Early Seedling Screening Reveals Unidentified Al Resistance Mechanisms in Lithuanian Barley Cultivars
Abstract
1. Introduction
2. Results
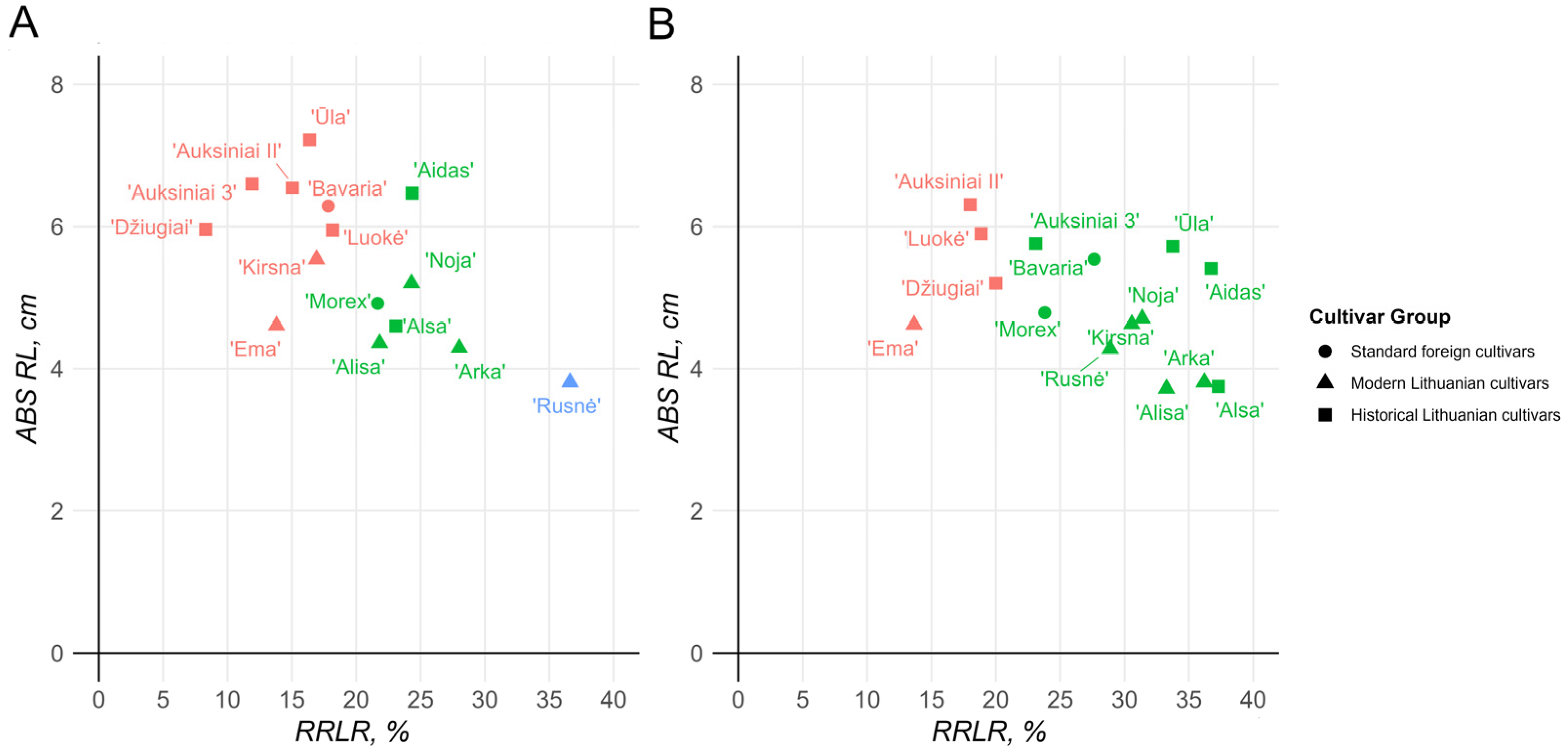
2.1. Evaluation of Al Resistance Using Morphometric Parameters
2.2. Evaluation of Al Resistance Using Biochemical Parameters
2.3. Investigation of Al-Resistance-Associated Genotypes in Lithuanian Barley Cultivars
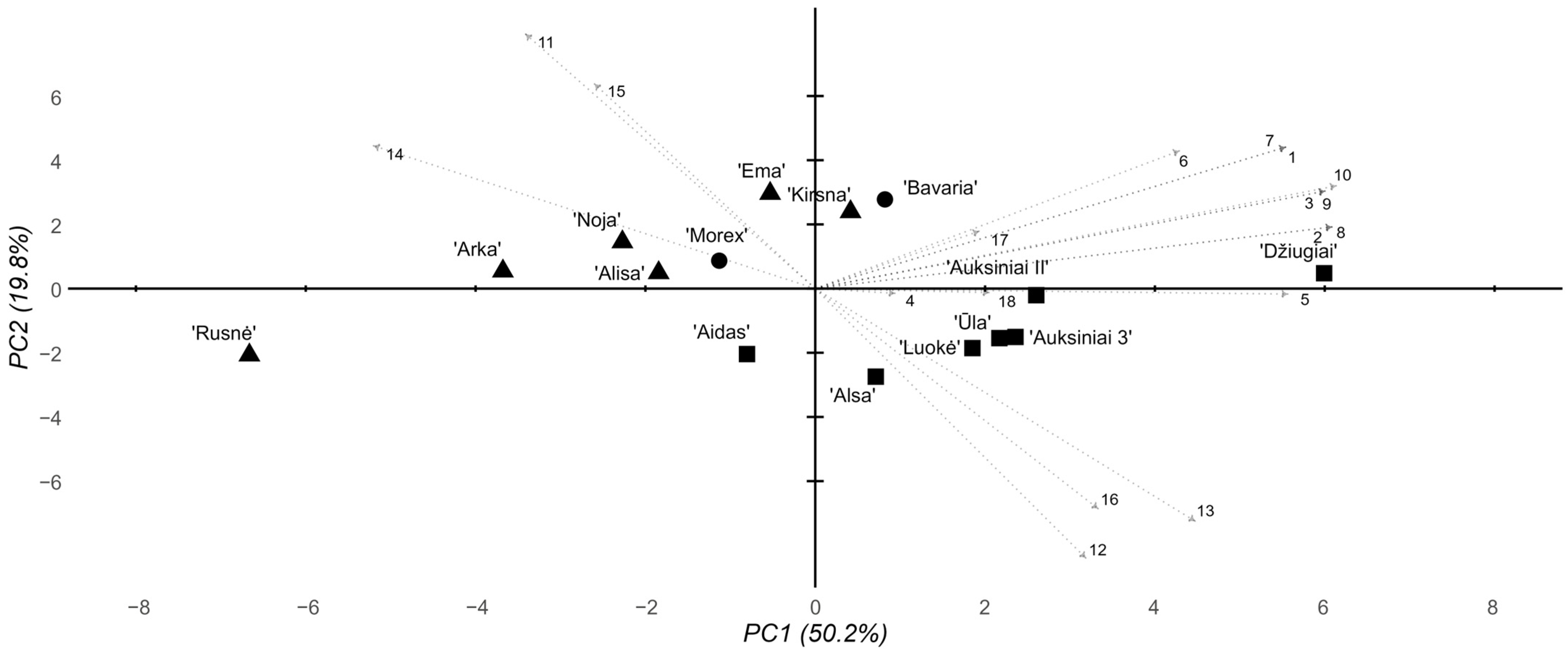
2.4. Multivariate Analyses
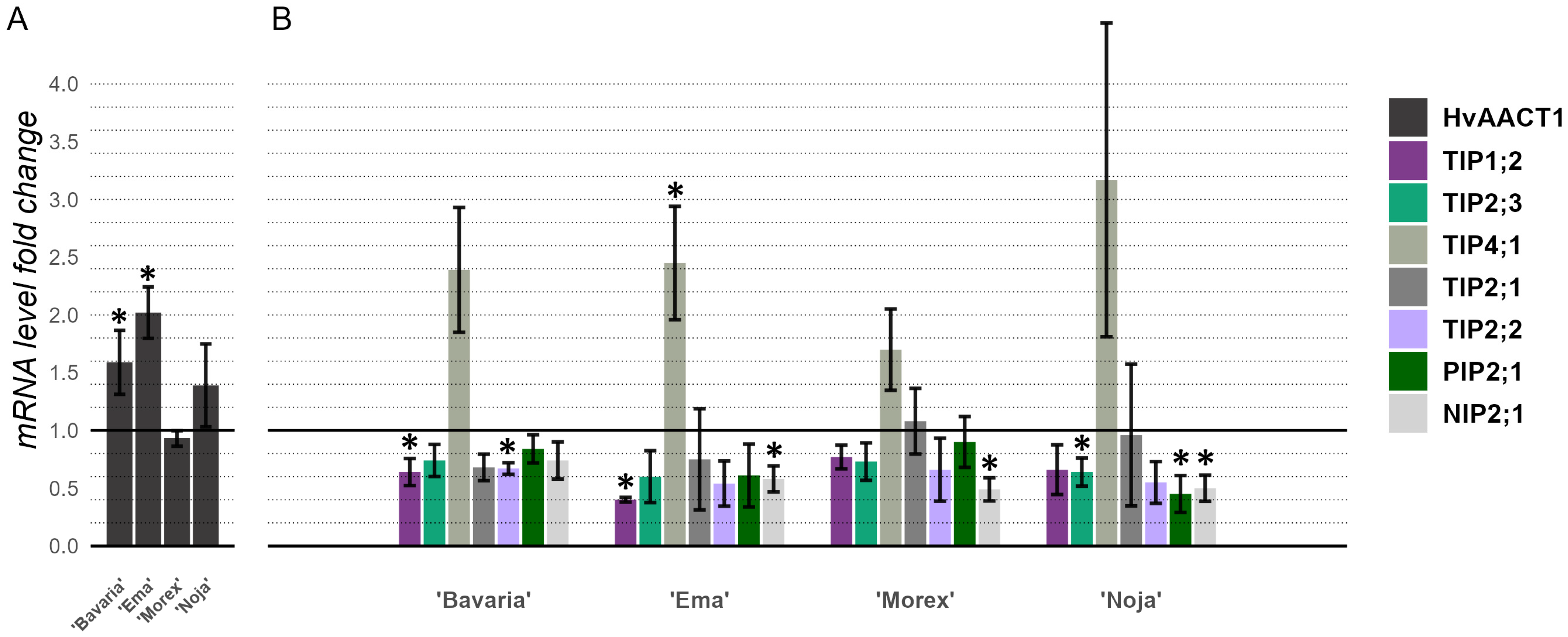
2.5. Expression Levels of Organic Acid Transporters and Aquaporins in Barley Roots
2.6. Sequence Analysis of the HvAACT1 Gene and 1 kb Upstream Region
2.7. Sequence Analysis of the HvALMT1 Gene
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Grain Sterilization and Al Stress Induction
4.3. Quantifying the Effects of Al Stress on Barley Seedling Morphology
4.4. Evaluation of Biochemical Markers Using Colorimetric Assays
4.5. Extraction of Barley Seedling Genomic DNA
4.6. HvAACT1 Sequence Analysis
4.7. RNA Extraction and cDNA Synthesis for Gene Expression Analysis
4.8. Quantitative PCR Assays for Gene Expression Changes Under Al Stress
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrigley, C.W.; Corke, H.; Walker, C.E. Encyclopedia of Grain Science, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004; ISBN 978-0-12-765490-4. [Google Scholar]
- Wang, J.; Raman, H.; Zhang, G.; Mendham, N.; Zhou, M. Aluminium tolerance in barley (Hordeum vulgare L.): Physiological mechanisms, genetics and screening methods. J. Zhejiang Univ. Sci. B 2006, 7, 769–787. [Google Scholar] [CrossRef]
- Tricase, C.; Amicarelli, V.; Lamonaca, E.; Leonardo Rana, R. Economic analysis of the barley market and related uses. In Grasses as Food and Feed; Tadele, Z., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-798-7. Available online: https://www.intechopen.com/books/grasses-as-food-and-feed/economic-analysis-of-the-barley-market-and-related-uses (accessed on 9 April 2025).
- Poehlman, J.M. Adaptation and Distribution. In Barley; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 1985; pp. 1–17. ISBN 978-0-89118-219-1. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Hebb, D.M.; Yamamoto, Y.; Sasaki, T.; Matsumoto, H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. USA 2004, 101, 15249–15254. [Google Scholar] [CrossRef]
- Sumner, M.; Noble, A. Soil acidification: The world story. In Handbook of Soil Acidity; Books in soils, plants, and the environment; Rengel, Z., Ed.; Dekker: New York, NY, USA; Basel, Switzerland, 2003; pp. 1–29. ISBN 978-0-8247-0890-0. [Google Scholar]
- Witcombe, J.R.; Hollington, P.A.; Howarth, C.J.; Reader, S.; Steele, K.A. Breeding for abiotic stresses for sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 703–716. [Google Scholar] [CrossRef]
- Borhannuddin Bhuyan, M.H.M.; Hasanuzzaman, M.; Nahar, K.; Mahmud, J.A.; Parvin, K.; Bhuiyan, T.F.; Fujita, M. Plants behavior under soil acidity stress: Insight into morphophysiological, biochemical, and molecular responses. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 35–82. ISBN 978-3-030-06118-0. [Google Scholar] [CrossRef]
- Shoghi Kalkhoran, S.; Pannell, D.J.; Thamo, T.; White, B.; Polyakov, M. Soil acidity, lime application, nitrogen fertility, and greenhouse gas emissions: Optimizing their joint economic management. Agric. Syst. 2019, 176, 102684. [Google Scholar] [CrossRef]
- Foy, C.D. Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In Soil Acidity and Liming; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; pp. 57–97. ISBN 978-0-89118-207-8. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Chwiałkowska, K.; Niemira, M.; Kwaśniewski, M.; Nawrot, M.; Gajecka, M.; Larsen, P.B.; Szarejko, I. Aluminum or low pH—Which is the bigger enemy of barley? Transcriptome analysis of barley root meristem under al and low pH stress. Front. Genet. 2021, 12, 675260. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Arunakumara, K.K.I.U.; Walpola, B.C.; Yoon, M.-H. Aluminum toxicity and tolerance mechanism in cereals and legumes—A review. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.F.; Sato, K.; Takeda, K. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 2003, 217, 794–800. [Google Scholar] [CrossRef]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef]
- Fujii, M.; Yokosho, K.; Yamaji, N.; Saisho, D.; Yamane, M.; Takahashi, H.; Sato, K.; Nakazono, M.; Ma, J.F. Acquisition of aluminium tolerance by modification of a single gene in barley. Nat. Commun. 2012, 3, 713. [Google Scholar] [CrossRef]
- Bian, M.; Waters, I.; Broughton, S.; Zhang, X.-Q.; Zhou, M.; Lance, R.; Sun, D.; Li, C. Development of gene-specific markers for acid soil/aluminium tolerance in barley (Hordeum vulgare L.). Mol. Breed. 2013, 32, 155–164. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Faria, B.F.; Comar Junior, M.; Delatorre, C.A.; Minella, E.; Pereira, J.F. Is a non-synonymous SNP in the HvAACT1 coding region associated with acidic soil tolerance in barley? Genet. Mol. Biol. 2017, 40, 480–490. [Google Scholar] [CrossRef]
- Mažvila, J.; Antanaitis, A.; Adomaitis, T.R.; Eitminavičius, L.; Lubytė, J.; Matusevičius, K.; Vaišvila, Z.J. Lietuvos Dirvožemių Agrocheminės Savybės ir jų Kaita; Lietuvos žemdirbystės institutas: Kaunas, Lithuania, 1998; ISBN 9986-527-47-3. [Google Scholar]
- Lietuvos Respublikos žemės ūkio ministerija. Dirvožemio Agrocheminių Savybių Stebėjimo Tyrimai, 2022–2023; Lietuvos agrarinių ir miškų mokslų centro Žemdirbystės instituto Agrocheminių tyrimų laboratorija: Kaunas, Lietuva, 2023; Available online: https://www.lammc.lt/data/public/uploads/2023/07/zum_ataskaita_dirvozemio-agrocheminiu-savybiu-stebejimo-tyrimai.pdf (accessed on 16 April 2025).
- Mohammadi, S.A.; Abdollahi Sisi, N.; Sadeghzadeh, B. The influence of breeding history, origin and growth type on population structure of barley as revealed by SSR markers. Sci. Rep. 2020, 10, 19165. [Google Scholar] [CrossRef]
- Larsson, M.N.A.; Leino, M.W.; Hagenblad, J. Genetic diversity in 19th century barley (Hordeum vulgare) reflects differing agricultural practices and seed trade in Jämtland, Sweden. Diversity 2021, 13, 315. [Google Scholar] [CrossRef]
- Nyiraguhirwa, S.; Grana, Z.; Henkrar, F.; Ouabbou, H.; Mohammed, I.; Udupa, S.M. Genetic diversity and structure of a barley collection predominatly from North African region. Cereal Res. Commun. 2022, 50, 647–654. [Google Scholar] [CrossRef]
- Capo-chichi, L.J.A.; Elakhdar, A.; Kubo, T.; Nyachiro, J.; Juskiw, P.; Capettini, F.; Slaski, J.J.; Ramirez, G.H.; Beattie, A.D. Genetic diversity and population structure assessment of Western Canadian barley cooperative trials. Front. Plant Sci. 2023, 13, 1006719. [Google Scholar] [CrossRef]
- Yang, C.J.; Russell, J.; Ramsay, L.; Thomas, W.; Powell, W.; Mackay, I. Overcoming barriers to the registration of new plant varieties under the DUS system. Commun. Biol. 2021, 4, 302. [Google Scholar] [CrossRef]
- Ruzgas, V. Plant breeding in Lithuania: 95 years for science and agriculture improvement. Žemės Ūkio Moksl. 2017, 4, 167–182. [Google Scholar] [CrossRef][Green Version]
- Lazauskas, J.; Dapkus, R. Lauko Augalų Selekcija Lietuvoje; Mokslas: Vilnius, Lithuania, 1992. [Google Scholar]
- Bivilienė, A. Lietuvoje Sukurtos Javų Veislės, Jų Genetinis Potencialas; Spaudvita: Kėdainiai, Lithuania, 2011. [Google Scholar]
- Roy, B.; Bhadra, S. Effects of toxic levels of aluminium on seedling parameters of rice under hydroponic culture. Rice Sci. 2014, 21, 217–223. [Google Scholar] [CrossRef]
- Phukunkamkaew, S.; Tisarum, R.; Pipatsitee, P.; Samphumphuang, T.; Maksup, S.; Cha-um, S. Morpho-physiological responses of indica rice (Oryza sativa sub. indica) to aluminum toxicity at seedling stage. Environ. Sci. Pollut. Res. 2021, 28, 29321–29331. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Ali, A.; Tuteja, N.; Gill, S.; Pattanayak, A. Identification and expression pattern of aluminium-responsive genes in roots of rice genotype with reference to Al-sensitivity. Sci. Rep. 2023, 13, 12184. [Google Scholar] [CrossRef]
- Kidd, P.S.; Proctor, J. Effects of aluminium on the growth and mineral composition of Betula pendula Roth. J. Exp. Bot. 2000, 51, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Rodrigues, J.S.; Carvalho, B.D.O.; Gavassi, M.A.; Bressan, A.C.G.; Habermann, G. Absence of aluminium compromises root integrity, reduces leaf hydration and Rubisco performance in Qualea grandiflora, an Al-accumulating species. Plant Biol. 2023, 25, 740–749. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Wu, Q.; Yue, K.; Yuan, J.; Yuan, C.; Peng, Y. Global characteristics and drivers of sodium and aluminum concentrations in freshly fallen plant litter. Front. Plant Sci. 2023, 14, 1174697. [Google Scholar] [CrossRef]
- Liu, W.; Xu, F.; Lv, T.; Zhou, W.; Chen, Y.; Jin, C.; Lu, L.; Lin, X. Spatial responses of antioxidative system to aluminum stress in roots of wheat (Triticum aestivum L.) plants. Sci. Total Environ. 2018, 627, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef]
- Mijiti, M.; Zhang, Y.; Zhang, C.; Wang, Y. Physiological and molecular responses of Betula platyphylla Suk to salt stress. Trees 2017, 31, 1653–1665. [Google Scholar] [CrossRef]
- Hossain, M.; Zhou, M.; Mendham, N. A reliable screening system for aluminium tolerance in barley cultivars. Aust. J. Agric. Res. 2005, 56, 475–482. [Google Scholar] [CrossRef]
- Giannakoula, A.; Moustakas, M.; Syros, T.; Yupsanis, T. Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ. Exp. Bot. 2010, 67, 487–494. [Google Scholar] [CrossRef]
- Martins, N.; Gonçalves, S.; Romano, A. Metabolism and aluminum accumulation in Plantago almogravensis and P. algarbiensis in response to low pH and aluminum stress. Biol. Plant. 2013, 57, 325–331. [Google Scholar] [CrossRef]
- Surapu, V.; Ediga, A.; Meriga, B. Salicylic acid alleviates aluminum toxicity in tomato seedlings (Lycopersicum esculentum mill.) through activation of antioxidant defense system and proline biosynthesis. Adv. Biosci. Biotechnol. 2014, 5, 777–789. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Knight, J.A.; Pieper, R.K.; McClellan, L. Specificity of the thiobarbituric acid reaction: Its use in studies of lipid peroxidation. Clin. Chem. 1988, 34, 2433–2438. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Oxidative Stress Triggered by Aluminum in Plant Roots; Springer: Berlin/Heidelberg, Germany, 2003; pp. 239–243. [Google Scholar]
- Kocjan, A.; Kwasniewska, J.; Szurman-Zubrzycka, M. Understanding plant tolerance to aluminum: Exploring mechanisms and perspectives. Plant Soil. 2024, 507, 195–219. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 2017, 12, e0176357. [Google Scholar] [CrossRef]
- Nowosielska, D.; Nowosielski, J. Morphological diversity and DNA polymorphism of common oat (Avena sativa L.) breeding varieties cultivated in Poland. Plant Breed. Seed Sci. 2009, 60, 31–44. [Google Scholar] [CrossRef]
- van de Wouw, M.; Hintum, T.; Kik, C.; Treuren, R.; Visser, B. Genetic diversity trends in twentieth century crop cultivars: A meta analysis. Theor. Appl. Genet. 2010, 120, 1241–1252. [Google Scholar] [CrossRef]
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J. Crop genetic erosion: Understanding and responding to loss of crop diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef]
- Leistrumaite, A.; Paplauskienė, V. Evaluation of photosynthetic pigments content and agronomically valuable traits in Lithuanian spring barley varieties. Žemės Ūkio Moksl. 2004, 4, 36–41. [Google Scholar]
- Palmer, A.J.; Baker, A.; Muench, S.P. The varied functions of aluminium-activated malate transporters–much more than aluminium resistance. Biochem. Soc. Trans. 2016, 44, 856–862. [Google Scholar] [CrossRef]
- Gruber, B.D.; Ryan, P.R.; Richardson, A.E.; Tyerman, S.D.; Ramesh, S.; Hebb, D.M.; Howitt, S.M.; Delhaize, E. HvALMT1 from barley is involved in the transport of organic anions. J. Exp. Bot. 2010, 61, 1455–1467. [Google Scholar] [CrossRef]
- Navakode, S.; Weidner, A.; Varshney, R.K.; Lohwasser, U.; Scholz, U.; Börner, A. A QTL analysis of aluminium tolerance in barley, using gene-based markers. CEREAL Res. Commun. 2009, 37, 531–540. [Google Scholar] [CrossRef]
- Cai, S.; Wu, D.; Jabeen, Z.; Huang, Y.; Huang, Y.; Zhang, G. Genome-wide association analysis of aluminum tolerance in cultivated and tibetan wild barley. PLoS ONE 2013, 8, 69776. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, J.; Shen, Q.; Huang, L.; Wu, D.; Zhang, G. Identification of microRNAs in response to aluminum stress in the roots of Tibetan wild barley and cultivated barley. BMC Genom. 2018, 19, 560. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Hussain, S.; Ashraf, U.; Ali, M.F.; Zulfiqar, U.; Khaliq, A. 4—Aquaporins: A potential weapon in plants for abiotic stress tolerance. In Transporters and Plant Osmotic Stress; Roychoudhury, A., Tripathi, D.K., Deshmukh, R., Eds.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2021; pp. 63–76. [Google Scholar]
- Vats, S.; Sudhakaran, S.; Bhardwaj, A.; Mandlik, R.; Sharma, Y.; Kumar, S.; Tripathi, D.K.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Targeting aquaporins to alleviate hazardous metal(loid)s imposed stress in plants. J. Hazard. Mater. 2021, 408, 124910. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Oshima, K.; Hattori, M.; Kanai, M.; Mano, S.; Nishimura, M.; Yoshida, K. Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS ONE 2012, 7, 43189. [Google Scholar] [CrossRef]
- Cavalheiro, M.F.; Gavassi, M.A.; Silva, G.S.; Nogueira, M.A.; Silva, C.M.S.; Domingues, D.S.; Habermann, G. Low root PIP1-1 and PIP2 aquaporins expression could be related to reduced hydration in ‘Rangpur’ lime plants exposed to aluminium. Funct. Plant Biol. 2019, 47, 112–121. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Han, J.-C.; Ahmad, M.; Ashraf, M.; Khaliq, M.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.; Khan, K. Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Hove, R.M.; Ziemann, M.; Bhave, M. Identification and expression analysis of the barley (Hordeum vulgare L.) aquaporin gene family. PLoS ONE 2015, 10, 0128025. [Google Scholar] [CrossRef] [PubMed]
- Ligaba, A.; Katsuhara, M.; Shibasaka, M.; Djira, G. Abiotic stresses modulate expression of major intrinsic proteins in barley (Hordeum vulgare). Comptes Rendus. Biol. 2011, 334, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M. Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ. 2016, 39, 2319–2328. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Dodd, I.C.; Puértolas, J.; Silva, G.S.; Carvalho, R.F.; Habermann, G. Aluminum-induced stomatal closure is related to low root hydraulic conductance and high ABA accumulation. Environ. Exp. Bot. 2020, 179, 104233. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant 2016, 38, 257. [Google Scholar] [CrossRef]
- Liu, W.; Feng, X.; Cao, F.; Wu, D.; Zhang, G.; Vincze, E.; Wang, Y.; Chen, Z.-H.; Wu, F. An ATP binding cassette transporter HvABCB25 confers aluminum detoxification in wild barley. J. Hazard. Mater. 2021, 401, 123371. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 5047–5052. [Google Scholar] [CrossRef]
- Gould, B.; McCouch, S.; Geber, M. Variation in soil aluminium tolerance genes is associated with local adaptation to soils at the Park Grass Experiment. Mol. Ecol. 2014, 23, 6058–6072. [Google Scholar] [CrossRef]
- Ramakrishna, N.; Lacey, J.; Smith, J.E. Effect of surface sterilization, fumigation and gamma irradiation on the microflora and germination of barley seeds. Int. J. Food Microbiol. 1991, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Huttová, J.; Tamás, L.; Mistrík, I. Aluminium induced acid phosphatase activity in roots of Al-sensitive and Al-tolerant barley varieties. Plant Soil. Environ. 2002, 48, 556–559. [Google Scholar] [CrossRef]
- Tamás, L.; Šimonovičová, M.; Huttová, J.; Mistrík, I. Aluminium stimulated hydrogen peroxide production of germinating barley seeds. Environ. Exp. Bot. 2004, 51, 281–288. [Google Scholar] [CrossRef]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, R.K.; Dubey, R.S. Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology 2013, 22, 656–670. [Google Scholar] [CrossRef]
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. ISBN 978-3-642-83964-1. Available online: http://link.springer.com/10.1007/978-3-642-83962-7_18 (accessed on 16 April 2025).
- Ferdous, J.; Li, Y.; Reid, N.; Langridge, P.; Shi, B.-J.; Tricker, P.J. Identification of reference genes for quantitative expression analysis of micrornas and mrnas in barley under various stress conditions. PLoS ONE 2015, 10, e0118503. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



| Cultivar | 2 mM | 8 mM | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| ‘Bavaria’ | 8.53 ± 0.23 | 1.13 ± 0.25 | 1.19 ± 0.35 | 2.04 ± 0.23 | −6.62 ± 0.25 | −6.38 ± 0.35 |
| ‘Morex’ | 8.62 ± 0.27 | −6.72 ± 0.33 | −8.66 ± 0.35 a | 9.76 ± 0.30 | 1.17 ± 0.35 | −0.64 ± 0.39 |
| ‘Alisa DS’ | −6.47 ± 0.24 | −11.93 ± 0.22 b | −9.28 ± 0.23 c | −10.83 ± 0.27 | −20.63 ± 0.33 c | −20.49 ± 0.37 c |
| ‘Arka DS’ | 1.50 ± 0.28 | −12.45 ± 0.39 | −11.96 ± 0.46 a | −3.54 ± 0.28 | −12.95 ± 0.36 b | −10.82 ± 0.47 b |
| ‘Ema DS’ | −8.70 ± 0.24 | −6.76 ± 0.31 a | −1.80 ± 0.44 a | −2.68 ± 0.24 | −6.34 ± 0.31 a | −0.27 ± 0.44 |
| ‘Kirsna DS’ | 0.35 ± 0.28 | −2.23 ± 0.36 | 0.22 ± 0.38 | 0.97 ± 0.28 | −6.65 ± 0.36 | −4.17 ± 0.38 |
| ‘Noja DS’ | −6.68 ± 0.28 | −8.58 ± 0.34 a | −0.79 ± 0.38 | −11.07 ± 0.28 | −9.84 ± 0.34 a | −4.47 ± 0.38 a |
| ‘Rusnė DS’ | −16.89 ± 0.22 c | −19.72 ± 0.28 c | −23.81 ± 0.35 c | −12.29 ± 0.18 c | −15.55 ± 0.16 c | −13.88 ± 0.17 c |
| ‘Aidas’ | 6.58 ± 0.34 | 2.16 ± 0.32 | 0.95 ± 0.33 | −2.05 ± 0.34 | −12.65 ± 0.32 | −13.85 ± 0.33 a |
| ‘Alsa’ | 5.52 ± 0.22 | 0.00 ± 0.34 | −7.97 ± 0.44 | 15.52 ± 0.31 | 2.20 ± 0.42 | −8.20 ± 0.52 |
| ‘Auksiniai II’ | −10.94 ± 0.27 | −6.97 ± 0.39 | −10.85 ± 0.43 | −1.50 ± 0.20 | −6.40 ± 0.20 | −6.25 ± 0.26 |
| ‘Auksiniai 3’ | 0.45 ± 0.16 | −2.82 ± 0.25 | −2.50 ± 0.34 | −13.79 ± 0.16 a | −18.06 ± 0.24 c | −14.25 ± 0.27 b |
| ‘Džiugiai’ | −9.82 ± 0.58 | 17.58 ± 1.17 | 4.24 ± 1.44 | −8.28 ± 0.58 | 11.42 ± 1.17 | 8.53 ± 1.44 |
| ‘Luokė’ | 2.91 ± 0.18 | 4.03 ± 0.31 | −5.52 ± 0.43 a | 8.89 ± 0.18 | 1.09 ± 0.31 | −2.52 ± 0.43 |
| ‘Ūla’ | −3.23 ± 0.20 | −4.13 ± 0.22 | −6.66 ± 0.29 | −7.38 ± 0.20 | −16.06 ± 0.22 b | −14.66 ± 0.29 c |
| Cultivar | 2 mM | 8 mM | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| ‘Bavaria’ | 0.07 ± 0.15 | −11.98 ± 0.19 b | −17.83 ± 0.26 c | −3.47 ± 0.15 | −21.01 ± 0.19 c | −27.64 ± 0.26 c |
| ‘Morex’ | −2.24 ± 0.15 | −17.18 ± 0.19 c | −21.67 ± 0.21 c | −5.69 ± 0.15 | −19.79 ± 0.19 c | −23.80 ± 0.21 c |
| ‘Alisa DS’ | −6.05 ± 0.13 | −16.52 ± 0.14 c | −21.82 ± 0.17 c | −13.65 ± 0.13 b | −29.15 ± 0.14 c | −33.26 ± 0.17 c |
| ‘Arka DS’ | −4.98 ± 0.15 | −24.15 ± 0.22 c | −28.01 ± 0.26 c | −14.78 ± 0.15 a | −30.83 ± 0.22 c | −36.20 ± 0.26 c |
| ‘Ema DS’ | −2.20 ± 0.18 | −14.84 ± 0.24 c | −13.81 ± 0.33 b | −4.93 ± 0.18 | −14.35 ± 0.24 c | −13.65 ± 0.33 c |
| ‘Kirsna DS’ | 0.33 ± 0.16 | −11.74 ± 0.21 b | −16.92 ± 0.24 c | −11.74 ± 0.16 a | −23.57 ± 0.21 c | −30.56 ± 0.24 c |
| ‘Noja DS’ | −7.01 ± 0.18 | −22.21 ± 0.22 c | −24.30 ± 0.24 c | −12.79 ± 0.18 b | −23.70 ± 0.22 c | −31.38 ± 0.24 c |
| ‘Rusnė DS’ | −17.48 ± 0.15 c | −26.73 ± 0.16 c | −36.62 ± 0.20 c | −18.49 ± 0.15 c | −25.86 ± 0.16 c | −28.89 ± 0.19 c |
| ‘Aidas’ | −10.30 ± 0.30 | −22.08 ± 0.28 c | −24.35 ± 0.36 c | −19.59 ± 0.30 b | −31.56 ± 0.28 c | −36.73 ± 0.36 c |
| ‘Alsa’ | −4.17 ± 0.48 | −16.00 ± 0.82 | −23.08 ± 1.01 | −6.02 ± 0.48 | −28.00 ± 0.82 | −37.29 ± 0.62 |
| ‘Auksiniai II’ | 1.19 ± 0.48 | −6.92 ± 0.45 | −15.04 ± 0.48 | −1.90 ± 0.48 | −9.87 ± 0.45 | −18.01 ± 0.24 a |
| ‘Auksiniai 3’ | −4.60 ± 0.26 | −13.08 ± 0.32 | −11.91 ± 0.35 | −11.50 ± 0.26 a | −21.76 ± 0.32 b | −23.10 ± 0.37 b |
| ‘Džiugiai’ | 7.82 ± 0.94 | −3.85 ± 1.21 | −8.31 ± 1.47 | −6.38 ± 0.94 | −12.88 ± 1.21 | −20.00 ± 1.47 |
| ‘Luokė’ | −3.89 ± 0.27 | −13.23 ± 0.33 | −18.17 ± 0.40 a | −1.04 ± 0.27 | −11.06 ± 0.33 | −18.86 ± 0.33 a |
| ‘Ūla’ | −0.38 ± 0.24 | −10.65 ± 0.33 a | −16.38 ± 0.43 b | −15.45 ± 0.24 | −26.81 ± 0.33 c | −33.75 ± 0.33 c |
| Cultivar | 1 kb Insertion in 5′UTR | 21 bp Deletion in 3′UTR | SNP at 1198 Position |
|---|---|---|---|
| ‘Bavaria’ | Not present | + | G |
| ‘Morex’ | − | T | |
| ‘Alisa DS’ | − | T | |
| ‘Arka DS’ | − | T | |
| ‘Ema DS’ | − | T | |
| ‘Kirsna DS’ | − | T | |
| ‘Noja DS’ | + | G | |
| ‘Rusnė DS’ | − | T | |
| ‘Aidas’ | + | G | |
| ‘Alsa’ | − | T | |
| ‘Auksiniai II’ | +/− | T/G | |
| ‘Auksiniai 3’ | +/− | T/G | |
| ‘Džiugiai’ | +/− | T/G | |
| ‘Luokė’ | +/− | T/G | |
| ‘Ūla’ | − | T/G |
| Cultivar | Position * | |||
|---|---|---|---|---|
| −169; 531, 411, 112 | 1198; 531, 412, 479 | 2283; 531, 413, 564 | 3644–3645; 531, 414, 925 | |
| ‘Morex’ | C | T | G | GA |
| ‘Bavaria’ | G | G | A | GA |
| ‘Noja DS’ | G | G | A | GATA |
| ‘Ema DS’ | C | T | G | GATA |
| Cultivar | Position * | |
|---|---|---|
| 1471; 469, 781, 055 | 2254; 469, 780, 278 | |
| ‘Morex’ | G | A |
| ‘Bavaria’ | A | G |
| All Lithuanian cultivars | A | G |
| Cultivar Name | Origin | Year of Development | Period of Registration | Status of Al Tolerance |
|---|---|---|---|---|
| ‘Morex’ * | ‘CI 15773’ | 1924 | Sensitive | |
| ‘Bavaria’ * | Local landraces | 1978 | Tolerant | |
| ‘Alisa DS’ | ‘Jacinta’ × ‘Vortex’ | 2008 | 2011– | Unknown |
| ‘Arka DS’ | ‘LŽI 7385’ × ‘Madona’ | 2009 | 2011– | Unknown |
| ‘Ema DS’ | ‘Mentor’ × ‘Annabell’ | 2010 | 2013– | Unknown |
| ‘Kirsna DS’ | ‘LŽI 7386’ × ‘Orlik’ | 2010 | 2013– | Unknown |
| ‘Noja DS’ | ‘LŽI 7386’ × ‘Pongo’ | 2009 | 2012– | Unknown |
| ‘Rusnė DS’ | ‘Mentor’ × ‘Annabell’ | 2013 | 2016– | Unknown |
| ‘Aidas’ | (KM-1192 × ‘Ofir’) × ‘Effendi’ | 1990 | 1994–2007 | Unknown |
| ‘Alsa’ | (‘Mirena’ × ‘Gintariniai’ mutant) × (‘Abava‘ × ‘Emir’) | 1993 | 1996–2007 | Unknown |
| ‘Auksiniai II’ | ‘Abed Kenia’ × ‘Ackermanns Isaria’ | 1947 | 1950–1990 | Suspected tolerant |
| ‘Auksiniai 3’ | ‘Carina’ × ‘Tarra 26’ | 1983 | 1987–2007 | Unknown |
| ‘Džiugiai’ | Local landraces | 1947 | 1950–1975 | Suspected tolerant |
| ‘Luokė’ | [‘Vega’ × (‘Ofir’ × ‘Berenice’)] × ‘Flare’ | 1999 | 2001– | Unknown |
| ‘Ūla’ | ‘Roland’ × Ca 33787 | 1992 | 1995–2007 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensonas, V.J.; Kleizaitė, V.; Leistrumaitė, A.; Šiukšta, R. Early Seedling Screening Reveals Unidentified Al Resistance Mechanisms in Lithuanian Barley Cultivars. Int. J. Mol. Sci. 2025, 26, 3803. https://doi.org/10.3390/ijms26083803
Mensonas VJ, Kleizaitė V, Leistrumaitė A, Šiukšta R. Early Seedling Screening Reveals Unidentified Al Resistance Mechanisms in Lithuanian Barley Cultivars. International Journal of Molecular Sciences. 2025; 26(8):3803. https://doi.org/10.3390/ijms26083803
Chicago/Turabian StyleMensonas, Vilius Jurgis, Violeta Kleizaitė, Algė Leistrumaitė, and Raimondas Šiukšta. 2025. "Early Seedling Screening Reveals Unidentified Al Resistance Mechanisms in Lithuanian Barley Cultivars" International Journal of Molecular Sciences 26, no. 8: 3803. https://doi.org/10.3390/ijms26083803
APA StyleMensonas, V. J., Kleizaitė, V., Leistrumaitė, A., & Šiukšta, R. (2025). Early Seedling Screening Reveals Unidentified Al Resistance Mechanisms in Lithuanian Barley Cultivars. International Journal of Molecular Sciences, 26(8), 3803. https://doi.org/10.3390/ijms26083803







