A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections
Abstract
:1. Introduction
2. Results
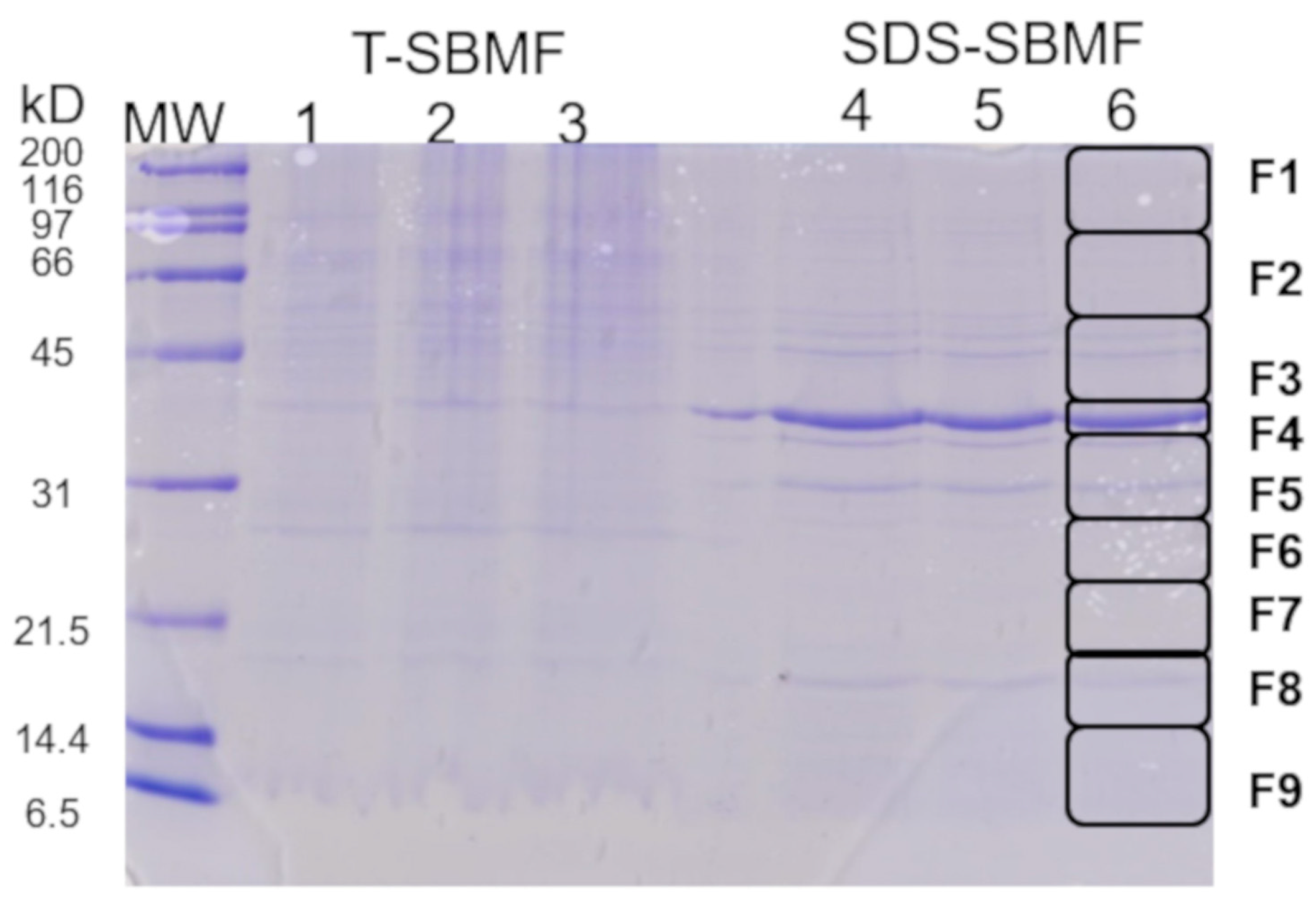
2.1. Obtaining a Fraction of Gram-Negative Bacteria E. coli for the Induction of Extracellular Vesicles from Macrophages
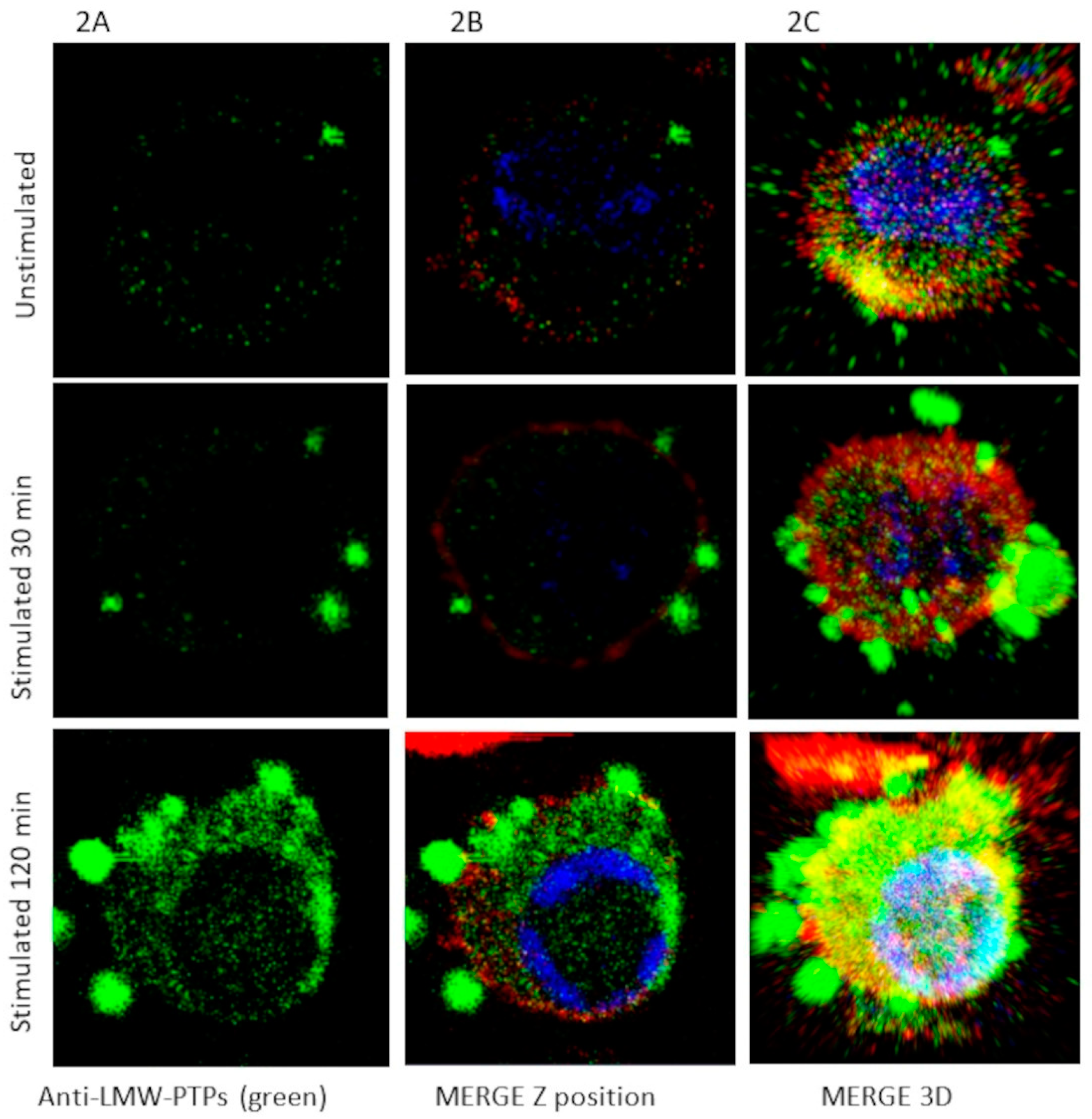
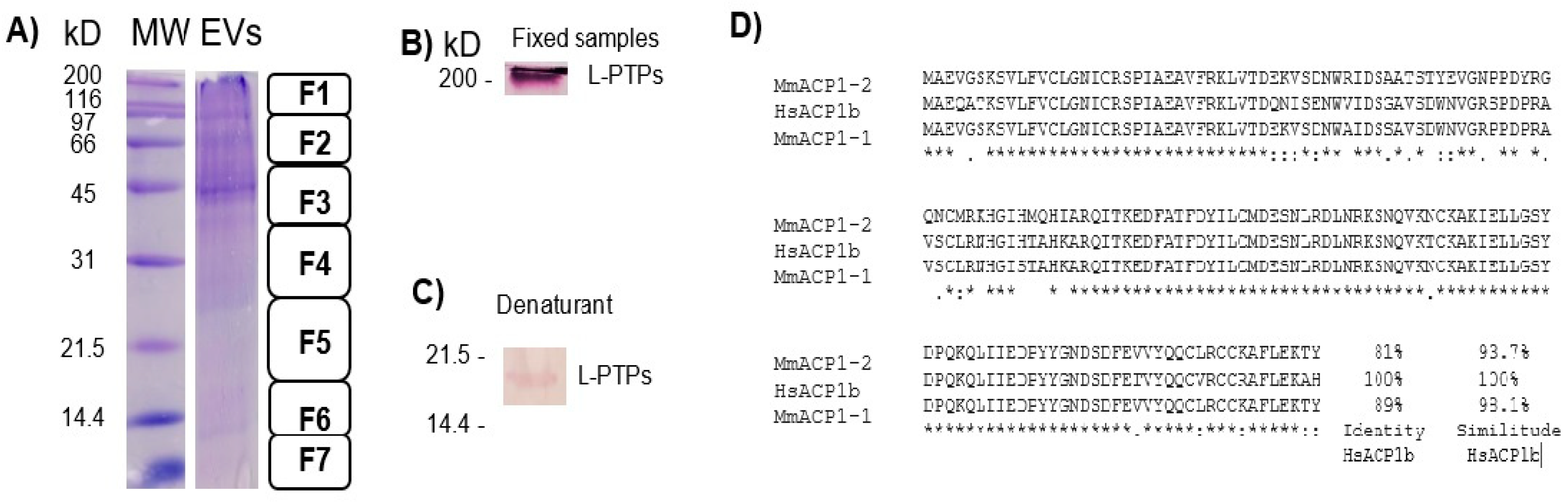
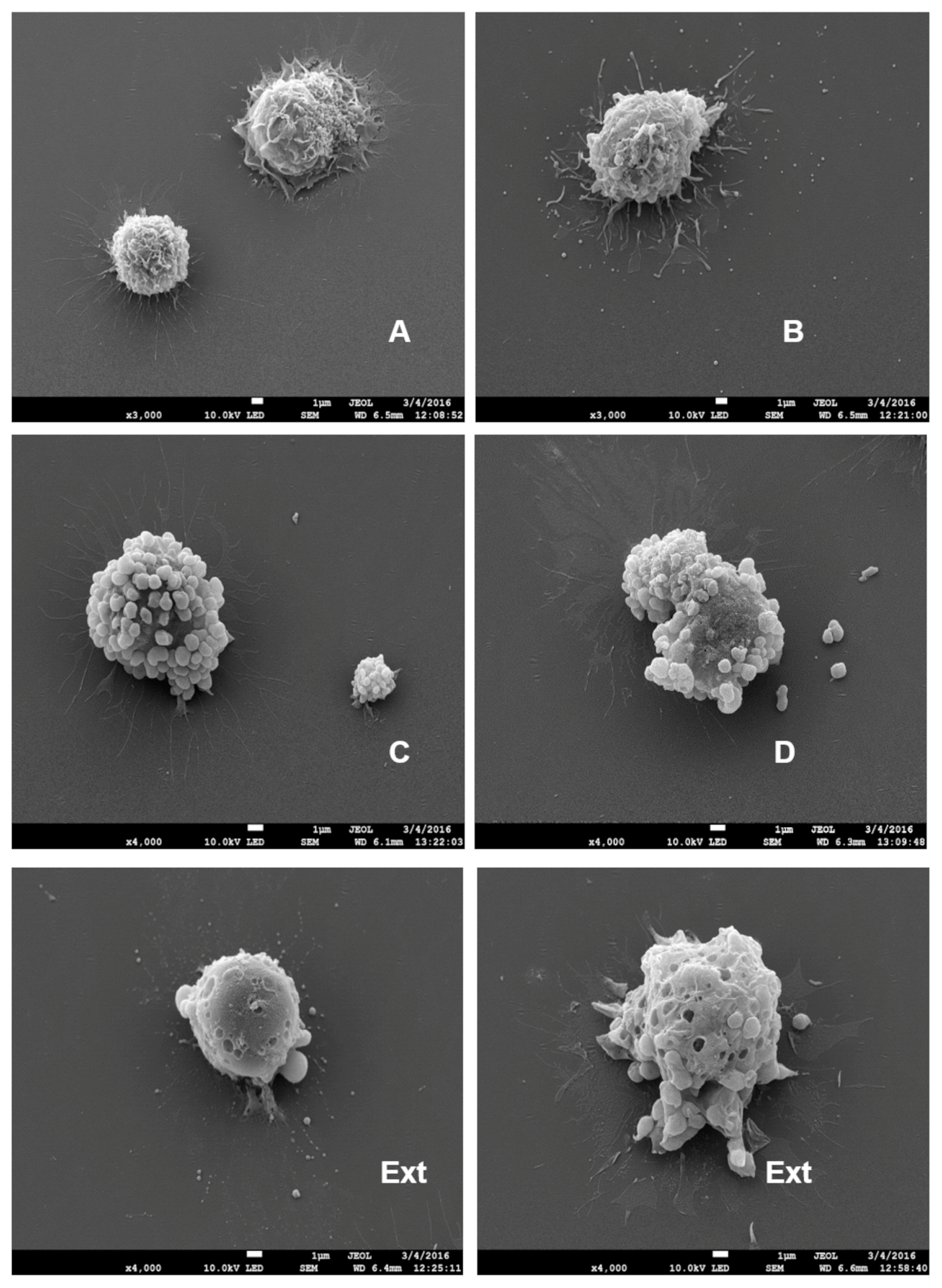
2.2. Induction of EV (Short and Large) Secretion from Macrophages with SDS-SBMF
2.3. Bacterial Co-Infection Analysis in COVID-19 Patients
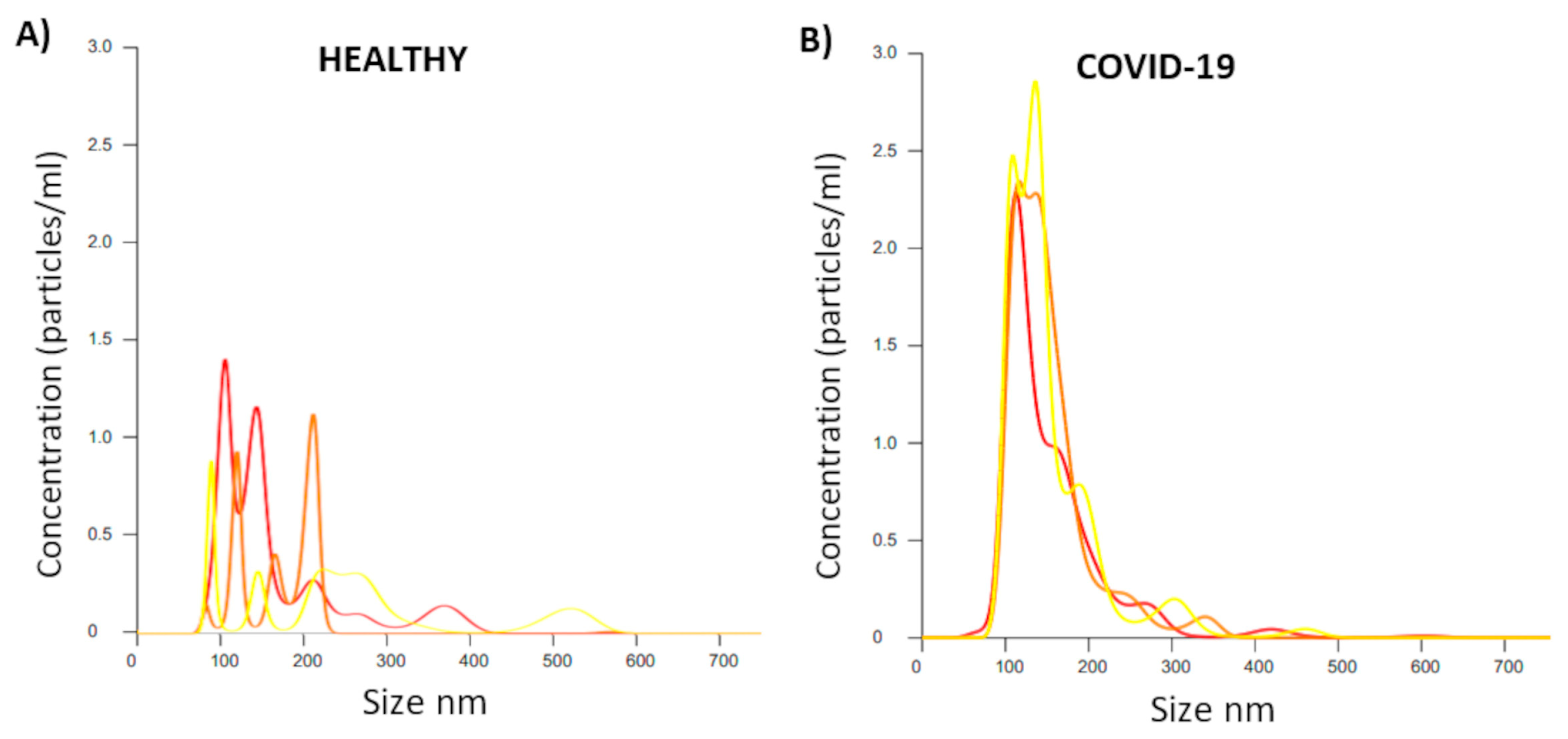
2.4. Analysis of EV Size and Concentration in Serum Applied to Patients with COVID-19
2.5. Detection of SARS-CoV-2 in EVs via PCR
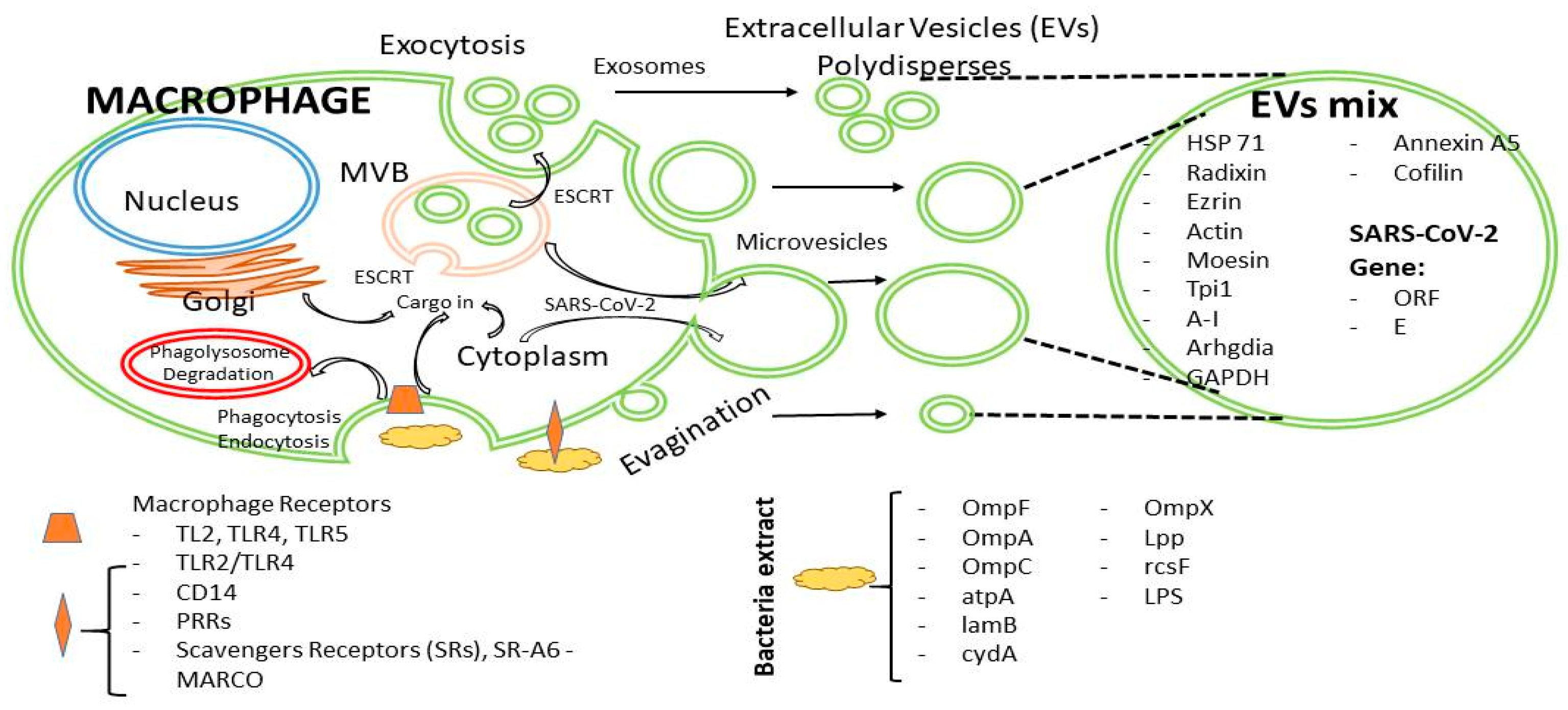
3. Discussion
4. Materials and Methods
4.1. Human Samples for Assays
4.2. Cell Culture
4.3. Cloning, Expression, and Purification of rHsLMW-PTP
4.4. Mice Immunization Protocol and Obtaining Anti-HsLMW-PTP Serum
4.5. Extraction of Escherichia coli Fraction
4.6. Standardization of Macrophage EV (Short and Large) Secretion and Isolation
4.7. SDS-PAGE and Western Blotting
4.8. Confocal Microscopy
4.9. Scanning Electron Microscopy (SEM)
4.10. Liquid Chromatography and MALDI-MS/MS
4.11. Detection of SARS-CoV-2 via qRT-PCR from Nasopharyngeal Swabs
4.12. Analysis of Bacterial Coinfection in Patients with COVID-19 via Real-Time PCR
4.13. Traditional Ultracentrifugation Method and Nano Sight Analysis of EV Size and Concentration in Serum Applied to Patients with COVID-19
4.14. Detection of SARS-CoV-2 in EVs Using Digital PCR
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, S.M.; Banyard, A.; Smith, C.; Mironov, A.; McCabe, M.G. Large Extracellular Vesicles Can be Characterised by Multiplex Labelling Using Imaging Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 8723. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzada, T.; Vijayan, A.; Vafaee, F.; Azimi, A.; Reid, G.; Clarke, S.; Kao, S.; Grau, G.E.; Hosseini-Beheshti, E. Small and Large Extracellular Vesicles Derived from Pleural Mesothelioma Cell Lines Offer Biomarker Potential. Cancers 2023, 15, 2364. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor. Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef]
- Moulin, C.; Crupi, M.J.F.; Ilkow, C.S.; Bell, J.C.; Boulton, S. Extracellular Vesicles and Viruses: Two Intertwined Entities. Int. J. Mol. Sci. 2023, 24, 1036. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, B.; Kim, O.Y.; Choi, D.S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Lee, J.M.; Park, G.W.; Lim, H.W.; Bang, J.Y.; Kim, Y.K.; Kwon, K.H.; Kwon, H.J.; Kim, K.P.; Gho, Y.S. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J. Proteome Res. 2007, 6, 4646–4655. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Liang, B.; Peng, P.; Chen, S.; Li, L.; Zhang, M.; Cao, D.; Yang, J.; Li, H.; Gui, T.; Li, X.; et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteomics 2013, 80, 171–182. [Google Scholar] [CrossRef]
- Oehmcke, S.; Westman, J.; Malmström, J.; Mörgelin, M.; Olin, A.I.; Kreikemeyer, B.; Herwald, H. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 2013, 9, e1003529. [Google Scholar] [CrossRef]
- Skogberg, G.; Gudmundsdottir, J.; van der Post, S.; Sandström, K.; Bruhn, S.; Benson, M.; Mincheva-Nilsson, L.; Baranov, V.; Telemo, E.; Ekwall, O. Characterization of human thymic exosomes. PLoS ONE 2013, 8, e67554. [Google Scholar] [CrossRef]
- Longatti, A. The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation. Viruses 2015, 17, 6707–6715. [Google Scholar] [CrossRef]
- Meng, B.; Ip, N.C.Y.; Abbink, T.E.M.; Kenyon, J.C.; Lever, A.M.L. ESCRT-II functions by linking to ESCRT-I in human immunodeficiency virus-1 budding. Cell Microbiol. 2020, 22, e13161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cai, X.; Yao, J.; Guo, H.; Yin, L.; Leung, W.; Xu, C. Role of Extracellular Vesicles in Influenza Virus Infection. Front. Cell Infect. Microbiol. 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Mullis, L.; Agnihothram, S. Viral and Bacterial Co-Infection and Its Implications. SciFed Virol. Res. J. 2017, 1. [Google Scholar]
- Meidaninikjeh, S.; Sabouni, N.; Marzouni, H.Z.; Bengar, S.; Khalili, A.; Jafari, R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. 2021, 269, 119010. [Google Scholar] [CrossRef]
- Abassi, Z.; Knaney, Y.; Karram, T.; Heyman, S.N. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020, 11, 1312. [Google Scholar] [CrossRef]
- Bernal, C.; How-Volkman, C.; Spencer, M.; El-Shamy, A.; Mohieldin, A.M. The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview. Life 2024, 14, 163. [Google Scholar] [CrossRef]
- Sun, R.; Cai, Y.; Zhou, Y.; Bai, G.; Zhu, A.; Kong, P.; Sun, J.; Li, Y.; Liu, Y.; Liao, W.; et al. Proteomic profiling of single extracellular vesicles reveals colocalization of SARS-CoV-2 with a CD81/integrin-rich EV subpopulation in sputum from COVID-19 severe patients. Front. Immunol. 2023, 14, 1052141. [Google Scholar] [CrossRef]
- Fazel, P.; Sedighian, H.; Behzadi, E.; Kachuei, R.; Imani, F.A.A. Interaction Between SARS-CoV-2 and Pathogenic Bacteria. Curr. Microbiol. 2023, 80, 223. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, G.; Sun, H.; Lyu, X. Clinical characteristics and risk factors for bacterial co-infections in COVID-19 patients: A retrospective study. J. Glob. Antimicrob. Resist. 2024, 38, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhou, L.; Lv, J.; Yang, S.; Chen, G.; Liu, X.; Han, C.; Tan, X.; Qian, S.; Wu, Z.; et al. Bacterial coinfections contribute to severe COVID-19 in winter. Cell Res. 2023, 33, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, M.; Vázquez-Flores, L.; Álvarez-Jiménez, V.D.; Castañeda-Casimiro, J.; Ibáñez-Hernández, M.; Sánchez-Torres, L.E.; Barrios-Payán, J.; Mata-Espinosa, D.; Estrada-Parra, S.; Chacón-Salinas, R.; et al. Extracellular vesicles released by J774A.1 macrophages reduce the bacterial load in macrophages and in an experimental mouse model of tuberculosis. Int. J. Nanomed. 2019, 14, 6707–6719. [Google Scholar] [CrossRef]
- Soni, S.; Wilson, M.R.; O’Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.S.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Zhu, Z.; Gho, Y.S.; Kim, Y.K. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy 2010, 65, 1256–1265. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.F.; Thakur, B.K. DNA in extracellular vesicles: From evolution to its current application in health and disease. Cell Biosci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sedger, L.M. Intercellular Vesicular Transfer by Exosomes, Microparticles and Oncosomes—Implications for Cancer Biology and Treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass. Spectrom. Rev. 2015, 34, 474–490. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Fu, Y.L.; Harrison, R.E. Microbial Phagocytic Receptors and Their Potential Involvement in Cytokine Induction in Macrophages. Front. Immunol. 2021, 12, 662063. [Google Scholar] [CrossRef] [PubMed]
- Stolk, M.; Seifert, M. Protein contaminations impact quantification and functional analysis of extracellular vesicle preparations from mesenchymal stromal cells. J. Stem Cells Regen. Med. 2015, 11, 44–47. [Google Scholar]
- Hassani, K.; Olivier, M. Immunomodulatory impact of leishmania-induced macrophage exosomes: A comparative proteomic and functional analysis. PLoS Negl. Trop. Dis. 2013, 7, e2185. [Google Scholar] [CrossRef] [PubMed]
- Bernimoulin, M.; Waters, E.K.; Foy, M.; Steele, B.M.; Sullivan, M.; Falet, H.; Walsh, M.T.; Barteneva, N.; Geng, J.G.; Hartwig, J.H.; et al. Differential stimulation of monocytic cells results in distinct populations of microparticles. J. Thromb. Haemost. 2009, 7, 1019–1028. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Lopez, F.; Baylón-Pacheco, L.; Vanegas-Villa, S.C.; Rosales-Encina, J.L. Characterization of low molecular weight protein tyrosine phosphatases of Entamoeba histolytica. Biochimie 2021, 180, 43–53. [Google Scholar] [CrossRef]
- Zepeda-Crestto, L.; Vidal-Vilches, S.; Sáenz-Iturriaga, L. Uso de Membranas Bacterianas como Fuente para la Elaboración de Liposomas como Adyuvantes. Avances en Ciencias Veterinarias 2012, 27, 16–24. [Google Scholar] [CrossRef]
- Sierra-Lopez, F.; Baylón-Pacheco, L.; Espíritu-Gordillo, P.; Lagunes-Guillén, A.; Chávez-Munguía, B.; Rosales-Encina, J.L. Influence of Micropatterned Grill Lines on Entamoeba histolytica Trophozoites Morphology and Migration. Front. Cell. Infect. Microbiol. 2018, 8, 295. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Chavez-Munguia, B.; Martinez-Palomo, A. High-resolution electron microscopical study of cyst walls of Entamoeba spp. J. Eukaryot. Microbiol. 2011, 58, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Rojas, J.; de la Vara, L.G.; Ríos-Castro, E.; Leyva-Castillo, L.E.; Gómez-Lojero, C. The distribution of divinyl chlorophylls a and b and the presence of ferredoxin-NADP+ reductase in Prochlorococcus marinus MIT9313 thylakoid membranes. Heliyon 2018, 4, e01100. [Google Scholar] [CrossRef] [PubMed]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell Proteomics 2007, 6, 1638–1655. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-López, F.; Iglesias-Vazquez, V.; Baylon-Pacheco, L.; Ríos-Castro, E.; Osorio-Trujillo, J.C.; Lagunes-Guillén, A.; Chávez-Munguía, B.; Hernández, S.B.; Acosta-Altamirano, G.; Talamás-Rohana, P.; et al. A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections. Int. J. Mol. Sci. 2025, 26, 3741. https://doi.org/10.3390/ijms26083741
Sierra-López F, Iglesias-Vazquez V, Baylon-Pacheco L, Ríos-Castro E, Osorio-Trujillo JC, Lagunes-Guillén A, Chávez-Munguía B, Hernández SB, Acosta-Altamirano G, Talamás-Rohana P, et al. A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections. International Journal of Molecular Sciences. 2025; 26(8):3741. https://doi.org/10.3390/ijms26083741
Chicago/Turabian StyleSierra-López, Francisco, Vanessa Iglesias-Vazquez, Lidia Baylon-Pacheco, Emmanuel Ríos-Castro, Juan Carlos Osorio-Trujillo, Anel Lagunes-Guillén, Bibiana Chávez-Munguía, Susana Bernardo Hernández, Gustavo Acosta-Altamirano, Patricia Talamás-Rohana, and et al. 2025. "A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections" International Journal of Molecular Sciences 26, no. 8: 3741. https://doi.org/10.3390/ijms26083741
APA StyleSierra-López, F., Iglesias-Vazquez, V., Baylon-Pacheco, L., Ríos-Castro, E., Osorio-Trujillo, J. C., Lagunes-Guillén, A., Chávez-Munguía, B., Hernández, S. B., Acosta-Altamirano, G., Talamás-Rohana, P., Rosales-Encina, J. L., & Sierra-Martínez, M. (2025). A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections. International Journal of Molecular Sciences, 26(8), 3741. https://doi.org/10.3390/ijms26083741






