Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARγ Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Effect of Luteolin on Body Weight, Organ Weight, and Blood Pressure
2.2. Effect of Luteolin on Glucose Homeostasis in HFD-Induced MAFLD Rats
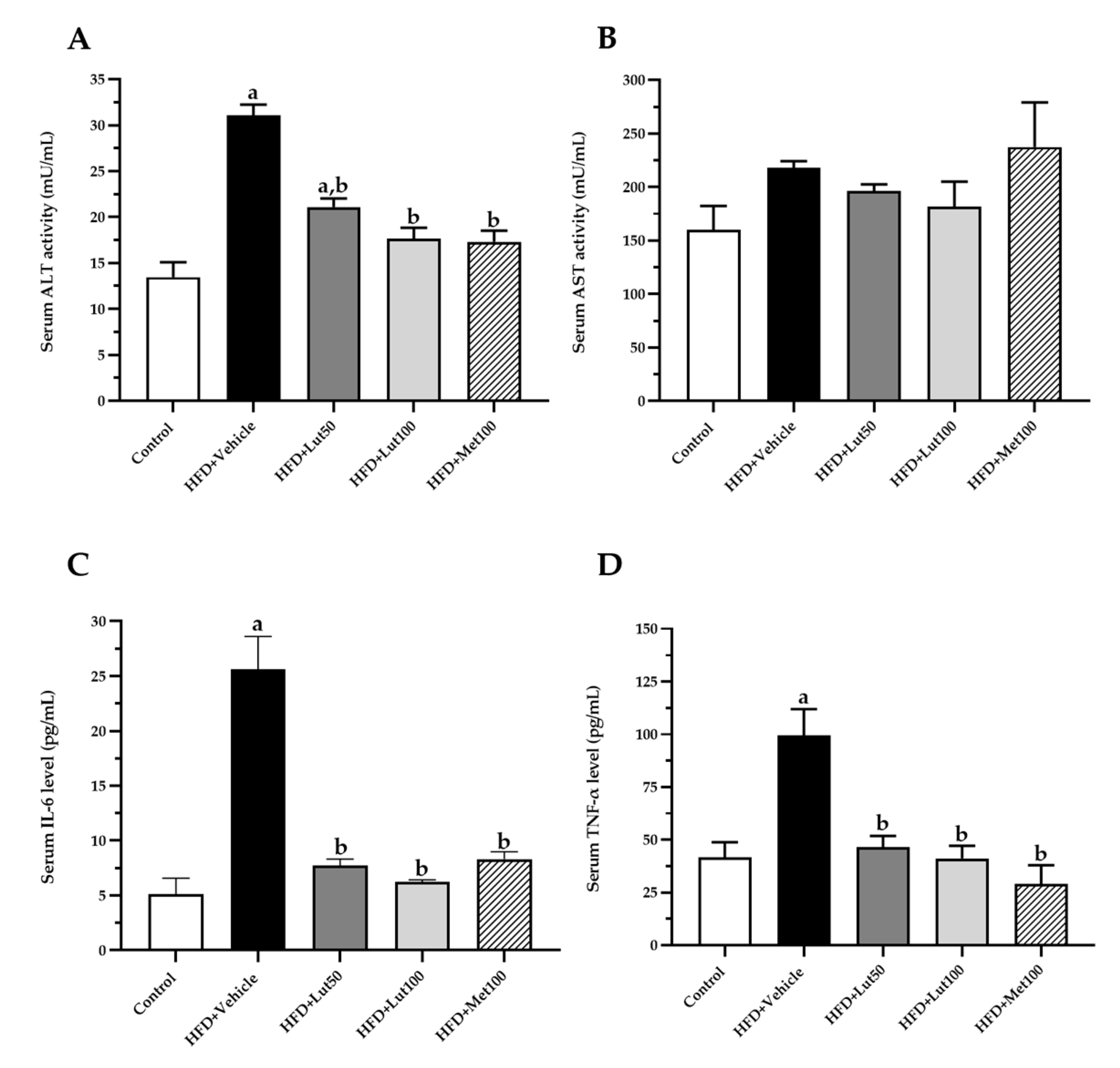
2.3. Effect of Luteolin on Lipid Profiles and Inflammatory Parameters
2.4. Effect of Luteolin on Oxidative Stress Markers
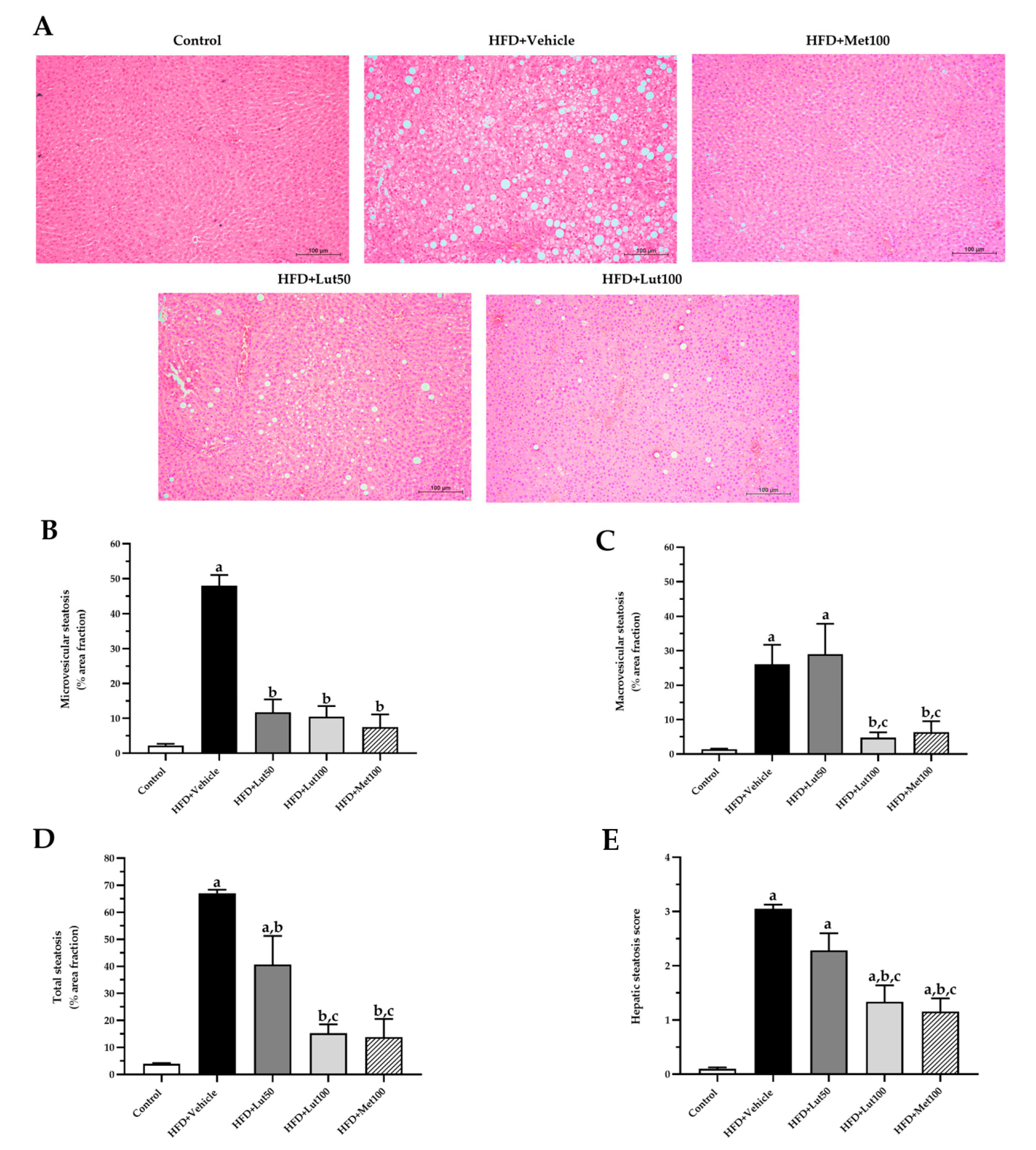
2.5. Effect of Luteolin on Histological Examination in Liver
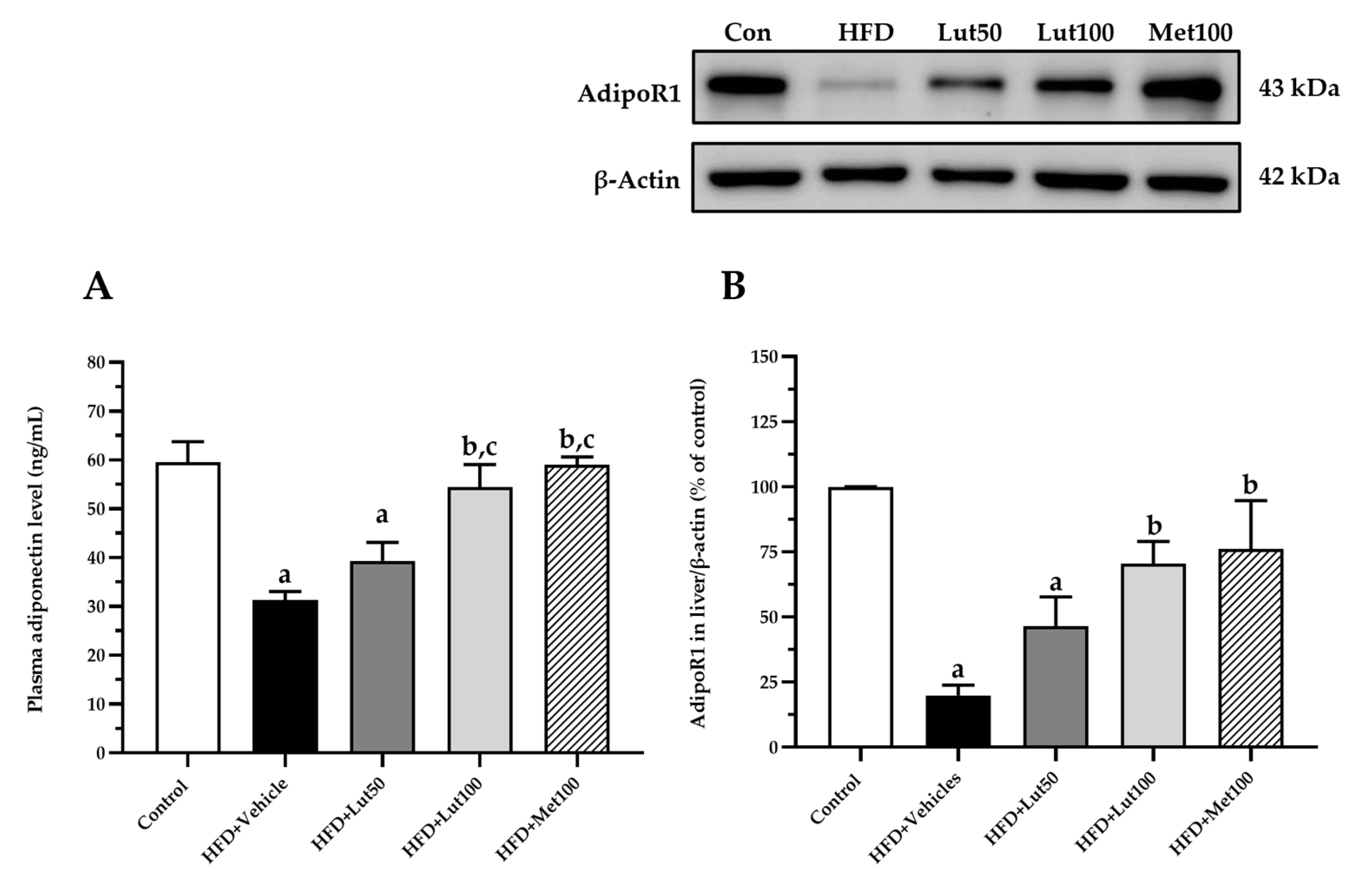
2.6. Effect of Luteolin on Adiponectin Levels and AdipoR1 Protein Expression in Liver
2.7. Effect of Luteolin on AMPK and PPARγ Protein Expression in Liver
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal and Experimental Groups
4.3. Systolic Blood Pressure Measurements
4.4. Glucose Metabolism Determination
4.5. Lipid Metabolism Determination
4.6. Inflammatory Markers, Plasma Aspartate Transaminase, Alanine Aminotransferase, and Adiponectin Analysis
4.7. Oxidative Stress and Antioxidant Biomarker Determination
4.8. Histopathology Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAFLD | Metabolic-dysfunction-associated fatty liver disease |
| HFD | High-fat diet |
| AdipoR1 | Adiponectin receptor 1 |
| AMPK | AMP-activated protein kinase |
| PPARγ | Peroxisome proliferator-activated receptor type γ |
| Lut | Luteolin |
| Met | Metformin |
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- James, M.; Varghese, T.P.; Sharma, R.; Chand, S. Association Between Metabolic Syndrome and Diabetes Mellitus According to International Diabetic Federation and National Cholesterol Education Program Adult Treatment Panel III Criteria: A Cross-Sectional Study. J. Diabetes Metab. Disord. 2020, 19, 437–443. [Google Scholar] [CrossRef]
- Mitrovic, B.; Obradovic, M.; Gluvic, Z.; Radunovic, M.; Rizzo, M.; Banach, M.; Isenovic, E. Non-Alcoholic Fatty Liver Disease, Metabolic Syndrome, and Type 2 Diabetes Mellitus: Where Do We Stand Today? Arch. Med. Sci. 2022, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Kemp, W.W.; Roberts, S.K. Nonalcoholic Fatty Liver Disease: Current Concepts, Epidemiology and Management Strategies. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.-H.; George, J. MAFLD: How Is It Different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E. Diabetes and Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolism 2021, 123, 154868. [Google Scholar] [CrossRef]
- Dongoran, R.A.; Tu, F.-C.; Liu, C.-H. Current Insights into the Interplay between Gut Microbiota-Derived Metabolites and Metabolic-Associated Fatty Liver Disease. Tzu. Chi Med. J. 2023, 35, 290–299. [Google Scholar] [CrossRef]
- Chua, D.; Low, Z.S.; Cheam, G.X.; Ng, A.S.; Tan, N.S. Utility of Human Relevant Preclinical Animal Models in Navigating NAFLD to MAFLD Paradigm. Int. J. Mol. Sci. 2022, 23, 14762. [Google Scholar] [CrossRef]
- Mato, J.M.; Alonso, C.; Noureddin, M.; Lu, S.C. Biomarkers and Subtypes of Deranged Lipid Metabolism in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2019, 25, 3009–3020. [Google Scholar] [CrossRef]
- Wayal, V.; Wang, S.-D.; Hsieh, C.-C. Novel Bioactive Peptides Alleviate Western Diet-Induced MAFLD in C57BL/6J Mice by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis via TLR4/NF-κB and Keap1/Nrf2/HO-1 Signaling Pathways. Int. Immunopharmacol. 2025, 148, 114177. [Google Scholar] [CrossRef]
- Jiang, L.; Yi, R.; Chen, H.; Wu, S. Quercetin Alleviates Metabolic-Associated Fatty Liver Disease by Tuning Hepatic Lipid Metabolism, Oxidative Stress and Inflammation. Anim. Biotechnol. 2025, 36, 2442351. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Bustamante, A.; Álvarez, D.; Ortiz, M.; Soto-Alarcon, S.A.; Muñoz, P.; Corbari, A.; Videla, L.A. Attenuation of High-Fat Diet-Induced Rat Liver Oxidative Stress and Steatosis by Combined Hydroxytyrosol-(HT-) Eicosapentaenoic Acid Supplementation Mainly Relies on HT. Oxidative Med. Cell. Longev. 2018, 2018, 5109503. [Google Scholar] [CrossRef]
- Vornoli, A.; Souid, A.; Lazzari, B.; Turri, F.; Pizzi, F.; Bramanti, E.; Campanella, B.; Trouki, C.; Raffaelli, A.; Wójcik, M.; et al. A Moderate Intake of Beer Improves Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in a High-Fat Diet (HFD)-Induced Mouse Model. Molecules 2024, 29, 5954. [Google Scholar] [CrossRef] [PubMed]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A High-fat Diet Leads to the Progression of Non-alcoholic Fatty Liver Disease in Obese Rats. FASEB J. 2005, 19, 136–138. [Google Scholar] [CrossRef]
- Noh, D.; Yoon, G.-A. Mulberry (Morus alba L.) Ethanol Extract Attenuates Lipid Metabolic Disturbance and Adipokine Imbalance in High-Fat Fed Rats. Nutr. Res. Pract. 2022, 16, 716. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma Concentrations of a Novel, Adipose-Specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Pan, J.; Ding, Y.; Sun, Y.; Li, Q.; Wei, T.; Gu, Y.; Zhou, Y.; Pang, N.; Pei, L.; Ma, S.; et al. Associations between Adipokines and Metabolic Dysfunction-Associated Fatty Liver Disease Using Three Different Diagnostic Criteria. J. Clin. Med. 2023, 12, 2126. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Dupont, J.; Chabrolle, C.; Ramé, C.; Tosca, L.; Coyral-Castel, S. Role of the Peroxisome Proliferator-Activated Receptors, Adenosine Monophosphate-Activated Kinase, and Adiponectin in the Ovary. PPAR Res. 2008, 2008, 176275. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis Through Adipose Tissue Crosstalk. Cell Metab. 2017, 23, 770–784. [Google Scholar] [CrossRef]
- Astapova, O.; Leff, T. Adiponectin and PPARg: Cooperative and Interdependent Actions of Two Key Regulators of Metabolism. Vitam. Horm. 2012, 90, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Rojas, Á.; Lara-Romero, C.; Muñoz-Hernández, R.; Gato, S.; Ampuero, J.; Romero-Gómez, M. Emerging Pharmacological Treatment Options for MAFLD. Ther. Adv. Endocrinol. 2022, 13, 20420188221142452. [Google Scholar] [CrossRef]
- Stefano, J.T.; Duarte, S.M.B.; Ribeiro Leite Altikes, R.G.; Oliveira, C.P. Non-Pharmacological Management Options for MAFLD: A Practical Guide. Ther. Adv. Endocrinol. 2023, 14, 20420188231160394. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Barbero-Becerra, V.J.; Santiago-Hernandez, J.J.; Villegas-Lopez, F.A.; Mendez-Sanchez, N.; Uribe, M.; Chavez-Tapia, N.C. Mechanisms Involved in the Protective Effects of Metformin Against Nonalcoholic Fatty Liver Disease. Curr. Med. Chem. 2012, 19, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, T.; Rahman, M.M.; Khan, F.; Kabir, F.; Nahar, K.; Lasker, S.; Islam, M.D.; Hossain, M.M.; Hasan, R.; Rana, S.; et al. Metformin Treatment Reverses High Fat Diet- Induced Non-Alcoholic Fatty Liver Diseases and Dyslipidemia by Stimulating Multiple Antioxidant and Anti-Inflammatory Pathways. Biochem. Biophys. Rep. 2021, 28, 101168. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Gogoi, R. Antioxidant and Antitumor Efficacy of Luteolin, a Dietary Flavone on Benzo(a)Pyrene-Induced Experimental Lung Carcinogenesis. Biomed. Pharmacother. 2016, 82, 568–577. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin Protects HUVECs from TNF-α-Induced Oxidative Stress and Inflammation via Its Effects on the Nox4/ROS-NF-κB and MAPK Pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin Prevents Cardiometabolic Alterations and Vascular Dysfunction in Mice with HFD-Induced Obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Dai, X.; Liang, B.; Sun, Y. Luteolin Ameliorates Rat Model of Metabolic Syndrome-Induced Cardiac Injury by Apoptosis Suppression and Autophagy Promotion via NR4A2/P53 Regulation. BMC Complement. Med. Ther. 2025, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Shehnaz, S.I.; Roy, A.; Vijayaraghavan, R.; Sivanesan, S. Luteolin Mitigates Diabetic Dyslipidemia in Rats by Modulating ACAT-2, PPARα, SREBP-2 Proteins, and Oxidative Stress. Appl. Biochem. Biotechnol. 2023, 195, 4893–4914. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and Nutritional Overview of Diet-Induced Metabolic Syndrome Models in Rats: What Is the Best Choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, Weight Gain, and the Insulin Resistance Syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; McNeill, J.H. Fructose-Induced Hypertension in Rats Is Concentration- and Duration-Dependent. J. Pharmacol. Toxicol. Methods 1995, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.E.; Gillis, E.E.; Musall, J.B.; Baban, B.; Sullivan, J.C. High-Fat Diet-Induced Hypertension Is Associated with a Proinflammatory T Cell Profile in Male and Female Dahl Salt-Sensitive Rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1713–H1723. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Chen, H.; Liu, T.; Chen, L.; Huang, Y.; Zeng, F.; Liu, B. Luteolin Cooperated with Metformin Hydrochloride Alleviates Lipid Metabolism Disorders and Optimizes Intestinal Flora Compositions of High-Fat Diet Mice. Food Funct. 2020, 11, 10033–10046. [Google Scholar] [CrossRef]
- Pal, S.C.; Méndez-Sánchez, N. Insulin Resistance and Adipose Tissue Interactions as the Cornerstone of Metabolic (Dysfunction)-Associated Fatty Liver Disease Pathogenesis. World J. Gastroenterol. 2023, 29, 3999–4008. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of Fatty Acids Stored in Liver and Secreted via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Kucera, O. Experimental Models of Non-Alcoholic Fatty Liver Disease in Rats. World J. Gastroenterol. 2014, 20, 8364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Zhang, Q.-Z.; Wang, Y.-R.; Fu, L.-N.; Han, J.-S.; Zhang, J.; Wang, B.-M. Astragaloside IV Improves High-Fat Diet–Induced Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Rats by Regulating Inflammatory Factors Level via TLR4/NF-κB Signaling Pathway. Front. Pharmacol. 2021, 11, 605064. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol Inhibits Nonalcoholic Fatty Liver Disease in Rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef]
- Qu, D.; Zhang, K.; Chen, L.; Wang, Q.; Wang, H. RNA-Sequencing Analysis of the Effect of Luteolin on Methamphetamine-Induced Hepatotoxicity in Rats: A Preliminary Study. PeerJ 2020, 8, e8529. [Google Scholar] [CrossRef] [PubMed]
- Fikry, H.; Saleh, L.A.; Sadek, D.R.; Alkhalek, H.A.A. The Possible Protective Effect of Luteolin on Cardiovascular and Hepatic Changes in Metabolic Syndrome Rat Model. Cell Tissue Res. 2025, 399, 27–60. [Google Scholar] [CrossRef]
- Madhesh, M.; Vaiyapuri, M. Effect of Luteolin on Lipid Peroxidation and Antioxidants in Acute and Chronic Periods of Isoproterenol Induced Myocardial Infarction in Rats. J. Acute Med. 2012, 2, 70–76. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin Inhibits IL-1β-Induced Inflammation in Rat Chondrocytes and Attenuates Osteoarthritis Progression in a Rat Model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Jeon, I.; Kim, H.; Kang, H.; Lee, H.-S.; Jeong, S.; Kim, S.; Jang, S. Anti-Inflammatory and Antipruritic Effects of Luteolin from Perilla (P. frutescens L.) Leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N. The Physiological and Pathophysiological Role of Adiponectin and Adiponectin Receptors in the Peripheral Tissues and CNS. FEBS Lett. 2008, 582, 74–80. [Google Scholar] [CrossRef]
- Qiu, Y.-Y.; Zhang, J.; Zeng, F.-Y.; Zhu, Y.Z. Roles of the Peroxisome Proliferator-Activated Receptors (PPARs) in the Pathogenesis of Nonalcoholic Fatty Liver Disease (NAFLD). Pharmacol. Res. 2023, 192, 106786. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Chen, S. The Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Pinostrobin in Rats: Ultra-High-Performance Liquid Chromatography Coupled with Linear Trap Quadrupole Orbitrap Mass Spectrometry Studies. Front. Pharmacol. 2020, 11, 574638. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, K.; Sharma, E.; Amara, V.R.; Pamulapati, H.; Dhawale, V.S. Metformin Improves Metabolic Memory in High Fat Diet (HFD)-Induced Renal Dysfunction. J. Biol. Chem. 2016, 291, 21848–21856. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, T.; Nekooeian, A.A.; Tanideh, N.; Koohi-Hosseinabadi, O.; Masoumi, S.J.; Amanat, S.; Azarpira, N.; Monabati, A. A Comparison of the Effects of Stevia Extract and Metformin on Metabolic Syndrome Indices in Rats Fed with a High-fat, High-sucrose Diet. J. Food Biochem. 2020, 44, e13242. [Google Scholar] [CrossRef]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Hesperidin Ameliorates Signs of the Metabolic Syndrome and Cardiac Dysfunction via IRS/Akt/GLUT4 Signaling Pathway in a Rat Model of Diet-Induced Metabolic Syndrome. Eur. J. Nutr. 2021, 60, 833–848. [Google Scholar] [CrossRef]
- Cheng, A.Y.Y. Oral Antihyperglycemic Therapy for Type 2 Diabetes Mellitus. Can. Med. Assoc. J. 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, S.M.; Rashid, H.; Hussain, Z.; Arshad, M.I.; Khan, J.A. Adiponectin and PPAR: A Setup for Intricate Crosstalk between Obesity and Non-Alcoholic Fatty Liver Disease. Rev. Endocr. Metab. Disord. 2019, 20, 253–261. [Google Scholar] [CrossRef]
- Chukwunonso Obi, B.; Chinwuba Okoye, T.; Okpashi, V.E.; Nonye Igwe, C.; Olisah Alumanah, E. Comparative Study of the Antioxidant Effects of Metformin, Glibenclamide, and Repaglinide in Alloxan-Induced Diabetic Rats. J. Diabetes Res. 2016, 2016, 1635361. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Y.; Lan, Y.; He, Q.; Xu, L.; Pei, Z. Luteolin Reduces Cardiac Damage Caused by Hyperlipidemia in Sprague-Dawley Rats. Heliyon 2023, 9, e17613. [Google Scholar] [CrossRef]
- Maneesai, P.; Jan-o, B.; Poasakate, A.; Rattanakanokchai, S.; Tong-Un, T.; Phuthong, S.; Pakdeechote, P. Limonin Mitigates Cardiometabolic Complications in Rats with Metabolic Syndrome through Regulation of the IRS-1/GLUT4 Signalling Pathway. Biomed. Pharmacother. 2023, 161, 114448. [Google Scholar] [CrossRef]
- Maneesai, P.; Wattanathorn, J.; Potue, P.; Khamseekaew, J.; Rattanakanokchai, S.; Thukham-Mee, W.; Muchimapura, S.; Pakdeechote, P. Cardiovascular Complications Are Resolved by Tuna Protein Hydrolysate Supplementation in Rats Fed with a High-Fat Diet. Sci. Rep. 2023, 13, 12280. [Google Scholar] [CrossRef]
- Apaijit, K.; Pakdeechote, P.; Maneesai, P.; Meephat, S.; Prasatthong, P.; Bunbupha, S. Hesperidin Alleviates Vascular Dysfunction and Remodelling in High-Fat/High-Fructose Diet-Fed Rats by Modulating Oxidative Stress, Inflammation, AdipoR1, and eNOS Expression. Tissue Cell 2022, 78, 101901. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Van Den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- AlNafea, H.M.; Korish, A.A. Activation of the Peroxisome Proliferator-Activated Receptors (PPAR-α/γ) and the Fatty Acid Metabolizing Enzyme Protein CPT1A by Camel Milk Treatment Counteracts the High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease. PPAR Res. 2021, 2021, 5558731. [Google Scholar] [CrossRef] [PubMed]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Khamseekaew, J.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Galangin Resolves Cardiometabolic Disorders through Modulation of AdipoR1, COX-2, and NF-κB Expression in Rats Fed a High-Fat Diet. Antioxidants 2021, 10, 769. [Google Scholar] [CrossRef]
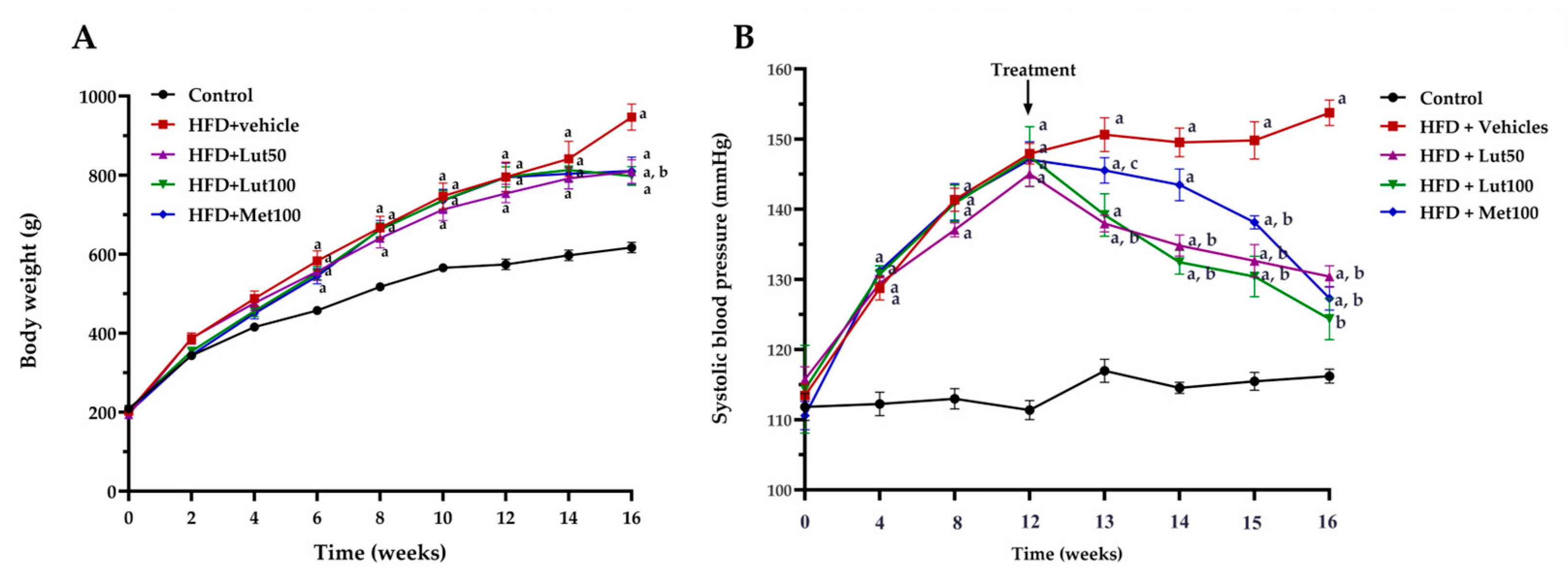
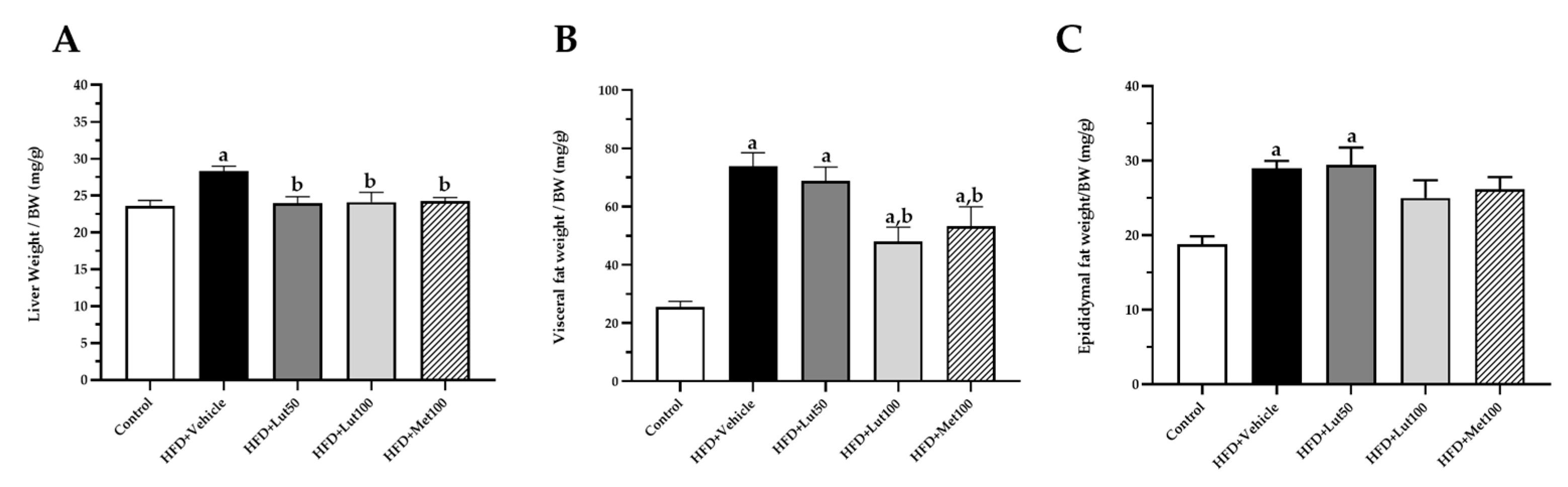
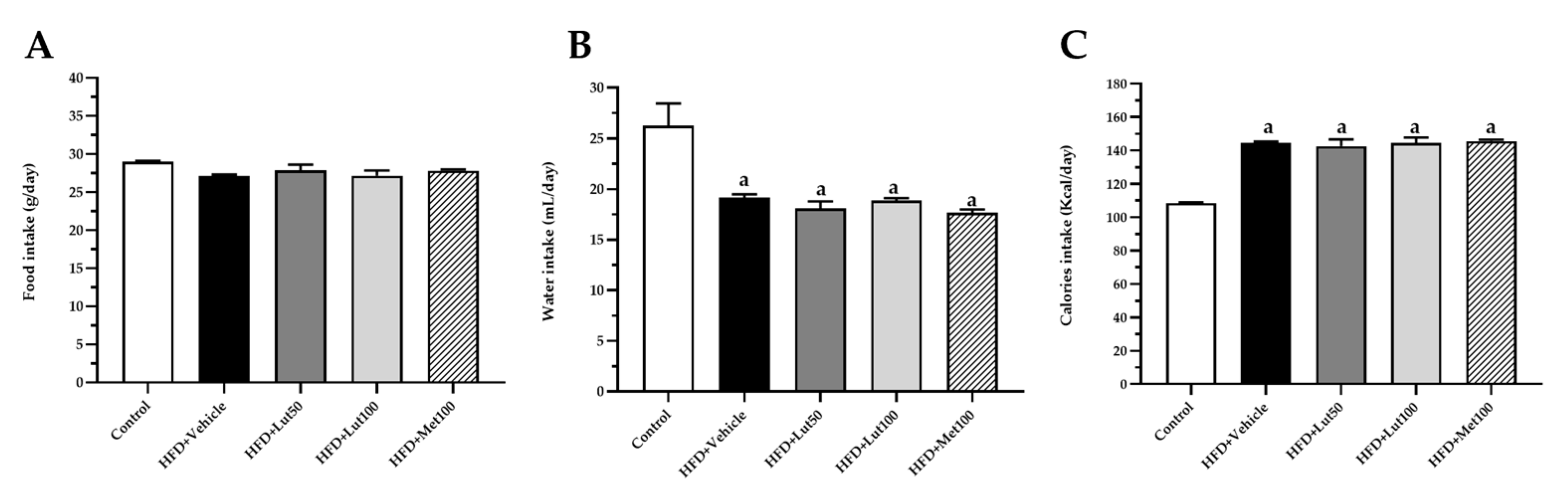
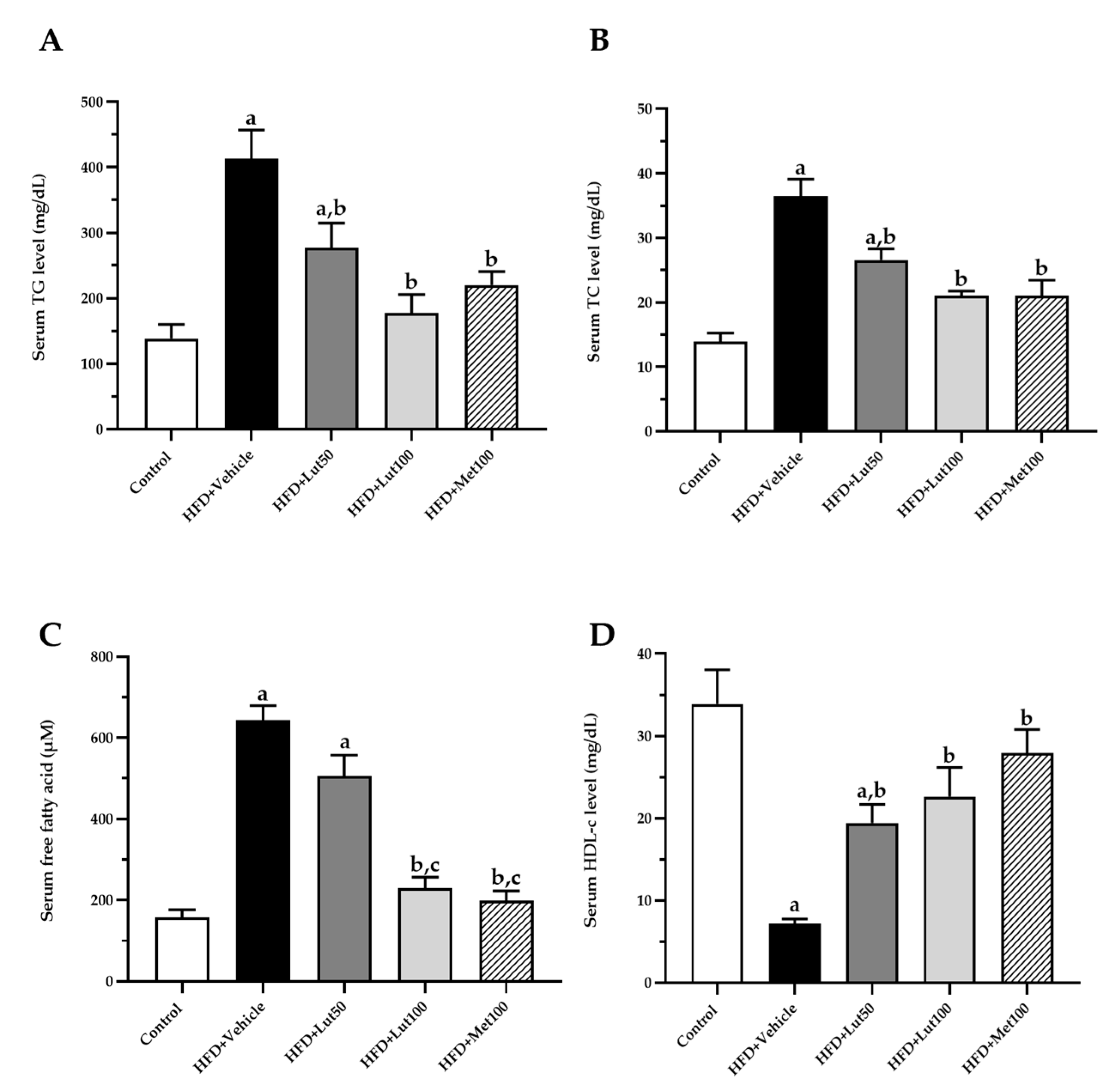

| Parameters | Control | HFD + Vehicle | HFD + Lut50 | HFD + Lut100 | HFD + Met100 |
|---|---|---|---|---|---|
| Fasting blood glucose (mg/dL) | 98.33 ± 0.92 | 127.17 ± 1.96 a | 112.83 ± 2.07 a | 99.57 ± 1.46 bc | 100.33 ± 3.83 bc |
| Fasting serum insulin (mg/dL) | 103.31 ± 11.64 | 260.21 ± 26.94 a | 190.04 ± 32.63 | 161.47 ± 14.72 b | 163.01 ± 17.55 b |
| HOMA-IR index | 23.82 ± 1.18 | 74.58 ± 9.72 a | 51.47 ± 9.69 | 39.72 ± 3.78 b | 44.84 ± 3.92 b |
| AUC of OGTT (mg/dL*min) | 22,170 ± 311.94 | 27,540 ± 468.09 a | 26,047.5 ± 291.98 a | 24,147.5 ± 392.73 abc | 24,285 ± 488.88 ab |
| Parameters | Control | HFD + Vehicle | HFD + Lut50 | HFD + Lut100 | HFD + Met100 |
|---|---|---|---|---|---|
| Plasma: | |||||
| Plasma MDA level (μM) | 4.15 ± 0.24 | 8.08 ± 0.29 a | 6.97 ± 0.36 a | 5.22 ± 0.66 b | 5.30 ± 0.77 b |
| Plasma CAT activity (U/mL) | 43.86 ± 7.27 | 8.22 ± 1.56 a | 31.87 ± 4.33 b | 35.20 ± 4.39 b | 40.94 ± 3.65 b |
| Liver tissue: | |||||
| Hepatic MDA level (nmol/g protein) | 360.58 ± 10.48 | 707.33 ± 34.93 a | 548.98 ± 69.52 | 394.07 ± 24.96 b | 397.73 ± 18.09 b |
| Hepatic O2•− production (count/mg dry weight/min) | 161.15 ± 24.24 | 573.92 ± 73.16 a | 467.36 ± 104.08 | 206.69 ± 32.68 b | 132.59 ± 56.18 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taweesap, P.; Potue, P.; Khamseekaew, J.; Iampanichakul, M.; Jan-O, B.; Pakdeechote, P.; Maneesai, P. Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARγ Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3804. https://doi.org/10.3390/ijms26083804
Taweesap P, Potue P, Khamseekaew J, Iampanichakul M, Jan-O B, Pakdeechote P, Maneesai P. Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARγ Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(8):3804. https://doi.org/10.3390/ijms26083804
Chicago/Turabian StyleTaweesap, Pongsakorn, Prapassorn Potue, Juthamas Khamseekaew, Metee Iampanichakul, Banyaphon Jan-O, Poungrat Pakdeechote, and Putcharawipa Maneesai. 2025. "Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARγ Signaling Pathway" International Journal of Molecular Sciences 26, no. 8: 3804. https://doi.org/10.3390/ijms26083804
APA StyleTaweesap, P., Potue, P., Khamseekaew, J., Iampanichakul, M., Jan-O, B., Pakdeechote, P., & Maneesai, P. (2025). Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARγ Signaling Pathway. International Journal of Molecular Sciences, 26(8), 3804. https://doi.org/10.3390/ijms26083804






