preparation and Sequencing
4.6. Raw Data Processing
4.7. Single Sample Analysis and Aggregation
4.8. Identification of DEGs and Gene Enrichment Analysis
4.9. Cell Differentiation Trajectory Analysis
4.10. hdWGCNA Constructed a Gene Co-Expression Network
4.11. Interactions Between Different Cell Types
4.12. In Vitro Ovarian Culture
4.13. In Vitro siRNA
4.14. Ovarian RNA Extraction and Real-Time Quantitative PCR
4.15. Ovarian Immunofluorescence
4.16. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, H.; Liu, B.; Wang, H.; Sun, G.; Feng, L.; Chen, Z.; Zhou, J.; Zhang, J.; Zhang, T.; He, M.; et al. SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice. J. Mol. Cell Biol. 2020, 12, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Byskov, A.G.; Himelstein-Braw, R.; Faber, M. Follicular growth: The basic event in the mouse and human ovary. J. Reprod. Fertil. 1975, 45, 559–566. [Google Scholar] [CrossRef]
- Tingen, C.; Kim, A.; Woodruff, T.K. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod. 2009, 15, 795–803. [Google Scholar] [CrossRef]
- Pepling, M.E. From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 2006, 44, 622–632. [Google Scholar] [CrossRef]
- Kerr, J.B.; Duckett, R.; Myers, M.; Britt, K.L.; Mladenovska, T.; Findlay, J.K. Quantification of healthy follicles in the neonatal and adult mouse ovary: Evidence for maintenance of primordial follicle supply. Reproduction 2006, 132, 95–109. [Google Scholar] [CrossRef]
- Pepling, M.E.; Spradling, A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001, 234, 339–351. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, C.; Li, X.; Li, J.; Chen, X.; Bin, L.; Hu, R. PAI-1 siRNA-loaded biomimetic nanoparticles for ameliorating diminished ovarian reserve and inhibiting ovarian fibrosis. Eur. J. Pharmacol. 2024, 983, 176948. [Google Scholar] [CrossRef]
- Rezaei, M.; Liang, M.; Yalcin, Z.; Martin, J.H.; Kazemi, P.; Bareke, E.; Ge, Z.J.; Fardaei, M.; Benadiva, C.; Hemida, R.; et al. Defects in meiosis I contribute to the genesis of androgenetic hydatidiform moles. J. Clin. Investig. 2024, 134, e170669. [Google Scholar] [CrossRef]
- Rajkovic, A.; Pangas, S.A.; Ballow, D.; Suzumori, N.; Matzuk, M.M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004, 305, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Bjarkadottir, B.D.; Nadjaja, D.; Sheikh, S.; Fatum, M.; Lane, S.; Williams, S.A. Effect of AMH on primordial follicle populations in mouse ovaries and human pre-pubertal ovarian xenografts during doxorubicin treatment. Front. Cell Dev. Biol. 2024, 12, 1449156. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Condrea, D.; Mayere, C.; Féret, B.; Klopfenstein, M.; Magnant, W.; Alunni, V.; Teletin, M.; Souali-Crespo, S.; Nef, S.; et al. Meiosis occurs normally in the fetal ovary of mice lacking all retinoic acid receptors. Sci. Adv. 2020, 6, eaaz1139. [Google Scholar] [CrossRef] [PubMed]
- Menke, D.B.; Koubova, J.; Page, D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 2003, 262, 303–312. [Google Scholar] [CrossRef]
- Adams, I.R.; McLaren, A. Sexually dimorphic development of mouse primordial germ cells: Switching from oogenesis to spermatogenesis. Development 2002, 129, 1155–1164. [Google Scholar] [CrossRef]
- Yao, H.H.; DiNapoli, L.; Capel, B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 2003, 130, 5895–5902. [Google Scholar] [CrossRef]
- Shimada, R.; Ishiguro, K.I. Female-specific mechanisms of meiotic initiation and progression in mammalian oocyte development. Genes Cells Devoted Mol. Cell. Mech. 2024, 29, 797–807. [Google Scholar] [CrossRef]
- Wu, Q.; Fukuda, K.; Kato, Y.; Zhou, Z.; Deng, C.X.; Saga, Y. Sexual Fate Change of XX Germ Cells Caused by the Deletion of SMAD4 and STRA8 Independent of Somatic Sex Reprogramming. PLoS Biol. 2016, 14, e1002553. [Google Scholar] [CrossRef]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef]
- Koubova, J.; Hu, Y.C.; Bhattacharyya, T.; Soh, Y.Q.; Gill, M.E.; Goodheart, M.L.; Hogarth, C.A.; Griswold, M.D.; Page, D.C. Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet. 2014, 10, e1004541. [Google Scholar] [CrossRef]
- Koubova, J.; Menke, D.B.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Shimada, R.; Kato, Y.; Takeda, N.; Fujimura, S.; Yasunaga, K.I.; Usuki, S.; Niwa, H.; Araki, K.; Ishiguro, K.I. STRA8-RB interaction is required for timely entry of meiosis in mouse female germ cells. Nat. Commun. 2023, 14, 6443. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.I.; Matsuura, K.; Tani, N.; Takeda, N.; Usuki, S.; Yamane, M.; Sugimoto, M.; Fujimura, S.; Hosokawa, M.; Chuma, S.; et al. MEIOSIN Directs the Switch from Mitosis to Meiosis in Mammalian Germ Cells. Dev. Cell 2020, 52, 429–445.e410. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Garcia-Rudaz, C.; Kerr, B.; Tapia, V.; Dissen, G.A.; Costa, M.E.; Cornea, A.; Ojeda, S.R. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology 2005, 146, 5267–5277. [Google Scholar] [CrossRef]
- Bai, L.; Li, P.; Xiang, Y.; Jiao, X.; Chen, J.; Song, L.; Liang, Z.; Liu, Y.; Zhu, Y.; Lu, L.Y. BRCA1 safeguards genome integrity by activating chromosome asynapsis checkpoint to eliminate recombination-defective oocytes. Proc. Natl. Acad. Sci. USA 2024, 121, e2401386121. [Google Scholar] [CrossRef]
- Rinaldi, V.D.; Bolcun-Filas, E.; Kogo, H.; Kurahashi, H.; Schimenti, J.C. The DNA Damage Checkpoint Eliminates Mouse Oocytes with Chromosome Synapsis Failure. Mol. Cell 2017, 67, 1026–1036.e1022. [Google Scholar] [CrossRef]
- Ravindranathan, R.; Raveendran, K.; Papanikos, F.; San-Segundo, P.A.; Tóth, A. Chromosomal synapsis defects can trigger oocyte apoptosis without elevating numbers of persistent DNA breaks above wild-type levels. Nucleic Acids Res. 2022, 50, 5617–5634. [Google Scholar] [CrossRef]
- Dokshin, G.A.; Baltus, A.E.; Eppig, J.J.; Page, D.C. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat. Genet. 2013, 45, 877–883. [Google Scholar] [CrossRef]
- Gao, D.; Jin, F.; Zhou, M.; Jiang, Y. Recent advances in single cell manipulation and biochemical analysis on microfluidics. Analyst 2019, 144, 766–781. [Google Scholar] [CrossRef]
- Ge, W.; Wang, J.J.; Zhang, R.Q.; Tan, S.J.; Zhang, F.L.; Liu, W.X.; Li, L.; Sun, X.F.; Cheng, S.F.; Dyce, P.W.; et al. Dissecting the initiation of female meiosis in the mouse at single-cell resolution. Cell. Mol. Life Sci. CMLS 2021, 78, 695–713. [Google Scholar] [CrossRef]
- Wang, J.J.; Ge, W.; Zhai, Q.Y.; Liu, J.C.; Sun, X.W.; Liu, W.X.; Li, L.; Lei, C.Z.; Dyce, P.W.; De Felici, M.; et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 2020, 18, e3001025. [Google Scholar] [CrossRef] [PubMed]
- Haston, K.M.; Tung, J.Y.; Reijo Pera, R.A. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS ONE 2009, 4, e5654. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Tilly, J.L. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat. Protoc. 2013, 8, 966–988. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A. Germ and somatic cell lineages in the developing gonad. Mol. Cell. Endocrinol. 2000, 163, 3–9. [Google Scholar] [CrossRef]
- Jameson, S.A.; Natarajan, A.; Cool, J.; DeFalco, T.; Maatouk, D.M.; Mork, L.; Munger, S.C.; Capel, B. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012, 8, e1002575. [Google Scholar] [CrossRef]
- Brennan, J.; Karl, J.; Capel, B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev. Biol. 2002, 244, 418–428. [Google Scholar] [CrossRef]
- Jeays-Ward, K.; Hoyle, C.; Brennan, J.; Dandonneau, M.; Alldus, G.; Capel, B.; Swain, A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 2003, 130, 3663–3670. [Google Scholar] [CrossRef]
- Shan, B.; Wang, X.; Wu, Y.; Xu, C.; Xia, Z.; Dai, J.; Shao, M.; Zhao, F.; He, S.; Yang, L.; et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017, 18, 519–529. [Google Scholar] [CrossRef]
- Son, M.; Porat, A.; He, M.; Suurmond, J.; Santiago-Schwarz, F.; Andersson, U.; Coleman, T.R.; Volpe, B.T.; Tracey, K.J.; Al-Abed, Y.; et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 2016, 128, 2218–2228. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.; Shen, X.; Du, J.; Zhang, X.; Zhao, Y. The immunological function of CD52 and its targeting in organ transplantation. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2017, 66, 571–578. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kolasa, M.; Podkowa, D.; Kloc, M.; Kubiak, J.Z. Transcriptional profiling validates involvement of extracellular matrix and proteinases genes in mouse gonad development. Mech. Dev. 2018, 149, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, D.A.; Arora, S.; Lim, Y.; Lim, A.R.; Carter, L.M.; Corrin, P.D.; Plaisier, C.L.; Basom, R.; Delrow, J.J.; Wang, S.; et al. N(6)-methyladenosine mRNA marking promotes selective translation of regulons required for human erythropoiesis. Nat. Commun. 2019, 10, 4596. [Google Scholar] [CrossRef] [PubMed]
- Seguin, A.; Jia, X.; Earl, A.M.; Li, L.; Wallace, J.; Qiu, A.; Bradley, T.; Shrestha, R.; Troadec, M.B.; Hockin, M.; et al. The mitochondrial metal transporters mitoferrin1 and mitoferrin2 are required for liver regeneration and cell proliferation in mice. J. Biol. Chem. 2020, 295, 11002–11020. [Google Scholar] [CrossRef] [PubMed]
- Tchaikovskii, V.; Desnick, R.J.; Bishop, D.F. Molecular expression, characterization and mechanism of ALAS2 gain-of-function mutants. Mol. Med. 2019, 25, 4. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Lorenzi, V.; Mazzeo, C.I.; Alves-Lopes, J.P.; Roberts, K.; Sancho-Serra, C.; Engelbert, J.; Marečková, M.; Gruhn, W.H.; Botting, R.A.; et al. Single-cell roadmap of human gonadal development. Nature 2022, 607, 540–547. [Google Scholar] [CrossRef]
- Ge, W.; Niu, Y.L.; Li, Y.K.; Li, L.; Wang, H.; Li, W.W.; Qiao, T.; Feng, Y.N.; Feng, Y.Q.; Liu, J.; et al. Spatiotemporal dynamics of early oogenesis in pigs. Genome Biol. 2025, 26, 2. [Google Scholar] [CrossRef]
- Bowles, J.; Koopman, P. Retinoic acid, meiosis and germ cell fate in mammals. Development 2007, 134, 3401–3411. [Google Scholar] [CrossRef]
- Lange, M.; Bergen, V.; Klein, M.; Setty, M.; Reuter, B.; Bakhti, M.; Lickert, H.; Ansari, M.; Schniering, J.; Schiller, H.B.; et al. CellRank for directed single-cell fate mapping. Nat. Methods 2022, 19, 159–170. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Wang, L.; Li, C.; Zhou, Y.; Zhu, J.; Ding, J.; Liu, S.; Cheng, Z. Sohlh1 and Lhx8 are prominent biomarkers to estimate the primordial follicle pool in mice. Reprod. Biol. Endocrinol. RBE 2023, 21, 46. [Google Scholar] [CrossRef]
- Hamazaki, N.; Kyogoku, H.; Araki, H.; Miura, F.; Horikawa, C.; Hamada, N.; Shimamoto, S.; Hikabe, O.; Nakashima, K.; Kitajima, T.S.; et al. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 2021, 589, 264–269. [Google Scholar] [CrossRef]
- Soyal, S.M.; Amleh, A.; Dean, J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000, 127, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Falender, A.E.; Shimada, M.; Lo, Y.K.; Richards, J.S. TAF4b, a TBP associated factor, is required for oocyte development and function. Dev. Biol. 2005, 288, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Grive, K.J.; Seymour, K.A.; Mehta, R.; Freiman, R.N. TAF4b promotes mouse primordial follicle assembly and oocyte survival. Dev. Biol. 2014, 392, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bilmez, Y.; Talibova, G.; Ozturk, S. Expression of the histone lysine methyltransferases SETD1B, SETDB1, SETD2, and CFP1 exhibits significant changes in the oocytes and granulosa cells of aged mouse ovaries. Histochem. Cell Biol. 2022, 158, 79–95. [Google Scholar] [CrossRef]
- Weng, L.; Hong, H.; Zhang, Q.; Xiao, C.; Zhang, Q.; Wang, Q.; Huang, J.; Lai, D. Sleep Deprivation Triggers the Excessive Activation of Ovarian Primordial Follicles via β2 Adrenergic Receptor Signaling. Adv. Sci. 2024, 11, e2402393. [Google Scholar] [CrossRef]
- Luan, Y.; So, W.; Dong, R.; Abazarikia, A.; Kim, S.Y. KIT in oocytes: A key factor for oocyte survival and reproductive lifespan. EBioMedicine 2024, 106, 105263. [Google Scholar] [CrossRef]
- Schmahl, J.; Rizzolo, K.; Soriano, P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008, 22, 3255–3267. [Google Scholar] [CrossRef]
- Chen, X.; Aravindakshan, J.; Yang, Y.; Tiwari-Pandey, R.; Sairam, M.R. Aberrant expression of PDGF ligands and receptors in the tumor prone ovary of follitropin receptor knockout (FORKO) mouse. Carcinogenesis 2006, 27, 903–915. [Google Scholar] [CrossRef]
- Annie, L.; Gurusubramanian, G.; Kumar Roy, V. Visfatin protein may be responsible for suppression of proliferation and apoptosis in the infantile mice ovary. Cytokine 2021, 140, 155422. [Google Scholar] [CrossRef]
- Annie, L.; Gurusubramanian, G.; Roy, V.K. Inhibition of visfatin/NAMPT affects ovarian proliferation, apoptosis, and steroidogenesis in pre-pubertal mice ovary. J. Steroid Biochem. Mol. Biol. 2020, 204, 105763. [Google Scholar] [CrossRef]
- Du, C.; Davis, J.S.; Chen, C.; Li, Z.; Cao, Y.; Sun, H.; Shao, B.S.; Lin, Y.X.; Wang, Y.S.; Yang, L.G.; et al. FGF2/FGFR signaling promotes cumulus-oocyte complex maturation in vitro. Reproduction 2021, 161, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kanke, T.; Fujii, W.; Naito, K.; Sugiura, K. Effect of fibroblast growth factor signaling on cumulus expansion in mice in vitro. Mol. Reprod. Dev. 2022, 89, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.D.; Frost, E.R.; Bagheri-Fam, S.; Croft, B.M.; Ryan, J.M.; Zhao, L.; Koopman, P.; Harley, V.R. Somatic FGFR2 is Required for Germ Cell Maintenance in the Mouse Ovary. Endocrinology 2023, 164, bqad031. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Li, K.; Wang, C.; Xie, Y.; Luo, N.; Wang, L.; Sun, Y.; Huang, W.; Cheng, Z.; et al. Identification of Biomarkers for Predicting Ovarian Reserve of Primordial Follicle via Transcriptomic Analysis. Front. Genet. 2022, 13, 879974. [Google Scholar] [CrossRef]
- Akimoto, Y.; Fujii, W.; Naito, K.; Sugiura, K. The effect of ACVR1B/TGFBR1/ACVR1C signaling inhibition on oocyte and granulosa cell development during in vitro growth culture. J. Reprod. Dev. 2023, 69, 270–278. [Google Scholar] [CrossRef]
- Bouillet, P.; Oulad-Abdelghani, M.; Vicaire, S.; Garnier, J.M.; Schuhbaur, B.; Dollé, P.; Chambon, P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev. Biol. 1995, 170, 420–433. [Google Scholar] [CrossRef]
- Chiba, H.; Clifford, J.; Metzger, D.; Chambon, P. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol. Cell. Biol. 1997, 17, 3013–3020. [Google Scholar] [CrossRef]
- Xu, H.; Beasley, M.D.; Warren, W.D.; van der Horst, G.T.; McKay, M.J. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 2005, 8, 949–961. [Google Scholar] [CrossRef]
- Handel, M.A.; Schimenti, J.C. Genetics of mammalian meiosis: Regulation, dynamics and impact on fertility. Nat. Rev. Genet. 2010, 11, 124–136. [Google Scholar] [CrossRef]
- Griswold, M.D.; Hogarth, C.A.; Bowles, J.; Koopman, P. Initiating meiosis: The case for retinoic acid. Biol. Reprod. 2012, 86, 35. [Google Scholar] [CrossRef]
- Rafiei, N.; Ronceret, A. The plant early recombinosome: A high security complex to break DNA during meiosis. Plant Reprod. 2024, 37, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Endo, T.; Nishimura, Y.; Kano, K.; Naito, K. Porcine SPDYA2 (RINGO A2) stimulates CDC2 activity and accelerates meiotic maturation of porcine oocytes. Biol. Reprod. 2007, 76, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A.; Choi, Y.; Ballow, D.J.; Zhao, Y.; Westphal, H.; Matzuk, M.M.; Rajkovic, A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. USA 2006, 103, 8090–8095. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yuan, D.; Rajkovic, A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol. Reprod. 2008, 79, 1176–1182. [Google Scholar] [CrossRef]
- Choi, Y.; Ballow, D.J.; Xin, Y.; Rajkovic, A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol. Reprod. 2008, 79, 442–449. [Google Scholar] [CrossRef]
- Griffith, G.J.; Trask, M.C.; Hiller, J.; Walentuk, M.; Pawlak, J.B.; Tremblay, K.D.; Mager, J. Yin-yang1 is required in the mammalian oocyte for follicle expansion. Biol. Reprod. 2011, 84, 654–663. [Google Scholar] [CrossRef]
- Gazdag, E.; Santenard, A.; Ziegler-Birling, C.; Altobelli, G.; Poch, O.; Tora, L.; Torres-Padilla, M.E. TBP2 is essential for germ cell development by regulating transcription and chromatin condensation in the oocyte. Genes Dev. 2009, 23, 2210–2223. [Google Scholar] [CrossRef]
- Muramatsu, T. Midkine and pleiotrophin: Two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002, 132, 359–371. [Google Scholar] [CrossRef]
- Kadomatsu, K.; Tomomura, M.; Muramatsu, T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 1988, 151, 1312–1318. [Google Scholar] [CrossRef]
- Shen, W.; Park, B.W.; Toms, D.; Li, J. Midkine promotes proliferation of primordial germ cells by inhibiting the expression of the deleted in azoospermia-like gene. Endocrinology 2012, 153, 3482–3492. [Google Scholar] [CrossRef]
- Tan, H.J.; Deng, Z.H.; Shen, H.; Deng, H.W.; Xiao, H.M. Single-cell RNA-seq identified novel genes involved in primordial follicle formation. Front. Endocrinol. 2023, 14, 1285667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Zhang, L.; Xu, G.; Yang, Z.; Xiang, L.; Jiao, K.; Chen, Z.; Zhang, X.; Liu, Y. Syndecan-1 inhibition promotes antitumor immune response and facilitates the efficacy of anti-PD1 checkpoint immunotherapy. Sci. Adv. 2024, 10, eadi7764. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Daley, W.P.; Bernstein, A.M.; Pal-Ghosh, S.; Tadvalkar, G.; Shashurin, A.; Palsen, S.; Jurjus, R.A.; Larsen, M. Syndecan-1 regulates cell migration and fibronectin fibril assembly. Exp. Cell Res. 2010, 316, 2322–2339. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- van de Haar, L.L.; Riga, D.; Boer, J.E.; Garritsen, O.; Adolfs, Y.; Sieburgh, T.E.; van Dijk, R.E.; Watanabe, K.; van Kronenburg, N.C.H.; Broekhoven, M.H.; et al. Molecular signatures and cellular diversity during mouse habenula development. Cell Rep. 2022, 40, 111029. [Google Scholar] [CrossRef]
- Reuter, B.; Fackeldey, K.; Weber, M. Generalized Markov modeling of nonreversible molecular kinetics. J. Chem. Phys. 2019, 150, 174103. [Google Scholar] [CrossRef]
- Reuter, B.; Weber, M.; Fackeldey, K.; Röblitz, S.; Garcia, M.E. Generalized Markov State Modeling Method for Nonequilibrium Biomolecular Dynamics: Exemplified on Amyloid β Conformational Dynamics Driven by an Oscillating Electric Field. J. Chem. Theory Comput. 2018, 14, 3579–3594. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Zhao, X.; Gan, L.; Yan, C.; Li, C.; Sun, Q.; Wang, J.; Yuan, C.; Zhang, H.; Shan, S.; Liu, J.N. Genome-Wide Identification and Characterization of Long Non-Coding RNAs in Peanut. Genes 2019, 10, 536. [Google Scholar] [CrossRef]
- Morabito, S.; Miyoshi, E.; Michael, N.; Shahin, S.; Martini, A.C.; Head, E.; Silva, J.; Leavy, K.; Perez-Rosendahl, M.; Swarup, V. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet. 2021, 53, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, Q.; Cheng, S.; Li, L.; Shen, W.; Ge, W. Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice. Int. J. Mol. Sci. 2025, 26, 3734. https://doi.org/10.3390/ijms26083734
Wang H, Liu Q, Cheng S, Li L, Shen W, Ge W. Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice. International Journal of Molecular Sciences. 2025; 26(8):3734. https://doi.org/10.3390/ijms26083734
Chicago/Turabian StyleWang, Han, Qingchun Liu, Shunfeng Cheng, Lan Li, Wei Shen, and Wei Ge. 2025. "Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice" International Journal of Molecular Sciences 26, no. 8: 3734. https://doi.org/10.3390/ijms26083734
APA StyleWang, H., Liu, Q., Cheng, S., Li, L., Shen, W., & Ge, W. (2025). Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice. International Journal of Molecular Sciences, 26(8), 3734. https://doi.org/10.3390/ijms26083734





