Cloning and Functional Analysis of ZFP5 from Amorpha fruticosa for Enhancing Drought and Saline–Alkali Resistance in Tobacco
Abstract
1. Introduction
2. Results
2.1. Cloning and Bioinformatics Analysis of the AfZFP5
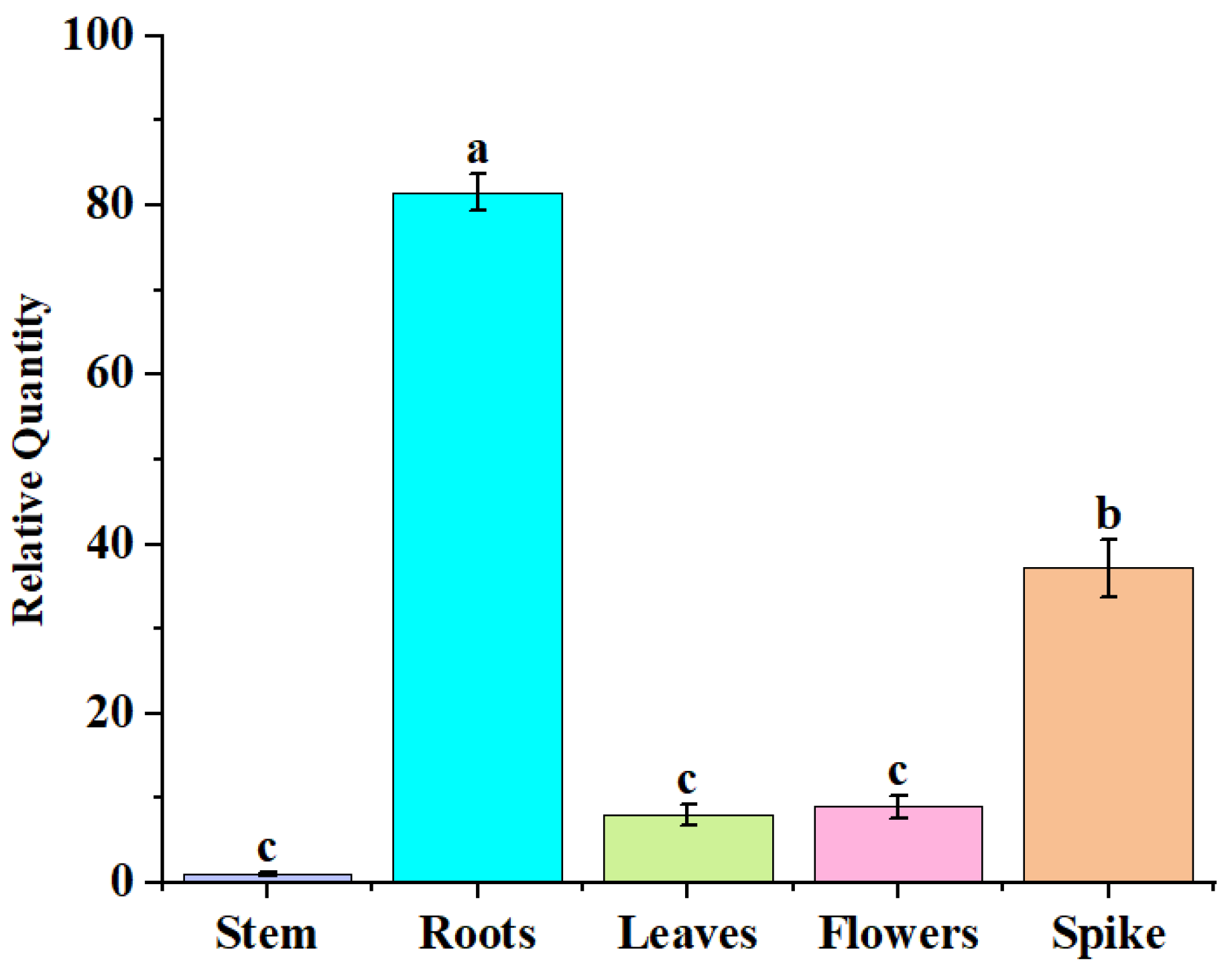
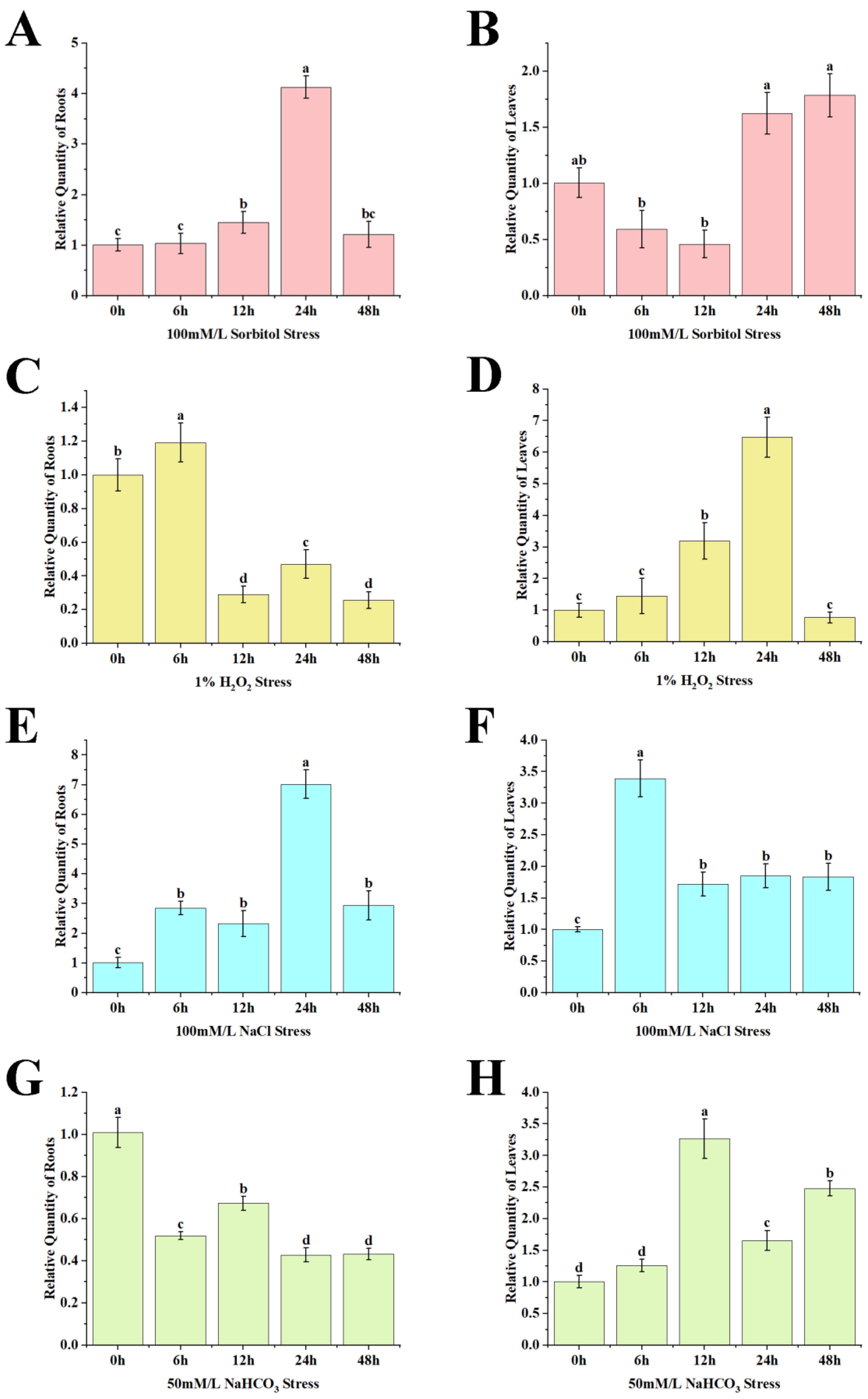
2.2. Analysis of AfZFP5 Expression Characteristics
2.3. Overexpression of AfZFP5 in Tobacco
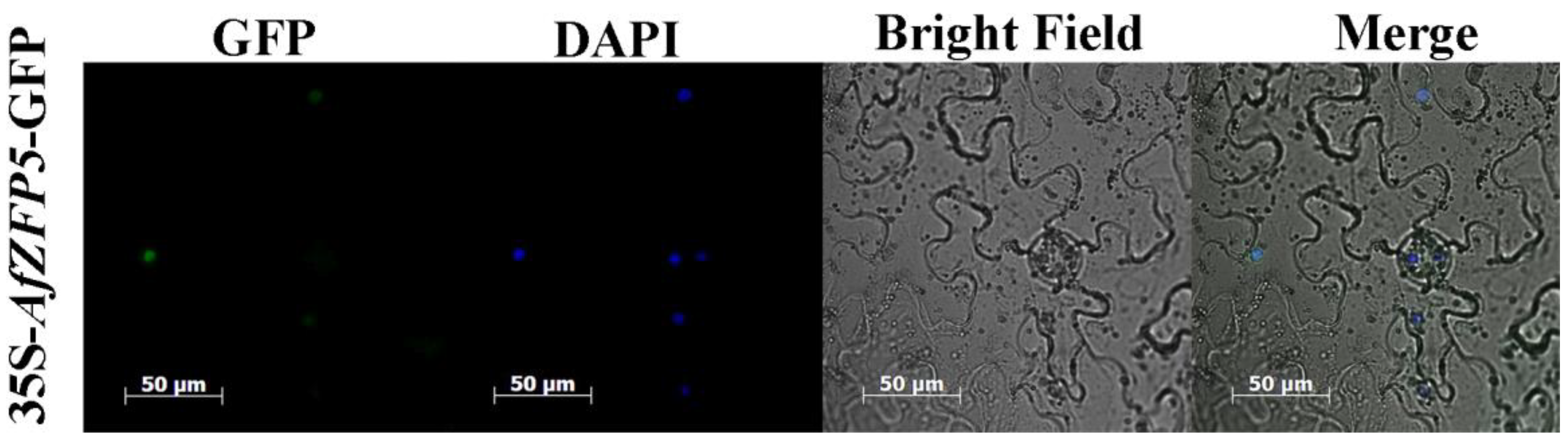
2.4. Subcellular Localization of AfZFP5 Gene-Encoded Protein
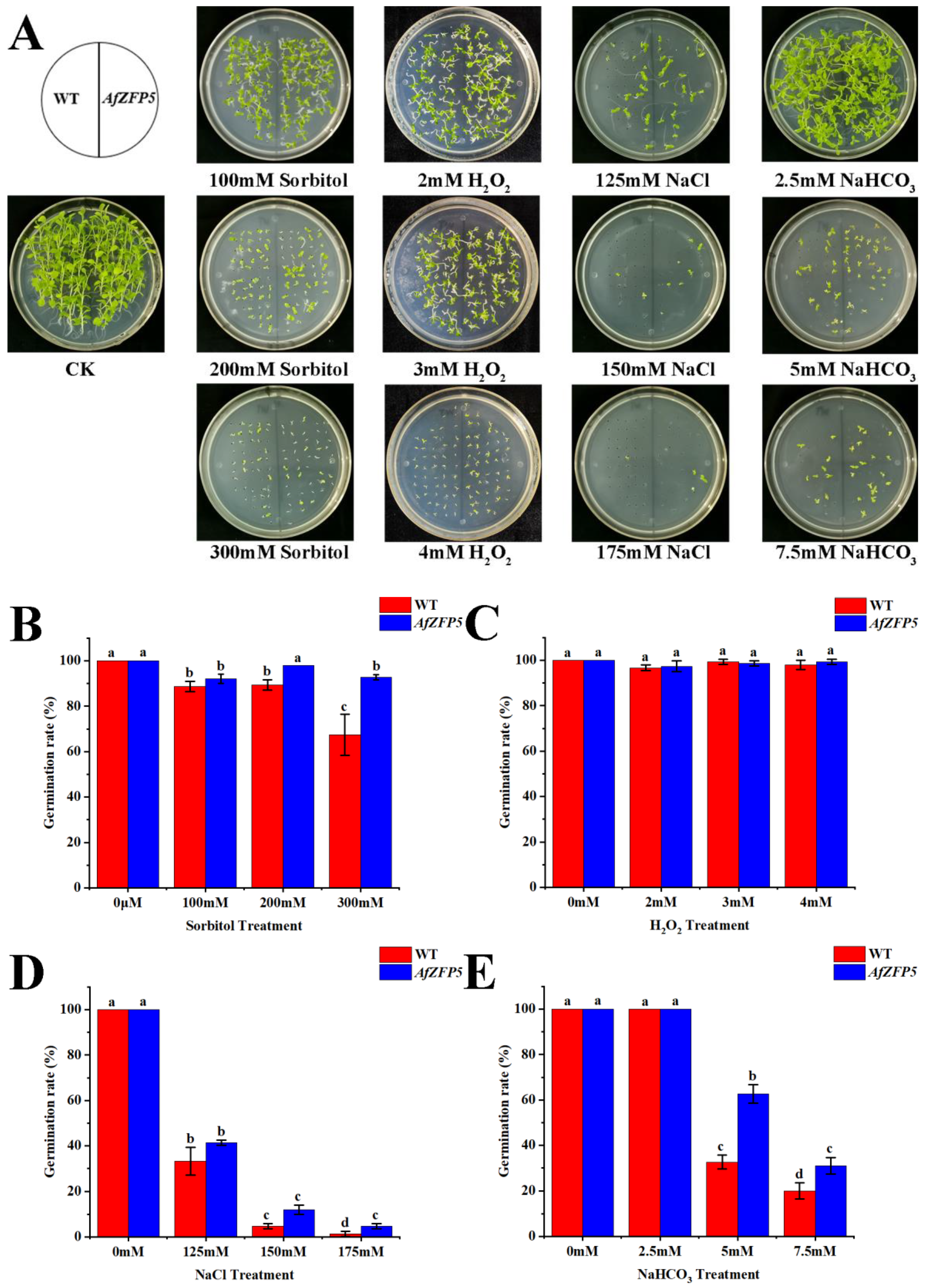
2.5. Tolerance of AfZFP5 Transgenic Tobacco to Drought and Salt–Alkali Stress During Germination
2.6. Tolerance of AfZFP5 Transgenic Tobacco to Drought and Salt–Alkali Stress During Seedling Stage
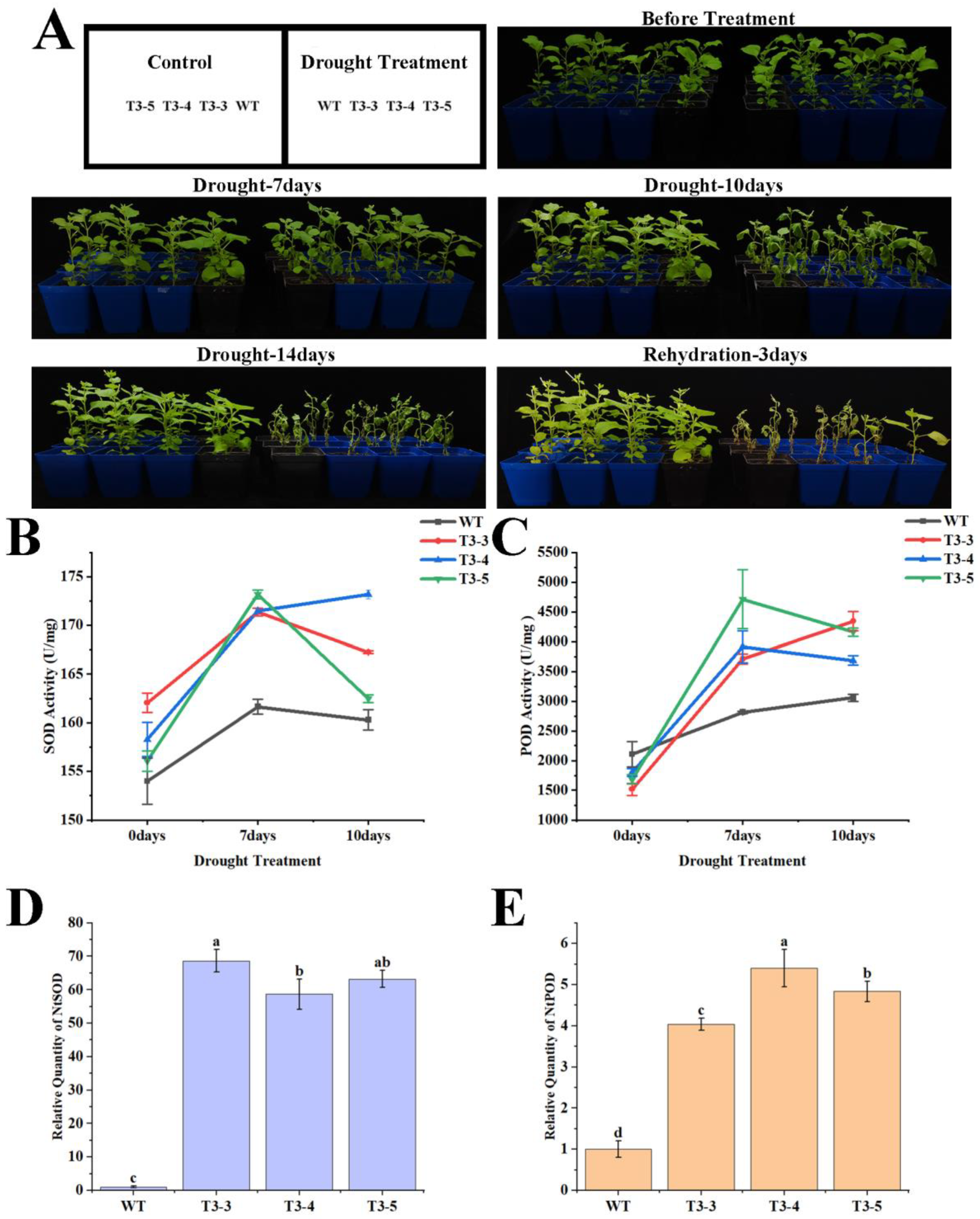
2.7. Tolerance of AfZFP5 Transgenic Tobacco to Drought
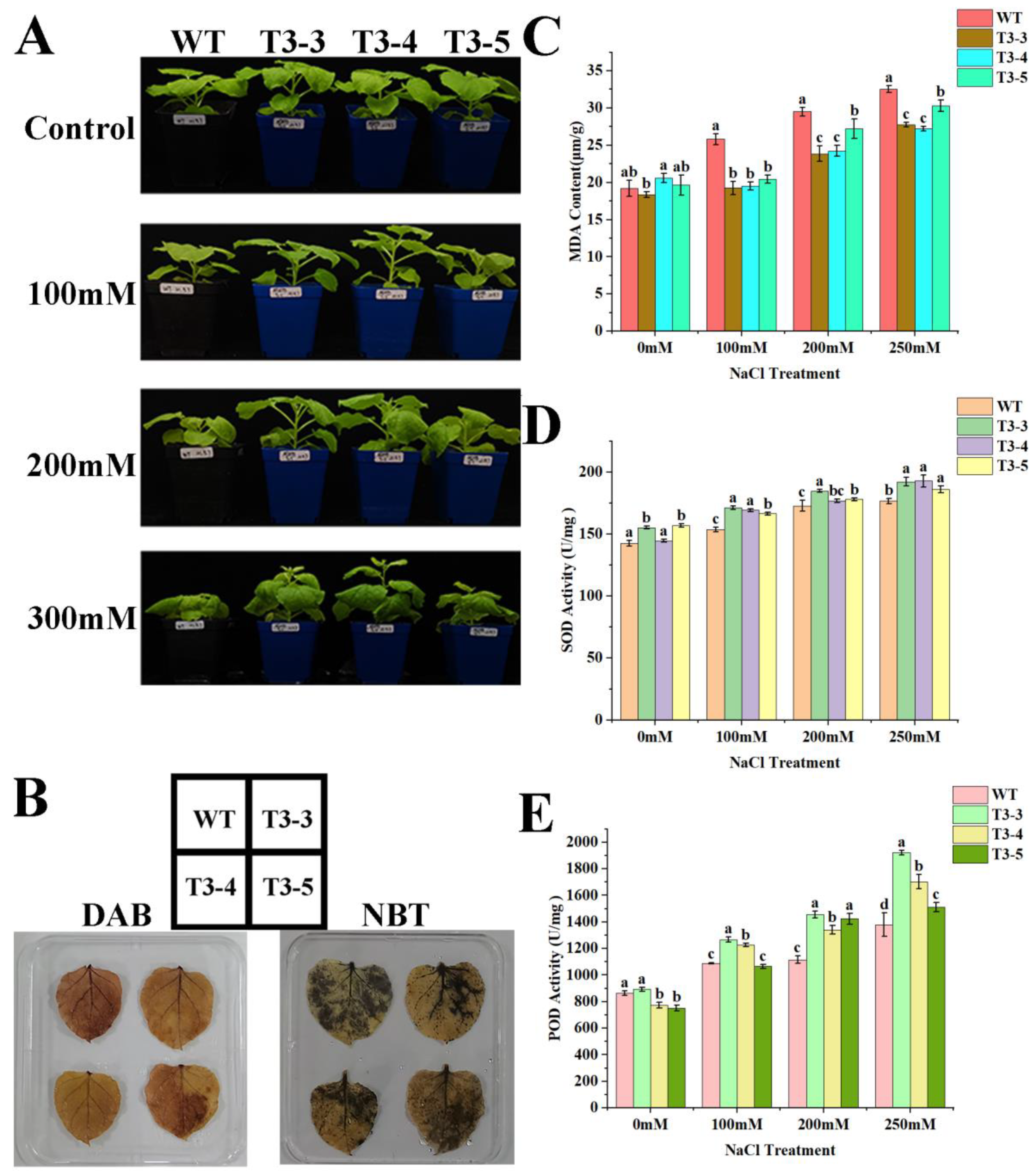
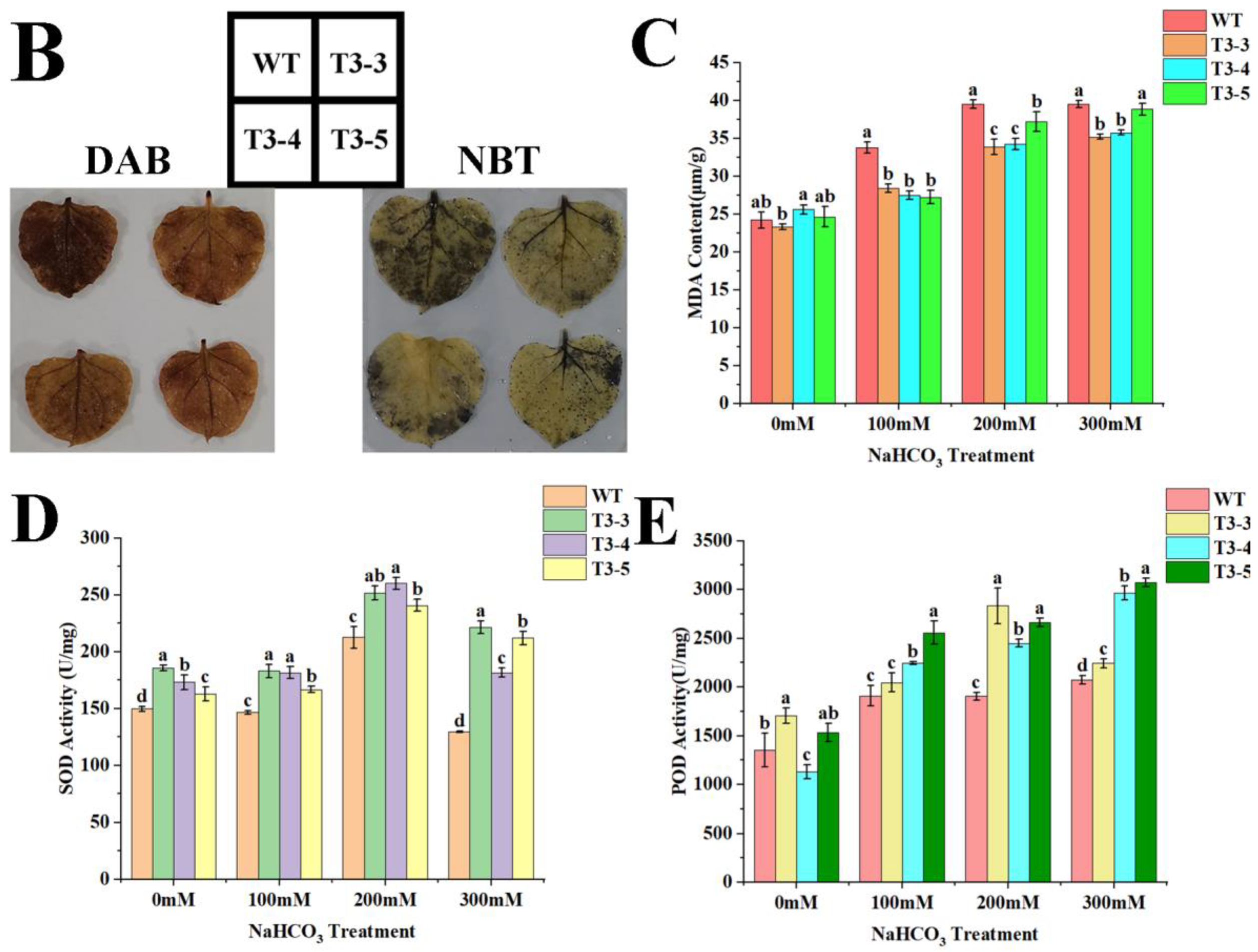
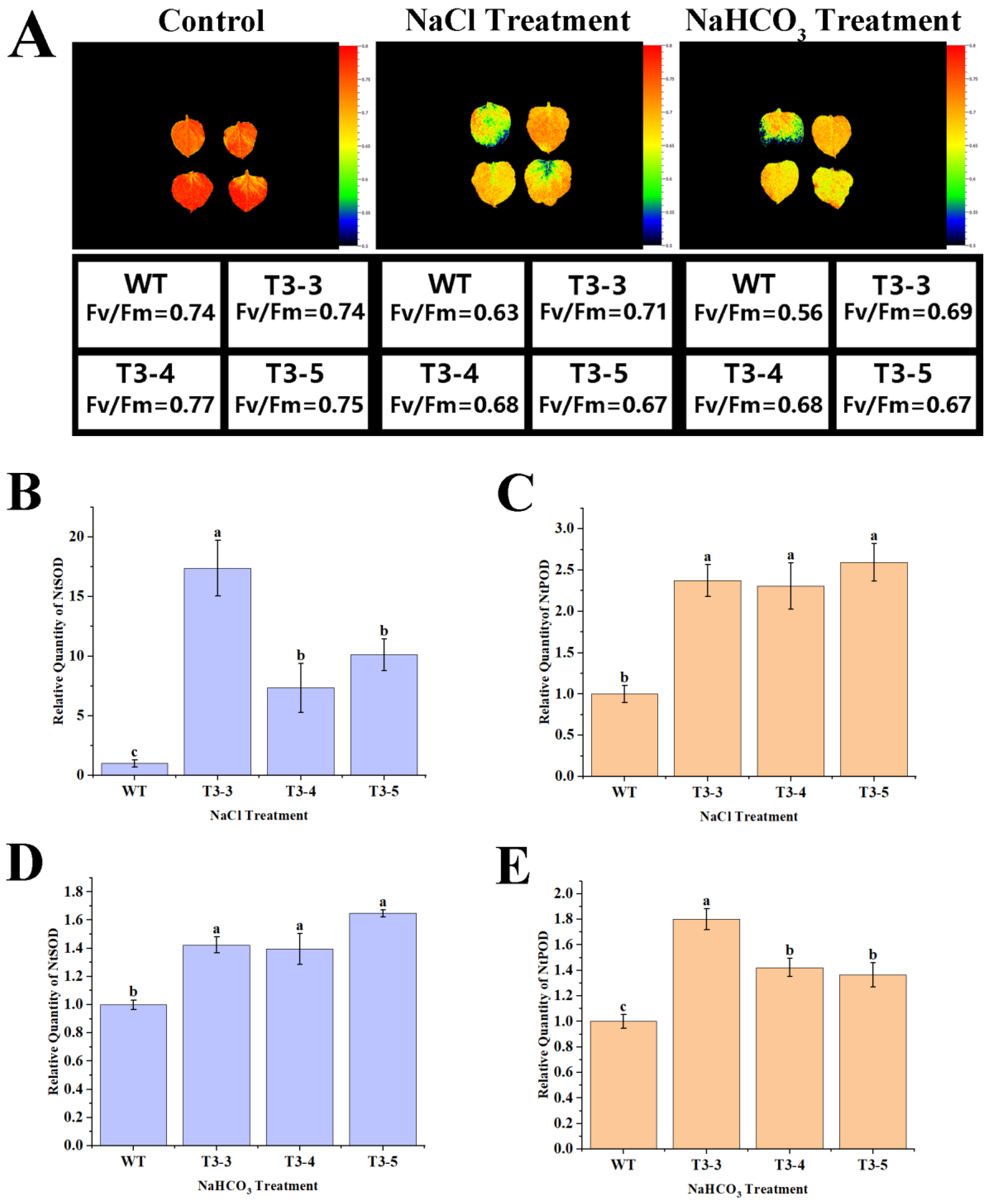
2.8. Tolerance of AfZFP5 Transgenic Tobacco to Salt–Alkali Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Strain, Vector, and Reagents
4.3. Cloning and Bioinformatics Analysis of AfZFP5
4.4. Expression Characteristics of AfZFP5
4.5. Subcellular Localization Analysis of AfZFP5
4.6. Acquisition of Transgenic Tobacco
4.7. Analysis of Drought and Salt–Alkali Stress Resistance in Tobacco Overexpressing AfZFP5
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sheng, L.; Sun, X.; Mo, C.; Hao, M.; Wei, X.; Ma, A. Relationship between antioxidant enzymes and sclerotial formation of Pleurotus tuber-regium under abiotic stress. Appl. Microbiol. Biotechnol. 2023, 107, 1391–1404. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Fang, Y.; Gao, Y.; Yang, S.; Su, J.; Ni, J.; Teng, Y.; Bai, S. Phosphorylated transcription factor PuHB40 mediates ROS-dependent anthocyanin biosynthesis in pear exposed to high light. Plant Cell 2024, 36, 3562–3583. [Google Scholar] [CrossRef]
- Guo, Z.; Dzinyela, R.; Yang, L.; Hwarari, D. bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement. Plants 2024, 13, 2058. [Google Scholar] [CrossRef]
- Li, D.; Gu, B.; Huang, C.; Shen, J.; Wang, X.; Guo, J.; Yu, R.; Mou, S.; Guan, Q. Functional Study of Amorpha fruticosa WRKY20 Gene in Response to Drought Stress. Int. J. Mol. Sci. 2023, 24, 12231. [Google Scholar] [CrossRef]
- Nikraftar, S.; Ebrahimzadegan, R.; Majdi, M.; Mirzaghaderi, G. Genome-wide analysis of the C2H2-ZFP gene family in Stevia rebaudiana reveals involvement in abiotic stress response. Sci. Rep. 2024, 14, 6164. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Fan, Y.; Zheng, C.; Yang, H.; Feng, L.; Chen, X.; Yang, Y.; Yao, X.; Weng, W.; Kong, L.; et al. bHLH transcription factor family identification, phylogeny, and its response to abiotic stress in Chenopodium quinoa. Front. Plant Sci. 2023, 14, 1171518. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Cai, J.; Bi, Y.; Li, D.; Song, F. Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol. 2019, 19, 278. [Google Scholar] [CrossRef]
- Ciftci-Yilmaz, S.; Mittler, R. The zinc finger network of plants. Cell. Mol. Life Sci. 2008, 65, 1150–1160. [Google Scholar] [CrossRef]
- Li, H.; Sheng, R.C.; Zhang, C.N.; Wang, L.C.; Li, M.; Wang, Y.H.; Qiao, Y.H.; Klosterman, S.J.; Chen, J.Y.; Kong, Z.Q.; et al. Two zinc finger proteins, VdZFP1 and VdZFP2, interact with VdCmr1 to promote melanized microsclerotia development and stress tolerance in Verticillium dahliae. BMC Biol. 2023, 21, 237. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Ngea, G.L.N.; Godana, E.A.; Ackah, M.; Dhanasekaran, S.; Zhang, Y.; Su, Y.; Yang, Q.; Zhang, H.; et al. Recent advances in the multifaceted functions of Cys2/His2-type zinc finger proteins in plant growth, development, and stress responses. J. Exp. Bot. 2024, 75, 5501–5520. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, H.; Wu, J.C.; Dai, G.X.; Zheng, H.P.; Liu, C.; Wang, Y.; Zhou, Z.K.; Tang, D.Y.; Deng, G.F.; et al. The zinc finger protein DHHC09 S-acylates the kinase STRK1 to regulate H2O2 homeostasis and promote salt tolerance in rice. Plant Cell 2024, 36, 919–940. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, W.; Wang, M.; Khan, M.; Liu, J.H. A C2H2-type zinc finger protein ZAT12 of Poncirus trifoliata acts downstream of CBF1 to regulate cold tolerance. Plant J. 2024, 117, 1317–1329. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, H.; Liang, L.; Zhang, Y.; Yang, H.; Li, W.; Choi, J.; Huang, J.; Deng, S. A C2H2-Type Zinc-Finger Protein from Millettia pinnata, MpZFP1, Enhances Salt Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10832. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tong, W.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 243, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Hovaneț, M.V.; Marinaș, I.C.; Dinu, M.; Oprea, E.; Chifiriuc, M.C.; Stavropoulou, E.; Lazǎr, V. The phytotoxicity and antimicrobial activity of Amorpha fruticosa L. leaves extract. Rom. Biotechnol. Lett. 2015, 20, 10670–10678. [Google Scholar]
- Muharini, R.; Díaz, A.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Rehberg, N.; Kalscheuer, R.; Hartmann, R.; Orfali, R.S.; Lin, W.; et al. Antibacterial and Cytotoxic Phenolic Metabolites from the Fruits of Amorpha fruticosa. J. Nat. Prod. 2017, 80, 169–180. [Google Scholar] [CrossRef]
- DeHaan, L.R.; Ehlke, N.J.; Sheaffer, C.C.; Wyse, D.L.; DeHaan, R.L. Evaluation of Diversity among North American Accessions of False Indigo (Amorpha fruticosa L.) for Forage and Biomass. Genet. Resour. Crop Evol. 2006, 53, 1463–1476. [Google Scholar] [CrossRef]
- Grabic, J.; Ljevnaic-Masic, B.; Zhan, A.; Benka, P.; Heilmeier, H. A review on invasive false indigo bush (Amorpha fruticosa L.): Nuisance plant with multiple benefits. Ecol. Evol. 2022, 12, e9290. [Google Scholar] [CrossRef]
- Cao, Q.; Li, J.; Xiao, H.; Cao, Y.; Xin, Z.; Yang, B.; Liu, T.; Yuan, M. Sap flow of Amorpha fruticosa: Implications of water use strategy in a semiarid system with secondary salinization. Sci. Rep. 2020, 10, 13504. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Liu, C.; Zhu, F.; Wang, K.; Wang, Z.; Li, X.; Lan, X.; Guan, Q. Drought resistance of tobacco overexpressing the AfNAC1 gene of Amorpha fruticosa Linn. Front. Plant Sci. 2022, 13, 980171. [Google Scholar] [CrossRef]
- González, E.M. Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2023, 24, 6562. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Liu, K.; Hou, Q.; Yu, R.; Deng, H.; Shen, L.; Wang, Q.; Wen, X. Genome-wide analysis of C2H2 zinc finger family and their response to abiotic stresses in apple. Gene 2024, 904, 148164. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.J.; Ma, H.Y.; Wang, Z.J.; Wang, Z.Y.; Bu, Q.Y.; Liu, S.K. A rice LSD1-like-type ZFP gene OsLOL5 enhances saline-alkaline tolerance in transgenic Arabidopsis thaliana, yeast and rice. BMC Genom. 2016, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis Drought Stress Response Using Kinetic Chlorophyll Fluorescence and Multicolor Fluorescence Imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef]
- Guan, Q.; Liao, X.; He, M.; Li, X.; Wang, Z.; Ma, H.; Yu, S.; Liu, S. Tolerance analysis of chloroplast OsCu/Zn-SOD overexpressing rice under NaCl and NaHCO3 stress. PLoS ONE 2017, 12, e0186052. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, Y.; Yang, L.; Wang, B.; Gao, X.; Huang, S.; Li, X.; Yang, C.; Guan, Q. Cloning and Functional Analysis of ZFP5 from Amorpha fruticosa for Enhancing Drought and Saline–Alkali Resistance in Tobacco. Int. J. Mol. Sci. 2025, 26, 3792. https://doi.org/10.3390/ijms26083792
Liu Z, Yang Y, Yang L, Wang B, Gao X, Huang S, Li X, Yang C, Guan Q. Cloning and Functional Analysis of ZFP5 from Amorpha fruticosa for Enhancing Drought and Saline–Alkali Resistance in Tobacco. International Journal of Molecular Sciences. 2025; 26(8):3792. https://doi.org/10.3390/ijms26083792
Chicago/Turabian StyleLiu, Ziang, Yu Yang, Lihua Yang, Bochun Wang, Xiaotong Gao, Shuchao Huang, Xiufeng Li, Chengjun Yang, and Qingjie Guan. 2025. "Cloning and Functional Analysis of ZFP5 from Amorpha fruticosa for Enhancing Drought and Saline–Alkali Resistance in Tobacco" International Journal of Molecular Sciences 26, no. 8: 3792. https://doi.org/10.3390/ijms26083792
APA StyleLiu, Z., Yang, Y., Yang, L., Wang, B., Gao, X., Huang, S., Li, X., Yang, C., & Guan, Q. (2025). Cloning and Functional Analysis of ZFP5 from Amorpha fruticosa for Enhancing Drought and Saline–Alkali Resistance in Tobacco. International Journal of Molecular Sciences, 26(8), 3792. https://doi.org/10.3390/ijms26083792





