Recent Strategies for Ni3S2-Based Electrocatalysts with Enhanced Hydrogen Evolution Performance: A Tutorial Review
Abstract
1. Introduction
2. Mechanism of HER and Performance Evaluation Parameters

2.1. Overpotential
2.2. Tafel Slope and Exchange Current Density
2.3. Electrochemical Active Specific Surface Area
2.4. EIS
2.5. Stability
2.6. Faradaic Efficiency
2.7. Mass Activity
2.8. Specific Activity
2.9. Turnover Frequency
3. Research Status of Ni-Based HER Electrocatalysts
3.1. Nickel-Based Oxides and Hydroxides
3.2. Nickel-Based Phosphide
3.3. Nickel-Based Sulfides
4. Synthesis Method for Ni3S2
4.1. Chemical Vapor Deposition Method
4.2. Electrochemical Deposition Method
4.3. Hydrothermal Reaction Method
4.4. Other Synthesis Methods
5. Strategies for Improving Electrocatalytic Hydrogen Evolution Performance of Ni3S2-Based Materials
5.1. Improving the Intrinsic Catalytic Activity of Ni3S2
5.1.1. Doping Heteroatoms
5.1.2. Introducing Defects
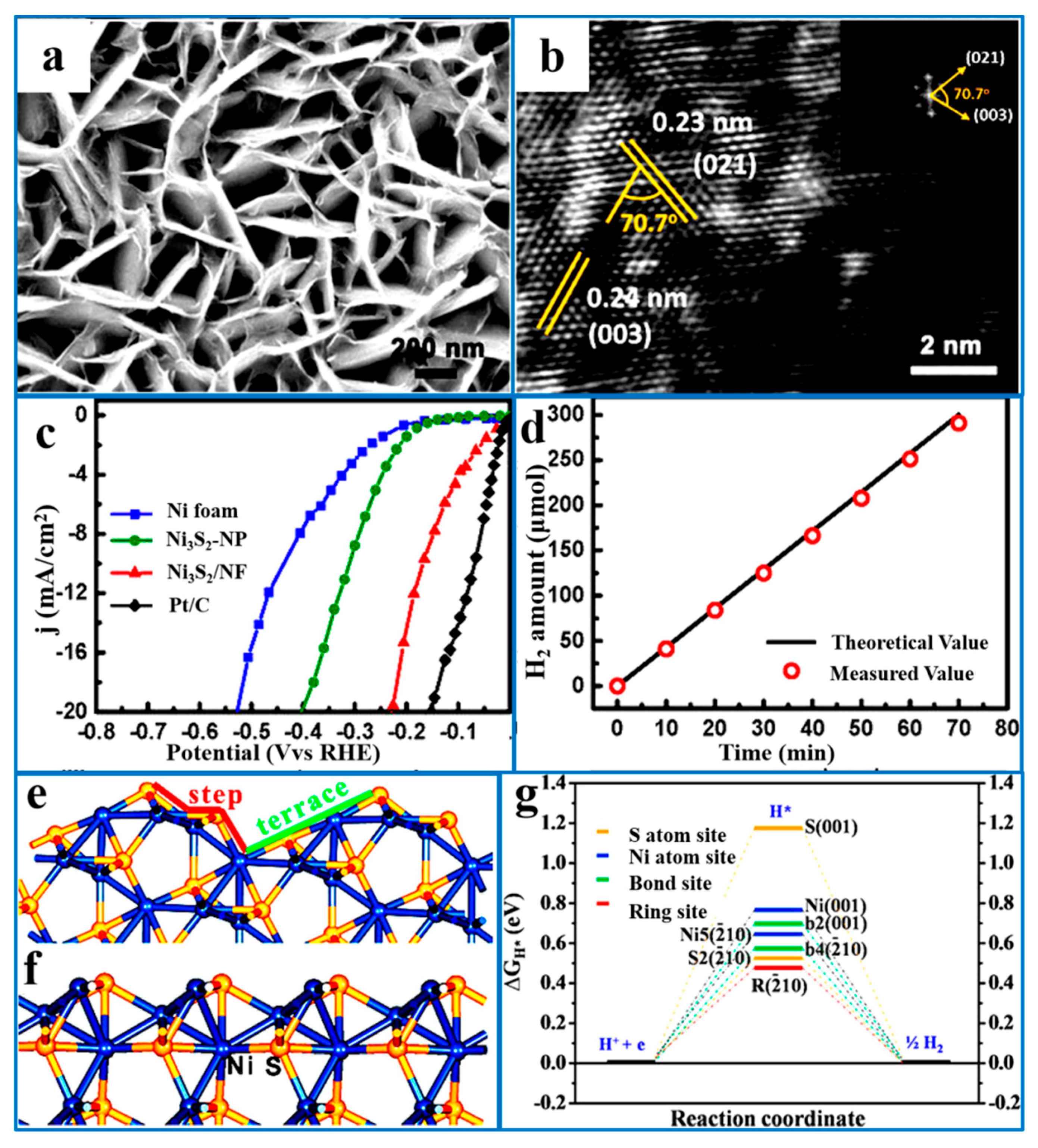
5.1.3. Crystal Facet Engineering
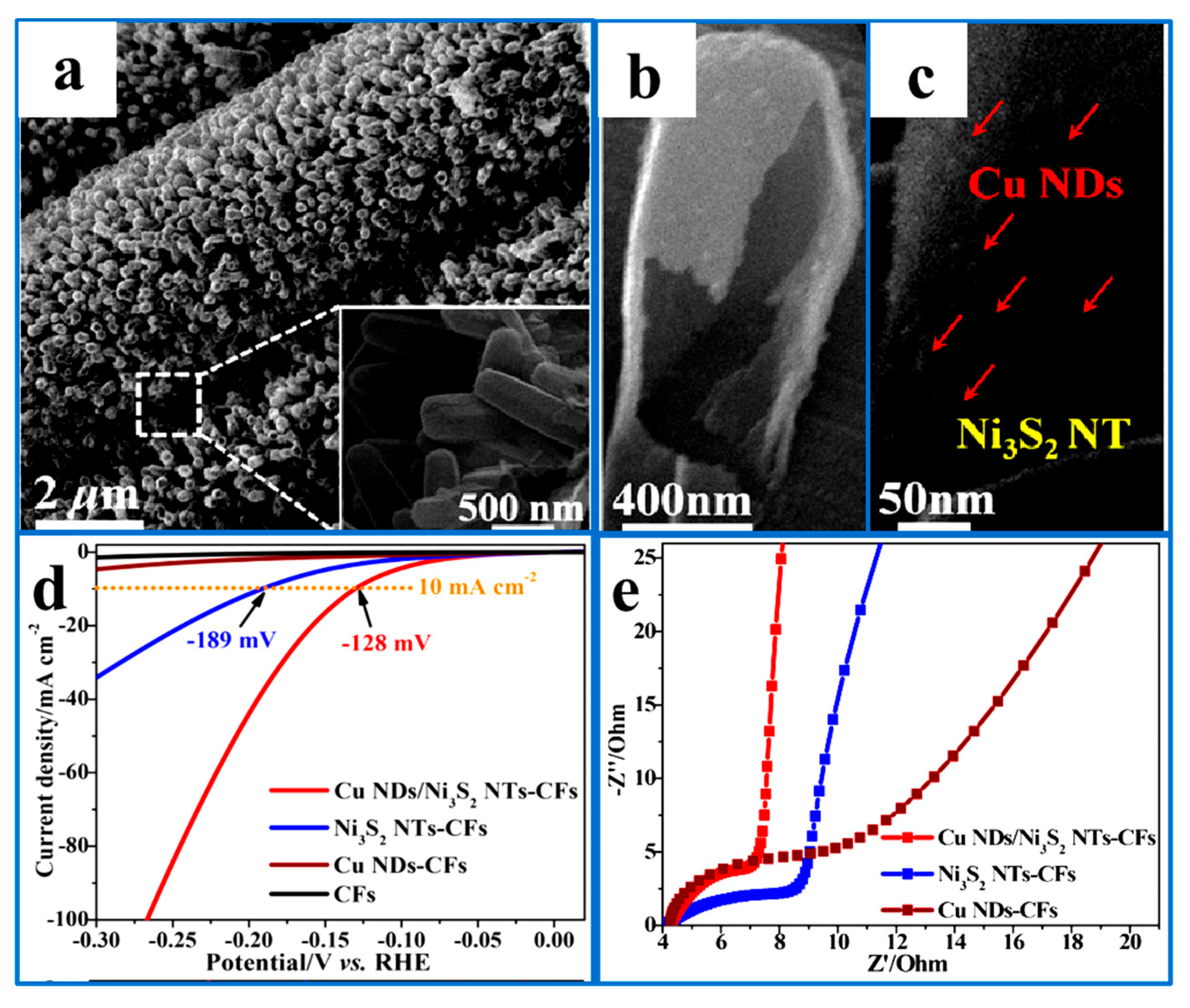
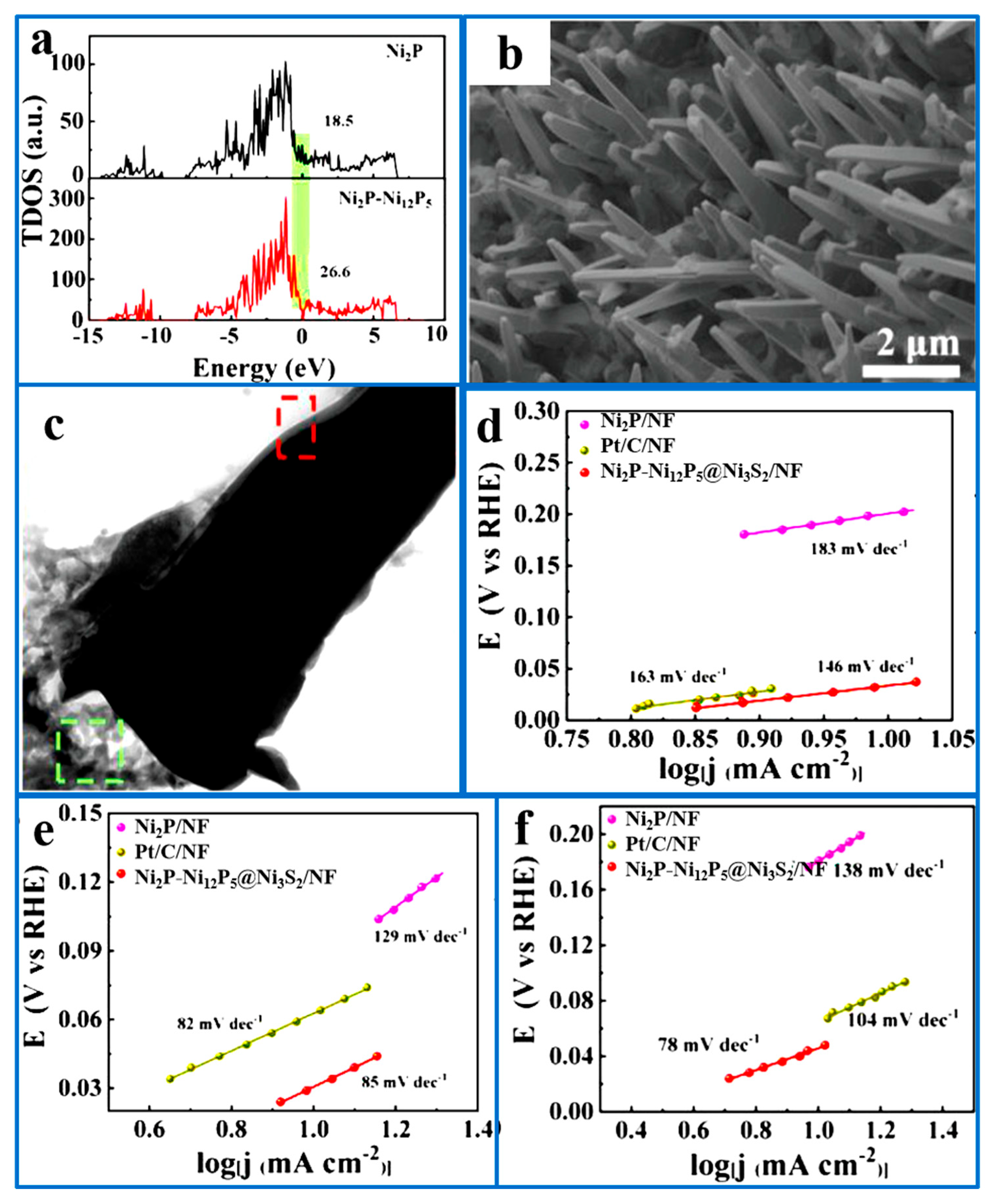
5.1.4. Synergistic Effects
5.2. Increase the Number of Catalytic Active Sites in Ni3S2-Based Materials
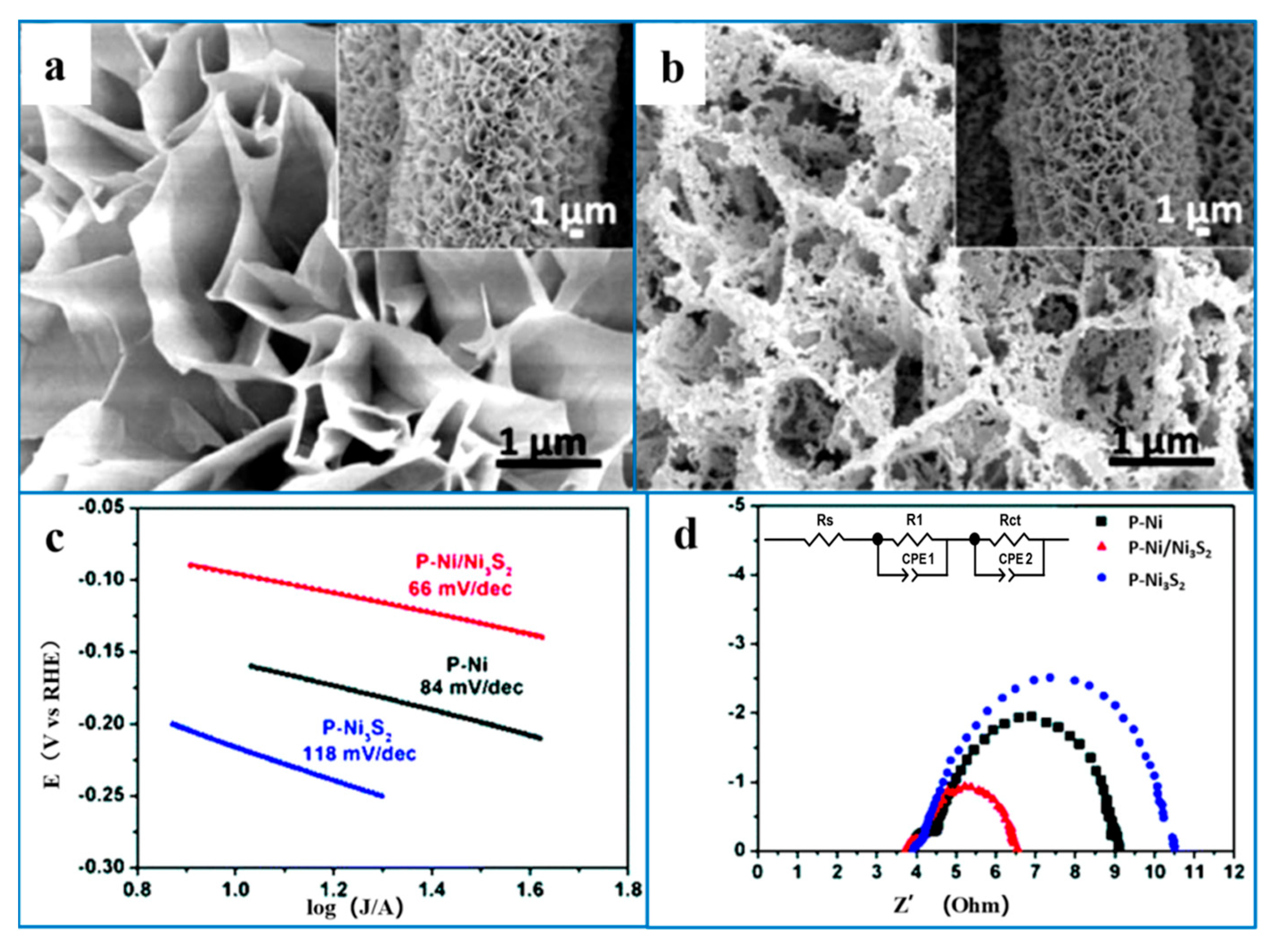
5.3. Combining Ni3S2 with Other Catalysts
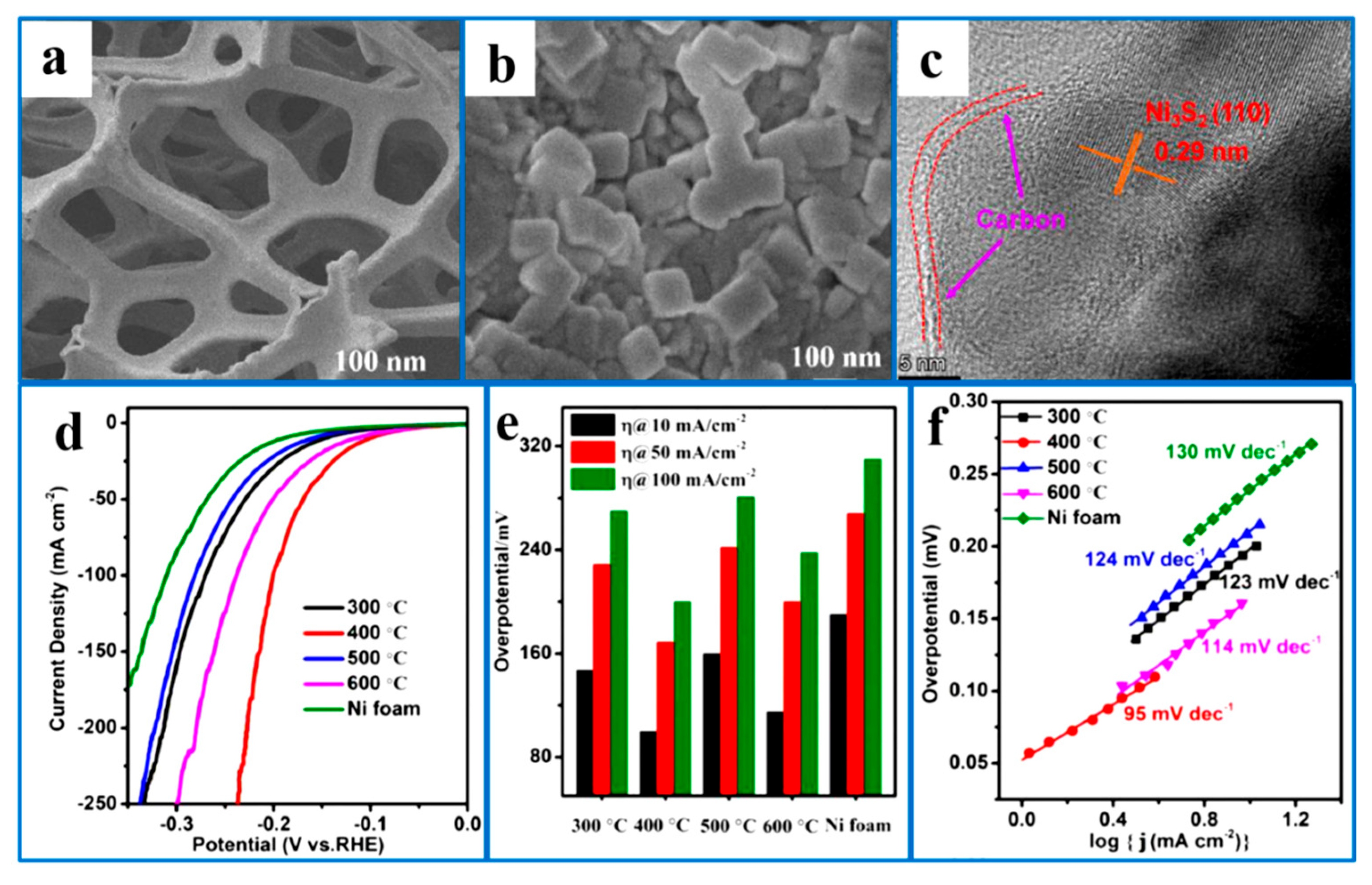
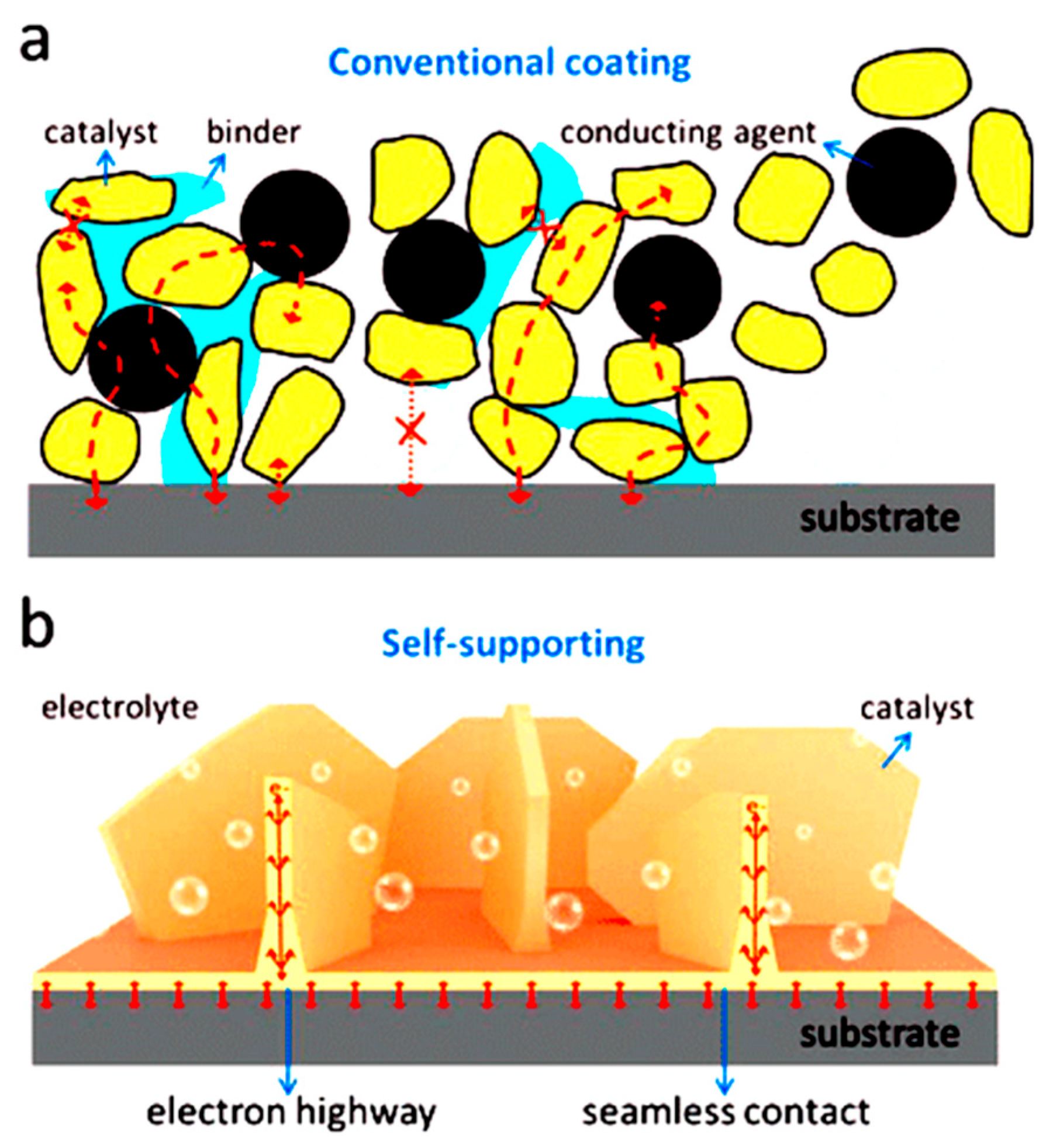
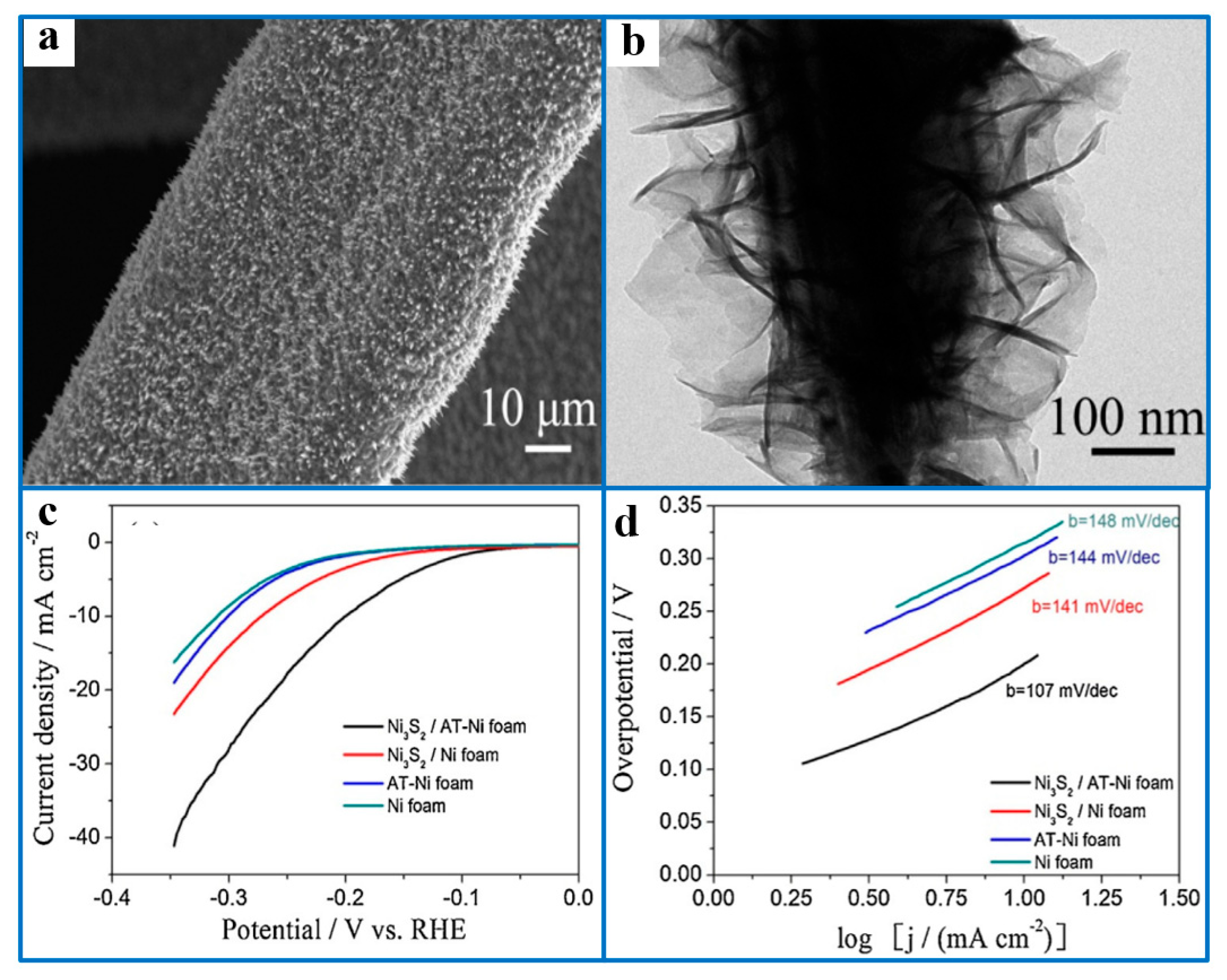
5.4. Loading Ni3S2 on a Self-Supporting Conductive Substrate
6. Conclusions, Challenges, and Perspectives
- The innovation of synthesis strategies will be crucial for enhancing the performance of Ni3S2 catalysts, particularly through advanced methods, such as in situ assembly and atomic layer deposition (ALD), which allow for precise control over the catalyst’s structure and composition. In addition, the development of synthesis methods suitable for large-scale industrial production remains to be further optimized. Among them, the electrodeposition method could be a promising approach due to its easily controlled operational conditions. However, the current lack of comprehensive technological procedures and standards limits its practical implementation, which warrants focused attention in future research.
- Additionally, the catalytic mechanism of Ni3S2-based catalysts remains a topic of ongoing debate, and identifying the true active centers or phases is of great significance for their further development. Future research should prioritize in-depth investigations using computational methods, such as DFT, to gain valuable insights into the catalytic mechanism, predict potential high-efficiency active sites, and provide theoretical guidance for experimental design, thereby accelerating the development and application of high-performance Ni3S2 catalysts. In addition, the advancement of in situ characterization techniques is essential for monitoring the dynamic evolution of active centers during electrochemical reactions, which will contribute to a deeper understanding of structure–activity relationships.
- The electrocatalytic activity and stability of Ni3S2-based catalysts still fall short of meeting industrial requirements, particularly under conditions of high current density, elevated temperatures, and strongly alkaline environments. Therefore, future research will focus on designing Ni3S2-based catalysts with excellent anti-corrosion and anti-degradation properties to enhance their durability and longevity in practical applications.
Author Contributions
Funding
Conflicts of Interest
References
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Shan, S.; Genç, S.Y.; Kamran, H.W.; Dinca, G. Role of green technology innovation and renewable energy in carbon neutrality: A sustainable investigation from Turkey. J. Environ. Manag. 2021, 294, 113004. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, C.H.; Li, N.W.; Luan, D.; Yu, L.; Lou, X.W. Design and synthesis of hollow nanostructures for electrochemical water splitting. Adv. Sci. 2022, 9, 2105135. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage. 2021, 40, 102676. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent advances in design of electrocatalysts for high-current-density water splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef]
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef]
- De Chialvo, M.R.G.; Chialvo, A.C. Kinetics of hydrogen evolution reaction with Frumkin adsorption: Re-examination of the Volmer-Heyrovsky and Volmer-Tafel routes. Electrochim. Acta 1998, 44, 841–851. [Google Scholar] [CrossRef]
- Bates, M.K.; Jia, Q.; Doan, H.; Liang, W.; Mukerjee, S. Charge-transfer effects in Ni-Fe and Ni-Fe-Co mixed-metal oxides for the alkaline oxygen evolution reaction. ACS Catal. 2016, 6, 155–161. [Google Scholar] [CrossRef]
- Gao, M.; Chen, L.; Zhang, Z.; Sun, X.; Zhang, S. Interface engineering of the Ni(OH)2–Ni3N nanoarray heterostructure for the alkaline hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 833–836. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Pitchai, C.; Vedanarayanan, M.; Chen, C.M.; Gopalakrishnan, S.M. Enhanced electrochemical efficiency of the open porous sandrose structured electrocatalyst for sustainable hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2024, 72, 755–763. [Google Scholar] [CrossRef]
- Pitchai, C.; Gopalakrishnan, S.M.; Chen, C.M. Ultra-efficient nitrogen-doped carbon dots-supported nickel sulfide as a platinum-free electrocatalyst for overall water splitting in basic medium. Energy Fuels 2024, 38, 2235–2247. [Google Scholar] [CrossRef]
- Zheng, L.; Lv, Z.; Xu, P.; Xu, H.; Zhu, M.; Zhao, Y.; Zheng, H. Defect engineering of Ni3S2 nanosheets with highly active (110) facets toward efficient electrochemical biomass valorization. J. Mater. Chem. A 2022, 10, 23244–23253. [Google Scholar] [CrossRef]
- Xia, X.; Wang, L.; Sui, N.; Colvin, V.L.; Yu, W.W. Recent progress in transition metal selenide electrocatalysts for water splitting. Nanoscale 2020, 12, 12249–12262. [Google Scholar] [CrossRef]
- Wu, B.; Qian, H.; Nie, Z.; Luo, Z.; Wu, Z.; Liu, P.; Zhang, F. Ni3S2 nanorods growing directly on Ni foam for all-solid-state asymmetric supercapacitor and efficient overall water splitting. J. Energy Chem. 2020, 46, 178–186. [Google Scholar] [CrossRef]
- Yoon, T.; Kim, K.S. One-Step Synthesis of CoS-Doped β-Co(OH)2@Amorphous MoS2+x Hybrid Catalyst Grown on Nickel Foam for High-Performance Electrochemical Overall Water Splitting. Adv. Funct. 2016, 26, 7386–7393. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Pehlivan, İ.B.; Arvizu, M.A.; Qiu, Z.; Niklasson, G.A.; Edvinsson, T. Impedance spectroscopy modeling of nickel–molybdenum alloys on porous and flat substrates for applications in water splitting. J. Phys. Chem. C 2019, 123, 23890–23897. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhang, H.; Ma, J.; Huang, Z.; Li, J.; Wang, Y. Transition-Metal Carbides as Hydrogen Evolution Reduction Electrocatalysts: Synthetic Methods and Optimization Strategies. Chem. Eur. J. 2021, 27, 5074–5090. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Tang, Q.; Jiang, D. CoP for hydrogen evolution: Implications from hydrogen adsorption. Phys. Chem. Chem. Phys. 2016, 18, 23864–23871. [Google Scholar] [CrossRef] [PubMed]
- Dastafkan, K.; Shen, X.; Hocking, R.K.; Meyer, Q.; Zhao, C. Monometallic interphasic synergy via nano-hetero-interfacing for hydrogen evolution in alkaline electrolytes. Nat. Commun. 2023, 14, 547. [Google Scholar] [CrossRef]
- Wang, H.; Choi, J.H. Hydrogen Adsorption on the Vertical Heterostructures of Graphene and Two-Dimensional Electrides: A First-Principles Study. ACS Omega 2022, 7, 16063–16069. [Google Scholar] [CrossRef]
- Nageswaran, G. Operando x-ray spectroscopic techniques: A focus on hydrogen and oxygen evolution reactions. Front. Chem. 2020, 23, 497887. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, M.; Long, A.; Xue, Y.; Liao, H.; Wei, C.; Xu, Z.J. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nanomicro Lett. 2018, 10, 75. [Google Scholar] [CrossRef]
- Fareza, A.R.; Nugroho, F.A.A.; Abdi, F.F.; Fauzia, V. Nanoscale metal oxides-2D materials heterostructures for photoelectrochemical water splitting-a review. J. Mater. Chem. A 2022, 10, 8656–8686. [Google Scholar] [CrossRef]
- Zhang, L.; Amiinu, I.S.; Ren, X.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. Surface modification of a NiS2 nanoarray with Ni(OH)2 toward superior water reduction electrocatalysis in alkaline media. Inorg. Chem. 2017, 56, 13651–13654. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.J. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 14971–15005. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Song, A.; Xia, M.; Li, Z.; Shao, G. The study on the active origin of electrocatalytic water splitting using Ni-MoS2 as example. Electrochim. 2018, 268, 268–275. [Google Scholar] [CrossRef]
- Zexing, W.; Junpo, G.; Jie, W.; Rong, L.; Weiping, X.; Cuijuan, X.; Deli, W. Hierarchically porous electrocatalyst with vertically aligned defect-rich CoMoS nanosheets for the hydrogen evolution reaction in an alkaline medium. ACS Appl. Mater. Interfaces 2017, 9, 5288–5294. [Google Scholar]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sankar, S.S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. NPJ 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.Y.; Yan, D.Y.; Mao, J.; Liu, H.; Hu, W.B.; Qiao, S.Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Yu, W.; Gao, Y.; Chen, Z.; Zhao, Y.; Wu, Z.; Wang, L. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chinese J. Catal. 2021, 42, 1876–1902. [Google Scholar] [CrossRef]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels. 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Hu, H.; Ou, J.Z.; Xu, X.; Lin, Y.; Zhang, Y.; Zhao, H.; Deng, L. Graphene-assisted construction of electrocatalysts for carbon dioxide reduction. Chem. Eng. J. 2021, 425, 130587. [Google Scholar] [CrossRef]
- Peng, S.; Gong, F.; Li, L.; Yu, D.; Ji, D.; Zhang, T.; Ramakrishna, S. Necklace-like Multishelled Hollow Spinel Oxides with Oxygen Vacancies for Efficient Water Electrolysis. J. Am. Chem. Soc. 2018, 140, 13644–13653. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.J.; Al-Anbari, R.; Sami, H.M.; Haider, M.J. Photocatalytic activity of nickel oxide. J. Mater. Chem. A 2019, 8, 2802–2808. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Jiang, L.; Lin, H.; An, Y.; Zhou, D.; Yang, S. Nanohybridization of MoS2 with layered double hydroxides efficiently synergizes the hydrogen evolution in alkaline media. Joule 2017, 1, 383–393. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Liu, C. Monodispersed nickel phosphide nanocrystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Carenco, S.; Portehault, D.; Boissiere, C.; Mezailles, N.; Sanchez, C. Nanoscaled metal borides and phosphides: Recent developments and perspectives. Chem. Rev. 2013, 113, 7981–8065. [Google Scholar]
- Yang, Z.; Lin, Y.; Jiao, F.; Li, J.; Wang, J.; Gong, Y. In situ growth of 3D walnut-like nano-architecture Mo-Ni2P@NiFe LDH/NF arrays for synergistically enhanced overall water splitting. J. Energy Chem. 2020, 49, 189–197. [Google Scholar] [CrossRef]
- Chu, S.; Chen, W.; Chen, G.; Huang, J.; Zhang, R.; Song, C.; Ostrikov, K.K. Holey Ni-Cu phosphide nanosheets as a highly efficient and stable electrocatalyst for hydrogen evolution. Appl. Catal. 2019, 243, 537–545. [Google Scholar] [CrossRef]
- Men, Y.; Li, P.; Zhou, J.; Cheng, G.; Chen, S.; Luo, W. Tailoring the electronic structure of Co2P by N doping for boosting hydrogen evolution reaction at all pH values. ACS Catal. 2019, 9, 3744–3752. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.I.; Lee, K. Morphology-controlled metal sulfides and phosphides for electrochemical water splitting. Adv. Mater. 2019, 31, 1806682. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Singh, G.; Sharma, R.K. Engineering multiple defects for active sites exposure towards enhancement of Ni3S2 charge storage characteristics. Chem. Eng. J. 2020, 384, 123364. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 5462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, X.; Zhang, Y.; Wang, J.; Zhong, C.; Deng, Y.; Hu, W. Controllable synthesis of nickel sulfide nanocatalysts and their phase-dependent performance for overall water splitting. Nanoscale 2019, 11, 5646–5654. [Google Scholar] [CrossRef]
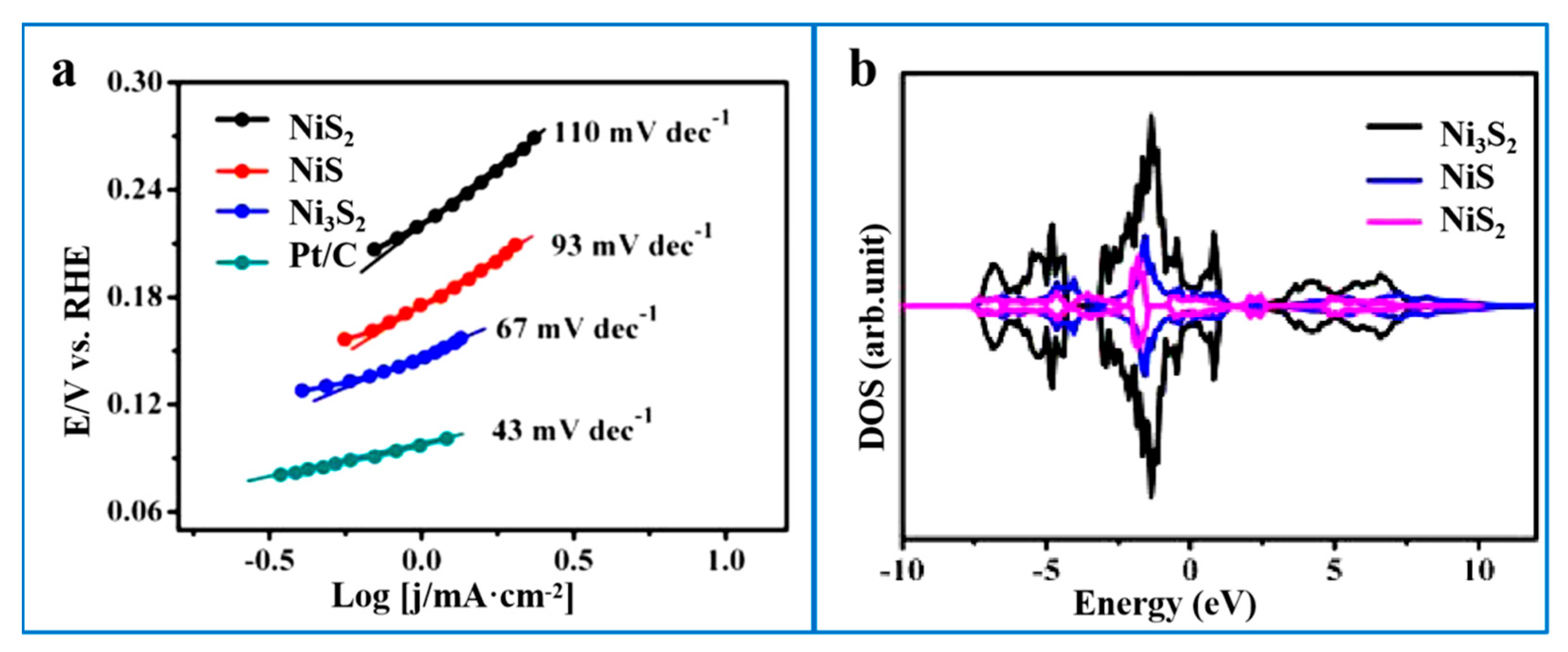
- Jiang, N.; Tang, Q.; Sheng, M.; You, B.; Jiang, D.E.; Sun, Y. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: A case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles. Catal. Sci. Technol. 2016, 6, 1077–1084. [Google Scholar] [CrossRef]
- Li, X.; Shang, X.; Rao, Y.; Dong, B.; Han, G.Q.; Hu, W.H.; Liu, C.G. Tuning crystal phase of NiSx through electro-oxidized nickel foam: A novel route for preparing efficient electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 2017, 396, 1034–1043. [Google Scholar] [CrossRef]
- Du, H.; Kong, R.; Qu, F.; Lu, L. Enhanced electrocatalysis for alkaline hydrogen evolution by Mn doping in a Ni3S2 nanosheet array. ChemComm. 2018, 54, 10100–10103. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Feng, D. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, T.; Zhang, M.; Tong, Y.; Zhong, C.; Zhang, N.; Xie, Y. 3D nitrogen-anion-decorated nickel sulfides for highly efficient overall water splitting. Adv. Mater. 2017, 29, 1701584. [Google Scholar] [CrossRef]
- Ye, L.; Du, Y.; Zhao, Y.; Zhao, L. W-doped Ni3S2 nanoparticles modified with NiFeLa hydroxide for hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 8372–8381. [Google Scholar] [CrossRef]
- Khan, R.; Samanta, M.; Ghosh, S.; Das, N.S.; Chattopadhyay, K.K. Co incorporated Ni3S2 hierarchical nano/micro cactus for electrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 21315–21323. [Google Scholar] [CrossRef]
- Tang, C.; Pu, Z.; Liu, Q.; Asiri, A.M.; Luo, Y.; Sun, X. Ni3S2 nanosheets array supported on Ni foam: A novel efficient three-dimensional hydrogen-evolving electrocatalyst in both neutral and basic solutions. Int. J. Hydrog. Energy 2015, 40, 4727–4732. [Google Scholar] [CrossRef]
- Sun, F.; Yue, C.; Wang, J.; Liu, Y.; Bao, W.; Liu, N.; Lu, Y. Lacunary polyoxometalate oriented construction of dispersed Ni3S2 confined in WO3 for electrocatalytic water splitting. J. Colloid Interface Sci. 2023, 645, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, M.Y.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Abbott, A.P. In-situ activation of self-supported 3D hierarchically porous Ni3S2 films grown on nanoporous copper as excellent pH-universal electrocatalysts for hydrogen evolution reaction. Nano Energy 2017, 36, 85–94. [Google Scholar] [CrossRef]
- Mohammadpour, E.; Asadpour-Zeynali, K. Ni3S2 nanosheets decorated on NiCo2O4 flakes-arrays directional growth of Ni foam for enhanced electrochemical hydrogen generation. J. Electroanal. Chem. 2022, 908, 116110. [Google Scholar] [CrossRef]
- Dhandapani, B.; Jagannathan, M.; AlSalhi, M.S.; Aljaafreh, M.J.; Prasad, S. N-doped carbon embedded Ni3S2 electrocatalyst material towards efficient hydrogen evolution reaction in broad pH range. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125194. [Google Scholar] [CrossRef]
- Yu, F.; Yao, H.; Wang, B.; Zhang, K.; Zhang, Z.; Xie, L.; Hao, J.; Mao, B.; Shen, H.; Shi, W. Nickel foam derived nitrogen doped nickel sulfide nanowires as an efficient electrocatalyst for the hydrogen evolution reaction. Dalton Trans. 2018, 47, 9871–9876. [Google Scholar] [CrossRef]
- Ryu, H.S.; Kim, J.S.; Park, J.; Park, J.Y.; Cho, G.B.; Liu, X.; Ahn, H.J. Degradation mechanism of room temperature Na/Ni3S2 cells using Ni3S2 electrodes prepared by mechanical alloying. J. Power Sources 2013, 244, 764–770. [Google Scholar] [CrossRef]
- Yao, M.; Sun, B.; He, L.; Wang, N.; Hu, W.; Komarneni, S. Self-assembled Ni3S2 nanosheets with mesoporous structure tightly held on Ni foam as a highly efficient and long-term electrocatalyst for water oxidation. ACS Sustain. Chem. Eng. 2019, 7, 5430–5439. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhu, T.; Wang, Y.; Cui, J.; Wu, J.; Wu, Y. 3D coral-like Ni3S2 on Ni foam as a bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2018, 10, 31330–31339. [Google Scholar] [CrossRef]
- Jiang, N.; Bogoev, L.; Popova, M.; Gul, S.; Yano, J.; Sun, Y. Electrodeposited nickel-sulfide films as competent hydrogen evolution catalysts in neutral water. J. Mater. Chem. A 2014, 2, 19407–19414. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, Y.; Chu, H.; Baines, R.; Dong, P.; Tang, J.; Shen, J. Controlled synthesis of Mo-doped Ni3S2 nano-rods: An efficient and stable electro-catalyst for water splitting. J. Mater. Chem. A 2017, 5, 1595–1602. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Hao, G.; Hu, Y.; Jiang, W. Synthesis of Iron–nickel sulfide porous nanosheets via a chemical etching/anion exchange method for efficient oxygen evolution reaction in alkaline media. Adv. Mater. Interfaces 2019, 6, 1900788. [Google Scholar] [CrossRef]
- Shriber, P.; Tkachev, M.; Atkins, A.; Perelshtein, I.; Bretler, S.; Schmerling, B.; Nessim, G.D. Synthesis of nickel sulfide dendrites from nickel foil using thermal annealing. Materialia 2022, 21, 101316. [Google Scholar] [CrossRef]
- Sun, K.; Qiao, F.; Yang, J.; Li, H.; Cui, Y.; Liu, P.F. Fluff spherical Co-Ni3S2/NF for enhanced hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 27986–27995. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Li, S.; Gao, Y.; Ge, L.; Song, W.; Liu, J. High-valent bimetal Ni3S2/Co3S4 induced by Cu doping for bifunctional electrocatalytic water splitting. Appl. Catal. 2021, 293, 120225. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, Y.; Huang, S.; He, S.; Li, X.; Tong, S.; Wu, M. Nitrogen-, oxygen-and sulfur-doped carbon-encapsulated Ni3S2 and NiS core-shell architectures: Bifunctional electrocatalysts for hydrogen evolution and oxygen reduction reactions. ACS Sustain. Chem. Eng. 2018, 6, 15582–15590. [Google Scholar] [CrossRef]
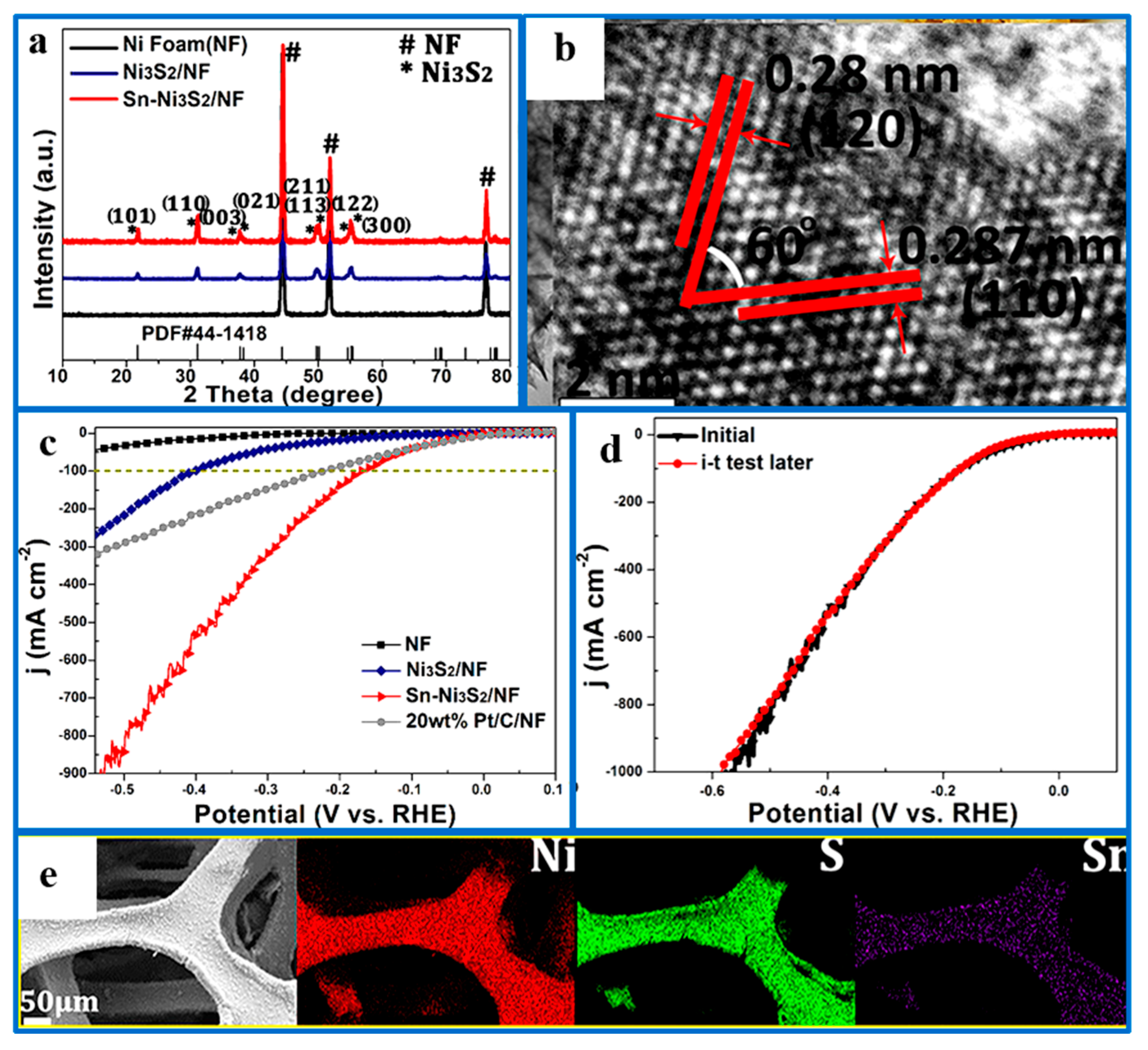
- Jian, J.; Yuan, L.; Qi, H.; Sun, X.; Zhang, L.; Li, H.; Feng, S. Sn-Ni3S2 ultrathin nanosheets as efficient bifunctional water-splitting catalysts with a large current density and low overpotential. ACS Appl. Mater. Interfaces 2018, 10, 40568–40576. [Google Scholar] [CrossRef]
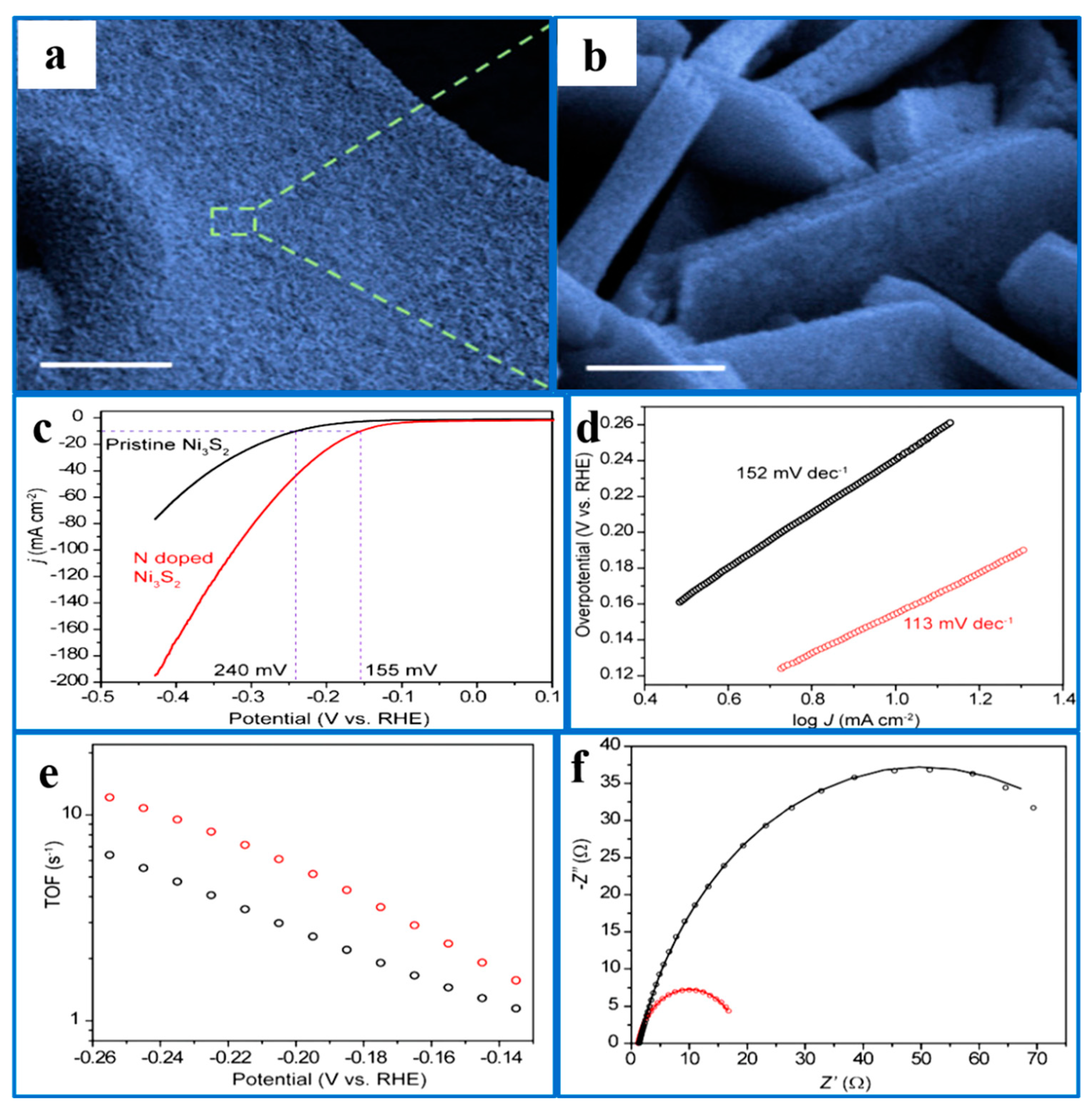
- Kou, T.; Smart, T.; Yao, B.; Chen, I.; Thota, D.; Ping, Y.; Li, Y. Theoretical and experimental insight into the effect of nitrogen doping on hydrogen evolution activity of Ni3S2 in alkaline medium. Adv. Energy Mater. 2018, 8, 1703538. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Tang, K.; Mao, Z.; Li, Q.; Wang, X.; Yan, C. Atom removal on the basal plane of layered MoS2 leading to extraordinarily enhanced electrocatalytic performance. Electrochimica Acta 2020, 336, 135740. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, R. Defect engineering of two-dimensional materials for efficient electrocatalysis. J. Materiomics. 2018, 4, 95–107. [Google Scholar] [CrossRef]
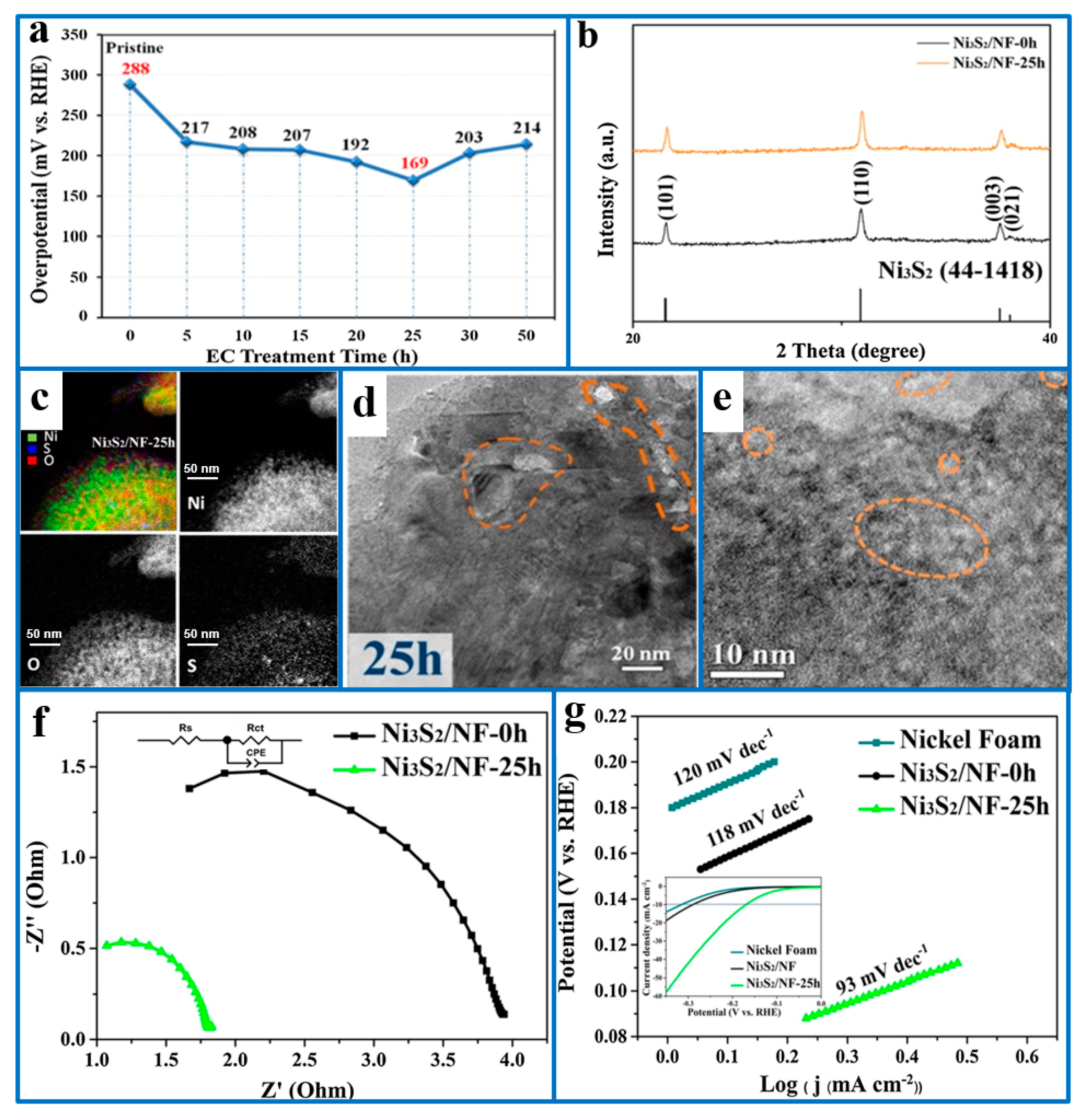
- Hao, S.; Liu, J.; Cao, Q.; Zhao, Y.; Zhao, X.; Pei, K.; Che, R. In-situ electrochemical pretreatment of hierarchical Ni3S2-based electrocatalyst towards promoted hydrogen evolution reaction with low overpotential. J. Colloid Interface Sci. 2020, 559, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.L.; Yu, G.; Wu, Y.; Li, G.D.; Li, H.; Sun, Y.; Zou, X. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, K.; Xie, D.; Zhang, Y.; Zhang, Y. High-index-faceted Ni3S2 branch arrays as bifunctional electrocatalysts for efficient water splitting. Nano-Micro Lett. 2019, 11, 12. [Google Scholar] [CrossRef]
- He, Y.; Shi, K.; Wang, X.; Zheng, X.; Luo, L.; Lin, L.; Sun, G. Synergistic Fe, Ce doping of Ni3S2 for enhancing oxygen evolution reaction performance. Inorg. Chem. Front. 2024, 11, 7623–7632. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Jiang, J.; Zhu, J.; Gao, H.; Zhao, T.; Zhang, L. Defect Engineering in Sn-doped NiS/Ni3S2 nanostructures for oxygen evolution reaction. ACS Appl. Nano Mater. 2024, 7, 15416–15424. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; He, W.; Li, Y.; Mao, J.; Zheng, X.; Hao, Q. Interfacial electronic modulation of Ni3S2 nanosheet arrays decorated with Au nanoparticles boosts overall water splitting. Appl. Catal. B Environ. 2022, 304, 120935. [Google Scholar] [CrossRef]
- Wang, S.; Geng, Z.; Bi, S.; Wang, Y.; Gao, Z.; Jin, L.; Zhang, C. Recent advances and future prospects on Ni3S2-Based electrocatalysts for efficient alkaline water electrolysis. Green Energy Environ. 2024, 9, 659–683. [Google Scholar] [CrossRef]
- Ding, X.; Liu, D.; Zhao, P.; Chen, X.; Wang, H.; Oropeza, F.E.; Zhang, K.H. Dynamic restructuring of nickel sulfides for electrocatalytic hydrogen evolution reaction. Nat. Commun. 2024, 15, 5336. [Google Scholar] [CrossRef]
- An, Y.; Huang, B.; Wang, Z.; Long, X.; Qiu, Y.; Hu, J.; Yang, S. Constructing three-dimensional porous Ni/Ni3S2 nano-interfaces for hydrogen evolution electrocatalysis under alkaline conditions. Dalton Trans. 2017, 46, 10700–10706. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wang, X.; Qu, Y.; Li, Y. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 4855. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guio, C.G.; Hu, X. Amorphous molybdenum sulfides as hydrogen evolution catalysts. Acc. Chem. Res. 2014, 47, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, Z.; Li, Y.; Gong, Y.; Zhao, M. Controlled synthesis of Ni(OH)2/Ni3S2 hybrid nanosheet arrays as highly active and stable electrocatalysts for water splitting. J. Mater. Chem. A 2018, 6, 6938–6946. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Gao, S.; Chen, A.; Qian, Q.; Kong, Q.; Xia, B.; Hu, G. Quenching-induced atom-stepped bimetallic sulfide heterointerface catalysts for industrial hydrogen generation. eScience 2025, 5, 100311. [Google Scholar] [CrossRef]
- Feng, J.X.; Wu, J.Q.; Tong, Y.X.; Li, G.R. Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption. J. Am. Chem. Soc. 2018, 140, 610–617. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q. Controllable preparation of N-doped Ni3S2 nanocubes@ N-doped graphene-like carbon layers for highly active electrocatalytic overall water splitting. Electrochimica Acta 2021, 399, 139408. [Google Scholar] [CrossRef]
- Fu, H.Q.; Zhou, M.; Liu, P.F.; Liu, P.; Yin, H.; Sun, K.Z.; Zhao, H. Hydrogen spillover-bridged Volmer/Tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039. [Google Scholar] [CrossRef]
- Yang, H.; Guo, P.; Wang, R.; Chen, Z.; Xu, H.; Pan, H.; Wu, R. Sequential phase conversion-Induced phosphides heteronanorod arrays for superior hydrogen evolution performance to Pt in wide pH media. Adv. Mater. 2022, 34, 2107548. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, L.; Li, M.; Tian, F.; Ren, X.; Jie, S.; Yu, Y. Accelerating the generation of NiOOH by in-situ surface phosphating nickel sulfide for promoting the proton-coupled electron transfer kinetics of urea electrolysis. Chem. Eng. J. 2024, 483, 149264. [Google Scholar] [CrossRef]
- Cheng, Y.; Fu, P.; Yu, Z.; Yang, X.; Zhang, Y.; Yuan, A.; Chen, L. Modulation of the multiphase phosphorus/sulfide heterogeneous interface via rare earth for solar-enhanced water splitting at industrial-level current densities. Carbon Neutralization 2024, 3, 873–887. [Google Scholar] [CrossRef]
- Wong, M.K.; Loh, J.Y.; Yap, F.M.; Ong, W.J. Fueling the future of clean energy with self-supported layered double hydroxides-based electrocatalysts: A step toward sustainability. InfoMat 2025, 7, e12639. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, X.; Wang, C.; Zhang, X.; Wu, J.; Ma, Z.; Wang, S. Hierarchically porous Ni3S2 nanorod array foam as highly efficient electrocatalyst for hydrogen evolution reaction and oxygen evolution reaction. Electrochim. Acta 2015, 174, 297–301. [Google Scholar] [CrossRef]

| Electrocatalyst | Advantage | Disadvantage | References |
|---|---|---|---|
| Nickel-based sulfide | Rich reserves; High conductivity; High stability. | Unbalanced hydrogen evolution performance. | [38] |
| Nickel-based oxide | Low toxicity; Low cost; Rich reserves. | Poor conductivity; Poor hydrogen evolution activity. | [39] |
| Nickel-based phosphide | Easy to synthesize; High conductivity. | Low stability; Slightly toxic. | [40] |
| Nickel-based nitride | Strong corrosion resistance; The structure is easy to control. | Difficult to synthesize. | [38,41] |
| Nickel-based carbide | Strong electrical conductivity; Good stability. | Polluted environment. | [42] |
| Catalyst | Electrolyte | Overpotential [mV]@Current Density [mA cm−2] | Stability Test | References |
|---|---|---|---|---|
| N-Ni3S2/NF | 1 M KOH | 110@10 | 10 h at ~50 mA cm−2 | [59] |
| W-doped Ni3S2-NiFeLaOH | 1 M KOH | 67@10 | 40 h at 10 mA cm−2 | [60] |
| Co-Ni3S2/NF | 0.5 M H2SO4 | 206@100 | 11 h at 100 mA cm−2 | [61] |
| Ni3S2/Ni foam | 1 M PBS 1 M KOH | 220@10, 396@100 123@10, 260@100 | 10 h at ~15mA cm−2 10 h at ~68 mA cm−2 | [62] |
| Ni3S2-WO3/NF-1 | 0.5 M H2SO4 1 M KOH | 86@10 107@10 | 12 h at 10 mA cm−2 12 h at 10 mA cm−2 | [63] |
| Ni3S2@NPC | 0.5 M H2SO4 1 M KOH 2 M PBS | 91.6@10 60.8@10 193.0@2 | 111 h at ~50 mA cm−2 28 h at ~50 mA cm−2 28 h at ~50 mA cm−2 | [64] |
| NiCo2O4/Ni3S2/NF | 0.5 M H2SO4 1 M KOH | 184@10 140@10 | 14 h at 10 mA cm−2 14 h at 10 mA cm−2 | [65] |
| Ni3S2/NC20 | 0.5 M H2SO4 1 M KOH | 174@10 199@10 | 15 h at 10 mA cm−2 15 h at 10 mA cm−2 | [66] |
| 1D N-Ni3S2 | 0.5 M H2SO4 1 M KOH | 196@10 105@10 | 12 h at 10 mA cm−2 12 h at 10 mA cm−2 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Bai, J.; Wei, H.; Gu, J.; Cao, Q. Recent Strategies for Ni3S2-Based Electrocatalysts with Enhanced Hydrogen Evolution Performance: A Tutorial Review. Int. J. Mol. Sci. 2025, 26, 3771. https://doi.org/10.3390/ijms26083771
Shen Y, Bai J, Wei H, Gu J, Cao Q. Recent Strategies for Ni3S2-Based Electrocatalysts with Enhanced Hydrogen Evolution Performance: A Tutorial Review. International Journal of Molecular Sciences. 2025; 26(8):3771. https://doi.org/10.3390/ijms26083771
Chicago/Turabian StyleShen, Yucheng, Jixing Bai, Huijie Wei, Jun Gu, and Qi Cao. 2025. "Recent Strategies for Ni3S2-Based Electrocatalysts with Enhanced Hydrogen Evolution Performance: A Tutorial Review" International Journal of Molecular Sciences 26, no. 8: 3771. https://doi.org/10.3390/ijms26083771
APA StyleShen, Y., Bai, J., Wei, H., Gu, J., & Cao, Q. (2025). Recent Strategies for Ni3S2-Based Electrocatalysts with Enhanced Hydrogen Evolution Performance: A Tutorial Review. International Journal of Molecular Sciences, 26(8), 3771. https://doi.org/10.3390/ijms26083771





