Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0)
Abstract
1. Introduction
2. Results
2.1. Evaluation of Aβ Plaques and Neuroinflammation in Dolphins
2.1.1. The Hippocampus Had the Greatest Variability of Aβ Plaque Density, and Older Dolphins Had the Highest Aβ Plaque Density
2.1.2. Neuroinflammation Was Present in Dolphin Brains, with the Greatest Microglial Activation in the Hippocampus of Dolphins
2.2. C15:0 Neuroprotective Mechanisms
2.2.1. Pentadecanoic Acid (C15:0) Is a Fatty Acid Amide Hydrolase (FAAH) Inhibitor
2.2.2. Pentadecanoic Acid (C15:0) Is a Monoamine Oxidase (MAO-B) Specific Inhibitor
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Study Population
4.3. Histology
4.3.1. Study Samples
4.3.2. Immunohistochemical (IHC) Staining
4.3.3. Whole Slide Imaging
4.3.4. Digital Image Analysis
4.3.5. Data Analysis
4.4. C15:0 Mechanistic Activities Relevant to Cognitive Health
4.4.1. Fatty Acid Amide Hydrolase (FAAH) Inhibition
4.4.2. Monoamine Oxidase B (MAO-B) Inhibition
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| C15:0 | Pentadecanoic acid |
| FAAH | Fatty acid amide hydrolase |
| MAO-B | Monoamine oxidase B |
| CD68+ | Cluster of differentiation 68 |
| MMP | U.S. Navy Marine Mammal Program |
| OCFA | Odd-chain saturated fatty acid |
| AMPK | AMP-activated protein kinase |
| AKT | PI3K-AKT signaling pathway |
| PPARα/δ | Peroxisome proliferator-activated receptors alpha/delta |
| mTOR | Mammalian target of rapamycin |
| JAK-STAT | Janus kinase—Signal transducer and activator of transcription |
| HDAC-6 | Histone deacetylase 6 |
| LDL | Low density lipoprotein |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| FFPE | Formalin-fixed paraffin-embedded |
| IHC | Immunohistochemical |
| DAB | 3, 3 -diaminobenzidine |
| IL-6 | Interleukin-6 |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor necrosis factor-alpha |
| TGF-β1 | Transforming growth factor-beta 1 |
| MoA | Mechanism of action |
| ROS | Reactive oxygen species |
| DIOS | Dysmetabolic iron overload syndrome |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| ECS | Endocannabinoid system |
| PD | Parkinson’s disease |
References
- A Blueprint for Dementia Research. World Health Organization: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/9789240058248 (accessed on 20 January 2025).
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Trans. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement. Geriatr. Cogn. Disord. 2007, 24, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAVMA 2004, 292, 2237–2242. [Google Scholar] [CrossRef]
- Raffaitin, C.; Gin, H.G.; Empana, J.P.; Helmer, C.; Berr, C.; Tzourio, C.; Portet, F.; Dartigues, J.F.; Alperovitch, A.; Barberger-Gateau, P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia. Diabetes Care 2008, 32, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Cairns, N.; Lantos, P.; Mann, A. Validity of current clinical criteria for Alzheimer’s disease, vascular dementia, and dementia with Lewy bodies. Br. J. Psychiatr. 1999, 174, 45–50. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ghaderi, S.; Fatehi, F. Iron accumulation/overload and Alzheimer’s disease risk factors in the precuneus region: A comprehensive narrative review. Aging Med. 2024, 7, 649–667. [Google Scholar] [CrossRef]
- Lanctot, K.L.; Hahn-Pedersen, J.H.; Eichinger, C.S.; Freeman, C.; Clark, A.; Tazazona, L.R.S.; Cummings, J. Burden of illness in people with Alzheimer’s disease: A systematic review of epidemiology, comorbidities, and mortality. J. Prev. Alzheimer’s Dis. 2024, 11, 97–107. [Google Scholar] [CrossRef]
- Nie, Y.; Chu, C.; Qin, Q.; Shen, H.; Wen, L.; Tang, Y.; Qu, M. Lipid metabolism and oxidative stress in patients with Alzheimer’s disease and amnestic mild cognitive impairment. Brain Pathol. 2023, 34, e13202. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yang, Y. Hearing loss promotes Alzheimer’s disease. Nat. Aging 2024, 4, 443–444. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Jensen, E.D.; Smith, C.R.; Xitco, M.; Ridgway, S.H. Annual survival, mortality, and longevity of bottlenose dolphins (Tursiops truncatus) at the U.S. Navy Marine Mammal Program, 2004–2013. J. Am. Vet. Med. Assoc. 2015, 246, 893–898. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Smith, C.R.; Gomez, F.; Jensen, E.D. Physiology of aging among healthy, older bottlenose dolphins (Tursiops truncatus): Comparisons with aging humans. J. Comp. Phys. B 2011, 181, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Smith, C.; Stevenson, S.; Parry, C.; Daniels, R.; Jensen, E.; Cendejas, V.; Balmer, B.; Janech, M.; Neely, B.A.; et al. Blood-based indicators of insulin resistance and metabolic syndrome in bottlenose dolphins (Tursiops truncatus). Front. Endocrinol. 2013, 4, 136. [Google Scholar] [CrossRef]
- Mazzaro, L.M.; Johnson, S.P.; Fair, P.A.; Bossart, G.; Carlin, K.P.; Jensen, E.D.; Smith, C.R.; Andrews, G.A.; Chavey, P.S.; Venn-Watson, S. Iron indices among bottlenose dolphins (Tursiops truncatus): Identifying populations at risk for iron overload. Comp. Med. 2012, 62, 508–515. [Google Scholar] [PubMed]
- Venn-Watson, S.; Benham, C.; Carlin, K.; Leger, J.S. Hemochromatosis and fatty change: Building evidence for insulin resistance in bottlenose dolphins (Tursiops truncatus). J. Zoo Wildl. Med. 2012, 43, S35–S47. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Carlin, K.; Ridgway, S. Dolphins as animal models for type 2 diabetes: Sustained, postprandial hyperglycemia and hyperinsulinemia. Gen. Comp. Endocrinol. 2011, 170, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Parry, C.; Baird, M.; Stevenson, S.; Carlin, K.; Daniels, R.; Smith, C.R.; Jones, R.; Wells, R.S.; Ridgway, S.; et al. Increased dietary intake of saturated fatty acid heptadecanoic acid (C17:0) associated with decreasing ferritin and alleviated metabolic syndrome in dolphins. PLoS ONE 2015, 10, e0132117. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Baird, M.; Novick, B.; Parry, C.; Jensen, E.D. Modified fish diet shifted serum metabolome and alleviated chronic anemia in bottlenose dolphins (Tursiops truncatus): Potential role of odd-chain saturated fatty acids. PLoS ONE 2020, 15, e0230769. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Dornan, K.; Gunenc, A.; Oomah, B.D.; Hosseinian, F. Odd chain fatty acids and odd chain phenolic lipids (alkylresorcinols) are essential for diet. J. Am. Chem. Soc. 2021, 98, 813–824. [Google Scholar] [CrossRef]
- Ciesielski, V.; Guerbette, T.; Fret, L.; Succar, M.; Launay, Y.; Dahirel, P.; Legrand, P.; Vlach, M.; Blat, S.; Rioux, V. Dietary pentadecanoic acid supplementation at weaning in essential fatty acid-deficient rats shed light on the new family of odd-chain n-8 PUFAs. J. Nutr. Biochem. 2025, 137, 109814. [Google Scholar] [CrossRef]
- Ciesielski, V.; Legrand, P.; Blat, S.; Rioux, V. New insights on pentadecanoic acid with special focus on its controversial essentiality: A mini-review. Biochimie 2024, 227, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Xu, F.; Li, N.; Yu, J.; Teng, F.; Tang, J.; Huang, C.; Zhu, H. Free long-chain fatty acids trigger early postembryonic development in starved Caenorhabditis elegans by suppressing mTORC1. PLoS Biol. 2024, 22, e3002841. [Google Scholar] [CrossRef]
- Fu, W.C.; Li, H.Y.; Li, T.T.; Yang, K.; Chen, J.X.; Want, S.J.; Liu, C.H.; Zhang, W. Pentadecanoic acid promotes basal and insulin-stimulated glucose uptake in C2C12 myotubes. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Bishop, C.A.; Machate, T.; Henkel, J.; Schulze, M.B.; Klaus, S.; Piepelow, K. Hepatadecanoic acid is not a key mediator in the prevention of diet-induced hepatic steatosis and insulin resistance in mice. Nutrients 2023, 15, 2052. [Google Scholar] [CrossRef] [PubMed]
- To, N.B.; Truong, V.N.P.; Ediriweera, M.K.; Cho, S.K. Effects of combined pentadecanoic acid and tamoxifen treatment on tamoxifen resistance in MCF-7/SC breast cancer cells. Int. J. Mol. Sci. 2022, 23, 11340. [Google Scholar] [CrossRef] [PubMed]
- To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K.; To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic acid, an odd-chain fatty acid, suppresses the stemness of MCF-7/SC human breast cancer stem-like cells through JAK2/STAT3 signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; To, N.B.; Lim, Y.; Cho, S.K. Odd-chain fatty acids as novel histone deacetylase 6 (HDAC6) inhibitors. Biochimie 2021, 186, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Schork, N. Pentadecanoic acid (C15:0), an essential fatty acid, shares clinically relevant cell-based activities with leading longevity-enhancing compounds. Nutrients 2023, 15, 4607. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef]
- Galdiero, E.; Ricciardelli, A.; D’Angelo, C.; de Alteriis, E.; Maione, A.; Albarano, L.; Casillo, A.; Corsaro, M.M.; Tutino, M.L.; Parrillii, E. Pentadecanoic acid against Candida albicans-Klebsiella pneunmoniae biofilm: Towards the development of an anti-biofilm coating to prevent polymicrobial infections. Res. Microbiol. 2021, 172, 103880. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, A.; Casillo, A.; Corsaro, M.M.; Tutino, M.L.; Parrilli, E.; Van Der Mei, H.C. Pentadecanal and pentadecanoic acid coatings reduce biofilm formation of Staphylococcus epidermidis on PDMS. Pathog. Dis. 2020, 78, ftaa012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Chen, Y.; Wang, K.; Luan, Y. Design, synthesis and antitumor activity study of a gemcitabine prodrug conjugated with a HDAC6 inhibitor. Bioorg. Med. Chem. Lett. 2022, 72, 128881. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D.; et al. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Lee, E.; Ugalde-Nicalo, P.A.; Skonieczny, J.W.; Chun, L.F.; Newton, K.P.; Schwimmer, J.B. Pentadecanoic acid supplementation in young adults with overweight and obesity: A randomized controlled trial. J. Nutr. 2024, 154, 2763–2771. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Zhang, Q.A.; Magkos, F.; Ng, M.; Michael, N.; Wu, X.; Volchanskaya, V.S.B.; Lai, X.; Wanjaya, E.R.; Elejalde, U.; et al. Effect of an Asian-adapted Mediterranean diet and pentadecanoic acid on fatty liver disease: The TANGO randomized controlled trial. Am. J. Clin. Nutr. 2024, 119, 788–799. [Google Scholar] [CrossRef]
- Shen, J.; Yu, H.; Li, K.; Ding, B.; Xiao, R.; Ma, W. The associations between plasma fatty acid and cognitive function mediated by inflammation in patients with type 2 diabetes melitus. Diabetes Metab. Syndr. Obes. 2022, 15, 1423–1436. [Google Scholar] [CrossRef]
- Brydges, C.R.; Bhattacharyya, S.; Dehkordi, S.M.; Milaneschi, Y.; Penninx, B.; Jansen, R.; Kristal, B.S.; Han, X.; Arnold, M.; Kastenmuller, G. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain Behav. Immun. 2022, 102, 42–52. [Google Scholar] [CrossRef]
- Gunn-Moore, D.; Kaidanovich-Beilin, O.; Iradi, M.C.G.; Gunn-Moore, F.; Lovestone, S. Alzheimer’s disease in humans and other animals: A consequence of postreproductive life span and longevity rather than aging. Alzheimer’s Dement. 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimer’s Dement. 2018, 14, 259–260. [Google Scholar] [CrossRef]
- Sacchini, S.; Diáz-Delgado, J.; De Los Monteros, A.E.; Paz, Y.; De Quirós, Y.B.; Sierra, E.; Arbelo, M.; Herraez, P.; Fernandez, A. Amyloid-beta peptide and phosphorylated tau in the frontopolar cerebral cortex and in the cerebellum of toothed whales: Aging versus hypoxia. Biol. Open 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.C.; Durrant, C.S.; Rose, J.; Hall, A.J.; Spires-Jones, T.L.; Gunn-Moore, F.; Dagleish, M.P. Alzheimer’s disease-like neuropathology in three species of oceanic dolphin. Eur. J. Neurosci. 2023, 57, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Garamszegi, S.P.; Brzostowicki, D.J.; Coyne, T.M.; Vontell, R.T.; Davis, D.A. TDP-43 and Alzheimer’s disease pathology in the brain of a harbor porpoise exposed to the cyanobacterial toxin BMAA. Toxins 2024, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, K.; Testori, C.; Giorda, F.; Grattarola, C.; Mattioda, V.; Di Guardo, G.; Corona, C.; Castagnaro, C.; Favole, A. Amyloid-β and phosphorylated tau screening in bottlenose dolphin (Tursiops truncatus) and striped dolphin (Stenella coeruleoalba) brains from Italy reveals distinct immunohistochemical patterns correlating with age and co-morbidity. PLoS ONE 2024, 19, e0314085. [Google Scholar] [CrossRef]
- Perez, S.E.; Raghanti, M.A.; Hof, P.R.; Kramer, L.; Ikonomovic, M.D.; Lacor, P.N.; Eriwin, J.M.; Sherwood, C.C.; Mufson, E.J. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J. Comp. Neurol. 2013, 521, 4318–4338. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murliamanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef]
- Bouras, C.; Hof, P.R.; Giannakopoulos, P.; Michel, J.P.; Morrison, J.H. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: A quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb. Cortex 1994, 4, 138–150. [Google Scholar] [CrossRef]
- Buckner, R.L. The role of the hippocampus in prediction and imagination. Ann. Rev. Psychol. 2010, 61, 27–48. [Google Scholar] [CrossRef]
- Lucena, P.B.; Heneka, M.T. Inflammatory aspects of Alzheimer’s disease. Acta Neuropathol. 2024, 148, 31. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Jensen, E.D.; Schork, N.J. A 25-y longitudinal dolphin cohort supports that long-lived individuals in same environment exhibit variation in aging rates. Proc. Natl. Acad. Sci. USA 2020, 117, 20950–20958. [Google Scholar] [CrossRef]
- Venn-Watson, S. The Cellular Stability Hypothesis: Evidence of ferroptosis and accelerated aging-associated diseases as newly identified nutritional pentadecanoic acid (C15:0) deficiency syndrome. Metabolites 2024, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; Xia, L.; Shi, Q.; Hou, Y.; Zhang, L.; Li, M.; Fan, C.; Sun, B. Ferroptosis mechanisms and Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Kaidery, N.A.; Sharma, S.M.; Gazaryan, I.; Nikulin, S.V.; Hushpulian, D.M.; Thomas, B. A critical appraisal of ferroptosis in Alzheimer’s and Parkinson’s disease: New insights into emerging mechanisms and therapeutic targets. Front. Pharmacol. 2024, 15, 1390798. [Google Scholar] [CrossRef]
- Majernikova, N.; Marmolejo-Garza, A.; Salinas, C.S.; Luu, M.D.A.; Zhang, Y.; Trombetta-Lima, M.; Tomin, T.; Birner-Gruenberger, R.; Lehtonen, S.; Koistinaho, J.; et al. The link between amyloid β and ferroptosis pathway in Alzheimer’s disease progression. Cell Death Dis. 2024, 15, 782. [Google Scholar] [CrossRef]
- Streit, W.J.; Phan, L.; Bechmann, I. Ferroptosis and pathogenesis of neuritic plaques in Alzheimer disease. Pharmacol. Rev. 2025, 77, 100005. [Google Scholar] [CrossRef] [PubMed]
- Egmond, N.V.; Straub, V.M.; van der Stelt, M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Ann. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, S.; Paliwal, A.; Dwivedi, J.; Paliwal, S.; Paliwal, V.; Paliwal, S.; Sharma, J. Discovery of novel fatty acid amide hydrolase (FAAH) inhibitors as anti-Alzheimer’s agents through pharmacophore-based virtual screening, molecule docking and experimental validation. Med. Chem. Res. 2024, 33, 136–150. [Google Scholar] [CrossRef]
- Armeli, F.; Coccurello, R.; Giacovazzo, G.; Mengoni, B.; Paoletti, I.; Oddi, S.; Maccorrone, M.; Businaro, R. FAAH inhibition counteracts neuroinflammation via autophagy recovery in AD models. Int. J. Mol. Sci. 2024, 25, 12044. [Google Scholar] [CrossRef]
- Rong, K.; Li, Z.; Wu, X.; Gao, S.; Zhao, J.; Yang, J.; Jiang, X.; Zhang, J.; Tang, W. Natural phenol carbamates: Selective BuChE/FAAH dual inhibitors show neuroprotection in an Alzheimer’s disease mouse model. Eur. J. Med. Chem. 2025, 281, 117003. [Google Scholar] [CrossRef]
- Brunetti, L.; Laghezza, A.; Fulvio, L.; Paolo, T.; Luca, P. Combining fatty acid amide hydrolase (FAAH) inhibition with peroxisome proliferator-activated receptor (PPAR) activation: A new potential multi-target therapeutic strategy for the treatment of Alzheimer’s disease. Neural Regen. Res. 2020, 15, 67–68. [Google Scholar] [PubMed]
- Jian, S.; Bisht, A.; Verma, K.; Negi, S.; Paliwal, S.; Sharma, S. The role of fatty acid amide hydrolase enzyme inhibitors in Alzheimer’s disease. Cell Biochem. Funct. 2021, 40, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Chen, L.L.; Fan, Y.G.; Zhao, L.X.; Zhang, Q.; Wang, Z.Y. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheimers Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Gabbouj, S.; Ryhanen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Happasalo, A.; Hiltunen, M.; et al. Altered insulin signaling in Alzheimer’s disease brain—Special emphasis on PI3K-Akt pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Querfurth, H.; Lee, H.K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegen. 2021, 16, 44. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Cai, N.; Liao, X.; Tang, L.; Wang, Y. Endocannabinoid hydrolase inhibitors: Potential novel anxiolytic drugs. Drug Des. Dev. Ther. 2024, 18, 2143–2167. [Google Scholar] [CrossRef]
- Portugalov, A.; Akirav, I. FAAH inhibition reverses depressive-like behavior and sex-specific neuroinflammatory alterations induced by early life stress. Cells 2024, 13, 1881. [Google Scholar] [CrossRef]
- Barbetti, M.; Mancabelli, L.; Vacondio, F.; Langhi, G.; Ferlenghi, F.; Viglioli, M.; Turroni, F.; Carnevali, L.; Mor, M.; Ventura, M.; et al. Social stress-induced depressive-like symptoms and changes in gut microbial and lipidomic profiles are prevented by pharmacological inhibition of FAAH activity in male rats. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2024, 131, 110963. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Liegowitz, M.R.; Stein, M.B.; Grunfeld, J.; Van Hove, I.; Simmons, W.K.; Van Der Ark, P.; Palmer, J.A.; Saad, Z.S.; Pemberton, D.J.; et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: A double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 2021, 46, 1004–1010. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Reiner, J.; Jensen, E.D. Pentadecanoylcarnitine is a newly discovered endocannabinoid with pleiotropic activities relevant to supporting physical and mental health. Sci. Rep. 2022, 23, 13717. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Nitulescu, G.; Mihai, D.P.; Nitulescu, G.M. Identifying FAAH inhibitors as new therapeutic options for the treatment of chronic pain through drug repurposing. Pharmaceut 2022, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Ha, C.; Million, M.; Arnett, S.; Germano, P.; Pearson, J.P.; Currie, M.G.; Tache, Y. FAAH inhibitor URB597 shows anti-hyperalgestic action and increases brain and intestinal tissues fatty acid amides in a model of CRF1 agonist mediated visceral hypersensitivity in male rats. Neurogastroenterol. Motil. 2024, 36, e14927. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, W.; Poklis, J.L.; Li, P.L.; Lichtman, A.H.; Gewirtz, D.A.; Li, N. Mitigation of cisplatin-induced acute kidney injury through oral administration of fatty acid amide hydrolase inhibitor PF-04457845. J. Pharmacol. Exp. Ther. 2025, 392, 100032. [Google Scholar] [CrossRef]
- Knoll, J. Deprenyl (selegiline): The history of its development and pharmacological action. Acta Neurol. Scan. Suppl. 1983, 95, 57–80. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z. Effects and safety of monoamine oxidase-B inhibitors for early Parkinson’s Disease: A network meta-analysis. Eur. Neurol. 2024, 87, 273–290. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Udi, O.A.; Atere, A.D.; Joseph, G.U.; Ogbutor, U.G. Molecular pathways: The quest for effective MAO-B inhibitors in neurodegenerative therapy. Mol. Biol. Rep. 2025, 52, 240. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Weihager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with ɣ-secretase and regulates neuronal amyloid ꞵ-peptide levels. Alzheimer’s Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Bar-Am, O.; Youdim, M.B.H. Neuroprotective effects of multifaceted hybrid agents targeting MAO, cholinesterase, iron and β-amyloid in again and Alzheimer’s disease. Br. J. Pharmacol. 2015, 173, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T. Monoamine oxidase-B inhibitors in the treatment of Alzheimer’s disease. Neurobiol. Aging 2000, 21, 343–348. [Google Scholar] [CrossRef]
- Mohamadpour, B.; Mirazi, N.; Komaki, A.; Basir, H.S.; Hosseini, A. Protective effects of selegiline against amyloid beta-induced anxiety-like behavior and memory impairment. Brain Behav. 2024, 14, e3599. [Google Scholar] [CrossRef]
- Rullo, M.; La Spada, G.; Catto, M.; Pisani, L. Monoamine oxidase inhibition for the treatment of neurodegenerative diseases: Rationale, assay methodologies, and reference compounds. Methods Neurodegener. Dis. Drug Discov. 2024, 216, 137–150. [Google Scholar]
- Naoi, M.; Maruyama, W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharm. Des. 2010, 16, 2799–2817. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.V.C.; Thomas, S.M.; Veerabathirn, R. An overview of the role of monoamine oxidase-B in Parkinson’s disease: Implications for neurodegeneration and therapy. Explor. Neuroprot. Ther. 2024, 4, 308–318. [Google Scholar] [CrossRef]
- Alborghetti, M.; Bianchini, E.; De Carolis, L.; Galli, S.; Pontieri, F.E.; Domiziana, R. Type-B monoamine oxidase inhibitors in neurological diseases: Clinical applications based on preclinical findings. Neural Regen. Res. 2024, 19, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Naio, M.; Maruyama, W.; Shamoto-Nagai, M. Type A and B monoamine oxidases distinctly modulate signal transduction pathway and gene expression to regulate brain function and survival of neurons. Neuro Preclin. Neurol. Stud. 2018, 125, 1635–1650. [Google Scholar] [CrossRef]
- Banerjee, C.; Tripathy, D.; Kumar, D.; Chakraborty, J. Monoamine oxidase and neurodegeneration: Mechanisms, inhibitors and natural compounds for therapeutic intervention. Neurochem. Int. 2024, 179, 105831. [Google Scholar] [CrossRef]
- Dormegny-Jeanjen, L.C.; Mainberger, O.A.E.; Crespin de Billy, C.; Obrecht, A.; Danila, V.; Erb, A.; Arcay, H.M.; Weibel, S.; Blanc, F.; Meyer, G.; et al. Safety and tolerance of combination of monoamine oxidase inhibitors and direct dopamine agonists in adults and older adults with highly resistant depression. L’enchephale 2024, 50, 137–142. [Google Scholar] [CrossRef]
- Karalija, N.; Papenberg, G.; Johansson, J.; Wahlin, A.; Salami, A.; Andersson, M.; Axelsson, J.; Kuznetsov, D.; Riklund, K.; Lövdén, D.; et al. Longitudinal support for the correlative triad among aging, dopamine D2-like receptor loss, and memory decline. Neurobiol. Aging 2024, 136, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.R.; Pallos, J.; Arreola-Bustos, A.; Martin, I. Natural variation in age-related dopamine neuron degeneration is glutathione dependent and linked to life span. Proc. Natl. Acad. Sci. USA 2024, 121, e2403450121. [Google Scholar] [CrossRef] [PubMed]
- Ruehl, W.W.; Enriken, T.L.; Muggenburg, B.A.; Bruyette, D.S.; Griffith, W.C.; Hahn, F.F. Treatment with L-deprenyl prolongs life in elderly dogs. Life Sci. 1997, 61, 1037–1044. [Google Scholar] [CrossRef]
- Knoll, J. The striatal dopamine dependency of life span in male rats. Longevity study with (−) deprenyl. Mech. Ageing Dev. 1988, 46, 237–262. [Google Scholar] [CrossRef]
- Knoll, J. Rationale for (−) deprenyl (selegiline) medication in Parkinson’s disease and in prevention of age-related nigral changes. Biomed. Pharmacol. 1995, 49, 187–195. [Google Scholar] [CrossRef] [PubMed]
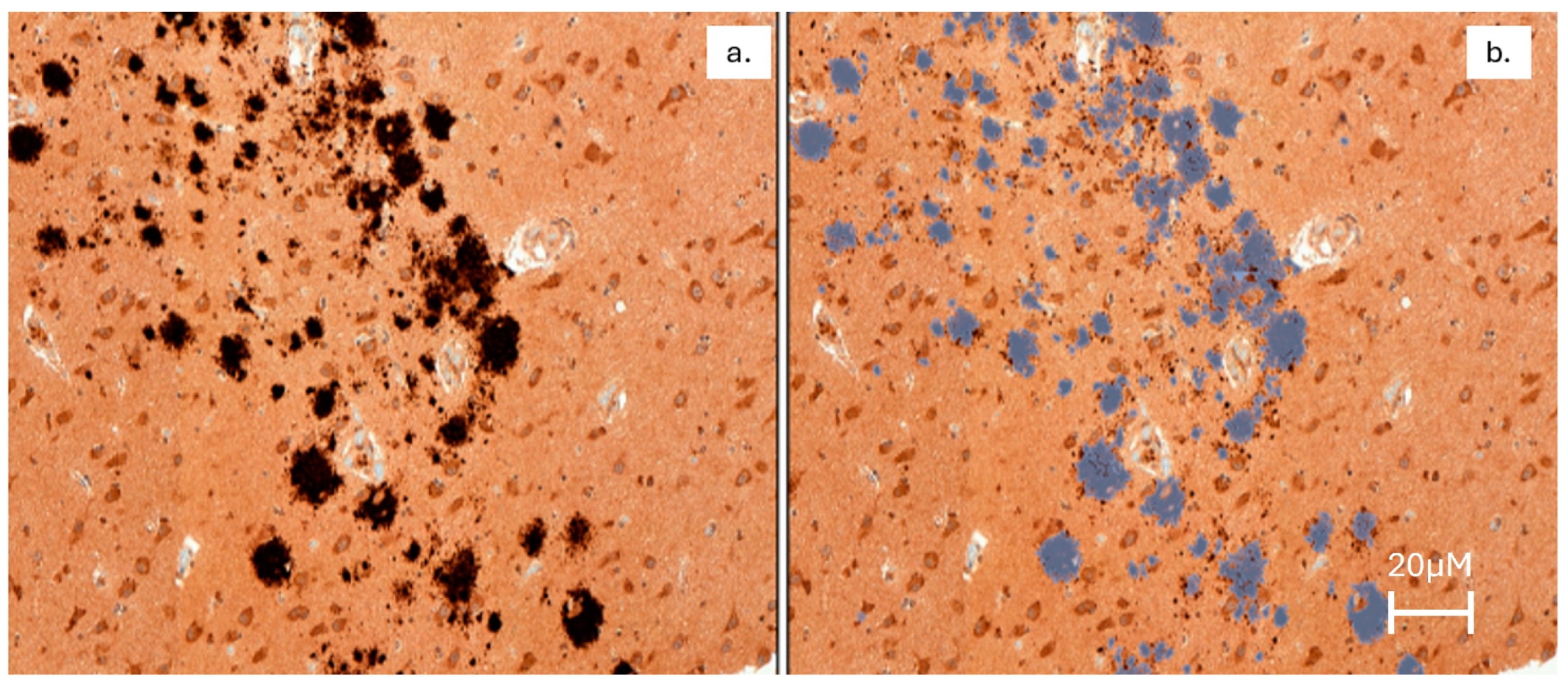

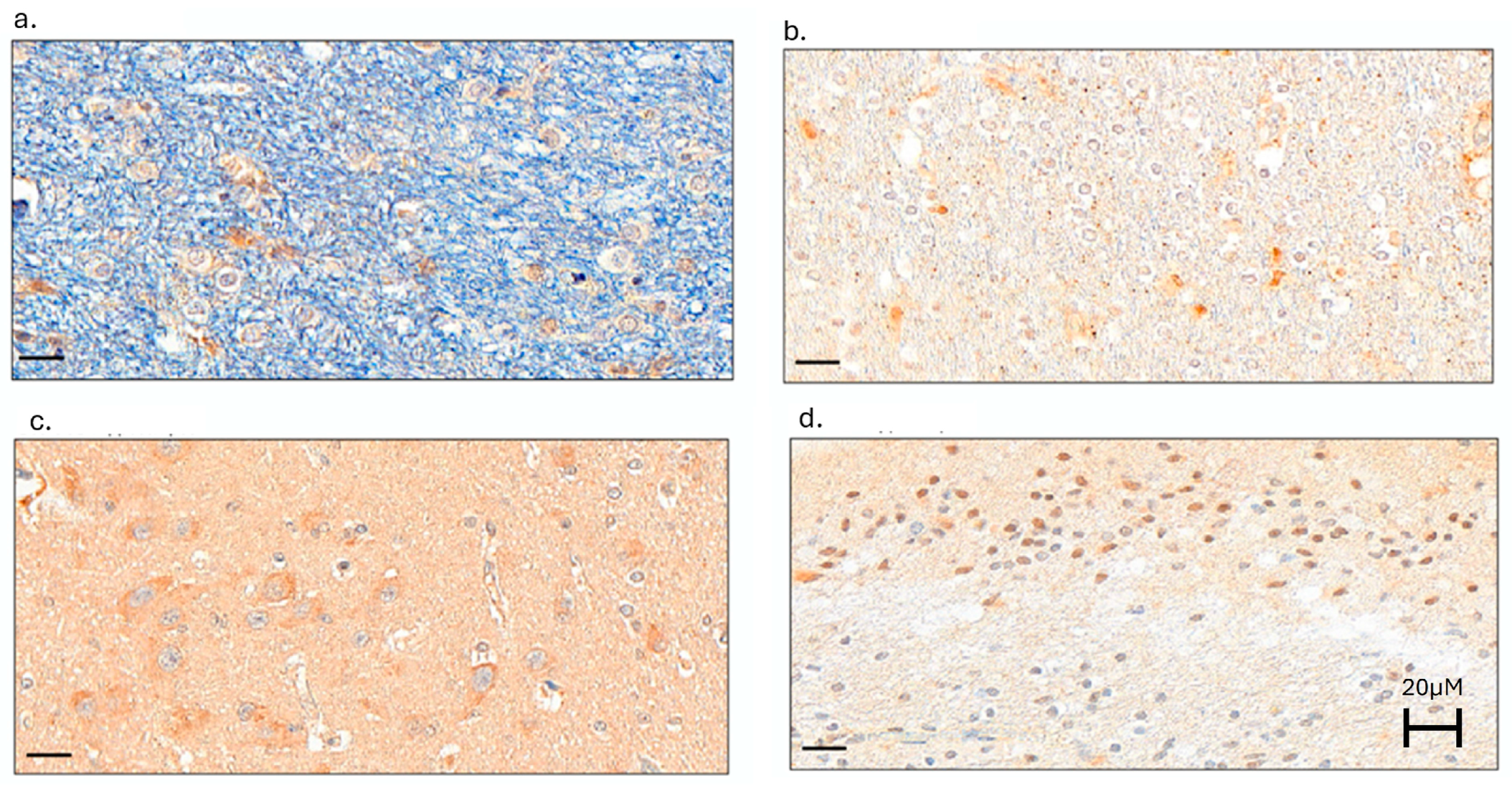

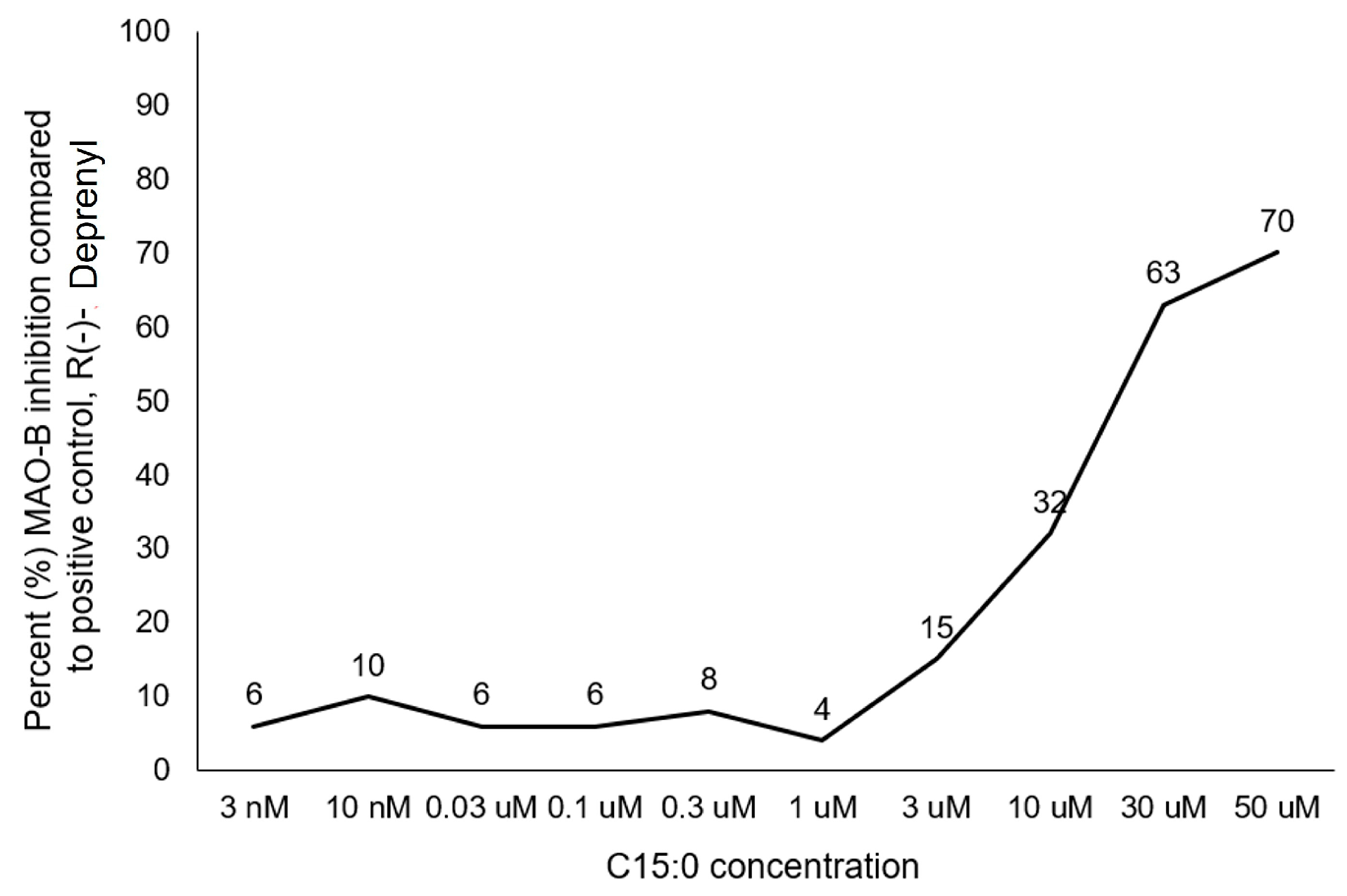
| Aβ Staining by Brain Section | Aβ Plaque Density (Plaques/mm2) | Aβ Plaques by Area (%) | Aβ Plaque Count | Mean Aβ Plaque Staining | Total Area of Aβ Plaques (mm2) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Frontal cortex (n = 18) | 1.4 ± 2.3 | 0.02 ± 0.03 | 219 ± 442 | 1.17 ± 0.44 | 0.03 ± 0.06 |
| Hippocampus (n = 15) | 3.8 ± 11.8 | 0.08 ± 0.25 | 595 ± 1809 | 1.24 ± 0.54 | 0.13 ± 0.38 |
| Red nucleus (n = 18) | 1.0 ± 1.3 | 0.01 ± 0.02 | 140 ± 236 | 1.57 ± 0.21 | 0.02 ± 0.03 |
| Substantia nigra (n = 17) | 1.5 ± 2.8 | 0.02 ± 0.05 | 183 ± 460 | 1.39 ± 0.41 | 0.03 ± 0.09 |
| p-value | p-value | p-value | p-value | p-value | |
| Differences by section | 0.91 | 0.76 | 0.59 | 0.003 | 0.49 |
| IHC Staining by Brain Section | CD68+ (Cells per mm2) | Mean Stain Intensity | |||
|---|---|---|---|---|---|
| IFN-γ | IL-6 | TGF-β | TNFα | ||
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| Frontal cortex (n = 18) | 0.34 ± 0.45 | 5.6 ± 2.5 | 5.0 ± 2.3 | 6.3 ± 2.9 | 22 ± 9 |
| Hippocampus (n = 15) | 0.46 ± 0.38 | 5.8 ± 2.8 | 4.9 ± 3.2 | 6.8 ± 3.1 | 21 ± 12 |
| Red nucleus (n = 18) | 0.18 ± 0.24 | 4.4 ± 1.7 | 3.4 ± 1.7 | 6.9 ± 2.1 | 18 ± 10 |
| Substantia nigra (n = 17) | 0.12 ± 0.14 | 4.2 ± 1.9 | 3.6 ± 2.2 | 5.4 ± 3.1 | 16 ± 9 |
| p-value | p-value | p-value | p-value | p-value | |
| Differences by section | 0.002 | 0.198 | 0.168 | 0.291 | 0.206 |
| Assay Name | Antagonist Response (Average % Inhibition Compared to Positive Control) | Met Antagonist Criteria (Where ≥50% Inhibition = Y, <50% Inhibition = N) |
|---|---|---|
| Cholinesterase, Acetyl, ACES | 44.7 | N |
| Cholinesterase, Butyryl, CHLE | 2.5 | N |
| Peptidase, CTSB (Cathepsin B) | −35.2 | N |
| Cyclooxygenase COX-1 | −17.0 | N |
| Cyclooxygenase COX-2 | 5.3 | N |
| Myeloperoxidase | 44.8 | N |
| IDO1 | 39.4 | N |
| Monoamine Oxidase MAO-A | 11.4 | N |
| Monoamine Oxidase MAO-B | 64.7 | Y |
| Peptidase, Endothelin Converting Enzyme-1 (ECE-1) | 7.1 | N |
| Peptidase, Metalloproteinase, Neutral Endopeptidase | 2.1 | N |
| Peptidase, BACE1 (beta-Secretase) | 2.4 | N |
| Protein Tyrosine Kinase, Fyn | −16.2 | N |
| Protein Serine/Threonine Kinase, GSK3B | 15.0 | N |
| Protein Serine/Threonine Kinase, MAPK14 (p38alpha) | −14.8 | N |
| Protein Tyrosine Phosphatase, DUSP22 | −28.1 | N |
| Fatty Acid Amide Hydrolase (FAAH) | 70.3 | Y |
| Nitric Oxide Synthetase, Inducible (iNOS) | 9.6 | N |
| Catechol-O-Methyl Transferase (COMT) | 1.5 | N |
| Lipoxygenase 15-LOX-2 | 8.0 | N |
| Adenosine A2A | 10.7 | N |
| Transporter, GABA | −3.3 | N |
| GABAA, Ro-15-1788, Hippocampus | −16.4 | N |
| Glutamate, AMPA | −1.7 | N |
| Glutamate, NMDA, MK-801 | −2.2 | N |
| Glutamate, NMDA, Polyamine | 0.4 | N |
| Glutamate, Metabotropic, mGlu5 | 0 | N |
| Histamine H3 | 1.7 | N |
| Chemokine CX3CR1 | 5.3 | N |
| Muscarinic M1 | 9.0 | N |
| Nicotinic Acetylcholine alpha7, Methyllycaconitine | −9.1 | N |
| Serotonin (5-Hydroxytryptamine) 5-HT1A, WAY-100635 | 3.9 | N |
| Serotonin (5-Hydroxytryptamine) 5-HT4B | −1.7 | N |
| Serotonin (5-Hydroxytryptamine) 5-HT6 | −16.1 | N |
| Sodium Channel, Site 1 | −1.3 | N |
| Somatostatin sst4 | −4.4 | N |
| Sigma 2 | 6.6 | N |
| Sigma1 | −18.6 | N |
| Platelet Activating Factor (PAF) | −6.6 | N |
| Antibody | Supplier and Catalog | Lot | Host | Dilution | Antigen Retrieval |
|---|---|---|---|---|---|
| Beta amyloid | Cell Signaling (Danvers, MA, USA) #2450 | 3 | Mouse | 1:200 | ER2(20) |
| IL-6 | Abcam (Cambridge, UK) #ab6672 | GR309805-5 | Rabbit | 1:100 | ER1(20) |
| IFN-γ | Kingfisher (Saint Paul, MN, USA) #PB0494-200 | DP1882KM | Goat | 1:1600 | ER1(20) |
| TNF-α | Abcam #ab1793 | GR32063-1 | Mouse | 1:100 | ER1(20) |
| TGF-β1 | Abcam #ab92486 | GR175622-2 | Rabbit | 1:250 | ER2(20) |
| CD68 | Thermo Fisher (Carlsbad, CA, USA) #MA5-13324 | SH2440231 | Mouse | 1:100 | ER1(20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venn-Watson, S.; Jensen, E.D. Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0). Int. J. Mol. Sci. 2025, 26, 3746. https://doi.org/10.3390/ijms26083746
Venn-Watson S, Jensen ED. Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0). International Journal of Molecular Sciences. 2025; 26(8):3746. https://doi.org/10.3390/ijms26083746
Chicago/Turabian StyleVenn-Watson, Stephanie, and Eric D. Jensen. 2025. "Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0)" International Journal of Molecular Sciences 26, no. 8: 3746. https://doi.org/10.3390/ijms26083746
APA StyleVenn-Watson, S., & Jensen, E. D. (2025). Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0). International Journal of Molecular Sciences, 26(8), 3746. https://doi.org/10.3390/ijms26083746






