Abstract
There are about 5000 species of Passeriformes birds, which are half of the extant ones. Their class I MHC molecules are found to be different from all other studied vertebrates, including other bird species; i.e., amino acid residues 10 and 96 are not the seven canonic residues extant in all other vertebrate molecules. Thus, the canonic residues in MHC class I vertebrate molecules are reduced to five. These differences have physical effects in MHC (Major Histocompatibility Complex) class I alpha chain interaction with beta-2-microglobulin but have yet unknown functional effects. Also, introns show specific Passeriformes distinction both in size and invariance. The studies reviewed in this paper on MHC structure have been done in wild birds that cover most of the world’s passerine habitats. In this context, we are going to expose the most commonly occurring bird diseases with the caveat that MHC and disease linkage pathogenesis is not resolved. In addition, this field is poorly studied in birds; however, common bird diseases like malaria and Marek’s disease are linked to MHC. On the other hand, the main established function of MHC molecules is presenting microbial and other antigens to T cells in order to start immune responses, and they also may modulate the immune system through NK receptors and other receptors (non-classical class I MHC molecules). Also, structural and polymorphic differences between classical class I molecules and non-classical class I molecules are at present not clear, and their definition is blurred. These passerine exceptional MHC class I molecules may influence linkage to diseases, transplantation, and other MHC presentation and self-protection functions. Further studies in more Passeriformes species are ongoing and needed.
1. Introduction
Dinosaurs and mammals were thriving on Earth in the Triassic Epoch (300 million years ago; MYA). Both groups survived the Cretaceous extinction about 65 MYA. At present, there are about 5500 mammal species and about 10,000 bird species, and 5700 species of the latter are Passeriformes or songbirds [1,2]. The class Aves is considered one of the most evolved forms of dinosaurs [1,2,3,4]. Class I MHC genes and their corresponding proteins in songbirds have been studied and compared to other non-passerine birds and to other available same-vertebrate genes [5]. Only passerines, among birds and other studied vertebrates, conserve Val and Leu amino acids at residues 10 and 96, respectively [5,6]. Also, class I MHC introns of canaries (Genus Serinus) and goldfinches (Genus Carduelis/Spinus) are studied in this work; they have been extant on Earth since the Miocene Epoch, 21 MYA [6]. Passeriformes have been calculated to be on Earth 55 million years ago (Eocene Epoch) [7].
The MHC class I and class II genes are very polymorphic in vertebrates, and their molecules are responsible for presenting peptides to clonotypic receptors on T-cell surfaces to start a specific immune response [8]. MHC class I genes have been widely sequenced and studied in chicken (Gallus gallus, order Galliformes), wild birds [9,10,11], and mammals [8,12,13,14]. The MHC was first described in chicken [15,16], specifically in the highly related variety Leghorn [12,17]. Chicken MHC-B is clearly different in both structure and function when compared with that of mammals like man (Figure 1); it is simpler and more compact because it has shorter introns, at least in chicken, and its genes are more tightly arranged than in mammals [12,18]. This fact supports the hypothesis that chicken MHC represents a ‘minimum essential MHC’ [12,13] that was also generally attributed to all birds. However, songbird MHC seems to be much more complex than that of chicken because (a) passerine birds have a larger number of genes [19,20]; (b) their introns are longer than those of chicken [5]); (c) pseudogenes have been reported in songbird MHC [19,21], like in humans [22,23]; and (d) passerine genes are highly polymorphic [20,24].
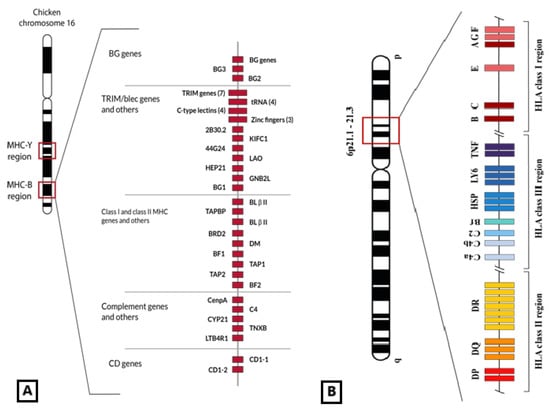
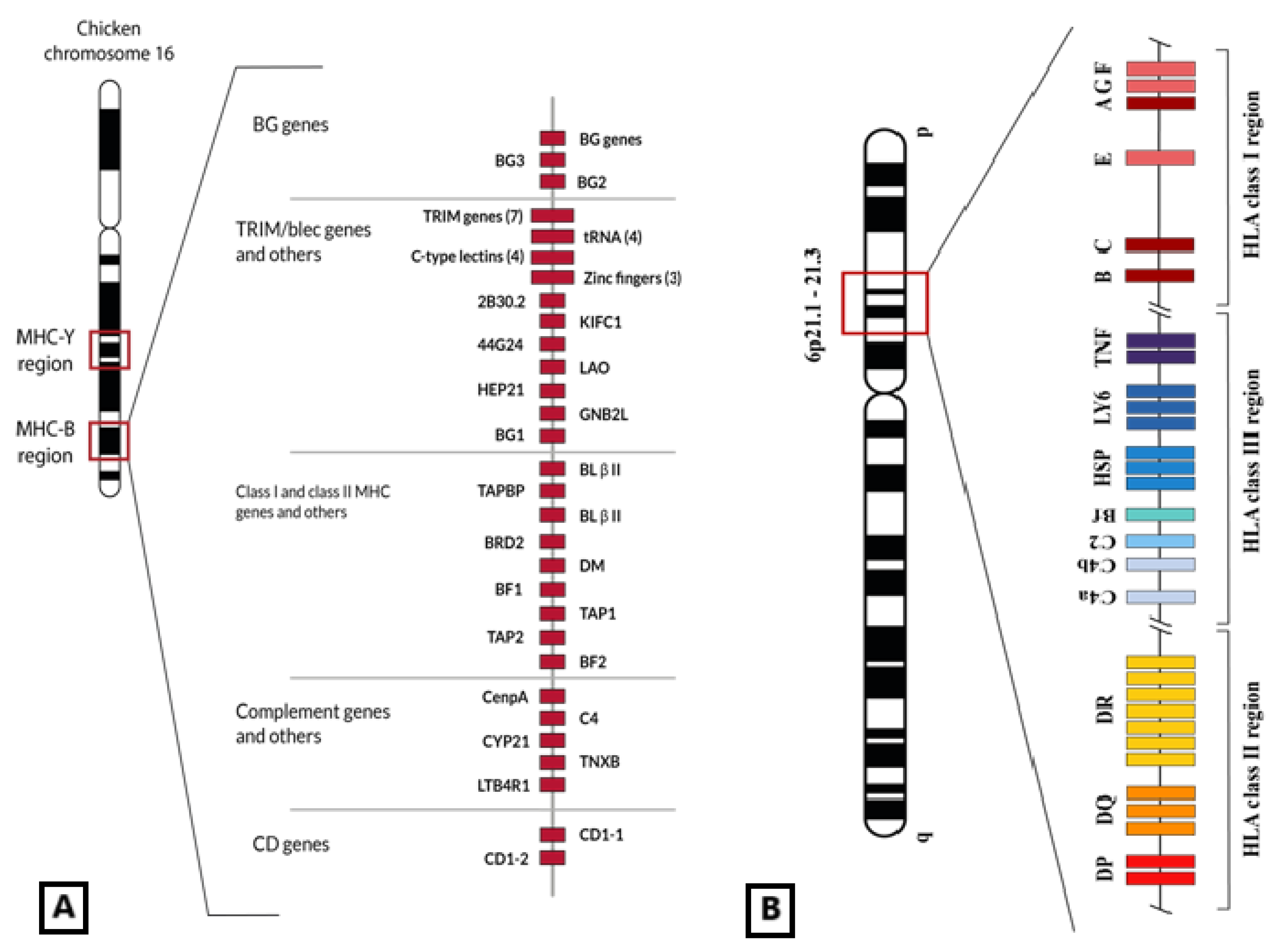
Figure 1.
(A) Genetic map of the chicken MHC-B region in which MHC-I genes (BF1 and BF2) are included, among others also related to the immune response (C4, TAP, CD1, class II genes or BL, and class II processing genes or DM). BG genes are also close to epithelial and immune cell expression [25]. This set of genes is located in the 16th micro-chromosome of the chicken genome. Figure adapted from [25]. (B) Representation of HLA genomic region placed in human chromosome 6 6p21.1–21.3 band. Non-classical class I HLA genes are located in the telomeric part of the HLA region. © by AA-V.
2. HLA Class I Region and Molecules
2.1. Classic Class I Molecules
HLA (i.e., Human Leukocyte Antigen) class I genes are split up into HLA class I classic genes (HLA-Ia) and class I non-classic genes (HLA-Ib) [26]. HLA-A, -B, and -C (HLA-Ia) were the first described and are expressed in all nucleated cells but mainly in macrophages, dendritic cells, and other immune system cells. HLA-E, -F, and -G (HLA-Ib) codify for very similar proteins to those of classical class I genes (Figure 2), although they differ in some characteristics such as expression location (only certain tissues like cornea or trophoblast), low polymorphism, and immunosuppressive function [27,28]. Structurally, HLA class I molecules are made up of a 270-amino-acid (aac) heavy chain (α domains) encoded in the HLA system and a light chain (beta-2-microglobulin; β2m) encoded in chromosome 15 [29,30]. α1 and α2 domains allow the formation of a three-dimensional valve in which the antigen is presented on the surface. α3 interacts with the CD8 co-receptor during lymphocyte T immune synapsis [18]. β2m has only a structural function.
2.2. The Non-Classical Class I HLA Genes: HLA-G, -E, and -F
HLA (Human Leukocyte Antigen) is the human Major Histocompatibility Complex, which is placed in a genomic region containing about 223 genes at chromosome 6p21.3 and encodes for the so-called HLA complex (equivalent to MHC in other species). It has a very important role in the immune response. Genes named classical class I genes for historical reasons (HLA-A, HLA-B, and HLA-C) encode for molecules whose function is presenting antigen peptides to clonotypic T-cell receptors placed on the surface of CD8+ cells; on the other hand, the non-classical class I molecules (HLA-G, HLA-E, and HLA-F) (Figure 1) are mainly related to control functions of the immune system cells [31,32,33]. The HLA-G function was assigned to be an immune control molecule, firstly found at the maternal–fetal interface and later in many other tissues, but its function was thought to be only inducing maternal–fetal tolerance [32,34,35,36]. Dan Geraghty et al. [37] named this protein HLA-6.0, the new gene that they discovered. The structure of the HLA-6.0 protein was similar to HLA-A, -B, and -C class I molecules, but a stop codon inhibited the translation of a large part of the cytoplasmatic region in the HLA-6.0 molecule. Regarding the promoter region of the HLA-6.0 gene, it was almost identical to that of the MHC-Qa mouse gene; in addition, both of these genes had equivalent substitutions, deletions, and other changes in their DNA sequences [37]. It was proposed [38] that MHC-Qa was a mouse HLA-G homologous with a similar gene and protein structure; MHC-Qa also has soluble isoforms similar to HLA-G5, G6, and G7 isoforms in man (Figure 1). In addition, it is proved that Qa-1b (MHC-Qa non-classical class I gene in mouse) is homologous to HLA-E (see HLA-E “Evolution” section). The full HLA-G protein has an extracellular structure almost identical to that of the classical HLA molecules, though its main function is not peptidic antigen presentation. Also, HLA-G hinders the cytotoxicity of T CD8+ and NK cells by interaction with leukocyte receptors, like LILRB1 (LIR1/ILT2), LILRB2 (ILT4), and KIR2DL4 (CD158d) [33,39,40,41,42,43,44].
However, the HLA-G gene and protein expression patterns are different in certain ways from those of classical HLA class I proteins, as follows: (a) It has a more restricted tissue expression in normal metabolic circumstances [45]. It has been found to be expressed on the maternal–fetal interface, particularly in the cellular line of extravillous cytotrophoblast [6], cornea, proximal nail matrix, thymus, hematopoietic stem cells, and pancreas [46,47,48,49,50,51]. HLA classical class I molecules are widely expressed in all body tissues. Non-classical class I HLA molecules are more restricted regarding tissue localization, and their function is different, as they are not antigen-presenting molecules [33]. The variation of presented antigens compared with those of classical class I MHC proteins is very reduced, possibly because of their lower polymorphism [52]. These non-classical class I proteins also modulate immune function through TCR-independent interactions (see below). (b) It has several membrane-bound and also soluble isoforms because there is an alternative splicing of the complete mRNA of HLA-G [32,33]. (c) A short cytoplasmic tail is found because there is a stop codon at exon 6 [32,33]. (d) At the moment, a relatively low HLA-G molecule polymorphism has been found, although it is quickly growing (Figure 2) [32,33,53]. (e) It has a peculiar 5′UTR (5′ upstream regulatory region), which differs from HLA classical class I genes [54,55]. (f) The 5′ promoter region [32,56,57,58,59,60] and the 3′UTR (3′ untranslated region) present some polymorphisms that are specifically linked to susceptibility to certain diseases [61]. However, it has been found that HLA-G presents endogenous peptides at the surface cells of the placenta trophoblast [62]; this does not occur with other HLA classical class I molecules, which may also be expressed on the same cell surface [63], except for HLA-C [64]. Therefore, HLA-G is extant on the outer placenta cytotrophoblast cell line of fetal and contacts,: (a) with maternal blood NK cells through their killer immunoglobulin-like receptors (KIRs), both inhibitory and activating receptors; (b) the so-called white blood cell LIRs (leukocyte immunoglobulin-like receptors); and (c) the complex of CD94-NKG2 receptors. This variety of HLA-G interactions results in achieving maternal tolerance to the fetus and a lack of rejection [43]. Also, HLA-G interacts with both cytolytic and regulatory [65,66] T cells through their respective surface clonotypic T-cell receptors [67]. On the other hand, the HLA-E molecule on the surface of cytotrophoblast placental cell polymorphisms sends signals to the mother’s immune system to enhance fetal tolerance: a complex of HLA-E allelic protein bound to peptides that come from leader peptides of class I HLA molecules. These HLA-E/peptide complexes interact with maternal CD94-NKG2 and clonotypic T-cell receptors [68,69].
In contrast with HLA-E and HLA-G physiology, HLA-F has not been so widely studied and has apparently deserved less attention. However, it is known that HLA-F also promotes fetal normal growth [70], and, in addition, this molecule modulates the immune system of the peripheral nervous system; it has been found in amyotrophic lateral sclerosis that HLA-F hinders motor neuron death by its binding to inhibitory KIR3DL2 receptors [71]. Also, HLA-F enhances the antiviral response to HIV (human immune deficiency virus-1) by binding to activating KIR3DS1 receptors of NK cells [72]. This is a very important HLA-F immune regulatory function because its binding to KIR3DS1 receptors has been found to be crucial in other diseases in which KIR3DS1 shows a pathogenetic function [73]. In summary, control or modulation by the HLA-F protein has been found to be critical in the pathogenesis of some diseases. Notwithstanding, HLA-F’s tertiary properties and structure need to be further studied in order to establish a relationship with its pathogenetic activity in diseases. Computational predictions conclude that HLA-F has only a partial open groove and normal MHC folding [67,74].
3. MHC Trans-Species Evolution
Mammals (apes) and birds have been shown to present MHC trans-species evolution [75,76]. This usually happens when speciation has rapidly occurred while MHC genes still remain. This fact is usually explained by MHC molecules’ adaptation to a specific environment with specific pathogens to work with, and evolutive forces for the invariance are strong enough to resist speciation timing. This trans-species evolution of MHC genes could also explain why fewer alleles are found in wild species than in “artificial“ (or more inbred) ones, like human, mouse, and domestic chicken [76,77]. Human, laboratory mouse, and domestic chicken are considered “artificial” models because they have undergone a bottleneck and subsequent inbreeding. This parental relatedness and relative chromatide similarities facilitate crossover at meiosis and, therefore, would lead to excessive MHC allelism since this has been shown to be the main MHC mechanism through which it generates diversity in this system—not through point mutations [76,77,78]. MHC class I DNA sequences of Passerine birds studied by us [79] clearly showed trans-species evolution: two DNA matches exist between MHC-I alleles of different South American siskin species. In addition, the Eurasian siskin shares an identical MHC protein allele with the pine siskin (Central North America radiation). The Eurasian siskin also shows a protein allele shared with three different South American siskin species. More protein allele sharing has been identified with one single MHC protein in eight South American siskin species, including the extant parental species C. notata (Figure 2). All MHC genes transmit the corresponding MHC allelic proteins to the descendant species if they have time (and no selective forces exist) before speciation occurs. South American siskins separated from the North American siskin radiation about 5-4 million years ago, and this study is detailed in Figure 2 [80]. The closest mt DNA genetic relationship occurs between the Eurasian siskin and the pine siskin [80,81,82,83]. This is further supported because these two species share one MHC class I protein allele. Apparently, the Eurasian siskin (or an extinct relative) was thriving around America, Asia, and Europe and has apparently given both North American and South American siskin radiations directly or through the North American radiation [80,81,82,83]. In summary, our own data with mitochondrial mt cyt b, together with the first described evidence of trans-species MHC evolution in birds, lead us to postulate that the Eurasian siskin is the extant ancestor of present-day North and South American siskins/goldfinches Carduelis species [80,81,82,83].
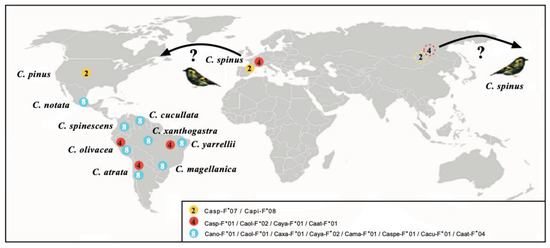
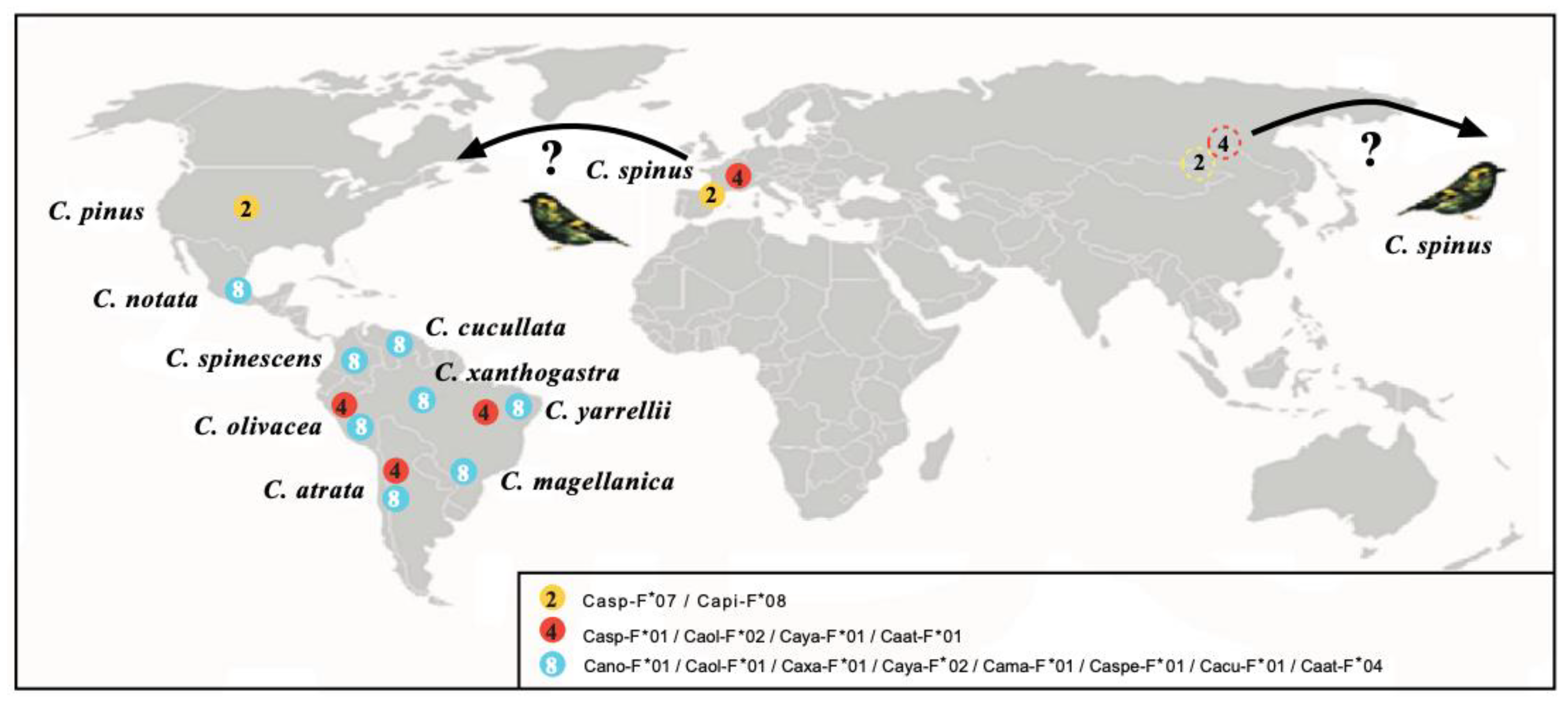
Figure 2.
Map showing the geographic location of species sharing MHC-I molecules. Proteins of bird species are depicted with the following names: Carduelis spinus (Casp-F*07, FJ266427; Casp-F*01, FJ266399); Carduelis pinus (Capi-F*08, FJ266391); Carduelis notata (Cano-F*01, DQ257468); Carduelis spinescens (Caspe-F*01, DQ257472); Carduelis olivacea (Caol-F*02, DQ257471); Carduelis atrata (Caat-F*01, FJ266350); Carduelis magellanica (Cama-F*01, DQ257467); Carduelis yarrellii (Caya-F*01, DQ257475; Caya-F*02, DQ257476); Carduelis xanthogastra (Caxa-F*01, DQ257473); and Carduelis cucullata (Cacu-F*01, DQ257465). The color of the circles indicates the three different proteins found; the number inside each circle indicates species that share each molecule. GenBank accession numbers are indicated for each allele [79,80,81,82,83]. North Asian dashed circles 3 and 4 mean alleles of Carduelis spinus, the probable parental species of North and South American radiations. Question marks mean that C.spinus bands have been reported in America outside of its present day range in Europe and Asia. See above indicated references © by AA-V.
Figure 2.
Map showing the geographic location of species sharing MHC-I molecules. Proteins of bird species are depicted with the following names: Carduelis spinus (Casp-F*07, FJ266427; Casp-F*01, FJ266399); Carduelis pinus (Capi-F*08, FJ266391); Carduelis notata (Cano-F*01, DQ257468); Carduelis spinescens (Caspe-F*01, DQ257472); Carduelis olivacea (Caol-F*02, DQ257471); Carduelis atrata (Caat-F*01, FJ266350); Carduelis magellanica (Cama-F*01, DQ257467); Carduelis yarrellii (Caya-F*01, DQ257475; Caya-F*02, DQ257476); Carduelis xanthogastra (Caxa-F*01, DQ257473); and Carduelis cucullata (Cacu-F*01, DQ257465). The color of the circles indicates the three different proteins found; the number inside each circle indicates species that share each molecule. GenBank accession numbers are indicated for each allele [79,80,81,82,83]. North Asian dashed circles 3 and 4 mean alleles of Carduelis spinus, the probable parental species of North and South American radiations. Question marks mean that C.spinus bands have been reported in America outside of its present day range in Europe and Asia. See above indicated references © by AA-V.
4. Songbirds Have at α1 Chain Val10 and Not the Canonic Vertebrates Thr10 Amino Acid and in α2 Chain Leu96 and Not the Canonic Vertebrates Gln96 Residue
Table 1 shows all vertebrates and bird species which are tested in this study and comprise most of Earth’s continents and thrive in various habitats. MHC class I molecules have been found to preserve seven canonical amino acid positions in vertebrates since the appearance of jawed fishes on Earth to humans (from Devonic Epoch, 300 MYA, until present) [83,84]. These conserved amino acids are Thr10, Asp29, and Asn86 in the α1 chain; and Gln96, Gly100, Cys101, and Cys164 in the α2 chain. The α1 and α2 protein domains form valve-like structures; antigenic molecules are accommodated inside this valve and are presented to the specific T-lymphocyte receptor to begin an immune response [8,85]. These conserved canonical residues must be under powerful evolutive forces to help keep the class I MHC valve-like structure. However, two of these permanent residues at such a wide time-evolutionary scale are NOT present in songbirds (Passeriformes), which account for about half (five thousand) of the extant birds: Val10 has been substituted for Thr10 on the α1 chain, and Leu96 has been substituted for Gln96 on the α2 chain (Figure 3; Table 2 and Table 3). Our research found these results in our MHC studies in wild passerine birds studied so far, including warbler and zebra finch predicted sequences [86].

Table 1.
Vertebrate English and Latin names of the species studied (including wild songbirds), and GenBank accession numbers are detailed. -: gap or unknown. *: predicted sequence. Passeriformes, other birds, primates, other mammals, reptiles, fishes, and wild passerines are thriving in an area that comprises all of Earth’s continents and sea ranges [43,44]. It is noteworthy that jawed fish (like zebra fish or carp) first had their appearance on Earth at least 400 MYA (see Ref. [3] from Table 1 at Ref. [5]).
Table 1.
Vertebrate English and Latin names of the species studied (including wild songbirds), and GenBank accession numbers are detailed. -: gap or unknown. *: predicted sequence. Passeriformes, other birds, primates, other mammals, reptiles, fishes, and wild passerines are thriving in an area that comprises all of Earth’s continents and sea ranges [43,44]. It is noteworthy that jawed fish (like zebra fish or carp) first had their appearance on Earth at least 400 MYA (see Ref. [3] from Table 1 at Ref. [5]).
| English Name | Name | GenBank No. | Position 10 | Position 96 |
|---|---|---|---|---|
| Zebra fish | Danio rerio | AAF20179 | - | Gln |
| Carp | Cyprinus carpio | [5] | Thr | Gln |
| African clawed frog | Xenopus laevis | [5] | Thr | Gln |
| Snake | Nerodiasipedon | [5] | - | - |
| Ameiva lizard | Ameiva ameiva | [5] | Thr | Gln |
| Cow | Bos taurus | ABW70136 | Thr | Gln |
| Dog | Canis familiaris | NP_001014767 | Thr | Gln |
| Horse | Equus caballus | NP_001075976 | Thr | Gln |
| Mouse | Mus musculus | AAY85367 | Thr | Gln |
| Sheep | Ovis aries | CAJ57269 | Thr | Gln |
| Rat | Rattus norvegicus | CAA74333 | Thr | Gln |
| Greater horseshoe bat | Rhinolophu sferrumequinum | ACC68844 * | Thr | Gln |
| Pig | Sus scrofa | ACA33862 | Thr | Gln |
| Short-beaked echidna | Tachyglossus aculeatus | AAM54212 | - | Gln |
| Commongibbon | Hylobates lar | AAB08074 | Thr | Gln |
| Orangutan | Pongo pygmaeus | AAK67485 | Thr | Gln |
| Western gorilla | Gorilla gorilla | CAA43100 | Thr | Gln |
| Chimpanzee | Pan troglodytes | BAC78189 | Thr | Gln |
| Bonobo | Pan paniscus | AAY59433 | Thr | Gln |
| Human (HLA-A2) | Homo sapiens (HLA-A2) | BAA07530 | Thr | Gln |
| Human (HLA-B) | Homo sapiens (HLA-B) | CAA06616 | Thr | Gln |
| Human (HLA-C) | Homo sapiens (HLA-C) | CAB02408 | Thr | Gln |
| Chicken | Gallus gallus | AY489160 | Thr | Gln |
| Japanese quail | Coturnix japonica | D29813 | Thr | Gln |
| Great reed warbler | Acrocephalus arundinaceus | CAA06566 | Val | Leu |
| Black siskin | Carduelis atrata | DQ257462 | Val | Leu |
| Black-capped siskin | Carduelis atriceps | FJ268821 | Val | Leu |
| European goldfinch | Carduelis carduelis | FJ266447 | Val | Leu |
| Citrilfinch | Carduelis citrinella | DQ257482 | Val | Leu |
| Lawrence’s goldfinch | Carduelis lawrencei | FJ314425 | Val | Leu |
| Pine siskin | Carduelis pinus | FJ266376 | Val | Leu |
| Eurasian siskin | Carduelis spinus | FJ266399 | Val | Leu |
| Common rosefinch | Carpodacus erythrinus | ACL31612.1 | - | Leu |
| Chaffinch | Fringilla coelebs | DQ257477 | Val | Leu |
| Yellow-rumped seedeater | Serinus atrogularis | DQ257479 | Val | Leu |
| Africancitril | Serinus citrinelloides | DQ257484 | Val | Leu |
| Lemon-breasted seedeater | Serinus citrinipectus | DQ257483 | Val | Leu |
| White-bellied canary | Serinus dorsostriatus | DQ257486 | Val | Leu |
| Yellow canary | Serinus flaviventris | DQ257487 | Val | Leu |
| Streaky-headed seedeater | Serinus gularis | DQ257489 | Val | Leu |
| Yellow-fronted canary | Serinus mozambicus | DQ257491 | Val | Leu |
| Streaky seedeater | Serinus striolatus | DQ257493 | Val | Leu |
| Tibetan serin | Serinus thibetanus | DQ257496 | Val | Leu |
| Zebra finch | Taeniopygia guttata | LOC100231469 * | Val | Leu |
In summary, songbirds have two different out of the seven canonic MHC class I amino acid residues that are present in vertebrates and also in other birds with more terrestrial thriving places, like chicken (G. gallus) and quail (Coturnix japonica) (Table 1 and Table 2). Sibley and Alquist offered a DNA classification not based on sequencing but on DNA RFLPs [3]. We have demonstrated that Passeriformes (accounting for 4600 species) of Serinus and Carduelis Genera have two residue changes in contrast with other bird species like chicken and quail (Table 1). Interactions of the two different songbird amino acid residues in tri-dimensional MHC proteins consist of the following:
- The amino acid residues Thr10 and Gln96 side chains that are canonic in all vertebrate MHC class I proteins do not present any interaction with each other or with the presented peptide within the molecular MHC class I valve.
- The side chains of these two exceptional songbird residues interact with the β2-microglobulin (B2M) molecule, and they provide security to maintain the tertiary structure of the complete molecule.
- In vertebrates other than birds (except chicken and quail), the canonic residues show these chemical peculiarities:
- B2M molecules have two hydrogen links to the Gln96 residue of the MHC class I chain: one link is to His31, and the second one is to the Trp60 amino acid of the B2M molecule.
- The Thr10 amino acid of the MHC chain is kept between residues Met54 and Phe62 of the B2M molecule; also, one H2O molecule is stuck to the Thr10 residue of the MHC peptide chain.
Table 1 and Table 2 and Figure 3 show which songbird species have these two canonic amino acid changes or exceptions and where the changes are in the MHC molecule. The effects of these changes are the following:
- Leu96 substitutes for Gln96 in the MHC chain, and this results in two hydrogen bonds to the B2M molecule disappearing, affecting the complete molecule stability. Also, the class I molecules of other Passeriformes, like Taeniopygia guttata (zebra finch), Acrocephalus arundinaceus (great reed warbler), and common rosefinch, have also been analyzed from the results in [38,87,88].
- The Val10 change in passerine birds from the canonical vertebrate Thr10 may counteract the effects of residue 96 change with regard to the B2M attachment affinity to the MHC class I α chain and the binding stability of the two molecules. This is due to the fact that van der Waals interactions with B2M appear at the level of residues Met54 and Phe62; in addition, the H2O molecule, which was trapped with the canonical vertebrate residue, is now released with an entropy change, i.e., an entropy gain.

Table 2.
MHC class I amino acid residue differences in sequences. Residue 10 in alpha-1 chain amino acid sequences of species MHC molecule. Residue 96 in MHC alpha-2 chain amino acid sequences of species. Passerine birds show exceptional changes on these two residues in contrast with other vertebrates. Passerines show Val10 instead of canonical Thr10 in vertebrates, which is replaced in the alpha-1 chain of MHC molecules, and they have Leu96 replacing canonical Leu96. This has been tested in passerine species thriving in a range that comprises most of the world. Non-passerine birds and other vertebrates show Thr and Gln at positions 10 and 96, respectively, which are found in non-passerine birds and all other analyzed vertebrates. The canonic vertebrate positions appeared in Earth vertebrates approximately 300 MYA, while passerines or songbirds appeared on Earth about 35 MYA in the Middle Eocene Epoch (see Figure 4, although some authors think that the passerine appearance on Earth was 30 MY before); thus, these are two exceptions out of the seven canonic residues in MHC class I molecules. The numbering of 10 and 96 residue positions is referred to as the human HLA-A2 molecule model [84,85,89]. The zebra finch (Taenopygia guttata) sequence is taken from [86]. The common rosefinch α2 domain sequence is referenced in [5,88]. The geographic origin of wild bird samples is detailed in Table 1 [79].
Table 2.
MHC class I amino acid residue differences in sequences. Residue 10 in alpha-1 chain amino acid sequences of species MHC molecule. Residue 96 in MHC alpha-2 chain amino acid sequences of species. Passerine birds show exceptional changes on these two residues in contrast with other vertebrates. Passerines show Val10 instead of canonical Thr10 in vertebrates, which is replaced in the alpha-1 chain of MHC molecules, and they have Leu96 replacing canonical Leu96. This has been tested in passerine species thriving in a range that comprises most of the world. Non-passerine birds and other vertebrates show Thr and Gln at positions 10 and 96, respectively, which are found in non-passerine birds and all other analyzed vertebrates. The canonic vertebrate positions appeared in Earth vertebrates approximately 300 MYA, while passerines or songbirds appeared on Earth about 35 MYA in the Middle Eocene Epoch (see Figure 4, although some authors think that the passerine appearance on Earth was 30 MY before); thus, these are two exceptions out of the seven canonic residues in MHC class I molecules. The numbering of 10 and 96 residue positions is referred to as the human HLA-A2 molecule model [84,85,89]. The zebra finch (Taenopygia guttata) sequence is taken from [86]. The common rosefinch α2 domain sequence is referenced in [5,88]. The geographic origin of wild bird samples is detailed in Table 1 [79].
| Model | 10 | 96 |
|---|---|---|
| HLA-A2 | Thr | Gln |
| Danio_rerio | - | - |
| Xenopus_laevis | - | - |
| Ameiva_ameiva | - | - |
| Bos_taurus | - | - |
| Canis_familiaris | - | - |
| Mus_musculus | - | - |
| Sus_scrofa | - | - |
| Pan_troglodytes | - | - |
| Coturnix_japonica | - | - |
| Gallus_gallus | - | - |
| Acrocephalus_arundinaceus | Val | Leu |
| Carduelis_atrata | Val | Leu |
| Carduelis_carduelis | Val | Leu |
| Carduelis_lawrencei | Val | Leu |
| Carduelis_pinus | Val | Leu |
| Carduelis_spinus | Val | Leu |
| Fringilla_coelebs | Val | Leu |
| Serinus_atrogularis | Val | Leu |
| Serinus_thibetanus | Val | Leu |
| Taeniopygia_guttata | Val | Leu |
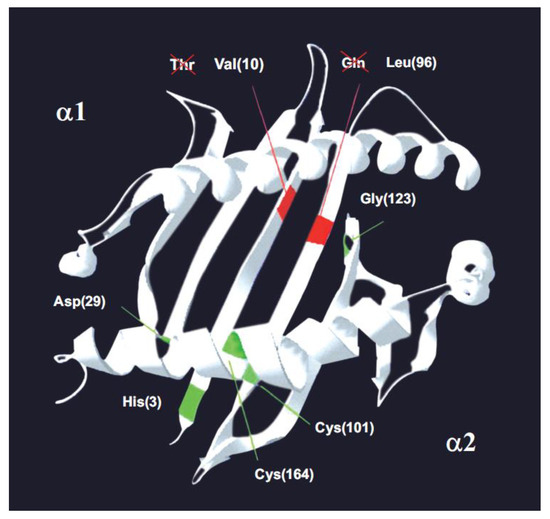
Figure 3.
The MHC-I molecule. A model of α-1 and α-2 domains on a songbird class I histocompatibility molecule. Green lines: the five positions conserved in vertebrates [84], including chicken and pheasant. Red lines: the two conserved positions (10 and 96) that have an amino acid change in songbirds–passerines compared to other vertebrates. Passerines bear Val10 and Leu96. In brackets: amino acid position number [5]. The 7 invariant or canonic amino acid positions in MHC class I molecules in vertebrates are shown in Passeriforme birds, where Val 10 and Gln 96 are observed instead of Thr 10 and Leu 96. The conclusion is that there are not seven conserved positions in vertebrates, but five conserved positions because, at the least, passerines have lost two out of the seven canonic positions. © by AA-V.
Figure 3.
The MHC-I molecule. A model of α-1 and α-2 domains on a songbird class I histocompatibility molecule. Green lines: the five positions conserved in vertebrates [84], including chicken and pheasant. Red lines: the two conserved positions (10 and 96) that have an amino acid change in songbirds–passerines compared to other vertebrates. Passerines bear Val10 and Leu96. In brackets: amino acid position number [5]. The 7 invariant or canonic amino acid positions in MHC class I molecules in vertebrates are shown in Passeriforme birds, where Val 10 and Gln 96 are observed instead of Thr 10 and Leu 96. The conclusion is that there are not seven conserved positions in vertebrates, but five conserved positions because, at the least, passerines have lost two out of the seven canonic positions. © by AA-V.
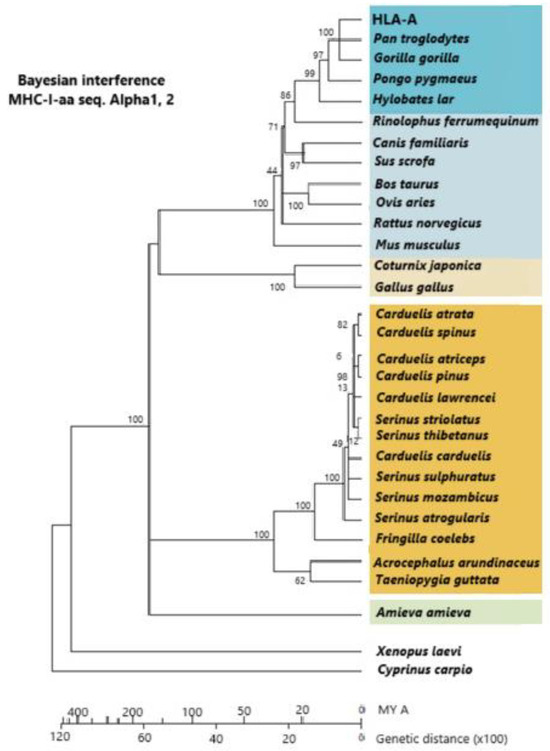
Figure 4.
A phylogram constructed with a linearized Bayesian inference methodology. It is built from alpha-1 and alpha-2 amino acid sequences of MHC class I molecules. Dark orange: Passeriformes; light orange: other birds; dark blue: primates; light blue: other mammals; green: Amieva lizard; white: other vertebrates. Vertebrates group together within groupal subdivisions. The vertebrate groupage is reptiles, mammals, more “terrestrial” birds, and more “aerial” birds. The use of molecules more reliable than MHCs (which are under selection pressure) is preferably carried out for calculating lineage time divergence (i.e., mt cyt b DNA molecules, which are subjected to a constant evolutive pressure). Passerine appearance on Earth is 35 MYA at the Middle Eocene Epoch, although some authors think that this date should be put back about 30 MY before. [5,79] © by AA-V.
Figure 4.
A phylogram constructed with a linearized Bayesian inference methodology. It is built from alpha-1 and alpha-2 amino acid sequences of MHC class I molecules. Dark orange: Passeriformes; light orange: other birds; dark blue: primates; light blue: other mammals; green: Amieva lizard; white: other vertebrates. Vertebrates group together within groupal subdivisions. The vertebrate groupage is reptiles, mammals, more “terrestrial” birds, and more “aerial” birds. The use of molecules more reliable than MHCs (which are under selection pressure) is preferably carried out for calculating lineage time divergence (i.e., mt cyt b DNA molecules, which are subjected to a constant evolutive pressure). Passerine appearance on Earth is 35 MYA at the Middle Eocene Epoch, although some authors think that this date should be put back about 30 MY before. [5,79] © by AA-V.
5. Relatedness of MHC Class I Molecules Among Passeriformes Birds and Other Birds and Vertebrates
Figure 4 shows how Passeriformes are closely related among themselves and separate from other birds (chicken and quail). The alpha-1 and alpha-2 domains of class I MHC molecules are used to construct the phylogenetic dendrogram. Since linearized Bayesian inference permits an approximate calculation of time (Figure 4, bottom line), it is also observed that Galliformes are older on Earth than Passeriformes [79]. It may be taken into account that half of the extant bird species are Passeriformes or songbirds, in contrast to all other extant birds, which are classified into 12 different species. Passeriformes are smaller and more aerial, and this may have helped them to pass more easily the 65 MYA dinosaur/bird extinction bottleneck, as the mainstream thinking is that birds and dinosaurs are closely related or that birds are extant surviving dinosaur lineages [79]. On the other hand, MHC class I introns of Passeriformes are substantially different from those of Galliformes (Arnaiz-Villena et al. unpublished, [79]; see Section 6).
6. Intron 2 of MHC Class I Molecules in Passeriformes
Wild Passeriformes were used throughout the studies presented in this work. Species were chosen whose living range and origin (Table 1) cover all world ranges. Table 3 shows that the geographically and genetically closest species, according to mitochondrial DNA analyses, have the biggest similarity percentage regarding similarity in nucleotide positions (Arnaiz-Villena et al., unpublished). Also, Passeriformes introns are larger than expected (in contrast to chicken smaller size): intron size is similar to that of human MHC class I intron 2. It was observed that 207 nucleotide positions out of 312 were conserved in the studied Passeriformes during approximately 15 million years (Figure 4, Table 3).

Table 3.
Similarity percentage between species studied by using nucleotide base positions of MHC class I intron 2. (79) © by AA-V.
Table 3.
Similarity percentage between species studied by using nucleotide base positions of MHC class I intron 2. (79) © by AA-V.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Carduelisatrata | ||||||||||||||||
| 2. Carduelisatriceps | 97.7 | |||||||||||||||
| 3. Carduelis carduelis | 97.1 | 97.4 | ||||||||||||||
| 4. C.ardueli scitrinella | 96.4 | 96.7 | 95.5 | |||||||||||||
| 5. Carduelis pinus | 98.1 | 99.7 | 97.7 | 97.1 | ||||||||||||
| 6. Carduelis spinus | 98.7 | 97.7 | 97.1 | 96.5 | 98.1 | |||||||||||
| 7. Serinus atrogularis | 97.1 | 97.0 | 96.1 | 95.5 | 97.1 | 97.1 | ||||||||||
| 8. Serinus citrinelloides | 97.4 | 97.1 | 97.1 | 95.8 | 97.4 | 97.4 | 98.4 | |||||||||
| 9. Serinus citrinipectus | 96.8 | 96.5 | 95.8 | 95.8 | 96.8 | 96.8 | 97.1 | 97.4 | ||||||||
| 10. Serinus dorsostriatus | 97.4 | 97.1 | 96.4 | 95.8 | 97.4 | 97.4 | 97.7 | 98.1 | 97.4 | |||||||
| 11. Serinus flaviventris | 96.4 | 96.1 | 95.5 | 94.8 | 96.4 | 96.5 | 97.4 | 97.7 | 96.5 | 97.1 | ||||||
| 12. Serinus gularis | 98.1 | 97.7 | 97.1 | 96.4 | 98.1 | 98.1 | 99.0 | 99.4 | 98.1 | 98.7 | 98.4 | |||||
| 13. Serinus mozambicus | 96.8 | 97.1 | 96.4 | 95.8 | 97.4 | 96.8 | 97.7 | 98.1 | 98.7 | 97.4 | 97,1 | 98.7 | ||||
| 14. Serinus striolatus | 97.4 | 97.1 | 97.1 | 95.8 | 97.4 | 97.4 | 97.7 | 98.7 | 96.8 | 97.4 | 97.1 | 98.7 | 97.4 | |||
| 15. Serinus thibetanus | 98.4 | 97.4 | 96.8 | 96.1 | 97.7 | 97.7 | 96.8 | 97.1 | 96.5 | 97.1 | 96.1 | 97.7 | 96.4 | 97.1 | ||
| 16. Fringilla coelebs | 84.5 | 84.5 | 84.2 | 83.5 | 84.5 | 84.2 | 83.5 | 84.5 | 83.9 | 84.2 | 82.4 | 84.5 | 83.9 | 83.5 | 83.5 |
7. MHC and Bird Diseases
MHC genes may also influence susceptibility or resistance to certain diseases. The research field on this topic in birds is yet undeveloped compared to studies in humans and mice. However, there are some bird diseases that have been described to be associated with MHC that are going to be briefly referred to in this section (see Table 3).
7.1. Marek’s Disease (MD)
Marek’s disease is a frequent disease in grange-bred chickens. It is caused by an oncogenic herpesvirus that causes serious immunosuppressive diseases, lymphoma, and other malignant diseases [90]. It is proved that chickens with resistance to MD have a specific size-determined (1.5 Kb) MHC class I transcript and no other [91]. The vaccine for MD is sometimes useless in certain breeding granges due to unknown reasons [90].
7.2. Malaria
MHC alleles or a group of neighbouring MHC genes/alleles, as measured by RFLP or other methodologies (haplotypes), have been found to be linked to malaria bird disease. Disease severity and prevalence have been associated with certain MHC-I alleles in Cyanistes caeruleus [92], Achricephalus arundinaceous [93], Geothlypistrichas [11], and Melospizamelodea [94]. The prevalence and severity of malaria in Parus major [95] and Achrocephalusschoenobacnus [96] are associated with MHC haplotypes. The prevalence of malaria has also been associated with MHC in Ficedulaalbicollis [97].
7.3. Bacterial Skeletal Disease
Chicken skeletal lesions, bacterial pathogens, and MHC genotype are apparently involved in the observed clinical signs. A4 and A12 MHC class I haplotypes are associated with this pathology. The disease causes lameness due to tenosynovitis, arthritis, and femoral and tibiotarsal osteomyelitis, and it is of significant importance in chicken breeding. Many times, it has been found to be associated with Staphylococcus aureus, but it also may be caused by other Staphyloccocus agents, such as S. agnetis, S. cohnii, S. epidermidis, S. hyicus, or S. simulans [98].
7.4. Avian Pox
Avian pox is a viral disease that affects wild and domestic birds. It is caused by the avipoxvirus, which is a member of the Poxviridae family. The disease is characterized by the formation of wart-like lesions on the skin, beak, and feet of affected birds. Avian pox is a common disease that affects a wide range of bird species, including songbirds, raptors, waterfowl, and game birds. In this article, we will explore the causes, impacts, and management of avian pox in wildlife populations. Susceptibility is also related to MHC [99].
7.5. Ectoparasite Infection
Four congenic lines of chickens, differing only at the MHC, were comparatively infested with a cosmopolitan ectoparasite of birds—Northern Fowl Mite (NFM)—which is also a serious pest species of poultry. Mite infestations were monitored over time, and mite densities (weekly and maximum) were compared among lines. Chickens with the MHC haplotype B21 were relatively resistant to NFM compared with birds in the B15 congenic line [96,100,101]. The NFM infestation produces a huge energetic cost in the individual due to a defective immune response causing less egg weight and other pathologies [102].
7.6. Infectious Bronchitis (IBV)
To study disease resistance in chickens, MHC congenic chicken lines that share the same genetic background with differences exclusively in their MHC B locus have been developed [103,104,105,106]. Using these chicken lines as animal models, associations between MHC haplotypes and disease resistance or susceptibility have been described for several infectious pathogens, including Coccidia [107,108], pathogenic bacteria [109,110,111,112], oncogenic viruses [113,114,115,116,117,118,119,120], and other viruses, including IBV [121,122,123,124,125,126,127,128,129,130]. The disease causes respiratory deficiency, leading to weight and egg production decreases [131].
Recent advances in MHC avian immunology and several infectious diseases are noteworthy: particular epitopes binding B2 haplotype chickens that confer resistance to infectious avian diseases have been identified [132]. Also, some of the CD8+ T responsive cell epitopes have also been discovered, and this may be crucial for effective vaccine development [133]. In addition, CD8+ T-cell-specific B haplotypes in duck and twelve CD8+ influenza virus T-cell epitopes have been reported in a progressive way to reach a fully useful vaccine [134].
8. Conclusions
1. The MHC class I molecules analyzed in songbirds or Passeriformes have only 5 out of 7 exceptional canonic or invariant residues (Figure 3): Passeriformes Val10 and Gln92 differ. Whether the tested MHC class I molecules are classical or non-classical is not known since the structural difference between both kinds of molecules is now blurred [135]. These two residues change the concept that MHC class I molecules have seven invariant canonic residues in vertebrates, as the canonic changes are only five when counting passerine birds.
2. These exceptional two residues have an effect on the MHC class I alpha chain joining to beta-2-microglobulin, whose entropic and physical interaction consequences are explained in the text, but no functional consequences may yet be inferred (Table 1 and Table 3). More studies are ongoing, and it is necessary that more species be searched.
3. Also, Passeriformes birds’ MHC class I intron 2 shows remarkable preservation over time; it has been quite stable for millions of years. All these studies have been performed with wild birds DNA (Figure 2, Table 1).
4. The linkage of MHC to diseases has been scanty in birds in comparison to the too many done (without a pathogenesis result) in man and mouse models. However, some microbes and parasites are associated with MHC in birds, like malaria, Marek’s disease, and mites. The susceptibility to these and other diseases has been related to both specific MHC alleles and MHC gene duplication.
Author Contributions
Conceptualization, writing of the paper, figure design, A.A.-V., T.L., and F.S.-T.; bibliography and references, T.L. and C.V.-Y.; review of the manuscript, I.J. and J.M.M.-V.; bird taxonomy, V.R.-d.-V.; funding, A.A.-V. and J.M.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
Universidad Complutense de Madrid grant number PR3/23-30834.
Acknowledgments
We want to thank the University Complutense of Madrid for its continuous support.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Barker, F.K.; Cibois, A.; Schikler, P.; Feinstein, J.; Cracraft, J. Phylogeny and diversification of the largest avian radiation. Proc. Natl. Acad. Sci. USA 2004, 101, 11040–11045. [Google Scholar] [CrossRef] [PubMed]
- Ericson, P.G.; Klopfstein, S.; Irestedt, M.; Nguyen, J.M.; Nylander, J.A. Dating the diversification of the major lineages of Passeriformes (Aves). BMC Evol. Biol. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.G.; Ahlquist, J. Phylogeny and Classification of Birds; Yale University Press: New Haven, CT, USA, 1990. [Google Scholar]
- Sanz, J. Los Dinosaurios Voladores: Historia Evolutiva de las Aves Primitivas; Ediciones Libertarias/Prodhufi: Madrid, Spain, 1999. [Google Scholar]
- Arnaiz-Villena, A.; Ruiz-del-Valle, V.; Reche, P.; Gomez-Prieto, P.; Lowry, E.; Zamora, J.; Areces, C.; Rey, D.; Parga, C.; Serrano-Vela, J.I. Songbirds conserved sites and intron size of MHC Class I molecules reveal a unique evolution in vertebrates. Open Ornithol. J. 2010, 3, 156–165. [Google Scholar] [CrossRef]
- Zamora, J.; Mosoco, J.; Ruiz-del-Valle, V.; Lowy, E.; Serrano-Vela, I.; Ira-Cachafeiro, J.; Arnaiz-Villena, A. Conjoint mitochondrial phylogenetic trees for canaries Serinus spp. and goldfinches Carduelis spp. show several specific polytomies. Ardeola 2006, 53, 1–17. [Google Scholar]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.L.; Harshman, J.; et al. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Natural History of the Major Histocompatibility Complex; John Willey and Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Hess, C.M.; Edwards, S. The Evolution of the Major Histocompatibility Complex in Birds. BioScience 2002, 52, 423–431. [Google Scholar] [CrossRef]
- Fleming-Canepa, X.; Jensen, S.M.; Mesa, C.M.; Diaz-Satizabal, L.; Roth, A.J.; Parks-Dely, J.A.; Moon, D.A.; Wong, J.P.; Evseev, D.; Gossen, D.A.; et al. Extensive Allelic Diversity of MHC Class I in Wild Mallard Ducks. J. Immunol. 2016, 197, 783–794. [Google Scholar] [CrossRef]
- Minias, P.; He, K.; Dunn, P.O. The strength of selection is consistent across both domains of the MHC class I peptide-binding groove in birds. BMC Ecol. Evol. 2021, 21, 80. [Google Scholar] [CrossRef]
- Kaufman, J.; Völk, H.; Wallny, H.J. A “minimal essential Mhc” and an “unrecognized Mhc”: Two extremes in selection for polymorphism. Immunol. Rev. 1995, 143, 63–88. [Google Scholar] [CrossRef]
- Kaufman, J.; Jacob, J.; Shaw, I.; Walker, B.; Milne, S.; Beck, S.; Salomonsen, J. Gene organisation determines evolution of function in the chicken, M.H.C. Immunol. Rev. 1999, 167, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, P.J.; Saper, M.A.; Samraoui, B.; Bennett, W.S.; Strominger, J.L.; Wiley, D.C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 1987, 329, 512–518. [Google Scholar] [CrossRef]
- Briles, W.E.; McGibbon, W.H. Heterozygosity of inbred lines of chickens of two loci effecting cellular antigens. Genetics 1948, 33, 605. [Google Scholar]
- Briles, W.; McGibbon, W.; Irwin, M. On multiple alleles effecting cellular antigens in the chicken. Genetics 1950, 35, 633–652. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.; Arnul, M.; Sorensen, P. The chicken MHC and its importance. In Improving Genetic Disease Resistance in Farm Animals; Van der Zijpp, A.J., Sybesma, W., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1989; pp. 42–62. [Google Scholar]
- Kroemer, G.; Zoorob, R.; Auffray, C. Structure and expression of a chicken MHC class I gene. Immunogenetics 1990, 31, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, H. Passerine MHC: Genetic variation and disease resistance in the wild. J. Ornithol. 2007, 148, 469–477. [Google Scholar] [CrossRef]
- Drews, A.; Westerdahl, H. Not all birds have a single dominantly expressed MHC-I gene: Transcription suggests that siskins have many highly expressed MHC-I genes. Sci. Rep. 2019, 9, 19506. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, H.; Mellinger, S.; Sigeman, H.; Kutschera, V.E.; Proux-Wéra, E.; Lundberg, M.; Weissensteiner, M.; Churcher, A.; Bunikis, I.; Hansson, B.; et al. The genomic architecture of the passerine MHC region: High repeat content and contrasting evolutionary histories of single copy and tandemly duplicated MHC genes. Mol. Ecol. Resour. 2022, 22, 2379–2395. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, Y.; Zheng, C.; Wei, Y.; Hou, J.; Zhang, P.; He, W.; Lv, X.; Ding, Y.; Liang, H.; et al. Systematic functional interrogation of human pseudogenes using CRISPRi. Genom. Biol. 2021, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.; Horton, R.; Humphray, S.; Rowen, L.; Trowsdale, J.; Beck, S. Gene organisation, sequence variation and isochore structure at the centromeric boundary of the human MHC. J. Mol. Biol. 1999, 291, 789–799. [Google Scholar] [CrossRef]
- Bonneaud, C.; Sorci, G.; Morin, V.; Westerdahl, H.; Zoorob, R.; Wittzell, H. Diversity of Mhc class I and IIB genes in house sparrows (Passer domesticus). Immunogenetics 2004, 55, 855–865. [Google Scholar] [CrossRef]
- Miller, M.M.; Taylor, R.L., Jr. Brief review of the chicken Major Histocompatibility Complex: The genes, their distribution on chromosome 16, and their contributions to disease resistance. Poult. Sci. 2016, 95, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.A.; Khodiyar, V.K.; Lush, M.J.; Povey, S.; Talbot, C.C., Jr.; Wright, M.W.; et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef]
- Gomez-Prieto, P.; Parga-Lozano, C.; Rey, D.; Moreno, E.; Arnaiz-Villena, A. HLA-G, -F and -E: Polymorphism, Function, and Evolution. In The HLA Complex in Biology and Medicine, A Resource Book, 1st ed.; Mehra, N.K., Ed.; Jaypee Brothers Medical Publisehrs: New Dehli, India, 2011; pp. 159–174. [Google Scholar]
- Robinson, J.; Halliwell, J.A.; Hayhurst, J.D.; Flicek, P.; Parham, P.; Marsh, S.G. The IPD and IMGT/HLA database: Allele variant databases. Nucleic Acids Res. 2015, 43, D423–D431. [Google Scholar] [CrossRef] [PubMed]
- Grey, H.M.; Kubo, R.T.; Colon, S.M.; Poulik, M.D.; Cresswell, P.; Springer, T.; Turner, M.; Strominger, J.L. The small subunit of HL-A antigens is beta 2-microglobulin. J. Exp. Med. 1973, 138, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Ploegh, H.L.; Orr, H.T.; Strominger, J.L. Major histocompatibility antigens: The human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell 1981, 24, 287–299. [Google Scholar] [CrossRef]
- Klein, J.; Sato, A. The HLA system. First Two Parts. N. Engl. J. Med. 2000, 343, 702–709. [Google Scholar] [CrossRef]
- Hviid, T.V. HLA-G in human reproduction: Aspects of genetics, function and pregnancy complications. Hum. Reprod. Update 2006, 12, 209–232. [Google Scholar] [CrossRef]
- Donadi, E.A.; Castelli, E.C.; Arnaiz-Villena, A.; Roger, M.; Rey, D.; Moreau, P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol. Life Sci. 2011, 68, 369–395. [Google Scholar] [CrossRef]
- Carosella, E.D.; Moreau, P.; LeMaoult, J.; Rouas-Freiss, N. HLA-G: From biology to clinical benefits. Trends Immunol. 2008, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Favier, B.; Rouas-Freiss, N.; Moreau, P.; Le Maoult, J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 2008, 111, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.S.; Hogge, W.A.; Barmada, M.M.; Ferrell, R.E. Comprehensive analysis of HLA-G: Implications for recurrent spontaneous abortion. Reprod. Sci. 2010, 17, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, D.E.; Koller, B.H.; Orr, H.T. A human histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc. Natl. Acad. Sci. USA 1987, 84, 9145. [Google Scholar] [CrossRef] [PubMed]
- Comiskey, M.; Goldstein, C.Y.; De Fazio, S.R.; Mammolenti, M.; Newmark, J.A.; Warner, C.M. Evidence that HLA-G is the functional homolog of mouse Qa-2, the Ped gene product. Hum. Immunol. 2003, 64, 999–1004. [Google Scholar] [CrossRef]
- Colonna, M. Specificity and function of immunoglobulin super- family NK cell inhibitory and stimulatory receptors. Immunol. Rev. 1997, 155, 127–133. [Google Scholar] [CrossRef]
- Ponte, M.; Cantoni, C.; Biassoni, R.; Tradori-Cappai, A.; Bentivoglio, G.; Vitale, C.; Bertone, S.; Moretta, A.; Moretta, L.; Mingari, M.C. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: Decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLAG1-specific receptor. Proc. Natl. Acad. Sci. USA 1996, 96, 5674–5679. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999, 189, 1093–1100. [Google Scholar] [CrossRef]
- Gao, G.F.; Willcox, B.E.; Wyer, J.R.; Boulter, J.M.; O’Callaghan, C.A.; Maenaka, K.; Stuart, D.I.; Jones, E.Y.; Van Der Merwe, P.A.; Bell, J.I.; et al. Classical and nonclassical class I major histocompatibility complex molecules exhibit subtle conformational differences that affect binding to CD8 α/α. J. Biol. Chem. 2000, 275, 15232–15238. [Google Scholar] [CrossRef] [PubMed]
- Contini, P.; Ghio, M.; Poggi, A.; Filaci, G.; Indiveri, F.; Ferrone, S.; Puppo, F. Soluble HLA-A, -B, -C and –G molecules induce apoptosis in T and NK CD8 (+) cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur. J. Immunol. 2003, 33, 125–134. [Google Scholar] [CrossRef]
- Shiroishi, M.; Kuroki, K.; Rasubala, L.; Tsumoto, K.; Kumagai, I.; Kurimoto, E.; Kato, K.; Kohda, D.; Maenaka, K. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc. Natl. Acad. Sci. USA 2006, 103, 16412–16417. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Malacko, A.R.; Ishitani, A.; Chen, M.-C.; Bajorath, J.; Marquardt, H.; Geraghty, D.E. The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity 1995, 3, 591–600. [Google Scholar] [CrossRef]
- Crisa, L.; McMaster, M.T.; Ishii, J.K.; Fisher, S.J.; Salomon, D.R. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J. Exp. Med. 1997, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Blaschitz, A.; Crisa, L.; Schmitt, C.; Fournel, S.; King, A.; Loke, Y.W.; Dohr, G.; Le Bouteiller, P. HLA-G in the human thymus: A subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int. Immunol. 1999, 11, 889–898. [Google Scholar] [CrossRef]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.M.; Carosella, E.D. Expression of HLA-G in human cornea, an immuneprivileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Menier, C.; Rabreau, M.; Challier, J.C.; Le Discorde, M.; Carosella, E.D.; Rouas-Freiss, N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood 2004, 104, 3153–3160. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saathoff, M.; Stampachiacchiere, B.; Bettermann, A.; Bulfone-Paus, S.; Takigawa, M.; Nickoloff, B.J.; Paus, R. Immunology of the human nail apparatus: The nail matrix is a site of relative immune privilege. J. Investig. Dermatol. 2005, 125, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, V.; Zalatan, J.; McMaster, M.; Prinsen, R.; Salomon, D.R.; Ricordi, C.; Torbett, B.E.; Meda, P.; Crisa, L. The class IHLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen, HLA-G. Diabetes 2006, 55, 1214–1222. [Google Scholar] [CrossRef]
- Braud, V.; Jones, E.Y.; McMichael, A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 1997, 27, 1164–1169. [Google Scholar] [CrossRef]
- Larsen, M.H.; Hviid, T.V. Human leukocyte antigen-G polymorphismin relation to expression, function, and disease. Hum. Immunol. 2009, 70, 1026–1034. [Google Scholar] [CrossRef]
- Solier, C.; Mallet, V.; Lenfant, F.; Bertrand, A.; Huchenq, A.; Le Bouteiller, P. HLA-G unique promoter region: Functional implications. Immunogenetics 2001, 53, 617–625. [Google Scholar] [CrossRef]
- Moreau, P.; Flajollet, S.; Carosella, E.D. Non-classical transcriptional regulation of HLA-G: An update. J. Cell Mol. Med. 2009, 13, 973–2989. [Google Scholar] [CrossRef]
- Hviid, T.V.; Sorensen, S.; Morling, N. Polymorphism in the regulatory region located more than 1.1 kilobases 50 to the start site of transcription, the promoter region, and exon 1 of the HLA-G gene. Hum. Immunol. 1999, 60, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Hviid, T.V.; Rizzo, R.; Christiansen, O.B.; Melchiorri, L.; Lindhard, A.; Baricordi, O.R. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics 2004, 56, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Shon, A.M.; Ober, C. Evidence of balancing selection at the HLA-G promoter region. Hum. Mol. Genet. 2005, 14, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.C.; Mendes-Junior, C.T.; Veiga-Castelli, L.C.; Roger, M.; Moreau, P.; Donadi, E.A. A comprehensive study of polymorphic sites along the HLA-G gene: Implication for gene regulation and evolution. Mol. Biol. Evol. 2011, 28, 3069–3086. [Google Scholar] [CrossRef]
- Martinez-Laso, J.; Herraiz, M.A.; Peñaloza, J.; Barbolla, M.L.; Jurado, M.L.; Macedo, J.; Vidart, J.; Cervera, I. Promoter sequences confirm the three different evolutionary lineages described for, H.L.A.-G. Hum. Immunol. 2013, 74, 383–388. [Google Scholar] [CrossRef]
- Castelli, E.C.; Mendes-Junior, C.T.; Deghaide, N.H.S.; de Albuquerque, R.S.; Muniz, Y.C.N.; Simões, R.T.; Carosella, E.D.; Moreau, P.; Donadi, E.A. The genetic structure of 3’ untranslated region of the HLA-G gene: Polymorphisms and haplotypes. Genes. Immun. 2010, 11, 134–141. [Google Scholar] [CrossRef]
- Clements, C.S.; Kjer-Nielsen, L.; Kostenko, L.; Hoare, H.L.; Dunstone, M.A.; Moses, E.; Freed, K.; Brooks, A.G.; Rossjohn, J.; McCluskey, J. Crystal structure of HLA-G: A nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc. Natl. Acad. Sci. USA 2005, 102, 3360–3365. [Google Scholar] [CrossRef]
- Ferreira, L.M.R.; Meissner, T.B.; Tilburgs, T.; Strominger, J.L. HLA-G: At the interface of maternal-fetal tolerance. Trends Immunol. 2017, 38, 272–286. [Google Scholar] [CrossRef]
- Papuchova, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The dual role of HLA-C in tolerance and immunity at the maternal-fetal interface. Front. Immunol. 2019, 10, 2730. [Google Scholar] [CrossRef]
- Canfield, S.M.; Gallagher, M.P.; Jiang, H.H.; Jiang, Y.; Zheng, Z.; Chess, L. HLA-E–restricted regulatory CD8+ T cells are involved in development and control of human autoimmune type 1 diabetes. J. Clin. Investig. 2010, 120, 3641–3650. [Google Scholar] [CrossRef]
- Romagnani, C.; Pietra, G.; Falco, M.; Millo, E.; Mazzarino, P.; Biassoni, R.; Moretta, A.; Moretta, L.; Mingari, M.C. Identification of HLA-E-specific alloreactive T lymphocytes: A cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc. Natl. Acad. Sci. USA 2002, 99, 11328–11333. [Google Scholar] [CrossRef]
- Dulberger, C.L.; McMurtrey, C.P.; Hölzemer, A.; Neu, K.E.; Liu, V.; Steinbach, A.M.; Garcia-Beltran, W.F.; Sulak, M.; Jabri, B.; Lynch, V.J.; et al. Human leukocyte antigen F presents peptides and regulates immunity through interactions with NK cell receptors. Immunity 2017, 46, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, T.; Blasczyk, R.; Bade-Doeding, B. HLAE: A novel player for histocompatibility. J. Immunol. Res. 2014, 2014, 352160. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.J.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors, C.D.9.4./.N.K.G.2.A. B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Burrows, C.K.; Kosova, G.; Herman, C.; Patterson, K.; Hartmann, K.E.; Edwards, D.R.V.; Stephenson, M.D.; Lynch, V.J.; Ober, C. Expression quantitative trait locus mapping studies in mid-secretory phase endometrial cells identifies HLA-F and TAP2 as fecundability-associated genes. PLoS Genet. 2016, 12, e1005858. [Google Scholar] [CrossRef]
- Song, S.; Miranda, C.J.; Braun, L.; Meyer, K.; Frakes, A.E.; Ferraiuolo, L.; Likhite, S.; Bevan, A.K.; Foust, K.D.; McConnell, M.J.; et al. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat. Med. 2016, 22, 397–403. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Hölzemer, A.; Martrus, G.; Chung, A.W.; Pacheco, Y.; Simoneau, C.R.; Rucevic, M.; Lamothe-Molina, P.A.; Pertel, T.; Kim, T.-E.; et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat. Immunol. 2016, 17, 1067–1074. [Google Scholar] [CrossRef]
- Korner, C.; Altfeld, M. Role of KIR3DS1 in human diseases. Front. Immunol. 2012, 3, 326. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F complex without peptide binds to MHC class I protein in the open conformer form. J. Immunol. 2010, 184, 6199–6208. [Google Scholar] [CrossRef]
- Suárez, B.; Morales, P.; Castro, M.; Fernández-Soria, V.; Recio, M.; Pérez-Bias, M.; Alvarez, M.; Díaz-Campos, N.; Arnaiz-Villena, A. Mhc-E polymorphism in Pongidae primates: The same allele is found in two different species. Tissue Antigens 1997, 50, 695–698. [Google Scholar] [CrossRef]
- Ferre, S. Evolución de los Genes de Histocompatibilidad de Clase I en la Radiación de Jilgueros (Lúganos) Sudamericanos. Doctoral Thesis, Facultad de Ciencias Biológicas, Madrid, Spain, 2001. [Google Scholar]
- Lowy, E. Evolución y Sistema Principal de Histocompatibilidad en Canarios (Género Serinus). Doctoral Thesis, Facultad de Medicina, Madrid, Spain, 2003. [Google Scholar]
- Klein, J.; Sato, A.; Nagl, S.; O’hUigin, C. Molecular trans-species polymorphism. Annu. Rev. Ecol. Syst. 1998, 29, 1–21. [Google Scholar] [CrossRef]
- Arnaiz-Villena, A.; Ruiz-Del-Valle, V.; Muñiz, E.; Palacio-Gruber, J.; Campos, C.; Gómez-Casado, E.; Villa, J.M.M.; Serrano-Vela, I. Major Histocompatibility Complex Allele Persistence in Eurasia and America in the Genus Carduelis (Spinus) During Million Years. Open Ornithol. J. 2017, 10, 92–104. [Google Scholar] [CrossRef]
- Arnaiz-Villena, A.; Areces, C.; Rey, D.; Enríquez-de-Salamanca, M.; Alonso-Rubio, J.; Ruiz-del-Valle, V. Three different North American Siskin/Goldfinch Evolutionary Radiations (Genus Carduelis): Pine Siskin Green Morphs and European siskins in America. Open Ornithol. J. 2012, 5, 73–81. [Google Scholar] [CrossRef]
- Arnaiz-Villena, A.; Álvarez-Tejado, M.; Ruíz-Del-Valle, V.; García-De-La-Torre, C.; Varela, P.; Recio, M.J.; Ferre, S.; Martínez-Laso, J. Phylogeny and rapid northern and southern hemisphere speciation of goldfinches during the Miocene and Pliocene epochs. Cell Mol. Life Sci. 1998, 54, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz-Villena, A.; Ruiz-del-Valle, V.; Moscoso, J.; Serrano-Vela, J.I.; Zamora, J. mtDNA phylogeny of North American Carduelis pinus group. Ardeola 2007, 54, 1–14. [Google Scholar]
- Arnaiz-Villena, A.; Ruiz-del-Valle, V.; Reguera, R.; Gomez-Prieto, P.; Serrano-Vela, J.I. What might have been the ancestor of New World siskins? Open Ornithol. J. 2008, 1, 46–47. [Google Scholar] [CrossRef]
- Grossberger, D.; Parham, P. Reptilian class I major histocompatibility complex genes reveal conserved elements in class I structure. Immunogenetics 1992, 36, 166–174. [Google Scholar] [CrossRef]
- Bjorkman, P.J.; Saper, M.A.; Samraoui, B.; Bennett, W.S. Structure of the human class I histocompatibility antigen, HLA-A 2. Nature 1987, 329, 506–512. [Google Scholar] [CrossRef]
- Stapley, J.; Birkhead, T.; Burke, T.; Slate, J. A linkage map of the zebra finch Taeniopygia guttata provides new insights into avian genome evolution. Genetics 2008, 179, 651–667. [Google Scholar] [CrossRef]
- Westerdahl, H.; Wittzell, H.; von Schantz, T. Polymorphism and transcription of MHC class I genes in a passerine bird, the great reed warbler. Immunogenetics 1999, 49, 158–170. [Google Scholar] [CrossRef]
- Promerová, M.; Albrecht, T.; Bryja, J. Extremely high MHC class I variation in a population of a long-distance migrant, the scarlet rosefinch (Carpodacus erythrinus). Immunogenetics 2009, 61, 451–461. [Google Scholar] [CrossRef]
- Parham, P.; Lomen, C.E.; Lawlor, D.A.; Ways, J.P.; Holmes, N.; Coppin, H.L.; Salter, R.D.; Wan, A.M.; Ennis, P.D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc. Natl. Acad. Sci. USA 1988, 85, 4005–4009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.J.; Teng, M.; Liu, Y.; Chen, F.J.; Yao, Y.; Li, E.Z.; Luo, J. Immune escape of avian oncogenic Marek’s disease herpesvirus and antagonistic host immune responses. npj Vaccines 2024, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, T.S.; Højsgaard, S.; Skjødt, K.; Juul-Madsen, H.R. Differences in chicken major histocompatibility complex (MHC) class Ialpha gene expression between Marek’s disease-resistant and -susceptible MHC haplotypes. Scand. J. Immunol. 2003, 57, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rivero de Aguilar, J.; Barroso, O.; Bonaccorso, E.; Cadena, H.; Hussing, L.; Jorquera, J.; Martinez, J.; Martínez-de la Puente, J.; Marzal, A.; León Miranda, F.; et al. Associations among MHC genes, latitude, and avian malaria infections in the rufous-collared sparrow (Zonotrichia capensis). Ecol. Evol. 2024, 14, e11634. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, H.; Waldenström, J.; Hansson, B.; Hasselquist, D.; von Schantz, T.; Bensch, S. Associations between malaria and MHC genes in a migratory songbird. Proc. Biol. Sci. 2005, 272, 1511–1518. [Google Scholar] [CrossRef]
- Slade, J.W.G.; Watson, M.J.; Kelly, T.R.; Gloor, G.B.; Bernards, M.A.; MacDougall-Shackleton, E.A. Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc. R. Soc. Lond. B Biol. Sci. 2016, 283, 20161966. [Google Scholar] [CrossRef] [PubMed]
- Sepil, I.; Lachish, S.; Hinks, A.E.; Sheldon, B.C. Mhc supertypes confer both qualitative and quantitative resistance to avian malaria infections in a wild bird population. Proc. Biol. Sci. 2013, 280, 20130134. [Google Scholar] [CrossRef]
- Ellis, V.A.; Collins, M.D.; Medeiros, M.C.; Sari, E.H.; Coffey, E.D.; Dickerson, R.C.; Lugarini, C.; Stratford, J.A.; Henry, D.R.; Merrill, L.; et al. Local host specializarion of avian haemosporidian parasites. Proc. Natl. Acad. Sci. USA 2015, 112, 11294–11299. [Google Scholar] [CrossRef] [PubMed]
- Radwan, J.; Zagalska-Neubauer, M.; Cichon, M.; Sendecka, J.; Kulma, K.; Gustafsson, L.; Babik, W. MHC diversity, malaria and lifetime reproductive success in collared flycatchers. Mol. Ecol. 2012, 21, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, G.M.; Szeleszczuk, P.; Dolka, B. Review on skeletal disorders caused by Staphylococcus spp. in poultry. Vet. Q. 2022, 42, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Gangoso, L.; Alcaide, M.; Grande, J.M.; Muñoz, J.; Talbot, S.L.; Sonsthagen, S.A.; Sage, G.K.; Figuerola, J. Colonizing the world in spite of reduced MHC variation. J. Evol. Biol. 2012, 25, 1438–1447. [Google Scholar] [CrossRef]
- Owen, J.P.; Delany, M.E.; Mullens, B.A. MHC haplotype involvement in avian resistance to an ectoparasite. Immunogenetics 2008, 60, 621–631. [Google Scholar] [CrossRef]
- Silva, A.P.D.; Gallardo, R.A. The Chicken MHC: Insights into Genetic Resistance, Immunity, and Inflammation Following Infectious Bronchitis Virus Infections. Vaccines 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.C.; Chappell, M.A.; Owen, J.P.; Mullens, B.A. Northern fowl mite (Ornithonyssussylviarum) effects on metabolism, body temperatures, skin condition, and egg production as a function of hen MHC haplotype. Poult. Sci. 2016, 95, 2536–2546. [Google Scholar] [CrossRef]
- Waters, N.F.; Lambert, W.V. Inbreeding in the White Leghorn fowl. Res. Bull. Iowa Agric. Home Econ. Exp. Stn. 1936, 18, 1. [Google Scholar]
- Delany, M.E.; Pisenti, J.M. Conservation of poultry genetic research resources: Consideration of the past, present and future. Poult. Avian Biol. Rev. 1998, 9, 25–42. [Google Scholar]
- Abplanalp, H. Inbred lines as genetic resources of chickens. Poult. Sci. Rev. 1992, 4, 29–39. [Google Scholar]
- Pisenti, J.M.; Delany, M.E.; Taylor, R.L., Jr.; Abbott, U.K.; Abplanalp, H.; Arthur, J.A.; Bakst, M.R.; Baxter-Jones, C.; Bitgood, J.J.; Bradley, F.A.; et al. Avian Genetics Resources at Risk: An Assessment and Proposal for Conservation of Genetic Stocks in the USA and Canada; McGuire, P.E., Ed.; UC Davis Genetic Resources Conservation Program: Davis, CA, USA, 1999. [Google Scholar]
- Clare, R.A.; Strout, R.G.; Taylor, R.L., Jr.; Collins, W.M.; Briles, W.E. Major histocompatibility (B) complex effects on acquired immunity to cecal coccidiosis. Immunogenetics 1985, 22, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Brake, D.A.; Fedor, C.H.; Werner, B.W.; Miller, T.J.; Taylor, R.L.; Clare, R.A. Characterization of immune response to Eimeria tenella antigens in a natural immunity model with hosts which di_er serologically at the B locus of the major histocompatibility complex. Infect. Immun. 1997, 65, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Lamont, S.J.; Bolin, C.; Cheville, N. Genetic resistance to fowl cholera is linked to the major histocompatibility complex. Immunogenetics 1987, 25, 284–289. [Google Scholar] [CrossRef]
- Cotter, P.F.; Taylor, R.L.; Abplanalp, H. Differential Resistance to Staphylococcus aureus Challenge in Major Histocompatibility (B) Complex Congenic Lines. Poult. Sci. 1992, 71, 1873–1878. [Google Scholar] [CrossRef]
- Cotter, P.F.; Taylor, R.L., Jr.; Abplanalp, H. B-complex associated immunity to Salmonella enteritidis challenge in congenic chickens. Poult. Sci. 1998, 77, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Miller, M.M.; Lamont, S.J. Association of MHC class I and class II gene polymorphisms with vaccine or challenge response to Salmonella enteritidis in young chicks. Immunogenetics 2002, 54, 582–590. [Google Scholar] [CrossRef]
- Collins, W.M.; Briles, W.E.; Zsigray, R.M.; Dunlop, W.R.; Corbett, A.C.; Clark, K.K.; Marks, J.L.; McGrail, T.P. The B locus (MHC) in the chicken: Association with the fate of RSV-induced tumors. Immunogenetics 1977, 5, 333–343. [Google Scholar] [CrossRef]
- Schierman, L.W.; Watanabe, D.H.; McBride, R.A. Increased growth of Rous sarcomas in chickens pretreated with formalinized syngeneic tumor cells. Eur. J. Immunol. 1977, 7, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Calnek, B.W.; Fabricant, J.; Abplanalp, H. Influence of oncogenicity of Marek’ disease virus on evaluation of genetic resistance. Poult. Sci. 1981, 60, 2559–2566. [Google Scholar] [CrossRef]
- Briles, W.E.; Briles, R.W.; Pollock, D.L.; Pattison, M. Marek’s disease resistance of B (MHC) heterozygotes in a cross of purebred Leghorn lines. Poult. Sci. 1982, 61, 205–211. [Google Scholar] [CrossRef]
- Bacon, L.D.; Crittenden, L.B.; Witter, R.L.; Fadly, A.; Motta, J. B5 and B15 associated with progressive Marek’s disease, Rous sarcoma, and avian leukosis virus-induced tumors in inbred 15I4 chickens. Poult. Sci. 1983, 62, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Witter, P.L.; Calnek, B.W. Resistance to Marek’s disease of congenic lines di_ering in major histocompatibility haplotypes to 3 virus strains. In International Symposium on Marek’s Disease, 2nd ed.; Abplanalp, H., Schat, K.A., Eds.; Springer: Ithaca, NY, USA, 1984; pp. 347–358. [Google Scholar]
- Wakenell, P.S.; Miller, M.M.; Goto, R.M.; Gauderman, W.J.; Briles, W.E. Association between the Rfp-Y haplotype and the incidence of Marek’s disease in chickens. Immunogenetics 1996, 44, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L. Major histocompatibility (B) complex control of responses against Rous sarcomas. Poult. Sci. 2004, 83, 638–664. [Google Scholar] [CrossRef] [PubMed]
- Bumstead, N.; Huggins, M.B.; Cook, J.K. Genetic differences in susceptibility to a mixture of avian infectious bronchitis virus and Escherichia coli. Br. Poult. Sci. 1989, 30, 39–48. [Google Scholar] [CrossRef]
- Cook, J.; Otsuki, K.; Huggins, M.; Bumstead, N. Investigations into resistance of chicken lines to infection with infectious bronchitis virus. Adv. Exp. Med. Biol. 1990, 276, 491–496. [Google Scholar] [PubMed]
- Otsuki, K.; Matsuo, K.; Maeda, N.; Sanekata, T.; Tsubokura, M. Selection of Variants of Avian Infectious Bronchitis Virus Showing Tropism for Different Organs; Springer: Boston, MA, USA, 1990; pp. 379–384. [Google Scholar]
- Ignjatovic, J.; Reece, R.; Ashton, F. Susceptibility of three genetic lines of chicks to infection with a nephropathogenic T strain of avian infectious bronchitis virus. J. Comp. Pathol. 2003, 128, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bacon, L.D.; Hunter, D.B.; Zhang, H.M.; Brand, K.; Etches, R. Retrospective evidence that the MHC (B haplotype) of chickens influences genetic resistance to attenuated infectious bronchitis vaccine strains in chickens. Avian Pathol. 2004, 33, 605–609. [Google Scholar] [CrossRef]
- Joiner, K.S.; Hoerr, F.J.; Ewald, S.J.; van Santen, V.L.; Wright, J.C.; van Ginkel, F.W.; Toro, H. Pathogenesis of infectious bronchitis virus in vaccinated chickens of two di_erent major histocompatibility B complex genotypes. Avian Dis. 2007, 51, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Banat, G.R.; Tkalcic, S.; Dzielawa, J.A.; Jackwood, M.W.; Saggese, M.D.; Yates, L.; Kopulos, R.; Briles, W.E.; Collisson, E.W. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev. Comp. Immunol. 2013, 39, 430–437. [Google Scholar] [CrossRef]
- Smith, J.; Sadeyen, J.R.; Cavanagh, D.; Kaiser, P.; Burt, D.W. The early immune response to infection of chickens with Infectious Bronchitis Virus (IBV) in susceptible and resistant birds. BMC Vet. Res. 2015, 11, 256. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Hauck, R.; Zhou, H.; Gallardo, R.A. Understanding immune resistance to infectious bronchitis using major histocompatibility complex chicken lines. Avian Dis. 2017, 61, 358–365. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Hauck, R.; Kern, C.; Wang, Y.; Zhou, H.; Gallardo, R.A. Effect of chicken MHC haplotype on resistance to distantly-related infectious bronchitis viruses. Avian Dis. 2019, 63, 310–317. [Google Scholar] [CrossRef]
- Falchieri, M.; Coward, V.J.; Reid, S.M.; Lewis, T.; Banyard, A.C. Infectious bronchitis virus: An overview of the “chicken coronavirus”. J. Med. Microbiol. 2024, 73, 001828. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, Q.; Li, Y.; Zhang, M.M.N.; Liao, M.; Song, W.; Chen, R.; Yao, Y.; Nair, V.; Dai, M. Revealing novel and conservative T-cell epitopes with MHC B2 restriction on H9N2 avian influenza virus (AIV). J. Biol. Chem. 2024, 300, 107395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Ma, M.; Yang, N.; Du, S.; Liao, M.; Dai, M. Revealing novel and conservative CD8+T-cell epitopes with MHC B2 restriction on ALV-J. Vet. Res. 2024, 55, 164. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Chen, Y.; Xie, Z.; Zhao, L.; Du, S.; Ma, M.; Liao, M.; Dai, M. Revealing novel CD8+ T-cell epitopes from the H5N1 avian influenza virus in HBW/B1 haplotype ducks. Vet. Res. 2024, 55, 169. [Google Scholar] [CrossRef]
- Arnaiz-Villena, A.; Suarez-Trujillo, F.; Juarez, I.; Rodríguez-Sainz, C.; Palacio-Gruber, J.; Vaquero-Yuste, C.; Molina-Alejandre, M.; Fernández-Cruz, E.; Martin-Villa, J.M. Evolution and molecular interactions of major histocompatibility complex (MHC)-G, -E and -F genes. Cell. Mol. Life Sci. 2022, 79, 464. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).