D-Serine Can Modify the Wall Teichoic Acid of MRSA via the dlt Pathway
Abstract
1. Introduction
2. Results
2.1. RNA Sequencing (RNA-Seq) Analysis
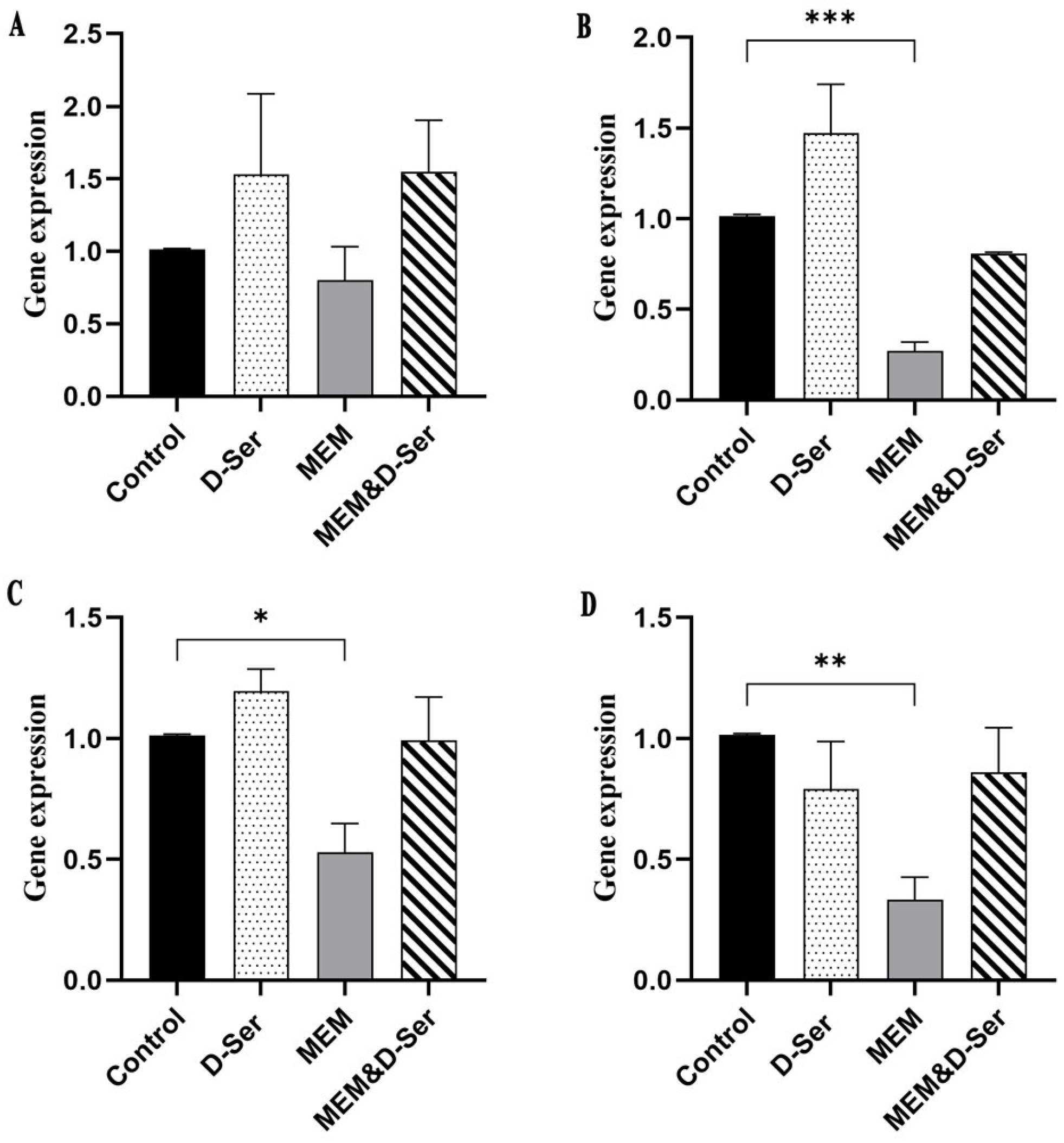
2.2. Quantitative Real-Time PCR (RT-qPCR) Result
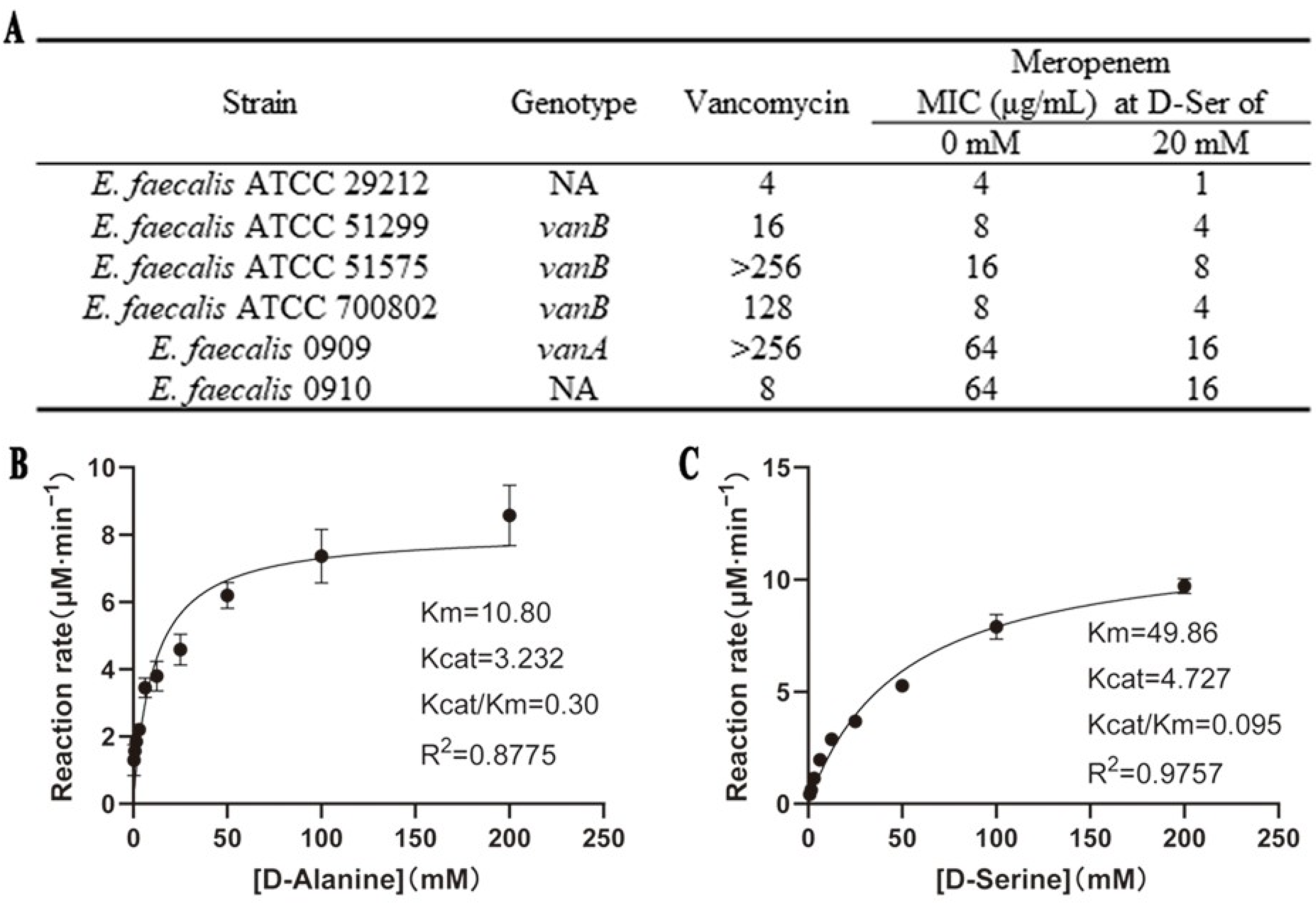
2.3. S. aureus DltA Protein Can Use D-Ser as a Substrate
2.4. D-Ser Can Sensitize E. faecalis to MEM with Effects on DltA
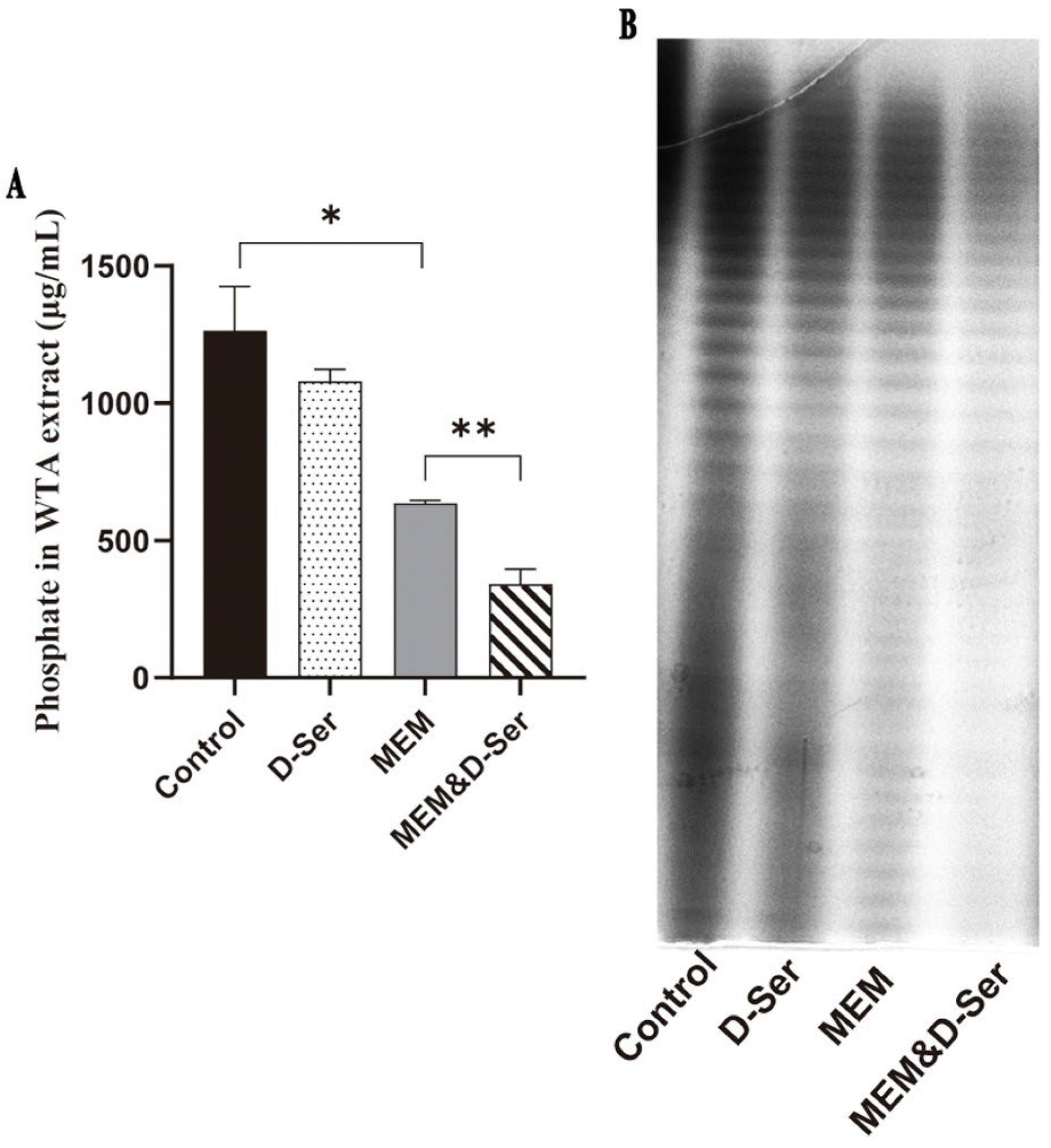
2.5. The Combination of D-Ser Can Reduce WTA Synthesis
2.6. In-Vitro Activity of D-Ser in Combination with Cationic Antimicrobial Peptides Against S. aureus
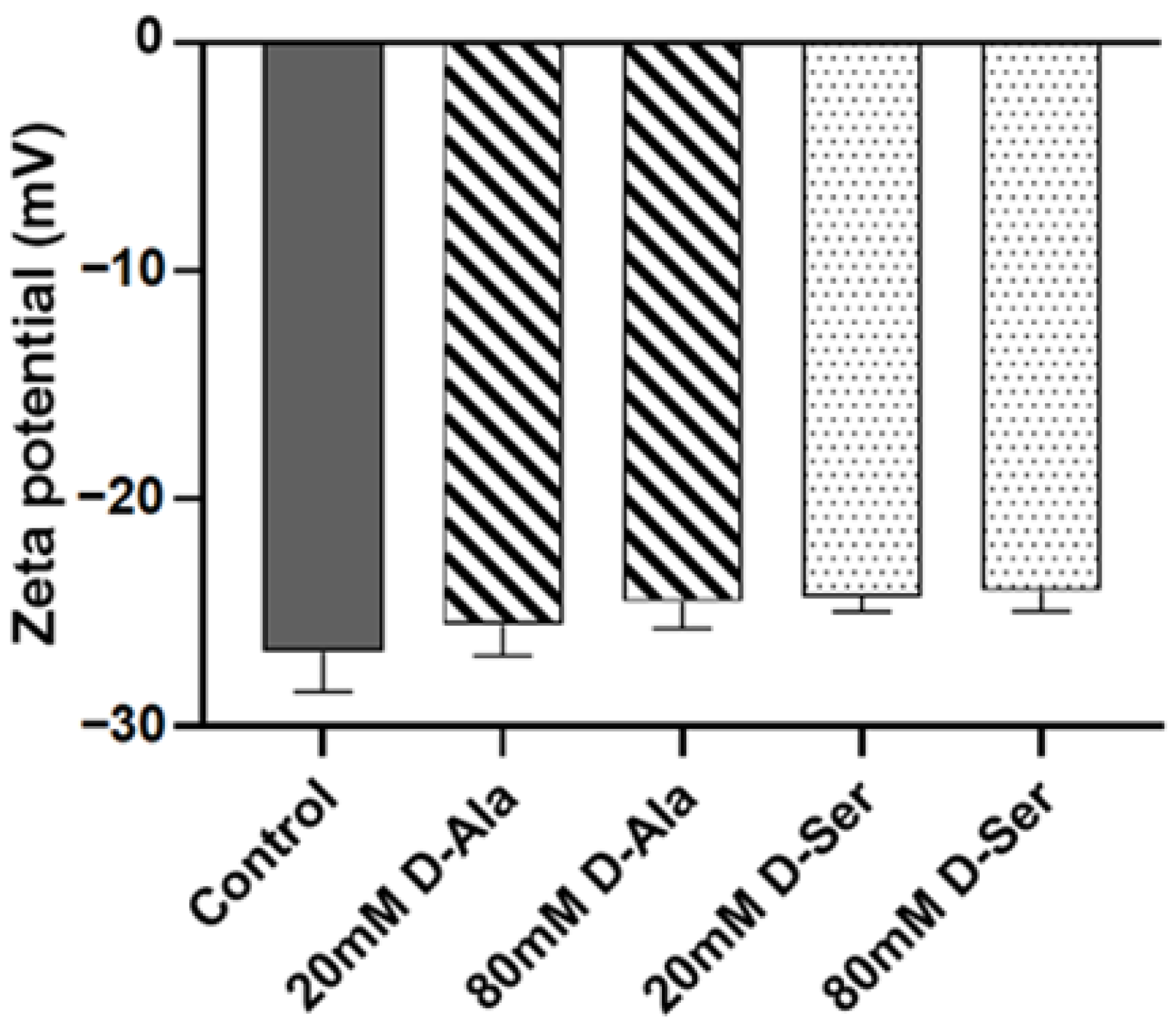
2.7. The Effect of D-Ser on the Surface Charge of MRSA N315
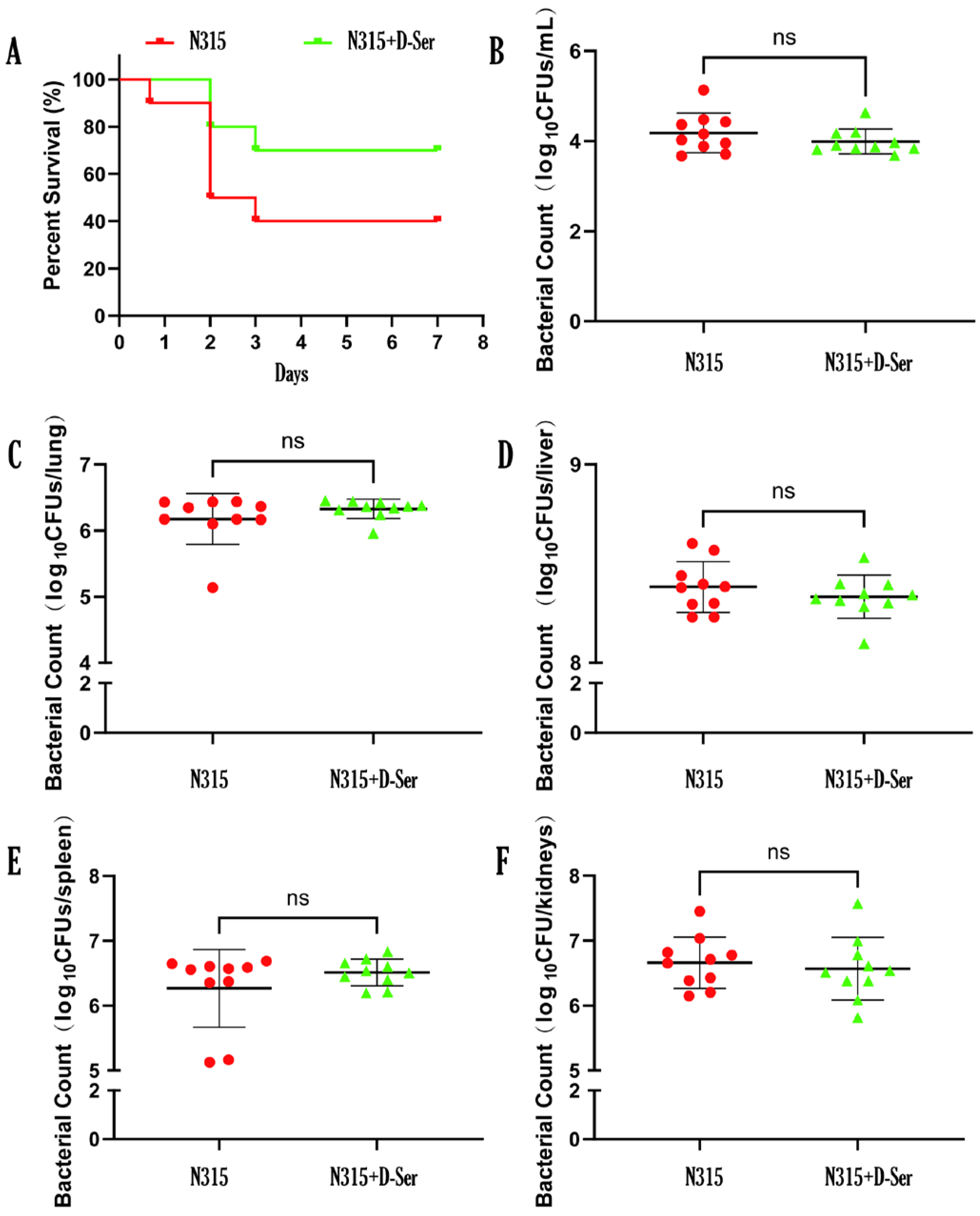
2.8. D-Ser Can Reduce the Virulence of MRSA N315 In Vivo
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media, Vectors, and Growth Parameters
4.2. RNA Extraction and RNA Sequencing (RNA-Seq)
4.3. Quantitative Real-Time PCR (RT-qPCR)
4.4. Antimicrobial Susceptibility Test
4.5. Cloning, Expression, and Purification of DltA and DltC
4.6. Enzyme Kinetics Assay
4.7. WTA Extraction and Quantification
4.8. Murine Systemic Infection Model
4.9. Zeta Potential Measurement
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAMP | Cationic antimicrobial peptide |
| D-Ser | D-Serine |
| D-Ala | D-Alanine |
| LTA | Lipoteichoic acids |
| MEM | Meropenem |
| MRSA | Methicillin-resistant S. aureus |
| WTA | Wall teichoic acid |
References
- Luo, Q.; Lu, P.; Chen, Y.; Shen, P.; Zheng, B.; Ji, J.; Ying, C.; Liu, Z.; Xiao, Y. ESKAPE in China: Epidemiology and characteristics of antibiotic resistance. Emerg. Microbes Infect. 2024, 13, 2317915. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Cava, F.; de Pedro, M.A.; Lam, H.; Davis, B.M.; Waldor, M.K. Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 2011, 30, 3442–3453. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Iwata, Y.; Sakai, N.; Yoneda, I.; Senda, Y.; Sakai-Takemori, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; et al. D-Serine inhibits the attachment and biofilm formation of methicillin-resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2021, 537, 50–56. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-positive bacterial cell wall. Microbiol Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Naumova, I.B.; Shashkov, A.S.; Tul’skaya, E.M.; Streshinskaya, G.M.; Kozlova, Y.I.; Potekhina, N.V.; Evtushenko, L.I.; Stackebrandt, E. Cell wall teichoic acids: Structural diversity, species specificity in the genus Nocardiopsis, and chemotaxonomic perspective. FEMS Microbiol. Rev. 2001, 25, 269–284. [Google Scholar] [CrossRef]
- van Dalen, R.; Peschel, A.; van Sorge, N.M. Wall teichoic acid in Staphylococcus aureus host interaction. Trends Microbiol. 2020, 28, 985–998. [Google Scholar] [CrossRef]
- Ersoy, S.C.; Proctor, R.A.; Rose, W.E.; Abdelhady, W.; Fan, S.H.; Madrigal, S.L.; Elsayed, A.M.; Chambers, H.F.; Sobral, R.G.; Bayer, A.S. Sensitizing methicillin-resistant Staphylococcus aureus (MRSA) to cefuroxime: The synergic effect of bicarbonate and the wall teichoic acid inhibitor ticlopidine. Antimicrob. Agents Chemother. 2024, 68, e0162723. [Google Scholar] [CrossRef]
- Hendriks, A.; Kerkman, P.F.; Varkila, M.R.J.; Haitsma Mulier, J.L.G.; Ali, S.; Ten Doesschate, T.; van der Vaart, T.W.; de Haas, C.J.C.; Aerts, P.C.; Cremer, O.L.; et al. Glycan-specific IgM is critical for human immunity to Staphylococcus aureus. Cell Rep. Med. 2024, 5, 101734. [Google Scholar] [CrossRef]
- Tamminga, S.M.; Völpel, S.L.; Schipper, K.; Stehle, T.; Pannekoek, Y.; van Sorge, N.M. Genetic diversity of Staphylococcus aureus wall teichoic acid glycosyltransferases affects immune recognition. Microb. Genom. 2022, 8, 000902. [Google Scholar] [CrossRef]
- Li, F.K.K.; Worrall, L.J.; Gale, R.T.; Brown, E.D.; Strynadka, N.C.J. Cryo-EM analysis of S. aureus TarL, a polymerase in wall teichoic acid biogenesis central to virulence and antibiotic resistance. Sci. Adv. 2024, 10, eadj3864. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, F.; Zhao, Q.; Cao, Q.; Chen, R.; Pan, H.; Wang, Y.; Huang, H.; Huang, R.; Liu, Q.; et al. Modulation of MRSA virulence gene expression by the wall teichoic acid enzyme TarO. Nat. Commun. 2023, 14, 1594. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Coupri, D.; Budin-Verneuil, A.; Hartke, A.; Benachour, A.; Léger, L.; Lequeux, T.; Pfund, E.; Verneuil, N. Genetic and pharmacological inactivation of D-alanylation of teichoic acids sensitizes pathogenic enterococci to β-lactams. J. Antimicrob. Chemother. 2019, 74, 3162–3169. [Google Scholar] [CrossRef]
- Lacotte, P.A.; Denis-Quanquin, S.; Chatonnat, E.; Le Bris, J.; Leparfait, D.; Lequeux, T.; Martin-Verstraete, I.; Candela, T. The absence of surface D-alanylation, localized on lipoteichoic acid, impacts the Clostridioides difficile way of life and antibiotic resistance. Front. Microbiol. 2023, 14, 1267662. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, Y.; Pang, J.; Li, X.; Lu, X.; Wang, X.; Hu, X.; Nie, T.; Yang, X.; Xiong, Y.Q.; et al. In vitro and in vivo activity of D-Serine in combination with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Acta Pharm. Sin. B 2019, 9, 496–504. [Google Scholar] [CrossRef]
- Shockman, G.D.; Barrett, J.F. Structure, function, and assembly of cell walls of Gram-positive bacteria. Annu. Rev. Microbiol. 1983, 37, 501–527. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Pollack, J.H.; Neuhaus, F.C. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J. Bacteriol. 1994, 176, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.; Meredith, T.C. Extraction and analysis of bacterial teichoic acids. Bio-protocol 2018, 8, e3078. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.V.; Kristian, S.A.; Weidenmaier, C.; Faigle, M.; Van Kessel, K.P.; Van Strijp, J.A.; Götz, F.; Neumeister, B.; Peschel, A. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 2002, 186, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhi, Z.; Wong, W.L.; Yuan, W.; Sun, N. Understanding the synergistic sensitization of natural products and antibiotics: An effective strategy to combat MRSA. Eur. J. Med. Chem. 2025, 281, 117012. [Google Scholar] [CrossRef]
- Tabashsum, Z.; Angeles-Solano, M.; Sidders, A.E.; Parsons, J.B.; Rowe, S.E. Palmitoleic acid sensitizes vancomycin-resistant Staphylococcus aureus to vancomycin by outpacing the expression of resistance genes. Microbiol. Spectr. 2024, 13, e0199624. [Google Scholar] [CrossRef]
- Gaudreau, A.; Watson, D.W.; Flannagan, R.S.; Roy, P.; Shen, C.; Abdelmoneim, A.; Beavers, W.N.; Gillies, E.R.; El-Halfawy, O.M.; Heinrichs, D.E. Mechanistic insights and in vivo efficacy of thiosemicarbazones against methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2024, 300, 107689. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synergistic antibacterial effects of exopolysaccharides/nickel-nanoparticles composites against multidrug-resistant bacteria. Sci. Rep. 2023, 13, 21519. [Google Scholar] [CrossRef]
- Lee, I.G.; Song, C.; Yang, S.; Jeon, H.; Park, J.; Yoon, H.J.; Im, H.; Kang, S.M.; Eun, H.J.; Lee, B.J. Structural and functional analysis of the D-alanyl carrier protein ligase DltA from Staphylococcus aureus Mu50. Acta Crystallogr. Sect. D Struct. Biol. 2022, 78, 424–434. [Google Scholar] [CrossRef]
- Heaton, M.P.; Neuhaus, F.C. Role of the D-alanyl carrier protein in the biosynthesis of D-alanyl-lipoteichoic acid. J. Bacteriol. 1994, 176, 681–690. [Google Scholar] [CrossRef]
- Perego, M.; Glaser, P.; Minutello, A.; Strauch, M.A.; Leopold, K.; Fischer, W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 1995, 270, 15598–15606. [Google Scholar] [CrossRef]
- Wood, B.M.; Santa Maria, J.P., Jr.; Matano, L.M.; Vickery, C.R.; Walker, S. A partial reconstitution implicates DltD in catalyzing lipoteichoic acid D-alanylation. J. Biol. Chem. 2018, 293, 17985–17996. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, X.; Zhao, X.; Li, P.; Gu, Q. Insight into the structure, biosynthesis, isolation method and biological function of teichoic acid in different Gram-positive microorganisms: A review. Int. J. Biol. Macromol. 2023, 253, 126825. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Meng, Q.; Cheng, D.; Fu, J.; Luo, Q.; Liu, Y.; Yu, Z. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 3771–3780. [Google Scholar] [CrossRef]
- Hyyryläinen, H.-L.; Vitikainen, M.; Thwaite, J.; Wu, H.; Sarvas, M.; Harwood, C.R.; Kontinen, V.P.; Stephenson, K. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 2000, 275, 26696–26703. [Google Scholar] [CrossRef]
- Rawlinson, L.B.; O’Gara, J.P.; Jones, D.S.; Brayden, D.J. Resistance of Staphylococcus aureus to the cationic antimicrobial agent poly(2-(dimethylamino ethyl)methacrylate) (pDMAEMA) is influenced by cell-surface charge and hydrophobicity. J. Med. Microbiol. 2011, 60, 968–976. [Google Scholar] [CrossRef]
- Bertsche, U.; Weidenmaier, C.; Kuehner, D.; Yang, S.J.; Baur, S.; Wanner, S.; Francois, P.; Schrenzel, J.; Yeaman, M.R.; Bayer, A.S. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and D-alanylation. Antimicrob. Agents Chemother. 2011, 55, 3922–3928. [Google Scholar] [CrossRef]
- Bertsche, U.; Yang, S.J.; Kuehner, D.; Wanner, S.; Mishra, N.N.; Roth, T.; Nega, M.; Schneider, A.; Mayer, C.; Grau, T.; et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS ONE 2013, 8, e67398. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Brignoli, T.; Douglas, E.; Duggan, S.; Fagunloye, O.G.; Adhikari, R.; Aman, M.J.; Massey, R.C. Wall teichoic acids facilitate the release of toxins from the surface of Staphylococcus aureus. Microbiol. Spectr. 2022, 10, e0101122. [Google Scholar] [CrossRef]
- Wu, X.; Han, J.; Gong, G.; Koffas, M.A.G.; Zha, J. Wall teichoic acids: Physiology and applications. FEMS Microbiol. Rev. 2021, 45, fuaa064. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, S.; Wu, M.; Chen, S.; Zhou, W.; Zhan, L.; Huang, X. Dlt operon regulates physiological function and cariogenic virulence in Streptococcus mutans. Future Microbiol. 2023, 18, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Ruiz-Bustos, E.; Courtney, H.S.; Dale, J.B.; Pence, M.A.; Nizet, V.; Aziz, R.K.; Gerling, I.; Price, S.M.; Hasty, D.L. Inactivation of DltA modulates virulence factor expression in Streptococcus pyogenes. PLoS ONE 2009, 4, e5366. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Kang, S.; Chang, J.; Tian, X.; Shi, C. Correlation analysis between GlpQ-regulated degradation of wall teichoic acid and biofilm formation triggered by lactobionic acid in Staphylococcus aureus. Foods 2022, 11, 3438. [Google Scholar] [CrossRef]
- McQuade, T.J.; Shallop, A.D.; Sheoran, A.; Delproposto, J.E.; Tsodikov, O.V.; Garneau-Tsodikova, S. A nonradioactive high-throughput assay for screening and characterization of adenylation domains for nonribosomal peptide combinatorial biosynthesis. Anal. Biochem. 2009, 386, 244–250. [Google Scholar] [CrossRef]
- Du, L.; Luo, Y. Thiolation-enhanced substrate recognition by D-alanyl carrier protein ligase DltA from Bacillus cereus. F1000Research 2014, 3, 106. [Google Scholar] [CrossRef]
- Xia, S.; Ma, Y.; Zhang, W.; Yang, Y.; Wu, S.; Zhu, M.; Deng, L.; Li, B.; Liu, Z.; Qi, C. Identification of Sare0718 as an alanine-activating adenylation domain in marine actinomycete Salinispora arenicola CNS-205. PLoS ONE 2012, 7, e37487. [Google Scholar] [CrossRef]
- May, J.J.; Finking, R.; Wiegeshoff, F.; Weber, T.T.; Bandur, N.; Koert, U.; Marahiel, M.A. Inhibition of the D-alanine:D-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium’s susceptibility to antibiotics that target the cell wall. FEBS J. 2005, 272, 2993–3003. [Google Scholar] [CrossRef]
- Chen, P.S.; Toribara, T.Y.; Warner, H. Microdetermination of phosphorus. Anal. Chem. 1956, 28, 1756–1758. [Google Scholar] [CrossRef]

| KEGG ID | Description | Padj | Gene name |
|---|---|---|---|
| sau01503 | Cationic antimicrobial peptide (CAMP) resistance | 0.02 | dltB/dltA/dltC/dltD |
| sau00340 | Histidine metabolism | 0.026 | hutU/hutG/hutI/hisG |
| sau02020 | Two-component system | 0.026 | lrgA/lrgB/uhpT/vraE/dltB/SA_RS00475/dltA/dltC/dltD |
| sau02060 | Phosphotransferase system (PTS) | 0.026 | SA_RS01835/SA_RS01830/SA_RS01840/SA_RS11260/SA_RS01110 |
| sau00552 | Teichoic acid biosynthesis | 0.130 | dltB/dltA/dltC/dltD |
| sau05150 | Staphylococcus aureus infection | 0.299 | sdrC/dltB/dltA/dltC/dltD |
| sau00330 | Arginine and proline metabolism | 0.349 | SA_RS08935/rocF |
| sau00470 | D-Amino acid metabolism | 0.497 | dltA/dltC |
| sau00561 | Glycerolipid metabolism | 0.497 | dhaL/dhaK |
| Gene Name | MEM vs. Control | D-Ser vs. Control | MEM & D-Ser vs. Control | MEM & D-Ser + vs. MEM | ||||
|---|---|---|---|---|---|---|---|---|
| Log2FC | Padj | Log2FC | Padj | Log2FC | Padj | Log2FC | Padj | |
| dltA | −0.541 | <0.01 | 0.578 | 0.211 | 0.578 | <0.05 | 1.129 | <0.001 |
| dltB | −0.798 | <0.001 | 0.517 | 0.299 | 0.220 | 0.478 | 1.040 | <0.001 |
| dltC | −1.059 | <0.001 | 0.480 | 0.469 | 0.227 | 0.546 | 1.301 | <0.001 |
| dltD | −1.093 | <0.001 | 0.458 | 0.448 | 0.245 | 0.467 | 1.343 | <0.001 |
| Strain | MIC (μg/mL) of Polypeptide at D-Ser of | |||||||
|---|---|---|---|---|---|---|---|---|
| Vancomycin | Daptomycin | Colistin | Polymyxin B | |||||
| 0 mM | 20 mM | 0 mM | 20 mM | 0 mM | 20 mM | 0 mM | 20 mM | |
| MSSA ATCC 29213 | 0.5 | 0.5 | 1 | 0.5 | 512 | 256 | 128 | 64 |
| MSSA12-02 | 1 | 1 | 1 | 1 | 1024 | 256 | 128 | 32 |
| MRSA 08-50 | 1 | 0.5 | 1 | 1 | 512 | 128 | 128 | 32 |
| MRSA N315 | 0.5 | 0.5 | 1 | 1 | 1024 | 256 | 128 | 64 |
| MRSA ATCC 43300 | 1 | 1 | 1 | 1 | >1024 | 256 | 256 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xie, J.; Wang, Q.; Wang, P.; Hu, X.; Nie, T.; Hou, L.; Yang, X.; Wang, X.; You, X.; et al. D-Serine Can Modify the Wall Teichoic Acid of MRSA via the dlt Pathway. Int. J. Mol. Sci. 2025, 26, 4110. https://doi.org/10.3390/ijms26094110
Wang L, Xie J, Wang Q, Wang P, Hu X, Nie T, Hou L, Yang X, Wang X, You X, et al. D-Serine Can Modify the Wall Teichoic Acid of MRSA via the dlt Pathway. International Journal of Molecular Sciences. 2025; 26(9):4110. https://doi.org/10.3390/ijms26094110
Chicago/Turabian StyleWang, Lei, Jinru Xie, Qing Wang, Penghe Wang, Xinxin Hu, Tongying Nie, Lei Hou, Xinyi Yang, Xiukun Wang, Xuefu You, and et al. 2025. "D-Serine Can Modify the Wall Teichoic Acid of MRSA via the dlt Pathway" International Journal of Molecular Sciences 26, no. 9: 4110. https://doi.org/10.3390/ijms26094110
APA StyleWang, L., Xie, J., Wang, Q., Wang, P., Hu, X., Nie, T., Hou, L., Yang, X., Wang, X., You, X., & Li, C. (2025). D-Serine Can Modify the Wall Teichoic Acid of MRSA via the dlt Pathway. International Journal of Molecular Sciences, 26(9), 4110. https://doi.org/10.3390/ijms26094110






