Abstract
The emergence of multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis strains poses serious challenges to global tuberculosis control, highlighting the urgent need to elucidate the mechanisms underlying multidrug resistance. In this study, we screened for spontaneous bortezomib (BTZ)-resistant Mycobacterium smegmatis (Msm) mutants and identified a strain, Msm-R1-2, exhibiting 16- and 64-fold increases in minimum inhibitory concentrations (MICs) to BTZ and linezolid (LZD), respectively, compared to the parental strain. Whole-genome sequencing revealed resistance-associated mutations in two functionally distinct genes: MSMEG_1380, encoding a transcriptional regulator involved in efflux pump expression, and MSMEG_0965, encoding a porin protein. CRISPR-Cpf1-assisted gene knockout and editing experiments confirmed that single mutations in either MSMEG_1380 or MSMEG_0965 caused low-level resistance (4-fold MIC increase) to BTZ and LZD, while dual mutations conferred resistance levels comparable to Msm-R1-2, with 16- and 64-fold increases in MICs for BTZ and LZD, respectively. An ethidium bromide accumulation assay demonstrated that mutations in MSMEG_0965 reduce cell wall permeability, contributing to multidrug resistance. Furthermore, quantitative real-time PCR showed that mutations in MSMEG_1380 upregulate the mmpS5-mmpL5 efflux system. Together, these dual mechanisms function synergistically: restricted drug entry combined with enhanced drug efflux confers robust multidrug resistance. These findings provide novel insights into the evolutionary mechanisms of resistance in mycobacteria.
1. Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a major global health challenge, especially due to the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains [1,2]. These resistant strains severely compromise current treatment regimens, underscoring the urgent need for novel therapeutic strategies [2,3]. Among the promising candidates, bortezomib (BTZ), a proteasome inhibitor originally approved for the treatment of multiple myeloma, has shown potent activity against Mtb [4]. Linezolid (LZD), an oxazolidinone antibiotic, is already a key component of TB treatment regimens [1,5]. However, the mechanisms underlying BTZ resistance remain poorly understood [6], and while LZD resistance has been primarily attributed to ribosomal mutations, non-ribosomal resistance mechanisms remain largely unexplored [7]. Thus, elucidating these resistance mechanisms is crucial for developing more effective therapeutic strategies.
Previous studies suggest that BTZ inhibits Mtb proteolysis by targeting ClpP1P2, a caseinolytic protease complex encoded by clpP1 and clpP2 [8,9,10], and may also target the Mtb proteasome complex PrcBA, encoded by prcB and prcA [11,12]. In contrast, LZD inhibits protein synthesis by binding to the 50S ribosomal subunit [13,14]. However, some resistant strains lack mutations in these established targets, implying the involvement of alternative resistance pathways, such as MmpL9, EmbB-EmbC, and GrcC1 [15,16,17,18]. Efflux pump systems, like the MmpS5-MmpL5 transporter, contribute to mycobacterial drug resistance by actively expelling antimicrobial compounds [19,20]. In Mycobacterium smegmatis (Msm), mutations in MSMEG_1380, encoding a TetR-family transcriptional regulator, upregulate efflux pump expression and confer resistance to multiple drugs including chrysomycin A [21,22]. Additionally, porins like MSMEG_0965 (MspA) are crucial for drug uptake by facilitating hydrophilic molecule transport across the mycobacterial cell wall [23,24]. Mutations in genes encoding porins can reduce membrane permeability, thereby diminishing the efficacy of antibiotics such as fluoroquinolones and potentially leading to treatment failure or prolonged therapy duration [25]. Although these resistance mechanisms have been individually characterized, their interactions in strains with multiple mutations remain unclear [26].
In this study, we used the nonpathogenic Msm mc2155 as a model organism [27] to investigate drug resistance mechanisms in mycobacteria. By screening spontaneous BTZ-resistant mutants and performing whole-genome sequencing (WGS), we identified multiple resistance-associated mutations, despite the absence of mutations in the known BTZ target, suggesting the involvement of alternative resistance mechanisms. Notably, among these mutants, we identified a strain exhibiting high-level resistance to both BTZ and LZD. CRISPR-Cpf1-assisted gene knockout, gene editing, overexpression, and complementation experiments revealed that MSMEG_1380 and MSMEG_0965 are key determinants of resistance. Single-gene mutations conferred low-level resistance (4-fold MIC increase), while double-gene mutations significantly enhanced resistance to BTZ, LZD, and other antibiotics (16- to 64-fold MIC increase). The ethidium bromide (EtBr) accumulation assay demonstrated that mutations in MSMEG_0965 led to a significant reduction in cell wall permeability, resulting in lower intracellular drug concentrations. Furthermore, quantitative real-time PCR (qRT-PCR) showed that mutations in MSMEG_1380 promote drug efflux through upregulation of the MmpS5-MmpL5 system. These results suggest that the synergy between reduced drug uptake (MSMEG_0965) and enhanced efflux (MmpS5-MmpL5) underscores a novel combinatorial resistance mechanism in mycobacteria, offering potential targets for countering MDR/XDR strains.
2. Results
2.1. High Frequency of Spontaneous Mutations Conferring BTZ Resistance in Msm
To investigate potential BTZ targets, we screened Msm mc2155 strains for spontaneous resistance to BTZ. First, we determined that the MIC of BTZ for Msm was 5 μg/mL. Msm strains were then plated on 7H10 agar plates containing increasing concentrations of BTZ (20 × MIC, 40 × MIC, and 60 × MIC) in four independent batches. From each batch, 10~20 single colonies were randomly selected, yielding a total of 57 resistant colonies. The MIC for each isolate was then determined to assess resistance levels. All isolates exhibited MIC ≥ 20 μg/mL (listed in Table S1), with a spontaneous mutation frequency ranging from 1.86 × 10−7 to 6.60 × 10−7. This frequency is higher than the reported spontaneous resistance mutation frequency of Mtb to LZD (2 × 10−8 to 5 × 10−9) [28], suggesting that Msm may exhibit increased mutability under BTZ exposure.
2.2. WGS of BTZ-Resistant Msm Strains Revealed a Variety of Mutations Across Different Genes
Sanger sequencing of prcA, prcB, clpP1, and clpP2, which encodes known BTZ targets, revealed no mutations in the resistant strains. To further explore potential resistance mechanisms, we randomly selected five resistant strains from the 57 isolates and the parent strain for WGS. WGS identified 35 unique mutations across various genes (Table S2). Notably, MSMEG_3244 was the most frequently mutated gene (4/5 strains), followed by MSMEG_5085 and MSMEG_3987 (2/5 strains). Mutations in MSMEG_1380 and MSMEG_0965 were detected in only one strain. Among the 57 isolates analyzed through Sanger sequencing, mutations were detected in 5 strains for MSMEG_3244, 24 strains for MSMEG_5085, 10 strains for MSMEG_3987, and 2 strains each for MSMEG_1380 and MSMEG_0965. Further validation is required to determine the association of these genes with BTZ resistance.
2.3. BTZ-Resistant Msm Strains Exhibited Cross-Resistance to Other Antibiotics
To determine whether the spontaneous drug-resistant mutants exhibited cross-resistance, we tested the susceptibility of WGS-analyzed strains to a range of antibiotics (Table 1). Most resistant strains showed no significant MIC changes (≤2-fold) in their sensitivity to antibiotics including levofloxacin (LEV), amikacin (AMK), and streptomycin (STR). However, some strains exhibited notable cross-resistance. For instance, Msm-R1-2 demonstrated high-level resistance to both BTZ and LZD, with MICs rising to 80 µg/mL and 128 µg/mL, respectively. Additionally, the MIC of clarithromycin (CLR) increased 4-fold. Similarly, Msm-R1-13 exhibited an 8-fold increase in MIC of CLR and a 4-fold increase in MIC of gentamicin (GEN), while Msm-R4-1 showed a 4-fold increase in MIC of ethambutol (EMB). These findings suggest that resistance mechanisms may involve shared pathways that regulate cross-resistance, warranting further investigation.

Table 1.
Minimum inhibitory concentrations (MICs) of various antibiotics against different spontaneous Mycobacterium smegmatis mc2155 strains (Msm).
2.4. Single Knockout of MSMEG_1380 or MSMEG_0965 in Msm Resulted in Low-Level Resistance to BTZ and LZD
To investigate the genes associated with BTZ resistance in Msm, we used CRISPR-Cpf1-assisted recombineering technology [29] to knock out MSMEG_3244, MSMEG_5085, MSMEG_3987, MSMEG_1380, and MSMEG_0965 genes. We then tested the MICs of BTZ against these knockout strains. The results showed that compared to Msm, Δ3244, Δ5085, and Δ3987 exhibited no significant changes in BTZ resistance, indicating that these genes are not involved in the resistance mechanism. In contrast, Δ1380 and Δ0965 showed a 4-fold increase in the MIC of BTZ compared to Msm, suggesting that MSMEG_1380 and MSMEG_0965 play crucial roles in Msm’s resistance to BTZ (Table 2).

Table 2.
MICs of BTZ against different gene knockout Msm strains.
Notably, Msm-R1-2 exhibited high-level resistance to both BTZ and LZD and carried mutations in MSMEG_1380 and MSMEG_0965 (Table 1 and Table S2). To determine their roles in resistance, we assessed the LZD susceptibility of Δ1380 and Δ0965. The results showed that, compared to wild-type Msm, both Δ1380 and Δ0965 exhibited a 4-fold increase in the MIC of LZD (Table 3). These findings suggest that MSMEG_1380 and MSMEG_0965 are key contributors to BTZ and LZD resistance in Msm. Based on the observed MIC shifts compared to the wild-type strain, we defined low-level resistance as a 4- to 8-fold increase in MIC (as observed in single mutants, Table 2 and Table 3), and high-level resistance as a ≥16-fold increase (as seen in Msm-R1-2, Table 1).

Table 3.
MICs of LZD against different gene knockout Msm strains.
2.5. Single Knockout of MSMEG_1380 or MSMEG_0965 in Msm Affected the Sensitivity to Other Antibiotics
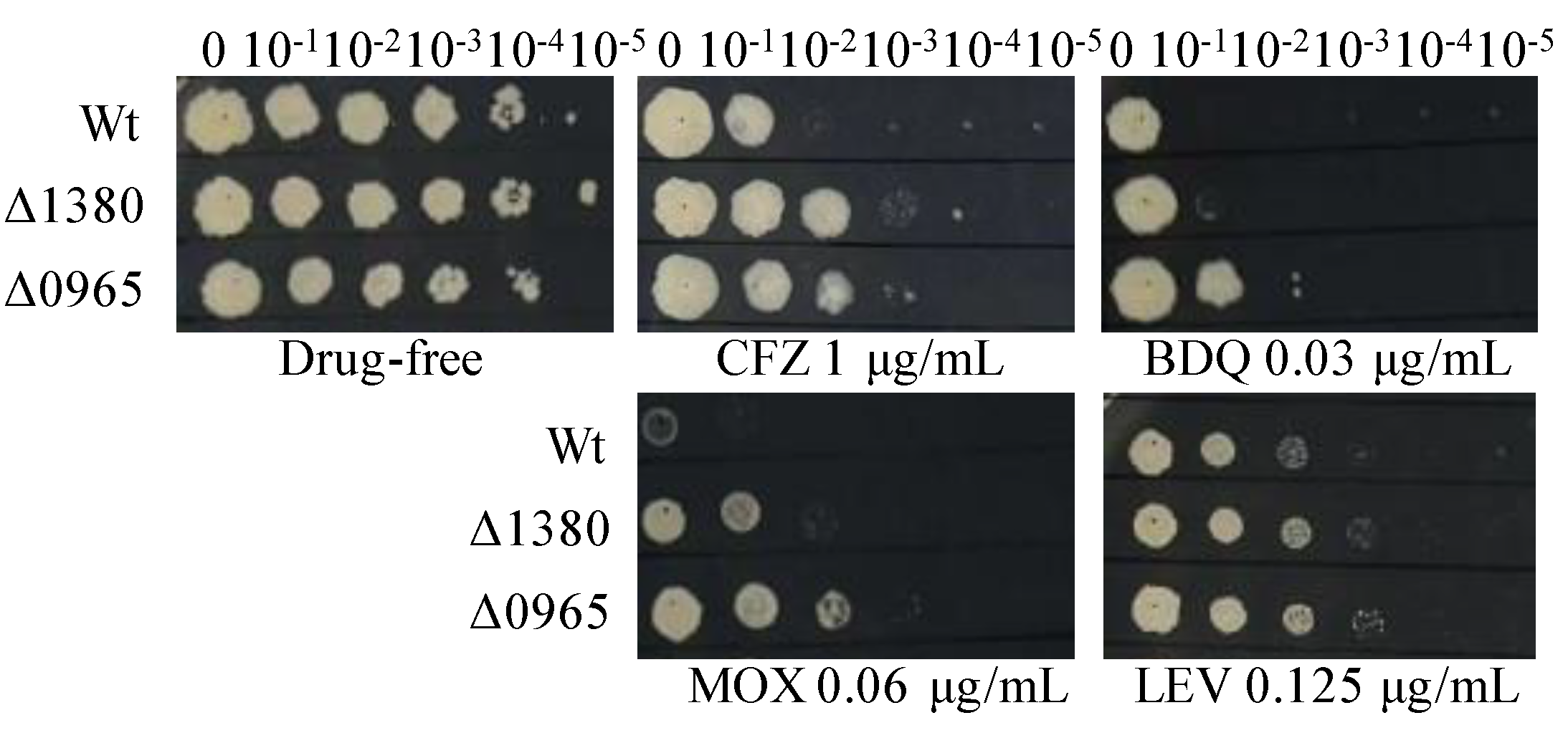
To investigate the antibiotic resistance profiles associated with MSMEG_1380 and MSMEG_0965, we tested the susceptibility of Δ1380 and Δ0965 to a range of antibiotics. Both Δ1380 and Δ0965 exhibited resistance to clofazimine (CFZ), moxifloxacin (MOX), and LEV. Interestingly, bedaquiline (BDQ) resistance was observed only in Δ0965, indicating that MSMEG_0965 plays a specific role in mediating BDQ resistance (Figure 1). In addition, both Δ1380 and Δ0965 displayed a 4-fold increase in MIC of vancomycin (VAN). Furthermore, Δ0965 also exhibited an 8-fold increase in MIC of CLR, a 4-fold increase in MIC of sulfadiazine (SDZ), and a 16-fold increase in MIC of sulfamethoxazole (SMX) (Table 4). In contrast, both knockout strains (Δ1380 and Δ0965) showed no significant differences in MICs for EMB, STR, GEN, and AMK compared to the wild-type Msm (Table S3). These findings suggest that the knockouts of MSMEG_1380 and MSMEG_0965 differentially affect antibiotic sensitivity, with gene-specific mechanisms such as BDQ resistance, which was mediated exclusively by MSMEG_0965.
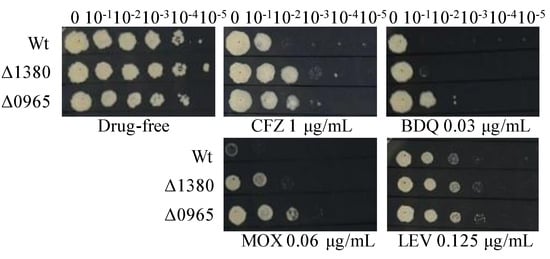
Figure 1.
The sensitivity of Wt, Δ1380, and Δ0965 to various antibiotics. Wt, wild-type Mycobacterium smegmatis mc2155 strain (Msm); Δ1380, MSMEG_1380 knockout Msm strain; Δ0965, MSMEG_0965 knockout Msm strain. CFZ, clofazimine; BDQ, bedaquiline; MOX, moxifloxacin; LEV, levofloxacin. The experiment was repeated three times.

Table 4.
MICs of multiple drugs against different gene knockout Msm strains.
2.6. Overexpression of MSMEG_1380 or MSMEG_0965 in Msm and Complementation of These Genes in Knockout Strains Affected the Drug Sensitivity to BTZ and LZD
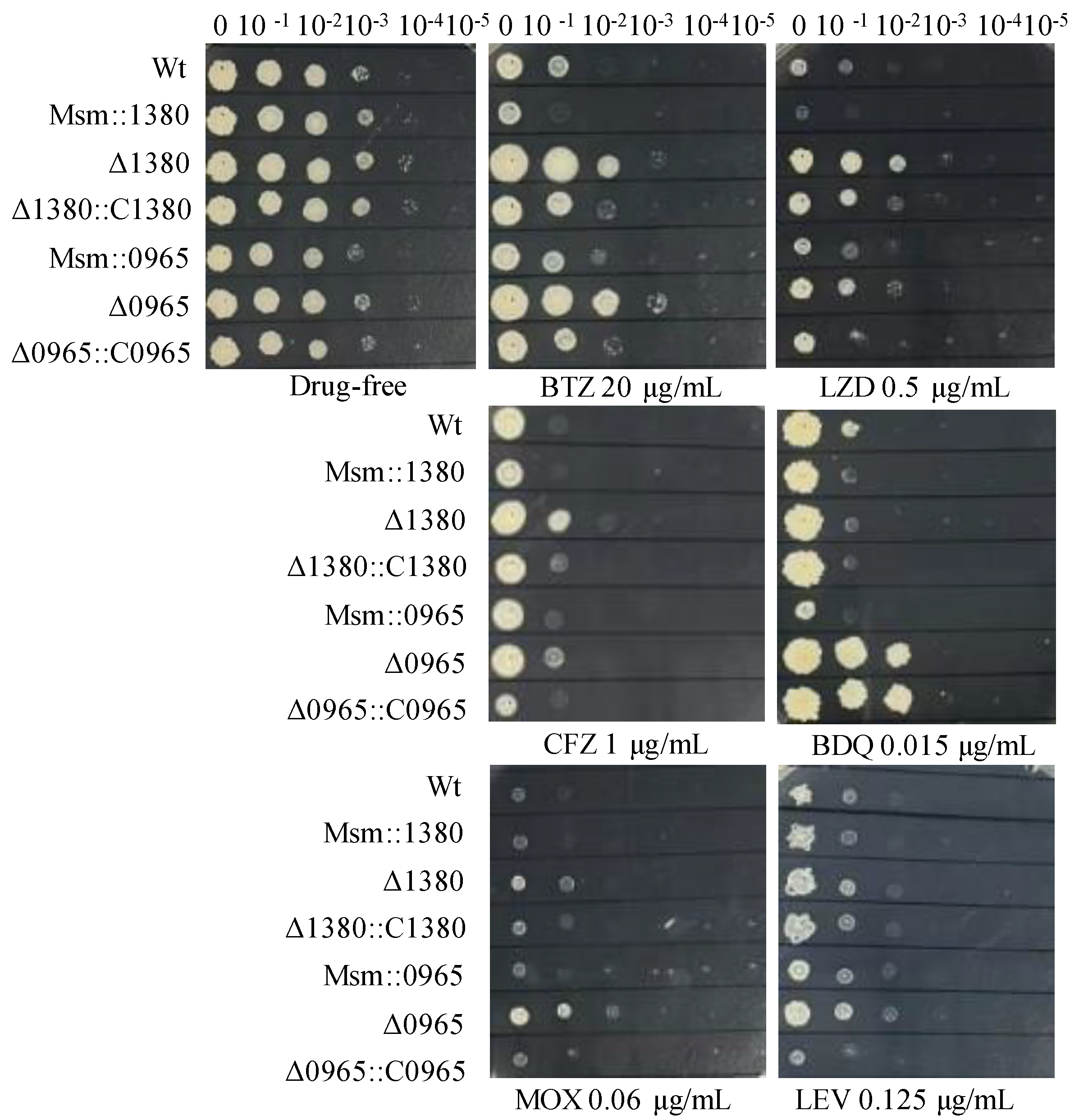
To further investigate the functional contributions of MSMEG_1380 and MSMEG_0965 to drug susceptibility phenotypes, we generated Msm recombinant strains harboring overexpression constructs for these genes and performed genetic complementation in their respective knockout backgrounds. We expressed MSMEG_1380 under the hsp60 promoter and MSMEG_0965 under its native promoter for overexpression and complementation studies. Drug susceptibility testing (DST) revealed that Msm::1380 and Msm::0965 strains displayed enhanced sensitivity to BTZ and LZD. The Δ1380::C1380 strain showed partially restored sensitivity to BTZ, LZD, and CFZ compared to Δ1380, while Δ0965::C0965 restored susceptibility to BTZ, LZD, MOX, and CFZ to the similar levels of wild-type Msm. Notably, BDQ susceptibility remained unaltered in the Δ0965::C0965 strain, suggesting a gene-specific interaction between MSMEG_0965 and intrinsic BDQ resistance mechanisms (Figure 2). We also evaluated the susceptibility of these strains to other antibiotics, including VAN and CLR. The MICs of these antibiotics in the overexpression strains remained equivalent to those of wild-type Msm, and the MICs in the complementation strains showed no significant changes compared to their respective knockout strains (Table S4). These results suggest that overexpression of MSMEG_1380 or MSMEG_0965, and complementation in the respective knockout strains affect specific drug sensitivity, but their underlying regulatory mechanisms differ, highlighting the complexity of the regulatory networks involved.
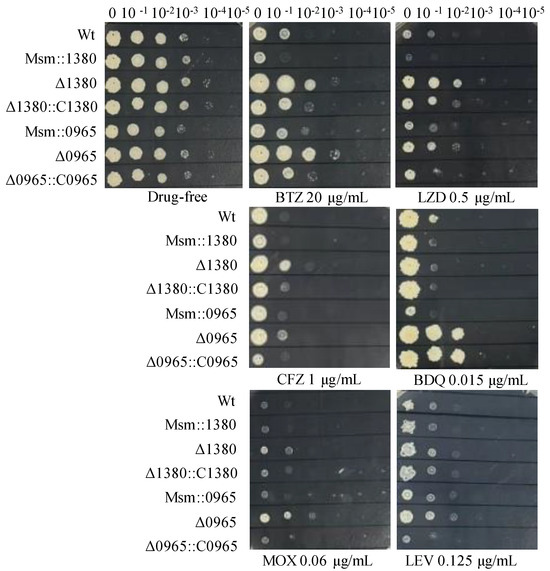
Figure 2.
The sensitivity of recombinant strains to various antibiotics. Wt, wild-type Msm strain; Msm::1380, MSMEG_1380 overexpression Msm strain; Δ1380, MSMEG_1380 knockout Msm strain; Δ1380::C1380, MSMEG_1380 complemented Δ1380 strain; Msm::0965, MSMEG_0965 overexpression Msm strain; Δ0965, MSMEG_0965 knockout Msm strain; Δ0965::C0965, MSMEG_0965 complemented Δ0965 strain. BTZ, bortezomib; LZD, linezolid; CFZ, clofazimine; BDQ, bedaquiline; MOX, moxifloxacin; LEV, levofloxacin. The experiment was repeated three times.
2.7. Roles of MSMEG_138012insC and MSMEG_0965400insCC in Multidrug Resistance Were Confirmed by Gene Editing in Msm
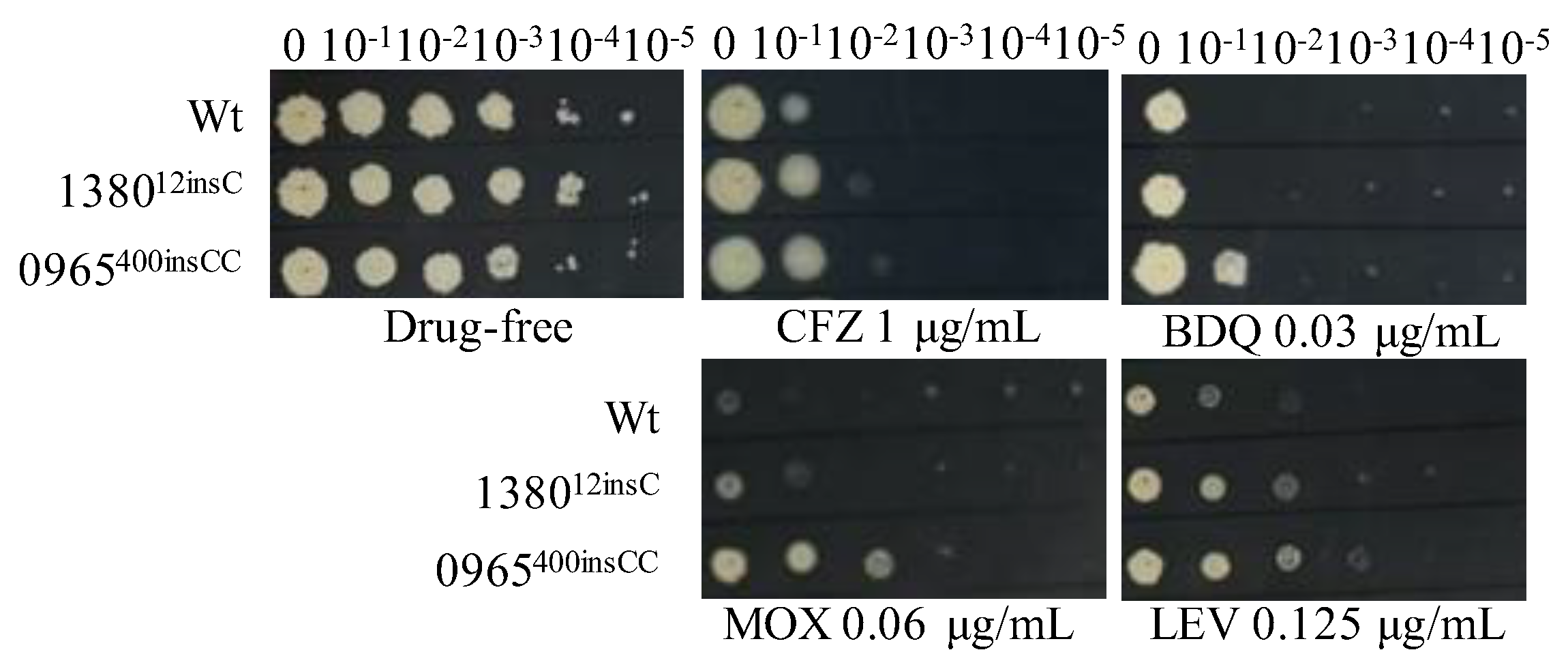
To verify that single mutations in MSMEG_1380 or MSMEG_0965 confer resistance to multiple drugs, we introduced these mutations using CRISPR-Cpf1-assisted recombineering technology [29]. The drug susceptibility profiles of the single gene-edited strains were consistent with those of Δ1380 and Δ0965 (Figure 1 and Figure 3, Table 4 and Table 5). These results indicated that single knockout or mutation of MSMEG_1380 or MSMEG_0965 in Msm conferred only low-level resistance to BTZ and LZD rather than the high-level resistance observed in the Msm-R1-2 strain. These findings confirm the important roles of MSMEG_1380 and MSMEG_0965 in conferring drug resistance in Msm. However, the precise mechanisms underlying the high-level resistance to BTZ and LZD in the Msm-R1-2 strain require further study.
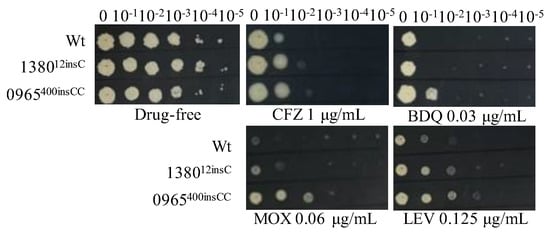
Figure 3.
The sensitivity of single gene-edited strains to various antibiotics. Wt, wild-type Msm strain; 138012insC, Msm strain with a 12insC insertion mutation in MSMEG_1380; 0965400insCC, Msm strain with a 400insCC insertion mutation in MSMEG_0965. CFZ, clofazimine; BDQ, bedaquiline; MOX, moxifloxacin; LEV, levofloxacin. The experiment was repeated three times.

Table 5.
MICs of multiple drugs against single gene-edited strains.
2.8. Dual Mutations of MSMEG_1380 and MSMEG_0965 in Msm Exhibited High-Level Resistance to BTZ and LZD
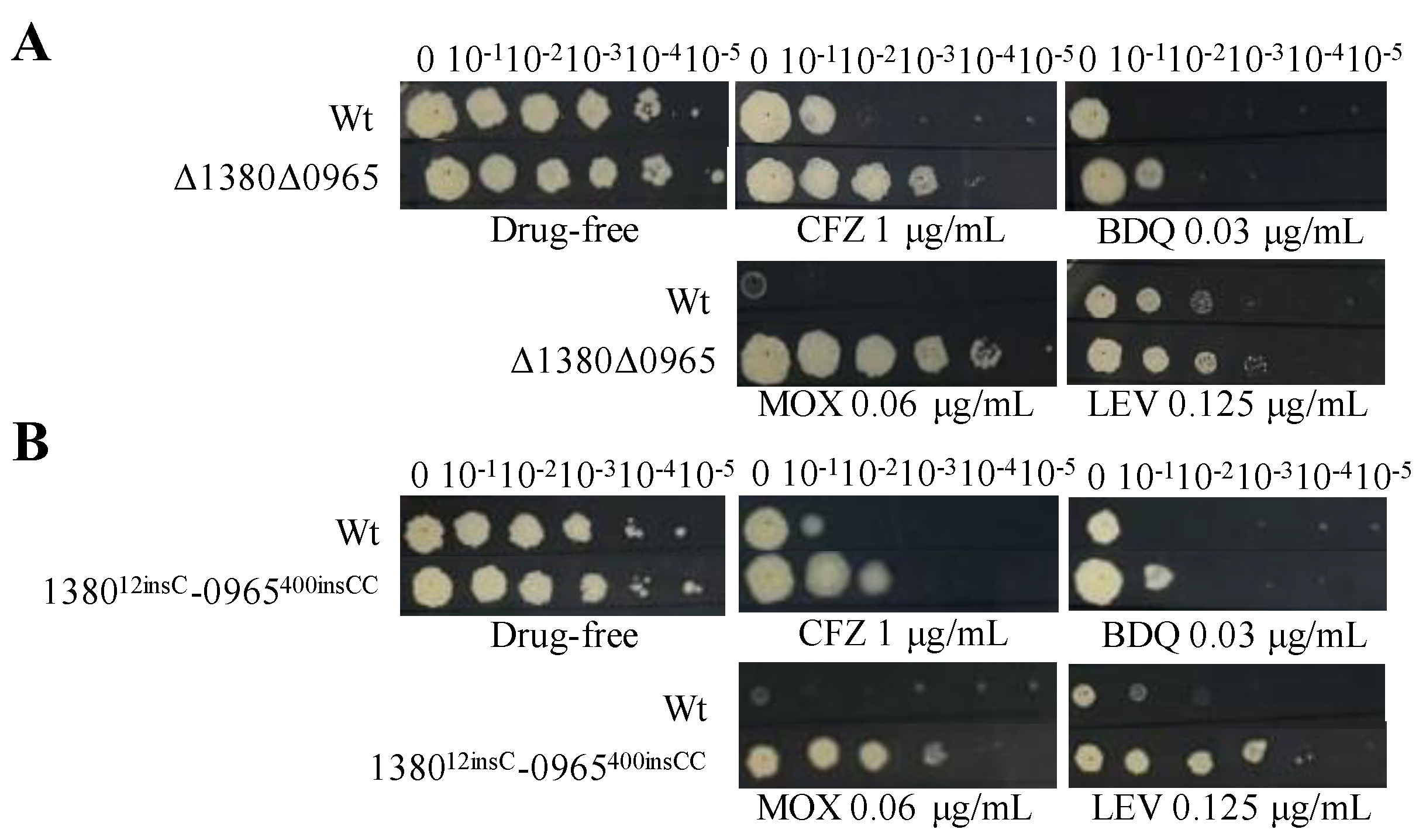
Based on the above results, we hypothesize that the dual mutations in MSMEG_1380 and MSMEG_0965 are the primary cause of high-level resistance in Msm [30,31]. To validate this hypothesis, we used CRISPR-Cpf1-assisted recombineering technology [29] to perform the dual knockouts and gene editing of these two genes in Msm. DST revealed that the MICs of BTZ and LZD against the dual mutation strains (Δ1380Δ0965 and 138012insC-0965400insCC) were 16-fold and 64-fold higher, respectively, than those to Msm (Table 6), which was consistent with the MIC values observed in Msm-R1-2 (Table 1). Compared to the single-gene mutant strains, the dual mutation strains also exhibited greater resistance to MOX and LEV (Figure 1, Figure 3 and Figure 4), and increased resistance to VAN (Table 4, Table 5 and Table 6). The resistance levels of the double mutation strains to CLR, SDZ, and SMX were comparable to those of Δ0965 and 0965400insCC (Table 4, Table 5 and Table 6). Interestingly, the complementation of MSMEG_1380 and MSMEG_0965 in the double-knockout strains did not alter the MICs of multiple drugs (Table 6). Based on the MIC results of the dual knockout and dual gene-edited strains, we propose that the dual mutations of MSMEG_1380 and MSMEG_0965 in Msm are the main reason for Msm’s high-level resistance to BTZ and LZD, and they also influence the sensitivity to other antibiotics.

Table 6.
MICs of multiple drugs against different strains.
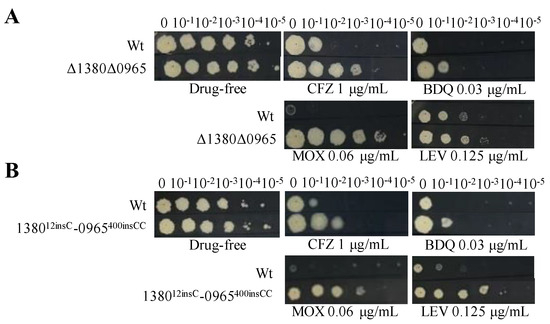
Figure 4.
Dual mutations of MSMEG_1380 and MSMEG_0965 affect Msm sensitivity to various antibiotics. (A) The sensitivity of Wt and Δ1380Δ0965 to various antibiotics. (B) The sensitivity of Wt and 138012insC-0965400insCC to various antibiotics. Wt, wild-type Msm strain; Δ1380Δ0965, MSMEG_1380 and MSMEG_0965 double-knockout Msm strain; 138012insC-0965400insCC, Msm strain with a 12insC insertion in MSMEG_1380 and a 400insCC insertion in MSMEG_0965. CFZ, clofazimine; BDQ, bedaquiline; MOX, moxifloxacin; LEV, levofloxacin. The experiment was repeated three times.
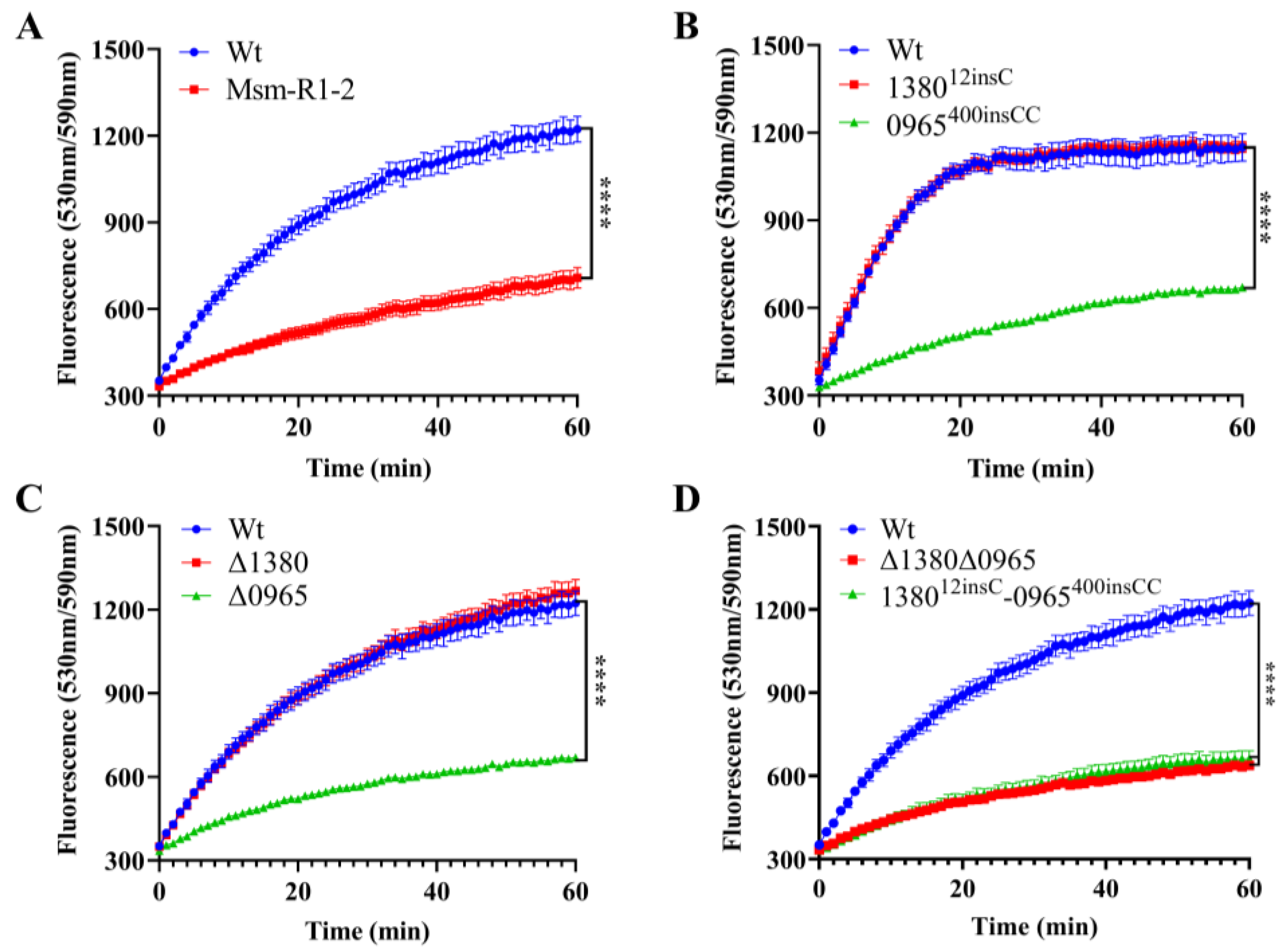
2.9. The Knockout and Mutation of MSMEG_0965 Reduced Cell Wall Permeability
To assess the impact of MSMEG_0965 on cell wall integrity [32,33], we performed the EtBr accumulation assay in Msm-R1-2, knockout and gene-edited Msm strains [34]. The results showed that Msm-R1-2, Δ0965 and 0965400insCC strains exhibited significantly reduced cell wall permeability, while the permeability of Δ1380 and 138012insC remained unchanged. Similarly, the permeability of Δ1380Δ0965 and 138012insC-0965400insCC was also significantly decreased (Figure 5). These results indicate that knockout and mutation of MSMEG_0965 reduce cell wall permeability, thereby contributing to drug resistance, while MSMEG_1380 does not appear to affect cell wall permeability.
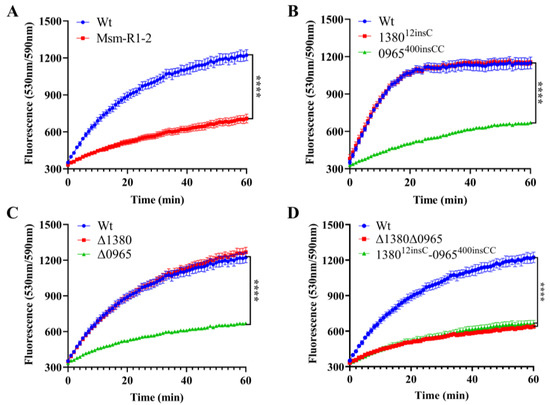
Figure 5.
MSMEG_0965 affects cell wall permeability in Msm. (A) EtBr accumulation in Wt and Msm-R1-2. (B) EtBr accumulation in single gene-edited strains. (C) EtBr accumulation in single knockout strains. (D) EtBr accumulation in double-knockout and double gene-edited strains. ****, p < 0.0001. Data are presented as mean ± standard deviation, n = 3. Wt, wild-type Msm strain; Msm-R1-2, BTZ-resistant strain; Δ1380, MSMEG_1380 knockout Msm strain; Δ0965, MSMEG_0965 knockout Msm strain; 138012insC, Msm strain with a 12insC insertion mutation in MSMEG_1380; 0965400insCC, Msm strain with a 400insCC insertion mutation in MSMEG_0965; Δ1380Δ0965, MSMEG_1380, and MSMEG_0965 double-knockout Msm strain; 138012insC-0965400insCC, Msm strain with a 12insC insertion in MSMEG_1380 and a 400insCC insertion in MSMEG_0965.
2.10. The Knockout and Mutation of MSMEG_1380 Upregulate the Expression of the mmpS5-mmpL5 Efflux System
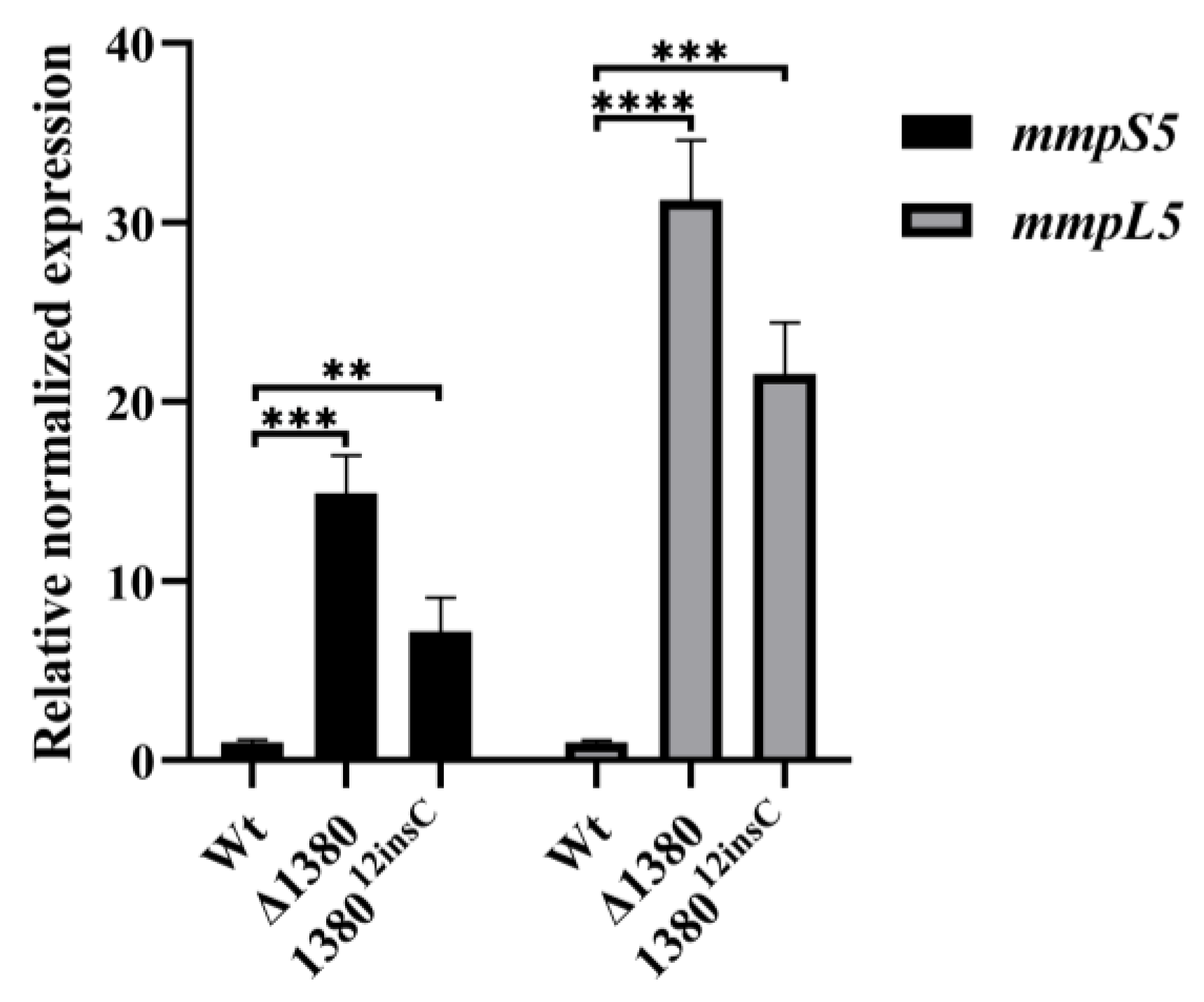
Given the observed resistance phenotype in MSMEG_1380 mutants, we examined the expression levels of mmpS5 and mmpL5 in the mutant strains [22]. The results demonstrated that compared to the wild-type Msm, mmpS5 expression increased 14.89-fold in Δ1380 and 7.16-fold in 138012insC, while mmpL5 expression increased 31.24-fold and 21.53-fold, respectively (Figure 6). These findings confirm that mutations in MSMEG_1380 significantly enhance the mmpS5-mmpL5 operon expression, thereby increasing drug efflux capacity.
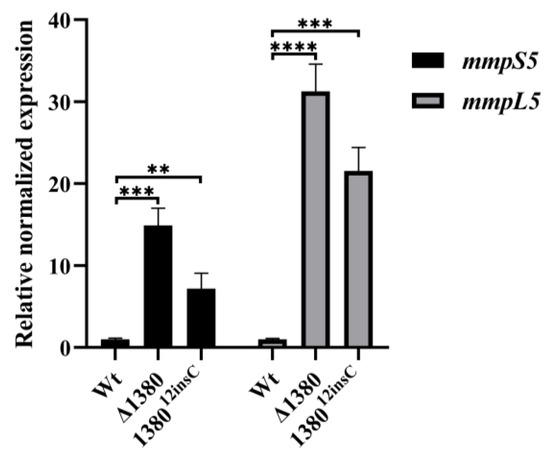
Figure 6.
MSMEG_1380 affects the relative expression levels of the mmpS5-mmpL5 operon genes in Msm. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Wt, wild-type Msm strain; Δ1380, MSMEG_1380 knockout Msm strain; 138012insC, Msm strain with a 12insC insertion mutation in MSMEG_1380.
3. Discussion
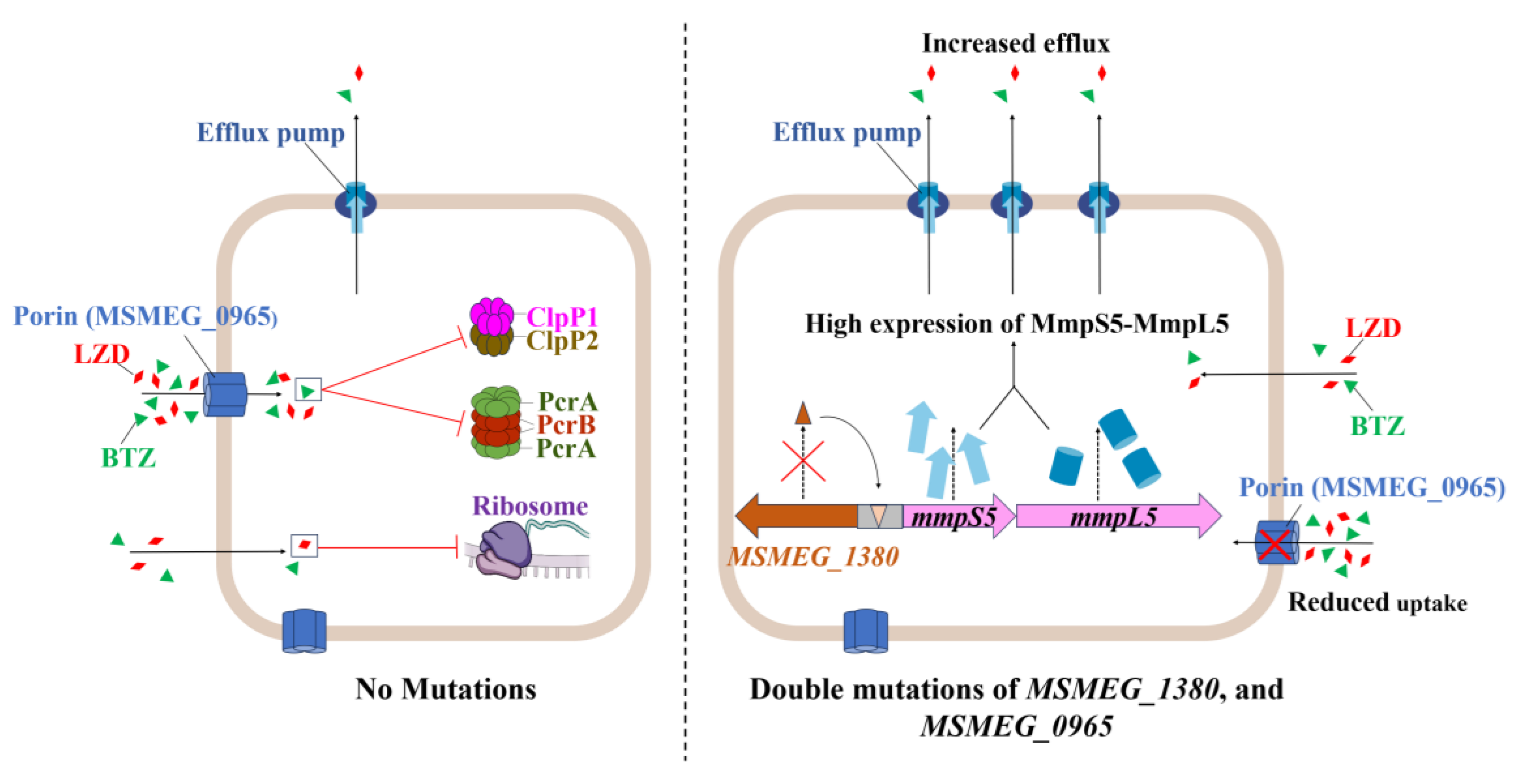
The emergence of MDR and XDR Mtb strains presents a critical barrier to global TB control, emphasizing the urgent need for new therapeutic strategies [1,35]. Our study demonstrates that dual mutations in MSMEG_1380 and MSMEG_0965 synergistically confer high-level resistance to BTZ and LZD through a coordinated modulation of efflux and uptake, revealing a previously unrecognized combinatorial mechanism in mycobacteria.
To dissect the genetic basis of resistance, we conducted a genomic analysis that validated the specificity of this mechanism. In the obtained BTZ-resistant Msm strains, neither Sanger sequencing nor WGS detected no mutations in known BTZ target genes (prcA, prcB, clpP1, and clpP2) [4,10] or in genes associated with LZD resistance (rplC, rrl, mmpL9, embB-embC, and grcC1) [15,16,17]. While we identified an rpoB mutation (associated with RIF resistance) in Msm-R1-2 [36], its phenotypic impact was minimal: only a 2-fold increase in RIF MIC, compared to 16- and 64-fold increases for BTZ and LZD, respectively. Moreover, the MIC shifts observed in MSMEG_0965 single mutants (Table S3) were consistent with this pattern. These results suggest that classical resistance pathways are not major contributors to the observed resistance.
Further phenotypic characterization of single-gene knockout strains revealed distinct resistance patterns, with Δ0965 but not Δ1380 conferring resistance to BDQ, CLR, SDZ, and SMX (Figure 1, Table 3, Table 4 and Table 5). The specific dependence of BDQ resistance on the MspA porin encoded by MSMEG_0965 [23,37,38,39] suggests this porin mediates in BDQ uptake. Meanwhile, the absence of CLR resistance in Δ1380 indicates that CLR efflux likely occurs through alternative systems such as ABC transporters rather than the MSMEG_1380-mediated MmpS5-MmpL5 system [40]. The particular sensitivity of MSMEG_0965 mutations strains to SMX and SDZ can be explained by their hydrophilic nature (logP = 0.79 and 0.25, respectively, obtained from https://go.drugbank.com/ (accessed on 31 March 2025), which makes them more dependent on porin-mediated uptake. In contrast, resistance to both BTZ and LZD appears to be dually influenced by both MSMEG_1380 and MSMEG_0965. In addition, complementation and overexpression experiments showed that MSMEG_1380 and MSMEG_0965 influence drug sensitivity to BTZ, LZD, and some other antibiotics, but their effects on drugs like LEV, VAN, and CLR remained unchanged. This aligns with studies showing that target overexpression or complementation can have drug-specific effects due to mechanistic differences [18,41].
Building on these findings, the synergistic resistance observed in the double mutant likely results from the combined disruption of uptake and efflux processes, as illustrated in Figure 7 [42,43,44]. To assess the broader relevance of this mechanism, we examined homologs in pathogenic mycobacteria. For example, the Rv0678 gene (the MSMEG_1380 homolog in Mtb) promotes efflux-mediated resistance to BDQ and CFZ [45,46]. Similarly, mutations in MAB_4384 (the homolog of MSMEG_1380 in Mycobacterium abscessus) are linked to LZD resistance [47]. Although Mtb lacks a homolog of MSMEG_0965 (MspA) [48], expressing MspA in Mtb enhances drug susceptibility [37], suggesting the presence of compensatory uptake mechanisms, such as the aquaporin Rv1698 [49]. Thus, despite molecular divergence, coordinated efflux–uptake regulation appears to be a conserved resistance strategy across mycobacteria.
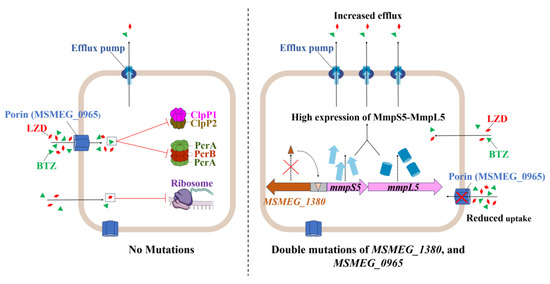
Figure 7.
Schematic representation of the mechanism underlying high-level resistance to BTZ and LZD in Msm harboring mutations in MSMEG_1380 and MSMEG_0965.
However, the interaction between MSMEG_1380 and MSMEG_0965 remains unclear. Their synergistic phenotype suggests potential coordination at transcriptional or post-translational levels. Such coordination between efflux and uptake is an evolutionarily conserved strategy for antibiotic resistance, as observed in Pseudomonas aeruginosa, where the MexAB-OprM efflux system cooperates with outer membrane permeability barriers to enhance drug resistance [50]. Based on this, we propose that reduced drug uptake due to MSMEG_0965 mutations may lower the intracellular antibiotic burden, thereby enhancing the efficiency of MSMEG_1380-mediated efflux. Conversely, increased efflux activity may impose selective pressure favoring porin mutations, further restricting drug entry. This interplay could establish a feedback loop, ultimately contributing to high-level resistance in dual mutants.
Despite these advances, this study has limitations. First, while our findings in Msm provide mechanistic insights, direct validation in clinical Mtb isolates remains essential to establish the translational relevance of this dual resistance model. Second, while the phenotypic effects of MSMEG_1380 and MSMEG_0965 mutations are well characterized, their precise molecular mechanisms are not yet fully understood. Functional studies, including protein structure–function analyses and investigations of regulatory pathways, are essential to further elucidate their roles. Finally, our study focused primarily on these two key determinants, leaving other potential resistance pathways and mutations identified in our screening (Table S2) unexplored, which warrants future investigation to obtain a more comprehensive understanding of mycobacterial drug resistance.
Future studies should focus on validating these findings in Mtb clinical isolates to establish clinical relevance, such as through heterologous expression of MSMEG_0965 or knockout of Rv0678 in Mtb, particularly to assess their roles in BTZ and LZD resistance. Further elucidation of MSMEG_1380 and MSMEG_0965 mechanisms is essential. Investigating their potential transcriptional co-regulation via RNA-seq or ChIP-seq and analyzing protein–protein interactions through pull-down assays or bacterial two-hybrid systems could clarify their regulatory relationships and physical associations. Additionally, a systematic analysis of other resistance-associated pathways identified in our screening data (Table S2) will help establish a more comprehensive model of mycobacterial drug resistance. From a therapeutic perspective, targeting the MmpS5-MmpL5 efflux system or restoring porin-mediated drug uptake may offer promising strategies to counteract resistance. Finally, systematic investigation of BTZ resistance mechanisms will be crucial for gaining deeper insights and providing the basis for the development of next-generation anti-TB therapies.
4. Materials and Methods
4.1. Strains, Plasmids, and Culture Conditions
All mycobacterial strains were cultured at 37 °C in 7H9 liquid medium (BD Difco, Sparks, MD, USA) supplemented with 0.05% Tween 80 (Amresco/VWR, Solon, OH, USA), 0.2% glycerol (Macklin, Shanghai, China), and 10% OADC (BD Difco, Sparks, MD, USA), or on 7H10/7H11 solid medium (BD Difco, Sparks, MD, USA). Antibiotics were added to the media as required: 50 μg/mL kanamycin (KAN, Solarbio, Beijing, China) and 30 μg/mL zeocin (ZEO, InvivoGen, Toulouse, France). For induced gene expression, 200 ng/mL anhydrotetracycline (aTc, Solarbio, Beijing, China) was added. All Escherichia coli strains were cultured in LB medium (prepared in-house using NaCl, agar, tryptone and yeast extract from Maikesi Biological Technology Co., Ltd., Shenzhen, China), supplemented with 50 μg/mL KAN, 30 μg/mL ZEO, and 100 μg/mL ampicillin as required.
4.2. Screening for Spontaneous BTZ-Resistant Msm Strains
Msm cultures at an OD600 of 1.0 were plated (500 μL) on 7H10 medium containing 100, 200, or 300 μg/mL BTZ (AbMole, Wuhan, China), with two replicates per concentration. Serial 10-fold dilutions were then plated on BTZ-free 7H10 agar to calculate colony-forming units (CFU). The mutation frequency was determined by counting CFU and the number of resistant colonies. Resistant strains were analyzed for mutations in prcA, prcB, clpP1, and clpP2 by Sanger sequencing using primers listed in Table S5. Genomic DNA from the parent strain and seven BTZ-resistant strains (which did not harbor mutations in prcA, prcB, clpP1, or clpP2) was sequenced by Shanghai Jingnuo Biotechnology Co., Ltd. (Shanghai, China). Mutations in resistant strains were identified by comparing their genomic profiles with those of the parent strain.
4.3. Construction of Knockout Strains
Gene knockout was performed using CRISPR-Cpf1-assisted recombineering technology [29]. First, the pJV53-Cpf1 plasmid was electroporated into Msm to obtain Msm::pJV53-cpf1 and then induced competent cells were prepared. The crRNA sequences were designed using the website (https://chopchop.cbu.uib.no/ (accessed on 27 April 2024), synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Guangzhou, China) (Table S6), and ligated into the linearized pCR-Zeo vector after annealing. We amplified ~800 bp homology arms (containing 15–36 bp of the target gene) and inserted them into pBlueSK. The assembled ~1600 bp fragment was amplified by PCR to generate the repair template. The pCR-Zeo plasmid containing the crRNA sequence and the double-stranded template were then electroporated into the Msm::pJV53-cpf1 inducible competent cells. The cells were plated on 7H11 agar containing 50 μg/mL KAN, 30 μg/mL ZEO, and 200 ng/mL aTc and incubated at 30 °C. Positive colonies were identified by PCR and Sanger sequencing.
4.4. Construction of Overexpression and Complementation Strains
The target genes were PCR-amplified from Msm and cloned into the linearized pRH2502 plasmid under the control of either the exogenous hsp60 promoter or the native promoter of the respective genes. Primers used in this study are listed in Table S5. The recombinant plasmids were electroporated into either wild-type Msm (for overexpression strains) or the corresponding knockout strains (for complementation strains). Transformants were plated on 7H10 agar containing 50 μg/mL KAN, and positive colonies were confirmed by PCR.
4.5. Construction of Gene Editing Strains
CRISPR-Cpf1-assisted recombineering [29] was used for gene editing. CrRNAs targeting the mutation sites were designed using the CHOPCHOP web tool (Table S6) and subsequently inserted into the linearized pCR-Zeo vector. The 500 bp flanking regions (~1000 bp total) of the mutation site were amplified from the resistant strains and used as repair templates. The crRNA-containing pCR-Zeo plasmid and repair templates were co-electroporated into Msm::pJV53-Cpf1 inducible competent cells. Transformants were plated on 7H11 agar containing 50 μg/mL KAN, 30 μg/mL ZEO, and 200 ng/mL aTc and incubated at 30 °C. Positive colonies were verified by PCR and Sanger sequencing.
4.6. DST
In this study, all drugs were prepared in solutions with DMSO (Xilong Scientific Co., Ltd., Shantou, China) or water at a final concentration of 10 mg/mL, except for CFZ (Meilunbio, Dalian, China), which was prepared at a final concentration of 5 mg/mL. Two methods were used to test the drug susceptibility of the strains. Knockout, overexpression, and complementation strains were grown in 7H9 medium at 37 °C to the logarithmic phase for both methods. All MIC assays were performed in three independent experiments.
According to CLSI guidelines [51], we performed the microplate broth dilution assay. Bacterial cultures were adjusted to an OD600 of 0.5 and subsequently diluted 1:1000. The MICs were determined using the broth microdilution method in 96-well plates. Cultures were incubated at 37 °C for three days, and the MIC was defined as the lowest concentration of antibiotic that visibly inhibited bacterial growth [17].
For the solid plate assay, cultures were adjusted to an OD600 of 0.9. Bacterial suspensions were serially diluted in 10-fold increments to obtain six different dilutions, ranging from the undiluted suspension to a 10−5 dilution. A 1 µL aliquot of each dilution was spotted onto solid media containing different concentrations of drugs, with 7H10 solid media without antibiotics serving as the control. The plates were incubated at 37 °C for three days, and colony growth was assessed visually and photographed.
4.7. Cell Wall Permeability Assay
The EtBr accumulation assay was performed as described previously [34]. We prepared the 4 μg/mL EtBr (Macklin, Shanghai, China) working solution in PBS (GENOM, Hangzhou, China) containing 0.08% glucose (Macklin, Shanghai, China) and 0.05% Tween 80. Cultures were grown to an OD600 of 0.6–0.8, centrifuged, and resuspended in PBS with 0.05% Tween 80 to an OD600 of 0.5. For each strain, 100 μL of the cell suspension was added to a white 96-well plate (3 experimental wells, 3 no-reagent controls, and 3 no-cell controls). Fluorescence was measured using a multimode microplate reader (excitation: 530 nm, emission: 590 nm) at 1 min intervals over 60 min. Background fluorescence was subtracted, and data were analyzed using GraphPad Prism 8.0.2. Results are presented as the mean ± SD, with n = 3 for each group. Statistical significance was assessed using an independent t-test, with p < 0.05 considered significant.
4.8. RNA Isolation and qRT-PCR
Total RNA extraction and qRT-PCR analysis were performed following standardized protocols [22]. Briefly, bacterial strains were cultured to an OD600 of 0.8–1.0 before harvesting the cells. Cell disruption was achieved through liquid nitrogen grinding, followed by total RNA extraction using the HiPure Bacterial RNA Kit (Magen, Guangzhou, China). For cDNA synthesis, 2 μg of total RNA was reverse transcribed using HiScript Q RT SuperMix for qPCR (Vazyme, Nanjing, China). The primers used for qRT-PCR are listed in Table S5. qRT-PCR was performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using 10-fold diluted cDNA as a template and Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The gene sigA served as a reference, and relative gene expression levels were calculated using the 2(−ΔΔCT) method. All data were analyzed using GraphPad Prism 8.0.2, with three biological replicates included for each sample to ensure experimental reliability. Statistical significance was determined by Student’s t-test, with p < 0.05 considered statistically significant.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26083779/s1.
Author Contributions
Conceptualization: Y.G. and T.Z.; methodology: H.Z.; software: H.Z. and B.Y.; validation: H.Z., Y.G. and T.Z.; formal analysis: B.Y.; investigation: H.Z. and C.F.; resources: H.A.H., Y.G. and T.Z.; data curation: B.Y.; writing—original draft preparation: H.Z. and C.F.; writing—review and editing: C.F., X.Z., S.W., H.A.H., Y.G. and T.Z.; visualization: H.Z.; supervision: Y.G. and T.Z.; project administration: Y.G. and T.Z.; funding acquisition: H.A.H., Y.G. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2021YFA1300904), the National Natural Science Foundation of China (NSFC 32300152), partially by the National Key R&D Program of China (2023YFF0713600), the State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Diseases, First Affiliated Hospital of Guangzhou Medical University (SKLRD-Z-202414, SKLRD-Z-202301), and Guangzhou Science and Technology Plan-Youth Doctoral “Sail” Project (2024A04J4273). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included within the paper and its Supplementary Materials.
Acknowledgments
We acknowledge the group of Yicheng Sun from the Institute of Pathogenic Biology, Chinese Academy of Medical Sciences, for kindly sending the pJV53-Cpf1 and pCR-Zeo plasmids as tools for gene knockout and gene editing.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Dheda, K.; Mirzayev, F.; Cirillo, D.M.; Udwadia, Z.; Dooley, K.E.; Chang, K.-C.; Omar, S.V.; Reuter, A.; Perumal, T.; Horsburgh, C.R., Jr.; et al. Multidrug-resistant tuberculosis. Nat. Rev. Dis. Primers 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, R.; Qi, Y.; Yan, X.; Qi, G.; Peng, Q. The progress of Mycobacterium tuberculosis drug targets. Front. Med. 2024, 11, 1455715. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Ngan Grace, J.Y.; Low Jian, L.; Poulsen, A.; Chia Brian, C.S.; Ang Melgious, J.Y.; Yap, A.; Fulwood, J.; Lakshmanan, U.; Lim, J.; et al. Target mechanism-based whole-cell screening identifies bortezomib as an inhibitor of caseinolytic protease in mycobacteria. mBio 2015, 6, e00253-15. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, L.; Ruiz-Serrano, M.J.; Pérez-Fernández Turégano, C.; García De Viedma, D.; Díaz-Infantes, M.; Marín-Arriaza, M.; Bouza, E. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 2003, 47, 416–417. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Aurooz, F.; Singh, V. Clp protease complex as a therapeutic target for tuberculosis. In Bacterial Enzymes as Targets for Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 363–385. [Google Scholar]
- Sander, P.; Belova, L.; Kidan, Y.G.; Pfister, P.; Mankin, A.S.; Böttger, E.C. Ribosomal and non-ribosomal resistance to oxazolidinones: Species-specific idiosyncrasy of ribosomal alterations. Mol. Microbiol. 2002, 46, 1295–1304. [Google Scholar] [CrossRef]
- Moreira, W.; Santhanakrishnan, S.; Dymock, B.W.; Dick, T. Bortezomib warhead-switch confers dual activity against mycobacterial caseinolytic protease and proteasome and selectivity against human proteasome. Front. Microbiol. 2017, 8, 746. [Google Scholar] [CrossRef]
- Lupoli, T.J.; Vaubourgeix, J.; Burns-Huang, K.; Gold, B. Targeting the proteostasis network for mycobacterial drug discovery. ACS Infect. Dis. 2018, 4, 478–498. [Google Scholar] [CrossRef]
- Hu, G.; Lin, G.; Wang, M.; Dick, L.; Xu, R.; Nathan, C.; Li, H. Structure of the Mycobacterium tuberculosis proteasome and mechanism of inhibition by a peptidyl boronate. Mol. Microbiol. 2006, 59, 1417–1428. [Google Scholar] [CrossRef]
- Moreira, W.; Santhanakrishnan, S.; Ngan, G.J.Y.; Low, C.B.; Sangthongpitag, K.; Poulsen, A.; Dymock, B.W.; Dick, T. Towards selective mycobacterial ClpP1P2 inhibitors with reduced activity against the human proteasome. Antimicrob. Agents Chemother. 2017, 61, e02307–e02316. [Google Scholar] [CrossRef]
- Lin, G.; Hu, G.; Tsu, C.; Kunes, Y.Z.; Li, H.; Dick, L.; Parsons, T.; Li, P.; Chen, Z.; Zwickl, P.; et al. Mycobacterium tuberculosis prcBA genes encode a gated proteasome with broad oligopeptide specificity. Mol. Microbiol. 2006, 59, 1405–1416. [Google Scholar] [CrossRef]
- Kadura, S.; King, N.; Nakhoul, M.; Zhu, H.; Theron, G.; Köser, C.U.; Farhat, M. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Ng, H.F.; Ngeow, Y.F. Mechanisms of linezolid resistance in mycobacteria. Pharmaceuticals 2023, 16, 784. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Xu, L.; Zou, Y.; Li, B.; Guo, Q.; Zhang, Y.; Zhan, M.; Xu, B.; Yu, F.; Zhang, Z.; et al. Molecular analysis of linezolid-resistant clinical isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e01842-18. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gao, Y.; Wang, J.; Hameed, H.M.A.; Wang, S.; Fang, C.; Tian, X.; Zhang, J.; Han, X.; Ju, Y.; et al. EmbB and EmbC regulate the sensitivity of Mycobacterium abscessus to echinomycin. mLife 2024, 3, 459–470. [Google Scholar] [CrossRef]
- Wang, S.; Cai, X.; Yu, W.; Zeng, S.; Zhang, J.; Guo, L.; Gao, Y.; Lu, Z.; Hameed, H.M.A.; Fang, C.; et al. Arabinosyltransferase C mediates multiple drugs intrinsic resistance by altering cell envelope permeability in Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0276321. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, H.; He, J.; Tian, X.; Zeng, S.; Han, X.; Wang, S.; Yusuf, B.; Hu, J.; Zhong, N.; et al. GrcC1 mediates low-level resistance to multiple drugs in M. marinum, M. abscessus, and M. smegmatis. Microbiol. Spectr. 2025, 13, e02289-24. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Haeili, M.; Ghazi, M.; Goudarzi, H.; Pormohammad, A.; Imani Fooladi, A.A.; Feizabadi, M.M. New insights in to the intrinsic and acquired drug resistance mechanisms in mycobacteria. Front. Microbiol. 2017, 8, 681. [Google Scholar] [CrossRef]
- Richard, M.; Gutiérrez, A.V.; Viljoen, A.J.; Ghigo, E.; Blaise, M.; Kremer, L. Mechanistic and structural insights into the unique TetR-dependent regulation of a drug efflux pump in Mycobacterium abscessus. Front. Microbiol. 2018, 9, 649. [Google Scholar] [CrossRef]
- Salini, S.; Muralikrishnan, B.; Bhat, S.G.; Ghate, S.D.; Rao, R.S.P.; Kumar, R.A.; Kurthkoti, K. Overexpression of a membrane transport system MSMEG_1381 and MSMEG_1382 confers multidrug resistance in Mycobacterium smegmatis. Microb. Pathog. 2023, 185, 106384. [Google Scholar] [CrossRef]
- Maslov, D.A.; Shur, K.V.; Vatlin, A.A.; Danilenko, V.N. MmpS5-MmpL5 transporters provide Mycobacterium smegmatis resistance to imidazo [1,2-b][1,2,4,5]tetrazines. Pathogens 2020, 9, 166. [Google Scholar] [CrossRef]
- Stahl, C.; Kubetzko, S.; Kaps, I.; Seeber, S.; Engelhardt, H.; Niederweis, M. MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 2001, 40, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.A. Cellular impermeability and uptake of biocides and antibiotics in gram-positive bacteria and mycobacteria. Symp. Ser. Soc. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Danilchanka, O.; Pavlenok, M.; Niederweis, M. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2008, 52, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial resistance in Mycobacterium tuberculosis: Mechanistic and evolutionary perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Snapper, S.B.; Melton, R.E.; Mustafa, S.; Kieser, T.; Jacobs, W.R., Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 1911, 4, 1911–1919. [Google Scholar] [CrossRef]
- Hillemann, D.; Rüsch-Gerdes, S.; Richter, E. In vitro-selected linezolid-resistant Mycobacterium tuberculosis mutants. Antimicrob. Agents Chemother. 2008, 52, 800–801. [Google Scholar] [CrossRef]
- Yan, M.; Yan, H.; Ren, G.; Zhao, J.; Guo, X.; Sun, Y. CRISPR-Cas12a-assisted recombineering in bacteria. Appl. Environ. Microbiol. 2017, 83, e00947-17. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Sui, X.; Guo, L.; Bao, Z.; Xian, M.; Zhao, G. Efflux pumps and porins enhance bacterial tolerance to phenolic compounds by inhibiting hydroxyl radical generation. Microorganisms 2025, 13, 202. [Google Scholar] [CrossRef]
- Niederweis, M. Mycobacterial porins—New channel proteins in unique outer membranes. Mol. Microbiol. 2003, 49, 1167–1177. [Google Scholar] [CrossRef]
- Purdy, G.E.; Niederweis, M.; Russell, D.G. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol. Microbiol. 2009, 73, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Ramos, J.; Couto, I.; Amaral, L.; Viveiros, M. Ethidium bromide transport across Mycobacterium smegmatis cell-wall: Correlation with antibiotic resistance. BMC Microbiol. 2011, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Gumbo, T.; Maartens, G.; Dooley, K.E.; McNerney, R.; Murray, M.; Furin, J.; Nardell, E.A.; London, L.; Lessem, E.; et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 2017, 5, P291–P360. [Google Scholar] [CrossRef] [PubMed]
- Hameed, H.M.A.; Fang, C.; Liu, Z.; Ju, Y.; Han, X.; Gao, Y.; Wang, S.; Chiwala, G.; Tan, Y.; Guan, P.; et al. Characterization of genetic variants associated with rifampicin resistance level in Mycobacterium tuberculosis clinical isolates collected in Guangzhou Chest Hospital, China. Infect. Drug Resist. 2022, 15, 5655–5666. [Google Scholar] [CrossRef]
- Mailaender, C.; Reiling, N.; Engelhardt, H.; Bossmann, S.; Ehlers, S.; Niederweis, M. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 2004, 150, 853–864. [Google Scholar] [CrossRef]
- Sonnenkalb, L.; Carter, J.; Spitaleri, A.; Iqbal, Z.; Hunt, M.; Malone, K.; Utpatel, C.; Cirillo, D.M.; Rodrigues, C.; Nilgiriwala, K.S.; et al. Deciphering bedaquiline and clofazimine resistance in tuberculosis: An evolutionary medicine approach. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ismail, N.; Rivière, E.; Limberis, J.; Huo, S.; Metcalfe, J.Z.; Warren, R.M.; Van Rie, A. Genetic variants and their association with phenotypic resistance to bedaquiline in Mycobacterium tuberculosis: A systematic review and individual isolate data analysis. Lancet Microbe 2021, 2, e604–e616. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, J.; Zhang, S.; Zou, Y.; Zhang, Y.; Huang, D.; Zhang, Z.; Li, B.; Chu, H. Efflux pumps contribute to intrinsic clarithromycin resistance in clinical, Mycobacterium abscessus isolates. Infect. Drug Resist. 2020, 13, 447–454. [Google Scholar] [CrossRef]
- Palmer, A.C.; Kishony, R. Opposing effects of target overexpression reveal drug mechanisms. Nat. Commun. 2014, 5, 4296. [Google Scholar] [CrossRef]
- Singh, R.; Dwivedi, S.P.; Gaharwar, U.S.; Meena, R.; Rajamani, P.; Prasad, T. Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef]
- Gifford, D.R.; Berríos-Caro, E.; Joerres, C.; Suñé, M.; Forsyth, J.H.; Bhattacharyya, A.; Galla, T.; Knight, C.G. Mutators can drive the evolution of multi-resistance to antibiotics. PLoS Genet. 2023, 19, e1010791. [Google Scholar] [CrossRef] [PubMed]
- Igler, C.; Rolff, J.; Regoes, R. Multi-step vs. single-step resistance evolution under different drugs, pharmacokinetics, and treatment regimens. eLife 2021, 10, e64116. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, C.; Ortiz, A.T.; Tan, C.C.S.; Pang, J.; Acman, M.; Millard, J.; Padayatchi, N.; Grant, A.D.; O’Donnell, M.; Pym, A.; et al. Detection of a historic reservoir of bedaquiline/clofazimine resistance-associated variants in Mycobacterium tuberculosis. Genome Med. 2024, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Hartkoorn, R.C.; Uplekar, S.; Cole, S.T. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 2979–2981. [Google Scholar] [CrossRef]
- Negatu, D.A.; Aragaw, W.W.; Dartois, V.; Dick, T. Characterization of in vitro resistance to linezolid in Mycobacterium abscessus. Microbiol. Spectr. 2023, 11, e02199-23. [Google Scholar] [CrossRef]
- Niederweis, M.; Ehrt, S.; Heinz, C.; Klöcker, U.; Karosi, S.; Swiderek, K.M.; Riley, L.W.; Benz, R. Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol. Microbiol. 1999, 33, 933–945. [Google Scholar] [CrossRef]
- Siroy, A.; Mailaender, C.; Harder, D.; Koerber, S.; Wolschendorf, F.; Danilchanka, O.; Wang, Y.; Heinz, C.; Niederweis, M. Rv1698 of Mycobacterium tuberculosis represents a new class of channel-forming outer membrane proteins. J. Biol. Chem. 2008, 283, 17827–17837. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Poole, K. Interplay between the MexA-MexB-OprM multidrug efflux system and the outer membrane barrier in the multiple antibiotic resistance of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 433–436. [Google Scholar] [CrossRef]
- Lewis, J.S., II; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).