Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material
Abstract
1. Introduction
2. Results
2.1. Bio-Oss® Collagen Modulates the Expression of Genes Involved in Skeletal and Cartilage Development in hBMSCs
2.2. Secretome Profile Analysis in hBMSCs: Proteins Involved in Immunomodulation
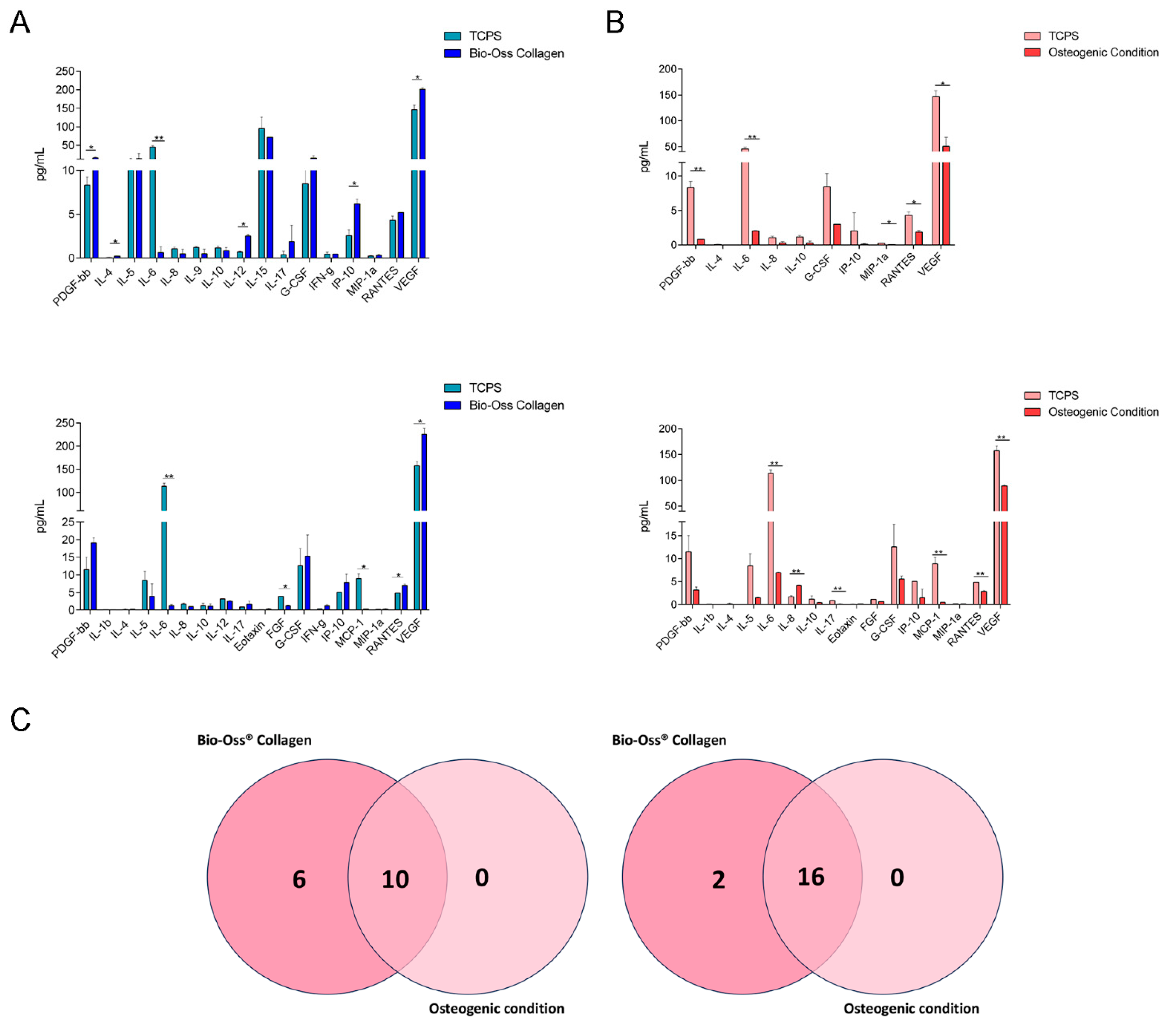
| TCPS | Bio-Oss® Collagen | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PDGF-bb | 8.320 | 0.905 | 16.095 | 1.850 |
| IL-4 | 0.045 | 0.049 | 0.220 | 0.000 |
| IL-5 | 10.705 | 3.854 | 14.000 | 14.000 |
| IL-6 | 45.290 | 3.960 | 0.620 | 0.679 |
| IL-8 | 1.050 | 0.198 | 0.490 | 0.490 |
| IL-9 | 1.220 | 0.100 | 0.510 | 0.500 |
| IL-10 | 1.155 | 0.219 | 0.840 | 0.354 |
| IL-12 | 0.680 | 0.100 | 2.510 | 0.200 |
| IL-15 | 95.130 | 30.745 | 71.450 | 0.100 |
| IL-17 | 0.400 | 0.400 | 1.880 | 1.880 |
| G-CSF | 8.455 | 1.945 | 15.375 | 6.003 |
| IFN-g | 0.445 | 0.219 | 0.460 | 0.000 |
| IP-10 | 2.550 | 0.673 | 6.160 | 0.555 |
| MIP-1a | 0.230 | 0.042 | 0.310 | 0.127 |
| RANTES | 4.300 | 0.467 | 5.190 | 0.000 |
| VEGF | 146.310 | 11.978 | 201.180 | 4.464 |
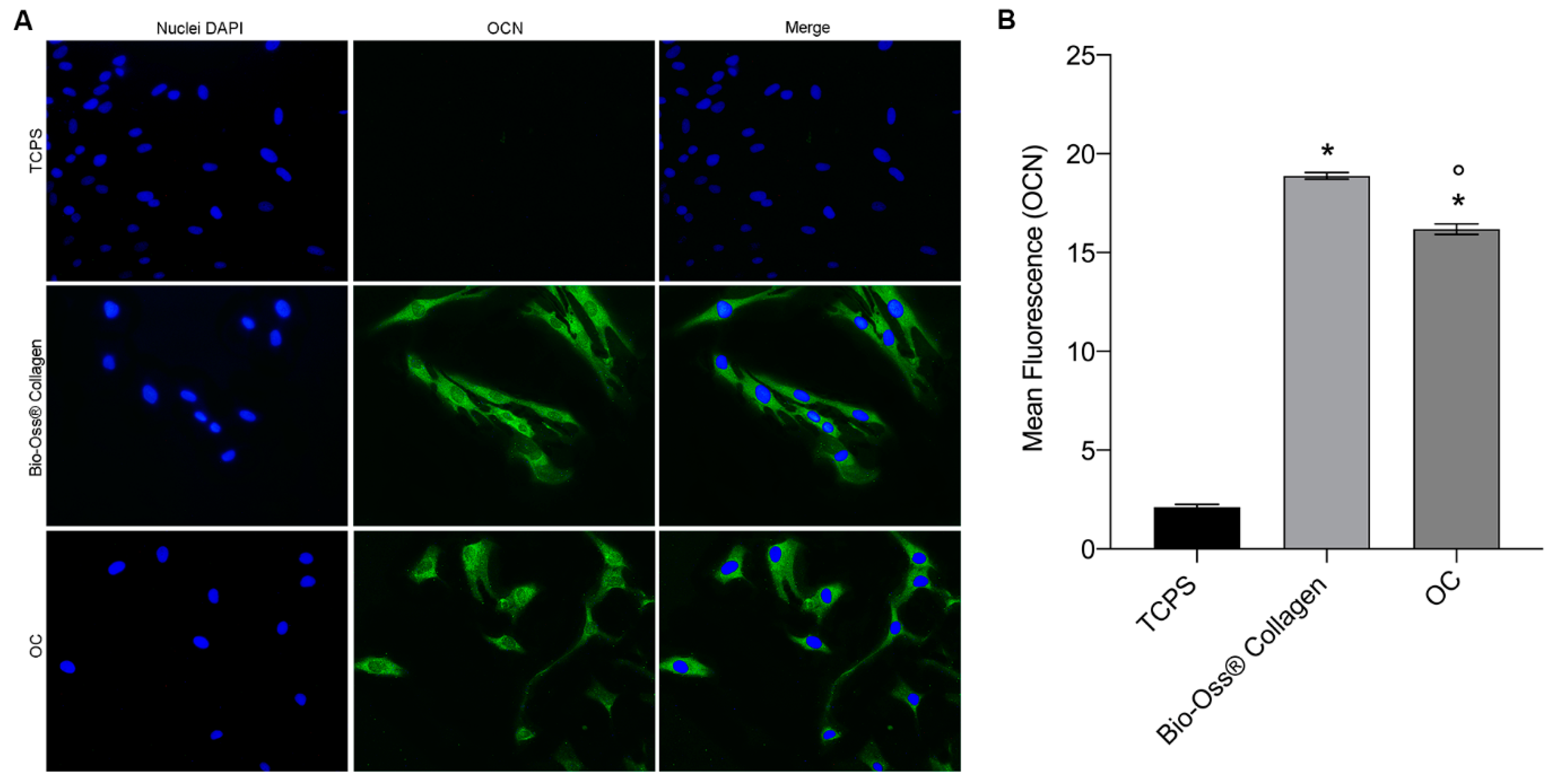
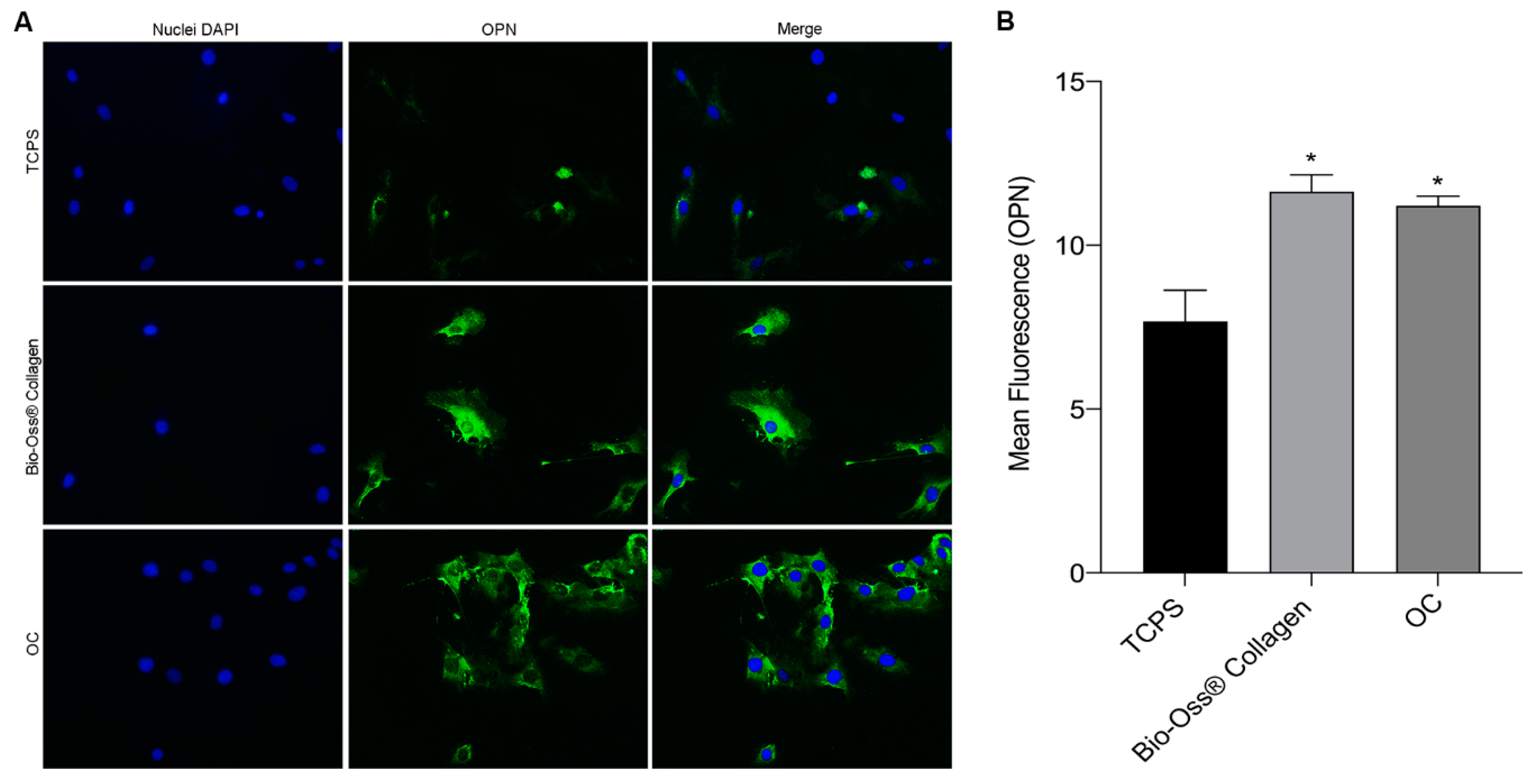
2.3. Osteocalcin and Osteopontin Expression as Osteogenic Markers
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Human BMSC Culture
- (i)
- hBMSCs seeded onto block-shaped Bio-Oss® Collagen (100 mg, approx. 5.0 mm × 5.0 mm × 7.0 mm) in 1 mL of α-MEM supplemented with 20% FBS and 2% Pen/Strep, in 24-well plates;
- (ii)
- OC group (positive control): hBMSCs were cultured in 1 mL of differentiation BullekitTM osteogenic medium (Lonza, Milan, Italy), which contains osteogenic basal medium (Lonza, Milan, Italy) and osteogenic SigleQuotesTM (dexamethasone, ascorbate, mesenchymal cell growth supplement, L-glutamine, and β-glycerophosphate) (Lonza, Milan, Italy), in 24-well plates;
- (iii)
- TCPS (negative control): hBMSCs were grown as a monolayer in standard culture plates using basal medium α-MEM, supplemented with 20% FBS and 2% Pen/Strep, in 24-well plates.
4.3. Bio-Oss® Collagen Material
4.4. RNA Isolation, cDNA Synthesis, and RT2 Profiler™ PCR Array Analyses of Human Osteogenesis Genes
4.5. Bio-Plex Pro Human Cytokine 27-Plex Assay
4.6. Immunofluorescence of the Osteogenic Markers—Osteopontin and Osteocalcin
4.7. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan Biomaterials Application in Dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The Role of microRNAs in the Osteogenic and Chondrogenic Differentiation of Mesenchymal Stem Cells and Bone Pathologies. Theranostics 2021, 11, 6573–6591. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Taraballi, F.; Torreggiani, E.; Rotondo, J.C.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I.; et al. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef]
- Mazzoni, E.; Mazziotta, C.; Iaquinta, M.R.; Lanzillotti, C.; Fortini, F.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Barbanti-Brodano, G.; Mescola, A.; et al. Enhanced Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells by a Hybrid Hydroxylapatite/Collagen Scaffold. Front. Cell Dev. Biol. 2021, 8, 610570. [Google Scholar] [CrossRef]
- Lynch, R.I.; Lavelle, E.C. Immuno-Modulatory Biomaterials as Anti-Inflammatory Therapeutics. Biochem. Pharmacol. 2022, 197, 114890. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef]
- Yu, Q.; Chang, J.; Wu, C. Silicate Bioceramics: From Soft Tissue Regeneration to Tumor Therapy. J. Mater. Chem. B 2019, 7, 5449–5460. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-Hydroxyapatite Use in Dentistry: A Systematic Review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Kushioka, J.; Chow, S.K.-H.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone Regeneration in Inflammation with Aging and Cell-Based Immunomodulatory Therapy. Inflamm. Regener. 2023, 43, 29. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Martini, F.; D’Agostino, A.; Trevisiol, L.; Bersani, M.; Torreggiani, E.; Tognon, M.; Rotondo, J.C.; Mazzoni, E. Stem Cell Fate and Immunomodulation Promote Bone Regeneration via Composite Bio-Oss®/AviteneTM Biomaterial. Front. Bioeng. Biotechnol. 2022, 10, 873814. [Google Scholar] [CrossRef]
- Galliera, E.; Locati, M.; Mantovani, A.; Corsi, M.M. Chemokines and Bone Remodeling. Int. J. Immunopathol. Pharmacol. 2008, 21, 485–491. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory Properties of Mesenchymal Stromal Cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, R.A.; Harley, B.A. Naturally Derived Biomaterials for Addressing Inflammation in Tissue Regeneration. Exp. Biol. Med. 2016, 241, 1015–1024. [Google Scholar] [CrossRef]
- D’Agostino, A.; Trevisiol, L.; Favero, V.; Gunson, M.J.; Pedica, F.; Nocini, P.F.; Arnett, G.W. Hydroxyapatite/Collagen Composite Is a Reliable Material for Malar Augmentation. J. Oral Maxillofac. Surg. 2016, 74, e1–e1238. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Manfrini, M.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Trevisiol, L.; Patergnani, S.; et al. Human Adipose Stem Cells Induced to Osteogenic Differentiation by an Innovative Collagen/Hydroxylapatite Hybrid Scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [CrossRef]
- Yahia, L. Natural Coral as a Biomaterial Revisited. Am. J. Biomed. Sci. Res. 2021, 13, 667–686. [Google Scholar] [CrossRef]
- Odusote, J.K.; Danyuo, Y.; Baruwa, A.D.; Azeez, A.A. Synthesis and Characterization of Hydroxyapatite from Bovine Bone for Production of Dental Implants. J. Appl. Biomater. 2019, 17, 228080001983682. [Google Scholar] [CrossRef]
- Gong, J.; Yang, L.; He, Q.; Jiao, T. In Vitro Evaluation of the Biological Compatibility and Antibacterial Activity of a Bone Substitute Material Consisting of Silver-Doped Hydroxyapatite and Bio-Oss®: Biological Compatibility and Antibacterial Activity of a Bone Substitute. J. Biomed. Mater. Res. 2018, 106, 410–420. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef]
- Kosinski, M.; Figiel-Dabrowska, A.; Lech, W.; Wieprzowski, L.; Strzalkowski, R.; Strzemecki, D.; Cheda, L.; Lenart, J.; Domanska-Janik, K.; Sarnowska, A. Bone Defect Repair Using a Bone Substitute Supported by Mesenchymal Stem Cells Derived from the Umbilical Cord. Stem Cells Int. 2020, 2020, e1321283. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, W.; Jiang, K.; Lin, Z.; Qian, C.; Wu, M.; Xia, Y.; Li, N.; Zhang, H.; Xiao, H.; et al. Regulation of Bone Homeostasis: Signaling Pathways and Therapeutic Targets. MedComm 2024, 5, e657. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of Osteopontin in Bone Remodeling and Orthodontic Tooth Movement: A Review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, S.; Tian, J.; Wang, L.; Dong, S.; Xia, T.; Wu, Z. Combination of Bone Marrow Concentrate and PGA Scaffolds Enhance Bone Marrow Stimulation in Rabbit Articular Cartilage Repair. J. Mater. Sci. Mater. Med. 2013, 24, 793–801. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, e2152435. [Google Scholar] [CrossRef]
- Thiagarajan, L.; Abu-Awwad, H.A.-D.M.; Dixon, J.E. Osteogenic Programming of Human Mesenchymal Stem Cells with Highly Efficient Intracellular Delivery of RUNX2. Stem Cells Transl. Med. 2017, 6, 2146–2159. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Nano-Hydroxyapatite Composite Biomaterials for Bone Tissue Engineering—A Review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Ficai, M.; Andronescu, E.; Ficai, D.; Voicu, G.; Ficai, A. Synthesis and Characterization of COLL–PVA/HA Hybrid Materials with Stratified Morphology. Colloids Surf. B Biointerfaces 2010, 81, 614–619. [Google Scholar] [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Ficai, A.; Marques, C.; Ferreira, J.M.F.; Andronescu, E.; Ficai, D.; Sonmez, M. Multifunctional Materials for Bone Cancer Treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and Current Interpretations of the Bone-Implant Interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.D.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis Imperfecta. Nat. Rev. Dis. Primers 2017, 3, 17052. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Acharya, C.; Christiansen, B.A.; Yik, J.H.N.; DiCesare, P.E.; Haudenschild, D.R. Cartilage Oligomeric Matrix Protein Enhances Osteogenesis by Directly Binding and Activating Bone Morphogenetic Protein-2. Bone 2013, 55, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Keupp, K.; Semler, O.; Wang, W.; Li, Y.; Thiele, H.; Yigit, G.; Pohl, E.; Becker, J.; Frommolt, P.; et al. Attenuated BMP1 Function Compromises Osteogenesis, Leading to Bone Fragility in Humans and Zebrafish. Am. J. Hum. Genet. 2012, 90, 661–674. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, T.; Wang, S.; Zhang, H.; Mues, G.; Qin, C.; Feng, J.Q.; D’Souza, R.N.; Lu, Y. Twist1- and Twist2-Haploinsufficiency Results in Reduced Bone Formation. PLoS ONE 2014, 9, e99331. [Google Scholar] [CrossRef]
- Tang, J.; Saito, T. A Novel Fragment Derived from Laminin-411 Facilitates Proliferation and Differentiation of Odontoblast-Like Cells. BioMed Res. Int. 2018, 2018, e9465383. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Wang, Y.; Shen, M.; Wang, S.; Tang, M.; Li, M.; Luo, Y.; Yang, K.; Wen, X. p75NTR Optimizes the Osteogenic Potential of Human Periodontal Ligament Stem Cells by Up-regulating A1 Integrin Expression. J. Cell Mol. Med. 2020, 24, 7563–7575. [Google Scholar] [CrossRef]
- Schmoldt, A.; Benthe, H.F.; Haberland, G. Digitoxin Metabolism by Rat Liver Microsomes. Biochem. Pharmacol. 1975, 24, 1639–1641. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Tan, Y. Different Roles of Matrix Metalloproteinase 2 in Osteolysis of Skeletal Dysplasia and Bone Metastasis (Review). Mol. Med. Rep. 2020, 23, 70. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between Bone and Immune Cells: Implications for Postmenopausal Osteoporosis. Semin. Cell Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and Matrix Gla Protein: Vitamin K-Dependent Proteins in Bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a Means to Cope with Environmental Insults: Regulation of Inflammation, Tissue Remodeling, and Cell Survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of Inflammation in Bone Regeneration: From Understandings to Therapeutic Applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- Bacevich, B.; Smith, R.; Reihl, A.; Mazzocca, A.; Hutchinson, I. Advances with Platelet-Rich Plasma for Bone Healing. Biologics 2024, 18, 29–59. [Google Scholar] [CrossRef]
- Bi, Z.; Cai, Y.; Shi, X.; Chen, J.; Li, D.; Zhang, P.; Liu, J. Macrophage-Mediated Immunomodulation in Biomaterial-Assisted Bone Repair: Molecular Insights and Therapeutic Prospects. Chem. Eng. J. 2024, 488, 150631. [Google Scholar] [CrossRef]
- Perera, P.-Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The Role of Interleukin-15 in Inflammation and Immune Responses to Infection: Implications for Its Therapeutic Use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in Vitro. Ann. Clin. Lab. Sci. 2016, 46, 65–71. [Google Scholar]
- Roseren, F.; Pithioux, M.; Robert, S.; Balasse, L.; Guillet, B.; Lamy, E.; Roffino, S. Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis. Int. J. Mol. Sci. 2021, 22, 3505. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Spilsbury, K.; Garrett, K.L.; Shen, W.Y.; Constable, I.J.; Rakoczy, P.E. Overexpression of Vascular Endothelial Growth Factor (VEGF) in the Retinal Pigment Epithelium Leads to the Development of Choroidal Neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The Dual Role of IL-6-Type Cytokines on Bone Remodeling and Bone Tumors. Cytokine Growth Factor. Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of Fibroblast Growth Factors in Bone Regeneration. Inflamm. Regener 2017, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.; Ke, K.; Kim, W.; Kim, S.; Lee, S.; Kim, H.; Kim, S.; Suh, J.; Choi, H. Absence of MCP-1 Leads to Elevated Bone Mass via Impaired Actin Ring Formation. J. Cell Physiol. 2012, 227, 1619–1627. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Xing, J.; Li, Z.; Yin, X.; Dou, C.; Dong, S.; Luo, F.; Xie, Z.; Hou, T.; et al. IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell Physiol. Biochem. 2018, 48, 361–370. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, D.; Luo, B.; Wang, L.; Li, H.; Wang, H. Regulation of Osteogenic Differentiation of hBMSCs by the Overlay Angles of Bone Lamellae-like Matrices. ACS Appl. Mater. Interfaces 2024, 16, 56801–56814. [Google Scholar] [CrossRef]
- Thalji, G.N.; Nares, S.; Cooper, L.F. Early Molecular Assessment of Osseointegration in Humans. Clin. Oral. Implants Res. 2014, 25, 1273–1285. [Google Scholar] [CrossRef]
- Manfrini, M.; Di Bona, C.; Canella, A.; Lucarelli, E.; Pellati, A.; D’Agostino, A.; Barbanti-Bròdano, G.; Tognon, M. Mesenchymal Stem Cells from Patients to Assay Bone Graft Substitutes. J. Cell Physiol. 2013, 228, 1229–1237. [Google Scholar] [CrossRef]
- Barbanti Brodano, G.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human Mesenchymal Stem Cells and Biomaterials Interaction: A Promising Synergy to Improve Spine Fusion. Eur. Spine J. 2012, 21, 3–9. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of Dexamethasone, Ascorbic Acid and β-Glycerophosphate on the Osteogenic Differentiation of Stem Cells in Vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Iaquinta, M.R.; Bononi, I.; Trevisiol, L.; Rotondo, J.C.; Patergnani, S.; Giorgi, C.; Gunson, M.J.; Arnett, G.W.; et al. Hydroxylapatite-Collagen Hybrid Scaffold Induces Human Adipose-Derived Mesenchymal Stem Cells to Osteogenic Differentiation in Vitro and Bone Regrowth in Patients. Stem Cells Transl. Med. 2020, 9, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Gasparello, J.; D’Aversa, E.; Papi, C.; Gambari, L.; Grigolo, B.; Borgatti, M.; Finotti, A.; Gambari, R. Sulforaphane Inhibits the Expression of Interleukin-6 and Interleukin-8 Induced in Bronchial Epithelial IB3-1 Cells by Exposure to the SARS-CoV-2 Spike Protein. Phytomedicine 2021, 87, 153583. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Montagner, G.; Bianchi, N.; Finotti, A.; Borgatti, M.; Lampronti, I.; Cabrini, G.; Gambari, R. MicroRNA miR-93-5p Regulates Expression of IL-8 and VEGF in Neuroblastoma SK-N-AS Cells. Oncol. Rep. 2016, 35, 2866–2872. [Google Scholar] [CrossRef] [PubMed]

| Upregulated Genes Bio-Oss® Collagen | Upregulated Genes OCs | ||||
| No. | Symbol | Fold Change (FC) | No. | Symbol | Fold Change (FC) |
| 1 | COMP | 2.17 | 1 | COMP | 1.19 |
| 2 | ITGA1 | 1.66 | 2 | ITGA1 | 0.41 |
| 3 | BMP1 | 1.48 | 3 | BMP1 | 0.02 |
| 4 | IGF1R | 1.28 | 4 | IGF1R | 0.16 |
| 5 | COL1A1 | 1.18 | 5 | COL1A1 | 0.46 |
| 6 | TWIST1 | 1.08 | 6 | TWIST1 | 0.51 |
| Downregulated genes Bio-Oss® Collagen | Downregulated genes OCs | ||||
| No. | Symbol | Fold change (FC) | No. | Symbol | Fold change (FC) |
| 1 | EGF | −5.64 | 1 | EGF | −4.64 |
| 2 | CSF3 | −5.06 | 2 | CSF3 | −3.06 |
| 3 | ITGA2 | −5.06 | 3 | ITGA2 | −5.64 |
| 4 | MMP2 | −2.94 | 4 | MMP2 | −3.64 |
| 5 | CTSK | −2.32 | 5 | CTSK | −3.06 |
| 6 | FGF2 | −1.43 | 6 | FGF2 | −2.39 |
| TCPS | Bio-Oss® Collagen | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PDGF-bb | 11.515 | 3.500 | 19.110 | 1.414 |
| IL-1b | 0.080 | 0.000 | 0.055 | 0.049 |
| IL-4 | 0.130 | 0.170 | 0.220 | 0.000 |
| IL-5 | 8.405 | 2.609 | 3.930 | 3.564 |
| IL-6 | 113.235 | 6.682 | 1.195 | 0.403 |
| IL-8 | 1.710 | 0.255 | 0.970 | 0.000 |
| IL-10 | 1.185 | 0.700 | 1.080 | 0.693 |
| IL-12 | 3.190 | 0.100 | 2.510 | 0.200 |
| IL-17 | 0.860 | 0.000 | 1.670 | 0.877 |
| Eotaxin | 0.075 | 0.064 | 0.290 | 0.198 |
| FGF | 3.900 | 0.100 | 1.150 | 0.200 |
| G-CSF | 12.585 | 4.914 | 15.375 | 6.003 |
| IFN-g | 0.370 | 0.000 | 1.180 | 0.339 |
| IP-10 | 5.040 | 0.000 | 7.820 | 2.348 |
| MCP-1 | 8.930 | 1.329 | 0.290 | 0.100 |
| MIP-1a | 0.180 | 0.100 | 0.220 | 0.200 |
| RANTES | 4.820 | 0.000 | 6.930 | 0.481 |
| VEGF | 157.330 | 8.895 | 225.280 | 13.630 |
| TCPS | Osteogenic Condition | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PDGF-bb | 8.320 | 0.905 | 0.810 | 0.000 |
| IL-4 | 0.045 | 0.049 | 0.010 | 0.000 |
| IL-6 | 45.290 | 3.960 | 2.025 | 0.064 |
| IL-8 | 1.050 | 0.198 | 0.275 | 0.233 |
| IL-10 | 1.155 | 0.219 | 0.265 | 0.318 |
| G-CSF | 8.455 | 1.945 | 3.000 | 0.000 |
| IP-10 | 2.030 | 2.673 | 0.100 | 0.100 |
| MIP-1a | 0.230 | 0.042 | 0.040 | 0.000 |
| RANTES | 4.300 | 0.467 | 1.865 | 0.233 |
| VEGF | 146.310 | 11.978 | 50.655 | 17.260 |
| TCPS | Osteogenic Conditions | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PDGF-bb | 11.515 | 3.500 | 3.165 | 0.658 |
| IL-1b | 0.080 | 0.000 | 0.025 | 0.021 |
| IL-4 | 0.130 | 0.170 | 0.010 | 0.000 |
| IL-5 | 8.405 | 2.609 | 1.450 | 0.100 |
| IL-6 | 113.235 | 6.682 | 6.910 | 0.127 |
| IL-8 | 1.710 | 0.255 | 4.110 | 0.127 |
| IL-10 | 1.185 | 0.700 | 0.435 | 0.078 |
| IL-17 | 0.860 | 0.100 | 0.080 | 0.100 |
| Eotaxin | 0.075 | 0.064 | 0.150 | 0.000 |
| FGF | 1.150 | 0.000 | 0.640 | 0.000 |
| G-CSF | 12.585 | 4.914 | 5.555 | 0.700 |
| IP-10 | 5.040 | 0.000 | 1.450 | 1.909 |
| MCP-1 | 8.930 | 1.329 | 0.470 | 0.000 |
| MIP-1a | 0.180 | 0.000 | 0.140 | 0.057 |
| RANTES | 4.820 | 0.000 | 2.835 | 0.219 |
| VEGF | 157.330 | 8.895 | 88.810 | 1.966 |
| Common Cytokines Secreted on Day 3: | Common Cytokines Secreted on Day 7: | ||
|---|---|---|---|
| Bio-Oss® Collagen | Osteogenic Conditions | Bio-Oss® Collagen | Osteogenic Conditions |
| PDGF-β | PDGF-β | PDGF-β | PDGF-β |
| IL-4 | IL-4 | IL-1β | IL-1β |
| IL-6 | IL-6 | IL-4 | IL-4 |
| IL-8 | IL-8 | IL-5 | IL-5 |
| IL-10 | IL-10 | IL-6 | IL-6 |
| G-CSF | G-CSF | IL-8 | IL-8 |
| IP-10 | IP-10 | IL-10 | IL-10 |
| MIP-1α | MIP-1α | IL-17 | IL-17 |
| RANTES | RANTES | Eotaxin | Eotaxin |
| VEGF | VEGF | FGF | FGF |
| Uncommon cytokines secreted on day 3: | G-CSF | G-CSF | |
| IL-5 | IP-10 | IP-10 | |
| IL-9 | MCP1 | MCP1 | |
| IL-12 | MIP-1α | MIP-1α | |
| IL-15 | RANTES | RANTES | |
| IL-17 | VEGF | VEGF | |
| INF-γ | Uncommon cytokines secreted on day 7: | ||
| IL-12 | |||
| INF-γ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaquinta, M.R.; De Pace, R.; Benkhalqui, A.; D’Agostino, A.; Trevisiol, L.; Finotti, A.; Breveglieri, G.; Tognon, M.; Martini, F.; Mazzoni, E. Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material. Int. J. Mol. Sci. 2025, 26, 3807. https://doi.org/10.3390/ijms26083807
Iaquinta MR, De Pace R, Benkhalqui A, D’Agostino A, Trevisiol L, Finotti A, Breveglieri G, Tognon M, Martini F, Mazzoni E. Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material. International Journal of Molecular Sciences. 2025; 26(8):3807. https://doi.org/10.3390/ijms26083807
Chicago/Turabian StyleIaquinta, Maria Rosa, Raffaella De Pace, Assia Benkhalqui, Antonio D’Agostino, Lorenzo Trevisiol, Alessia Finotti, Giulia Breveglieri, Mauro Tognon, Fernanda Martini, and Elisa Mazzoni. 2025. "Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material" International Journal of Molecular Sciences 26, no. 8: 3807. https://doi.org/10.3390/ijms26083807
APA StyleIaquinta, M. R., De Pace, R., Benkhalqui, A., D’Agostino, A., Trevisiol, L., Finotti, A., Breveglieri, G., Tognon, M., Martini, F., & Mazzoni, E. (2025). Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material. International Journal of Molecular Sciences, 26(8), 3807. https://doi.org/10.3390/ijms26083807









