Epithelial–Mesenchymal Transitions Leading to Conceptus Adhesion in Ruminants: Early Pregnancy Events in Cattle
Abstract
1. Introduction
2. Trophoblast–Endometrium Interaction
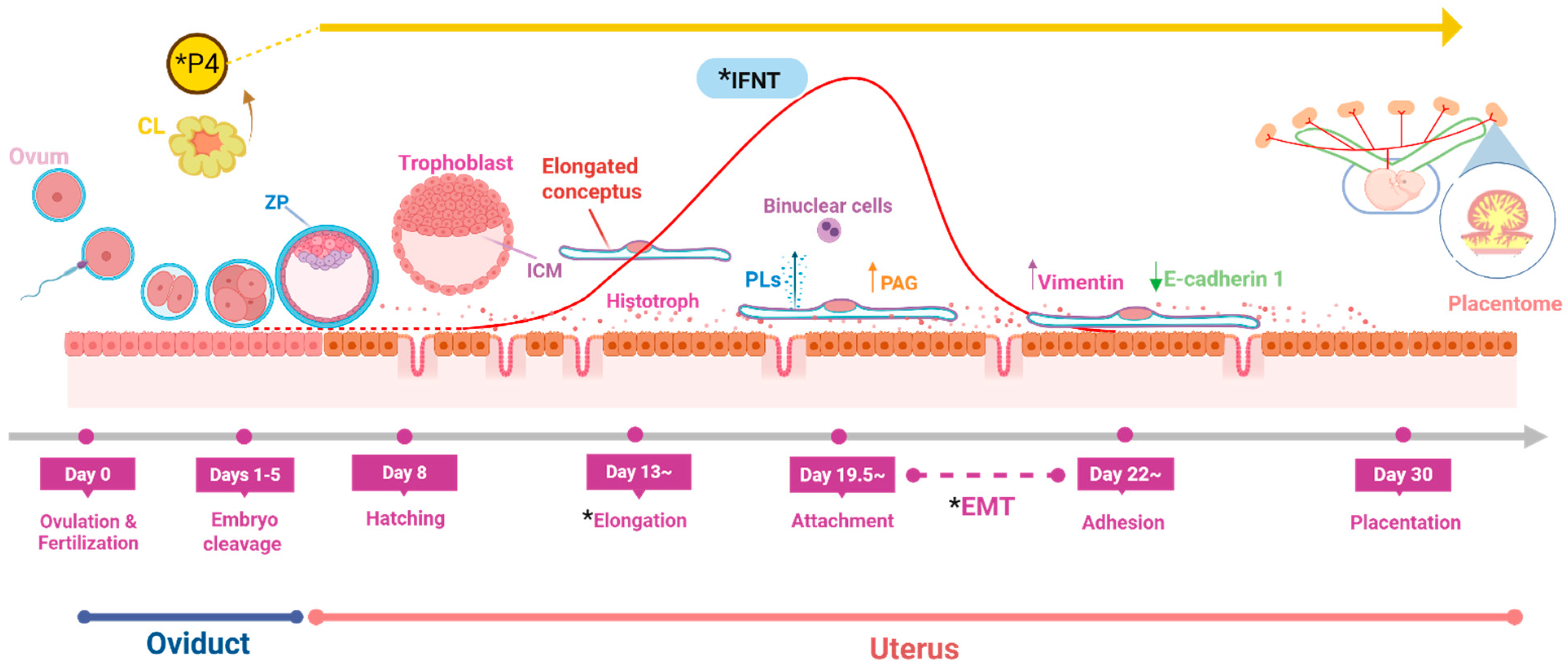
3. Conceptus Elongation and Attachment to Endometrium
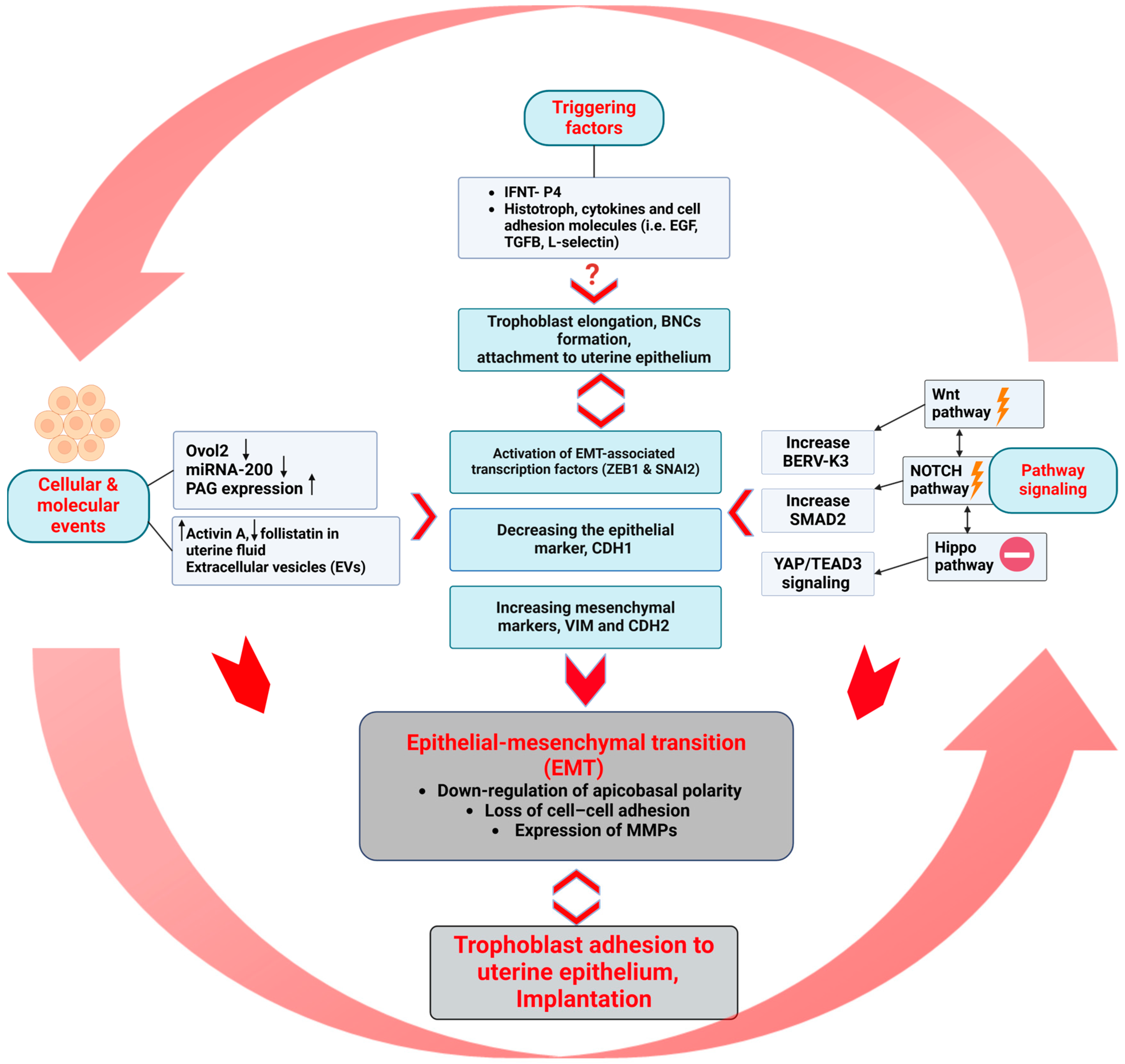
3.1. Triggering Agents
3.2. Cellular and Molecular Events
3.3. Pathway Signaling
4. Insights from Mouse and Human Models
4.1. Immune Modulation at the Maternal-Fetal Interface
4.2. Retrovirus Gene Expression in Placenta
4.3. Hypoxia and Oxygen-Sensing
4.4. Circulating Placental RNAs to Predict Pregnancy Complications
5. Research Challenge
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivera, J.E.; Chará, J. CH4 and N2O Emissions from Cattle Excreta: A Review of main drivers and mitigation strategies in grazing systems. Front. Sustain. Food Syst. 2021, 5, 657936. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Murphy, J.J.; Sreenan, J.M. Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 2006, 96, 297–311. [Google Scholar] [CrossRef]
- Andrade, M.F.; Simões, J. Embryonic and fetal mortality in dairy cows: Incidence, relevance, and diagnosis approach in field conditions. Dairy 2024, 5, 526–541. [Google Scholar] [CrossRef]
- Mee, J.F. Investigation of bovine abortion and stillbirth/perinatal mortality-similar diagnostic challenges, different approaches. Ir. Vet. J. 2020, 73, 20. [Google Scholar] [CrossRef]
- Casarotto, L.T.; Jones, H.N.; Chavatte-Palmer, P.; Dahl, G.E. Placental physiology and fetal programming in ruminants under heat stress. Biol. Reprod. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Liu, B.; Ren, S.; An, H.; Liang, Y.; Sheng, X.; Qi, X.; Xiao, L.; Wang, X. Establishment of functional trophoblast organoids from trophoblast cells of bovine placenta. Cells Dev. 2024, 180, 203970. [Google Scholar] [CrossRef]
- Green, J.A.; Geisert, R.D.; Johnson, G.A.; Spencer, T.E. Implantation and Placentation in Ruminants. In Placentation in Mammals: Tribute to E.C. Amoroso’s Lifetime Contributions to Viviparity; Geisert, R.D., Spencer, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–154. [Google Scholar]
- Jia, G.X.; Ma, W.J.; Wu, Z.B.; Li, S.; Zhang, X.Q.; He, Z.; Wu, S.X.; Tao, H.P.; Fang, Y.; Song, Y.W.; et al. Single-cell transcriptomic characterization of sheep conceptus elongation and implantation. Cell Rep. 2023, 42, 112860. [Google Scholar] [CrossRef] [PubMed]
- Barnwell, C.; Farin, P.; Whisnant, C.; Alexander, J.; Farin, C. Maternal serum progesterone concentration and early conceptus development of bovine embryos produced in vivo or in vitro. Adv. Anat. Embryol. Cell Biol. 2015, 52, 75–81. [Google Scholar] [CrossRef]
- Spencer, T.E.; Hansen, T.R. Implantation and establishment of pregnancy in ruminants. Adv. Anat. Embryol. Cell Biol. 2015, 216, 105–135. [Google Scholar] [CrossRef]
- Moraes, J.G.; Behura, S.K.; Geary, T.W.; Hansen, P.J.; Neibergs, H.L.; Spencer, T.E. Uterine influences on conceptus development in fertility-classified animals. Proc. Natl. Acad. Sci. USA 2018, 115, E1749–E1758. [Google Scholar] [CrossRef]
- Hansen, P.J. Embryonic mortality in cattle from the embryo’s perspective. J. Anim. Sci. 2002, 80 (Suppl. S2), E33–E44. [Google Scholar] [CrossRef]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 260–267. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.A.; Leme, R.A.; Agnol, A.M.D.; Alfieri, A.F. Sanitary program to reduce embryonic mortality associated with infectious diseases in cattle. Anim. Reprod. 2019, 16, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Slimane-Bureau, W.C.; King, W.A. Chromosomal abnormalities: A potential quality issue for cloned cattle embryos. Cloning Stem Cells 2002, 4, 319–329. [Google Scholar] [CrossRef]
- Davenport, K.M.; Ortega, M.S.; Johnson, G.A.; Seo, H.; Spencer, T.E. Review: Implantation and placentation in ruminants. Animal 2023, 17, 100796. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Bazer, F.W.; Seo, H.; Burghardt, R.C.; Wu, G.; Pohler, K.G.; Cain, J.W. Understanding placentation in ruminants: A review focusing on cows and sheep. Reprod. Fertil. Dev. 2024, 36, 93–111. [Google Scholar] [CrossRef]
- Khorami-Sarvestani, S.; Vanaki, N.; Shojaeian, S.; Zarnani, K.; Stensballe, A.; Jeddi-Tehrani, M.; Zarnani, A.H. Placenta: An old organ with new functions. Front. Immunol. 2024, 15, 1385762. [Google Scholar] [CrossRef]
- Bappoo, N.; Tongpob, Y.; Hakim, M.; Myers, J.; Panting, E.; Chapman, K.E.; Thomson, A.J.W.; Moran, C.M.; Kelsey, L.J.; Srinivasan, V.; et al. Feto-placental vascular structure and in silico haemodynamics: Of mice, rats, and human. Placenta 2024, 158, 175–184. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Čížková, D.; Hudák, R.; Dosedla, E.; Findrik-Balogová, A.; Živčák, J. In vitro model of human trophoblast in early placentation. Biomedicines 2022, 10, 904. [Google Scholar] [CrossRef]
- Aguilera, N.; Salas-Pérez, F.; Ortíz, M.; Álvarez, D.; Echiburú, B.; Maliqueo, M. Rodent models in placental research. Implications for fetal origins of adult disease. Anim. Reprod. 2022, 19, e20210134. [Google Scholar] [CrossRef]
- Igwebuike, U.M. Trophoblast cells of ruminant placentas—A minireview. Anim. Reprod. Sci. 2006, 93, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; Fisher, P.J.; Davies, C.J. The bovine placenta before and after birth: Placental development and function in health and disease. Anim. Reprod. Sci. 2000, 60–61, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T. Bovine placenta: A review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology 2013, 80, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K. A historical review of blastocyst implantation research. Biol. Reprod. 2018, 99, 175–195. [Google Scholar] [CrossRef]
- Seo, H.; Melo, G.D.; Oliveira, R.V.; Franco-Johannsen, G.; Bazer, F.W.; Pohler, K.; Johnson, G. Immunohistochemical examination of the utero-placental interface of cows on days 21, 31, 40, and 67 of gestation. Reproduction 2023, 167, e230444. [Google Scholar] [CrossRef]
- Black, S.G.; Arnaud, F.; Palmarini, M.; Spencer, T.E. Endogenous Retroviruses in trophoblast differentiation and placental development. Am. J. Reprod. Immunol. 2010, 64, 255–264. [Google Scholar] [CrossRef]
- Wooding, F.; Morgan, G.; Adam, C.L. Structure and function in the ruminant synepitheliochorial placenta: Central role of the trophoblast binucleate cell in deer. Microsc. Res. Tech. 1997, 38, 88–99. [Google Scholar] [CrossRef]
- Wooding, F.B.P. The ruminant placental trophoblast binucleate cell: An evolutionary breakthrough. Biol. Reprod. 2022, 107, 705–716. [Google Scholar] [CrossRef]
- Klisch, K.; Schraner, E.M. Intraluminal vesicles of binucleate trophoblast cell granules are a possible source of placental exosomes in ruminants. Placenta 2020, 90, 58–61. [Google Scholar] [CrossRef]
- Wooding, P.; Burton, G. Comparative Placentation: Structures, Functions and Evolution; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Seo, H.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Immunohistochemical examination of trophoblast syncytialization during early placentation in sheep. Int. J. Mol. Sci. 2019, 20, 4530. [Google Scholar] [CrossRef]
- Yamada, A.; Ohtsuki, K.; Shiga, N.; Green, J.A.; Matsuno, Y.; Imakawa, K. Epithelial-mesenchymal transition and bi- and multi-nucleated trophoblast cell formation in ovine conceptuses during the peri-implantation period. J. Reprod. Dev. 2022, 68, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Degrelle, S.A.; Liu, F.; Laloe, D.; Richard, C.; Le Bourhis, D.; Rossignol, M.N.; Hue, I. Understanding bovine embryo elongation: A transcriptomic study of trophoblastic vesicles. Front. Physiol. 2024, 15, 1331098. [Google Scholar] [CrossRef]
- Mathew, D.J.; Peterson, K.D.; Senn, L.K.; Oliver, M.A.; Ealy, A.D. Ruminant conceptus-maternal interactions: Interferon-tau and beyond. J. Anim. Sci. 2022, 100, skac123. [Google Scholar] [CrossRef]
- Hue, I.; Degrelle, S.A.; Campion, E.; Renard, J.P. Gene expression in elongating and gastrulating embryos from ruminants. Soc. Reprod. Fertil. Suppl. 2007, 64, 365–377. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Sánchez, J.M.; Mathew, D.J.; Passaro, C.; Fair, T. Embryo development in cattle and interactions with the reproductive tract. Reprod. Fertil. Dev. 2018, 31, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.K.; van Leeuwen, J.; Beaumont, S.; Berg, M.; Pfeffer, P.L. Embryo loss in cattle between Days 7 and 16 of pregnancy. Theriogenology 2010, 73, 250–260. [Google Scholar] [CrossRef]
- Grealy, M.; Diskin, M.G.; Sreenan, J.M. Protein content of cattle oocytes and embryos from the two-cell to the elongated blastocyst stage at day 16. J. Reprod. Fertil. 1996, 107, 229–233. [Google Scholar] [CrossRef]
- Lonergan, P. New insights into the function of progesterone in early pregnancy. Anim. Front. 2015, 5, 12–17. [Google Scholar] [CrossRef]
- Hernandez-Ledezma, J.J.; Sikes, J.D.; Murphy, C.N.; Watson, A.J.; Schultz, G.A.; Roberts, R.M. Expression of bovine trophoblast interferon in conceptuses derived by in vitro techniques. Biol. Reprod. 1992, 47, 374–380. [Google Scholar] [CrossRef]
- Rabaglino, M.B. Review: Overview of the transcriptomic landscape in bovine blastocysts and elongated conceptuses driving developmental competence. Animal 2023, 17, 100733. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Forde, N.; Lonergan, P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod. Fertil. Dev. 2017, 29, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Burghardt, R.; Johnson, G.; Bazer, F.; Spencer, T. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002, 124, 289–300. [Google Scholar] [CrossRef]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Martínez-Campa, C.; Álvarez-García, V.; Alonso-González, C.; González, A.; Cos, S. Melatonin and its role in the epithelial-to-mesenchymal transition (EMT) in cancer. Cancers 2024, 16, 956. [Google Scholar] [CrossRef]
- Bai, R.; Kusama, K.; Nakamura, K.; Sakurai, T.; Kimura, K.; Ideta, A.; Aoyagi, Y.; Imakawa, K. Down-regulation of transcription factor OVOL2 contributes to epithelial-mesenchymal transition in a noninvasive type of trophoblast implantation to the maternal endometrium. FASEB J. 2018, 32, 3371–3384. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Bai, R.; Ideta, A.; Aoyagi, Y.; Okuda, K.; Imakawa, K. Regulation of epithelial to mesenchymal transition in bovine conceptuses through the interaction between follistatin and activin A. Mol. Cell. Endocrinol. 2016, 434, 81–92. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusama, K.; Ideta, A.; Imakawa, K.; Hori, M. IFNT-independent effects of intrauterine extracellular vesicles (EVs) in cattle. Reproduction 2020, 159, 503–511. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, S.; Bai, H.; Bai, R.; Kusama, K.; Ideta, A.; Aoyagi, Y.; Kaneko, K.; Iga, K.; Yasuda, J.; et al. Novel endogenous retrovirus-derived transcript expressed in the bovine placenta is regulated by WNT signaling. Biochem. J. 2017, 474, 3499–3512. [Google Scholar] [CrossRef]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell Oncol. 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Bai, R.; Fujiwara, H.; Ideta, A.; Aoyagi, Y.; Kusama, K. Continuous model of conceptus implantation to the maternal endometrium. J. Endocrinol. 2017, 233, R53–R65. [Google Scholar] [CrossRef]
- Spencer, T.E.; Sandra, O.; Wolf, E. Genes involved in conceptus-endometrial interactions in ruminants: Insights from reductionism and thoughts on holistic approaches. Reproduction 2008, 135, 165–179. [Google Scholar] [CrossRef]
- Bai, R.; Kusama, K.; Matsuno, Y.; Bai, H.; Sakurai, T.; Kimura, K.; Imakawa, K. Expression of NFIL3 and CEBPA regulated by IFNT induced-PGE2 in bovine endometrial stromal cells during the pre-implantation period. Front. Endocrinol. 2023, 14, 1075030. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Burzyn, D.; Mundiñano, J.; Berguer, P.; Bekinschtein, P.; Costa, H.; Castillo, L.F.; Goldman, A.; Meiss, R.; Piazzon, I.; et al. Cathepsin-L influences the expression of extracellular matrix in lymphoid organs and plays a role in the regulation of thymic output and of peripheral T cell number. J. Immunol. 2005, 174, 7022–7032. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Wada, Y.; Tabassum, S.; Inagaki, S.; Mitaki, S.; Yano, S.; Nagai, A. Aggregation of cystatin C changes its inhibitory functions on protease activities and amyloid β fibril formation. Int. J. Mol. Sci. 2021, 22, 9682. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.L.; Burghardt, R.C.; Jousan, F.D.; Hansen, P.J.; Bazer, F.W.; Spencer, T.E. Galectin 15 (LGALS15) functions in trophectoderm migration and attachment. FASEB J. 2008, 22, 548–560. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Nakamura, K.; Okada, S.; Yasuda, J.; Imakawa, K. Endometrial factors similarly induced by IFNT2 and IFNTc1 through transcription factor FOXS1. PLoS ONE 2017, 12, e0171858. [Google Scholar] [CrossRef]
- Sakurai, T.; Bai, H.; Bai, R.; Arai, M.; Iwazawa, M.; Zhang, J.; Konno, T.; Godkin, J.D.; Okuda, K.; Imakawa, K. Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol. Reprod. 2012, 87, 60. [Google Scholar] [CrossRef]
- Matsuno, Y.; Amin, Y.A.; Kusama, K.; Imakawa, K. Formation of fibrin at sights of conceptus adhesion in the ewe. Reproduction 2021, 161, 709–720. [Google Scholar] [CrossRef]
- Yamakoshi, S.; Bai, R.; Chaen, T.; Ideta, A.; Aoyagi, Y.; Sakurai, T.; Konno, T.; Imakawa, K. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction 2012, 143, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, S.; Yu, T.J.; Zhang, F.L.; Yang, F.; Huang, Y.N.; Ma, D.; Liu, G.Y.; Shao, Z.M.; Li, D.Q. Pregnancy-specific glycoprotein 9 acts as both a transcriptional target and a regulator of the canonical TGF-β/Smad signaling to drive breast cancer progression. Clin. Transl. Med. 2020, 10, e245. [Google Scholar] [CrossRef] [PubMed]
- Poddar, A.; Ahmady, F.; Rao, S.R.; Sharma, R.; Kannourakis, G.; Prithviraj, P.; Jayachandran, A. The role of pregnancy associated plasma protein-A in triple negative breast cancer: A promising target for achieving clinical benefits. J. Biomed. Sci. 2024, 31, 23. [Google Scholar] [CrossRef]
- Kagawa, S.; Hayashi, Y.; Bai, H.; Takahashi, M.; Kawahara, M. In utero morphological and functional properties of bovine trophoblastic vesicles. Mol. Reprod. Dev. 2024, 91, e23767. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Matsuno, Y.; Fujiwara, H. New Roles for EVs, miRNA and lncRNA in Bovine Embryo Implantation. Front. Vet. Sci. 2022, 9, 944370. [Google Scholar] [CrossRef]
- Matsuno, Y.; Kusama, K.; Imakawa, K. Characterization of lncRNA functioning in ovine conceptuses and endometria during the peri-implantation period. Biochem. Biophys. Res. Commun. 2022, 594, 22–30. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusama, K.; Hori, M.; Imakawa, K. Global analyses and potential effects of extracellular vesicles on the establishment of conceptus implantation during the peri-implantation period. J. Reprod. Dev. 2023, 69, 246–253. [Google Scholar] [CrossRef]
- Lu, W.; Tu, Z.; Wang, S.; Lu, J.; Wang, Q.; Wang, W.; Wang, B.; Wang, H.; Ni, H.; Guo, Y. Spatiotemporal expression of Wnt signaling pathway components during bovine placental development. Theriogenology 2013, 80, 893–902. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Li, C.; Yang, B.; Feng, X.; Cao, J.; Du, W.; Shahzad, M.; Khan, A.; Sun, S.-C.; et al. Effects of regulating Hippo and Wnt on the development and fate differentiation of bovine embryo. Int. J. Mol. Sci. 2024, 25, 3912. [Google Scholar] [CrossRef]
- Nakagawa, S.; Bai, H.; Sakurai, T.; Nakaya, Y.; Konno, T.; Miyazawa, T.; Gojobori, T.; Imakawa, K. Dynamic Evolution of Endogenous Retrovirus-Derived Genes Expressed in Bovine Conceptuses during the Period of Placentation. Genome Biol. Evol. 2013, 5, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Burghardt, R.C.; Bazer, F.W.; Spencer, T.E. WNTs in the ovine uterus: Potential regulation of periimplantation ovine conceptus development. Endocrinology 2007, 148, 3496–3506. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Guo, Y.; Simoneau, A.R.; Hope, C.; Xie, J.; Holcombe, R.F.; Hoang, B.H. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 2005, 65, 9762–9770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Sakurai, T.; Bai, H.; Ideta, A.; Aoyagi, Y.; Imakawa, K. A transcriptional cofactor YAP regulates IFNT expression via transcription factor TEAD in bovine conceptuses. Domest. Anim. Endocrinol. 2016, 57, 21–30. [Google Scholar] [CrossRef]
- Choi, Y.; Johnson, G.A.; Spencer, T.E.; Bazer, F.W. Pregnancy and interferon tau regulate major histocompatibility complex class I and beta2-microglobulin expression in the ovine uterus. Biol. Reprod. 2003, 68, 1703–1710. [Google Scholar] [CrossRef]
- Wattegedera, S.R.; Doull, L.E.; Goncheva, M.I.; Wheelhouse, N.M.; Watson, D.M.; Pearce, J.; Benavides, J.; Palarea-Albaladejo, J.; McInnes, C.J.; Ballingall, K.; et al. Immunological homeostasis at the ovine placenta may reflect the degree of maternal fetal interaction. Front. Immunol. 2019, 9, 3025. [Google Scholar] [CrossRef]
- Benedictus, L.; Koets, A.P.; Rutten, V.P.M.G. The role of placental MHC class I expression in immune-assisted separation of the fetal membranes in cattle. J. Reprod. Immunol. 2015, 112, 11–19. [Google Scholar] [CrossRef]
- Madeja, Z.; Yadi, H.; Apps, R.; Boulenouar, S.; Roper, S.J.; Gardner, L.; Moffett, A.; Colucci, F.; Hemberger, M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4012–4017. [Google Scholar] [CrossRef]
- Davies, C.J. Why is the fetal allograft not rejected? J. Anim. Sci. 2007, 85 (Suppl. S13), E32–E35. [Google Scholar] [CrossRef]
- Hunt, J.S.; Banerjee, S.; Pace, J.L. Differential expression and regulation of a human transgene, HLA-B27, in mouse placental and embryonic cell lines. Mol. Hum. Reprod. 1998, 4, 817–825. [Google Scholar] [CrossRef]
- Comiskey, M.; Warner, C.M.; Schust, D.J. MHC Molecules of the preimplantation embryo and trophoblast. In Immunology of Pregnancy; Mor, G., Ed.; Springer: New York, NY, USA, 2006; pp. 130–147. [Google Scholar]
- da Silva, M.I.; Oli, N.; Gambonini, F.; Ott, T. Effects of parity and early pregnancy on peripheral blood leukocytes in dairy cattle. J. Dairy Sci. 2024, 107, 11728–11743. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, Z.; Jafarpour, R.; Mehdizadeh, S.; Bayatipoor, H.; Pashangzadeh, S.; Motallebnezhad, M. Functional prominence of natural killer cells and natural killer T cells in pregnancy and infertility: A comprehensive review and update. Pathol. Res. Pract. 2022, 238, 154062. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Sakai, A.; Nojima, H.; Suda, Y.; Yokomizo, Y.; Imakawa, K.; Sakai, S.; Christenson, R.K. A chemokine, interferon (IFN)-gamma-inducible protein 10 kDa, is stimulated by IFN-tau and recruits immune cells in the ovine endometrium. Biol. Reprod. 2003, 68, 1413–1421. [Google Scholar] [CrossRef]
- Imakawa, K.; Imai, M.; Sakai, A.; Suzuki, M.; Nagaoka, K.; Sakai, S.; Lee, S.R.; Chang, K.T.; Echternkamp, S.E.; Christenson, R.K. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol. Reprod. Dev. 2006, 73, 850–858. [Google Scholar] [CrossRef]
- Imakawa, K.; Nagaoka, K.; Nojima, H.; Hara, Y.; Christenson, R.K. Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus. Am. J. Reprod. Immunol. 2005, 53, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Dupressoir, A.; Marceau, G.; Vernochet, C.; Bénit, L.; Kanellopoulos, C.; Sapin, V.; Heidmann, T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA 2005, 102, 725–730. [Google Scholar] [CrossRef]
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837. [Google Scholar] [CrossRef]
- Imakawa, K.; Kusama, K.; Kaneko-Ishino, T.; Nakagawa, S.; Kitao, K.; Miyazawa, T.; Ishino, F. Endogenous Retroviruses and placental evolution, development, and diversity. Cells 2022, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Almiñana, C.; Wiesendanger, M.; Schuler, G.; Kowalewski, M.P.; Klisch, K. Bovine placental extracellular vesicles carry the fusogenic syncytin BERV-K1. Theriogenology 2024, 223, 59–69. [Google Scholar] [CrossRef]
- Nakaya, Y.; Miyazawa, T. The roles of syncytin-like proteins in ruminant placentation. Viruses 2015, 7, 2928–2942. [Google Scholar] [CrossRef]
- Baba, K.; Nakaya, Y.; Shojima, T.; Muroi, Y.; Kizaki, K.; Hashizume, K.; Imakawa, K.; Miyazawa, T. Identification of novel endogenous betaretroviruses which are transcribed in the bovine placenta. J. Virol. 2011, 85, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Koshi, K.; Nakagawa, S.; Hashizume, K.; Miyazawa, T. Fematrin-1 is involved in fetomaternal cell-to-cell fusion in bovinae placenta and has contributed to diversity of ruminant placentation. J. Virol. 2013, 87, 10563–10572. [Google Scholar] [CrossRef]
- Bagchi, I.C.; Bagchi, M.K. Maternal–fetal mechanisms underlying adaptation to hypoxia during early pregnancy. Trends Endocrinol. Metab. 2024, 35, 1091–1099. [Google Scholar] [CrossRef]
- Cowden Dahl, K.D.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol. Cell. Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and placental development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediat. 2010, 2010, 401323. [Google Scholar] [CrossRef]
- Li, C.; Yang, D.; Yang, W.; Wang, Y.; Li, D.; Li, Y.; Xiao, B.; Zhang, H.; Zhao, H.; Dong, H.; et al. Hypoxia activation attenuates progesterone synthesis in goat trophoblast cells via NR1D1 inhibition of StAR expression†. Biol. Reprod. 2023, 109, 720–735. [Google Scholar] [CrossRef]
- Autiero, M.; Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: Novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J. Thromb. Haemost. 2003, 1, 1356–1370. [Google Scholar] [CrossRef]
- Saw, K.E.E.; Thann, T. Association Between Vascular Endothelial Growth Factor (VEGF) +936C/T Polymorphism (rs3025039) and Preeclampsia Among Myanmar Pregnant Women. J. Pregnancy 2024, 2024, 7608096. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Vonnahme, K.A.; Johnson, M.L.; Grazul-Bilska, A.T.; Redmer, D.A.; Caton, J.S. Placental angiogenesis in sheep models of compromised pregnancy. J. Physiol. 2005, 565 Pt 1, 43–58. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Johnson, M.L.; Borowicz, P.P.; Bilski, J.J.; Cymbaluk, T.; Norberg, S.; Redmer, D.A.; Reynolds, L.P. Placental development during early pregnancy in sheep: Effects of embryo origin on vascularization. Reproduction 2014, 147, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.R.; Farin, C.E.; Rodriguez, K.F.; Alexander, J.E.; Farin, P.W. Effects of embryo culture on angiogenesis and morphometry of bovine placentas during early gestation. Biol. Reprod. 2005, 73, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Whitehead, C.L.; Walker, S.P.; Tong, S. Measuring circulating placental RNAs to non-invasively assess the placental transcriptome and to predict pregnancy complications. Prenat. Diagn. 2016, 36, 997–1008. [Google Scholar] [CrossRef]
- Romero, J.J.; Antoniazzi, A.Q.; Nett, T.M.; Ashley, R.L.; Webb, B.T.; Smirnova, N.P.; Bott, R.C.; Bruemmer, J.E.; Bazer, F.W.; Anthony, R.V.; et al. Temporal release, paracrine and endocrine actions of ovine conceptus-derived interferon-tau during early pregnancy. Biol. Reprod. 2015, 93, 146. [Google Scholar] [CrossRef]
- Mauffré, V.; Grimard, B.; Eozenou, C.; Inghels, S.; Silva, L.; Giraud-Delville, C.; Capo, D.; Sandra, O.; Constant, F. Interferon stimulated genes as peripheral diagnostic markers of early pregnancy in sheep: A critical assessment. Animal 2016, 10, 1856–1863. [Google Scholar] [CrossRef]
- Soumya, N.P.; Das, D.N.; Jeyakumar, S.; Mondal, S.; Mor, A.; Mundhe, U.T. Differential expression of ISG 15 mRNA in peripheral blood mononuclear cells of nulliparous and multiparous pregnant versus non-pregnant Bos indicus cattle. Reprod. Domest. Anim. 2017, 52, 97–106. [Google Scholar] [CrossRef]
- Gualtieri, R.; De Gregorio, V.; Candela, A.; Travaglione, A.; Genovese, V.; Barbato, V.; Talevi, R. In vitro culture of mammalian embryos: Is there room for improvement? Cells 2024, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.S.; Rizo, J.A.; Drum, J.N.; O’Neil, E.V.; Pohler, K.G.; Kerns, K.; Schmelze, A.; Green, J.; Spencer, T.E. Development of an improved in vitro model of bovine trophectoderm differentiation. Front. Anim. Sci. 2022, 3, 898808. [Google Scholar] [CrossRef]
- Nakano, H.; Shimada, A.; Imai, K.; Takezawa, T.; Takahashi, T.; Hashizume, K. Bovine trophoblastic cell differentiation on collagen substrata: Formation of binucleate cells expressing placental lactogen. Cell Tissue Res. 2002, 307, 225–235. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular signaling regulating endometrium-blastocyst crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef]
- Tinning, H.; Edge, J.C.; DeBem, T.H.C.; Deligianni, F.; Giovanardi, G.; Pensabene, V.; Meirelles, F.V.; Forde, N. Review: Endometrial function in pregnancy establishment in cattle. Animal 2023, 17, 100751. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, M.S.; Imakawa, K. Epithelial–Mesenchymal Transitions Leading to Conceptus Adhesion in Ruminants: Early Pregnancy Events in Cattle. Int. J. Mol. Sci. 2025, 26, 3772. https://doi.org/10.3390/ijms26083772
Yousef MS, Imakawa K. Epithelial–Mesenchymal Transitions Leading to Conceptus Adhesion in Ruminants: Early Pregnancy Events in Cattle. International Journal of Molecular Sciences. 2025; 26(8):3772. https://doi.org/10.3390/ijms26083772
Chicago/Turabian StyleYousef, Mohamed Samy, and Kazuhiko Imakawa. 2025. "Epithelial–Mesenchymal Transitions Leading to Conceptus Adhesion in Ruminants: Early Pregnancy Events in Cattle" International Journal of Molecular Sciences 26, no. 8: 3772. https://doi.org/10.3390/ijms26083772
APA StyleYousef, M. S., & Imakawa, K. (2025). Epithelial–Mesenchymal Transitions Leading to Conceptus Adhesion in Ruminants: Early Pregnancy Events in Cattle. International Journal of Molecular Sciences, 26(8), 3772. https://doi.org/10.3390/ijms26083772





