The Current Roadmap of Lung Cancer Biology, Genomics and Racial Disparity
Abstract
:1. Introduction
2. Epidemiology and Risk Factors: An Ethnic and Ancestral View
2.1. Histological Subtypes of Lung Cancer
2.2. Environmental and Lifestyle Risk Factors
2.3. Genetic and Racial Disparities and Lung Cancer Susceptibility
3. Current Detection and Treatment Modalities of Lung Cancer
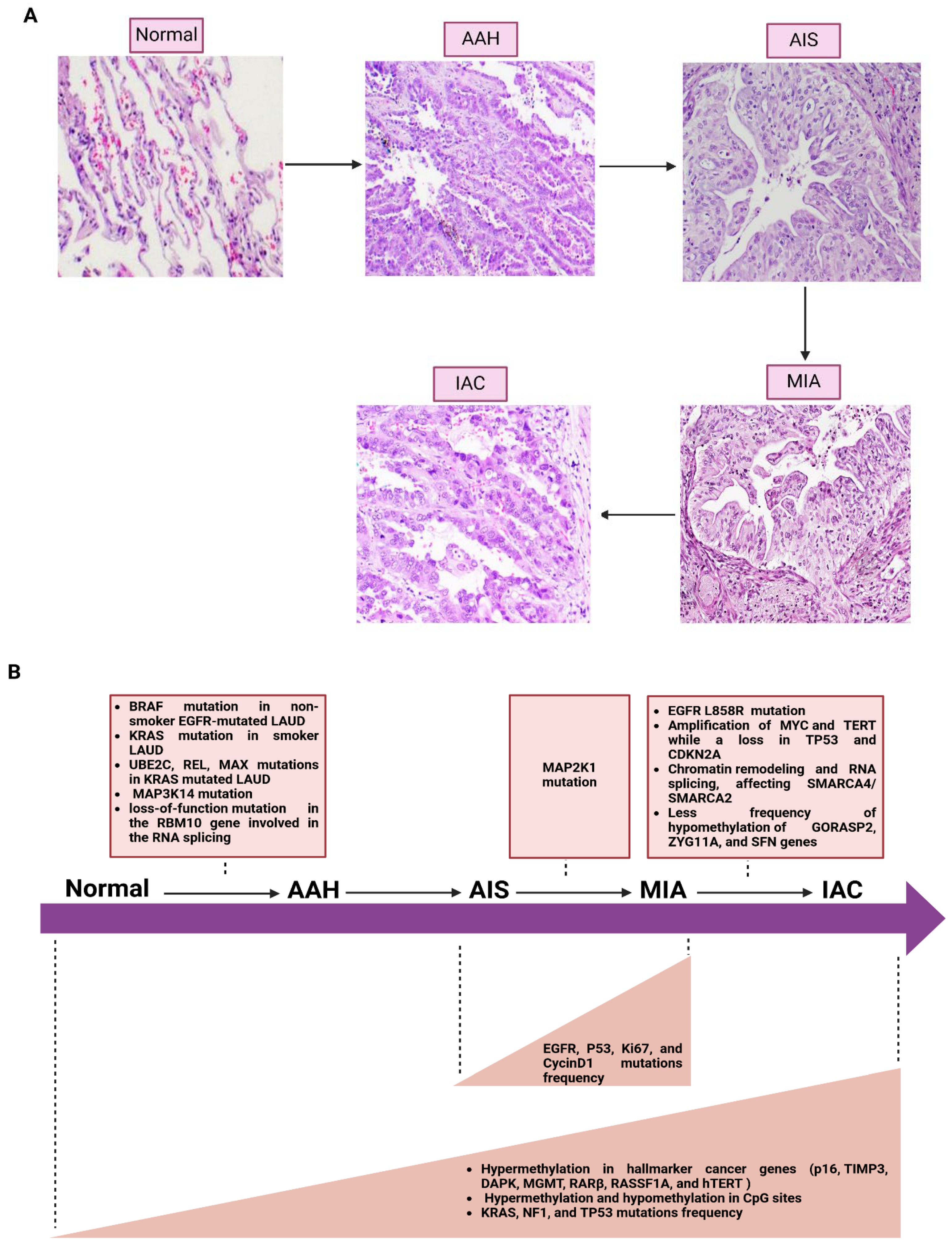
4. Molecular Progression of Lung Cancer
5. The Nuclear Genetic Alterations in Lung Cancer Subtypes and Their Racial Distribution
5.1. The Nuclear Genetic Alterations in LUAD Subtype and Its Racial Distribution
5.2. The Nuclear Genetic Alterations in LUSC Subtype and Its Racial Distribution
5.3. The Nuclear Genetic Alterations in Adenosquamous Carcinoma Subtype and Its Racial Distribution
5.4. The Nuclear Genetic Alterations in SCLC and Its Racial Distribution
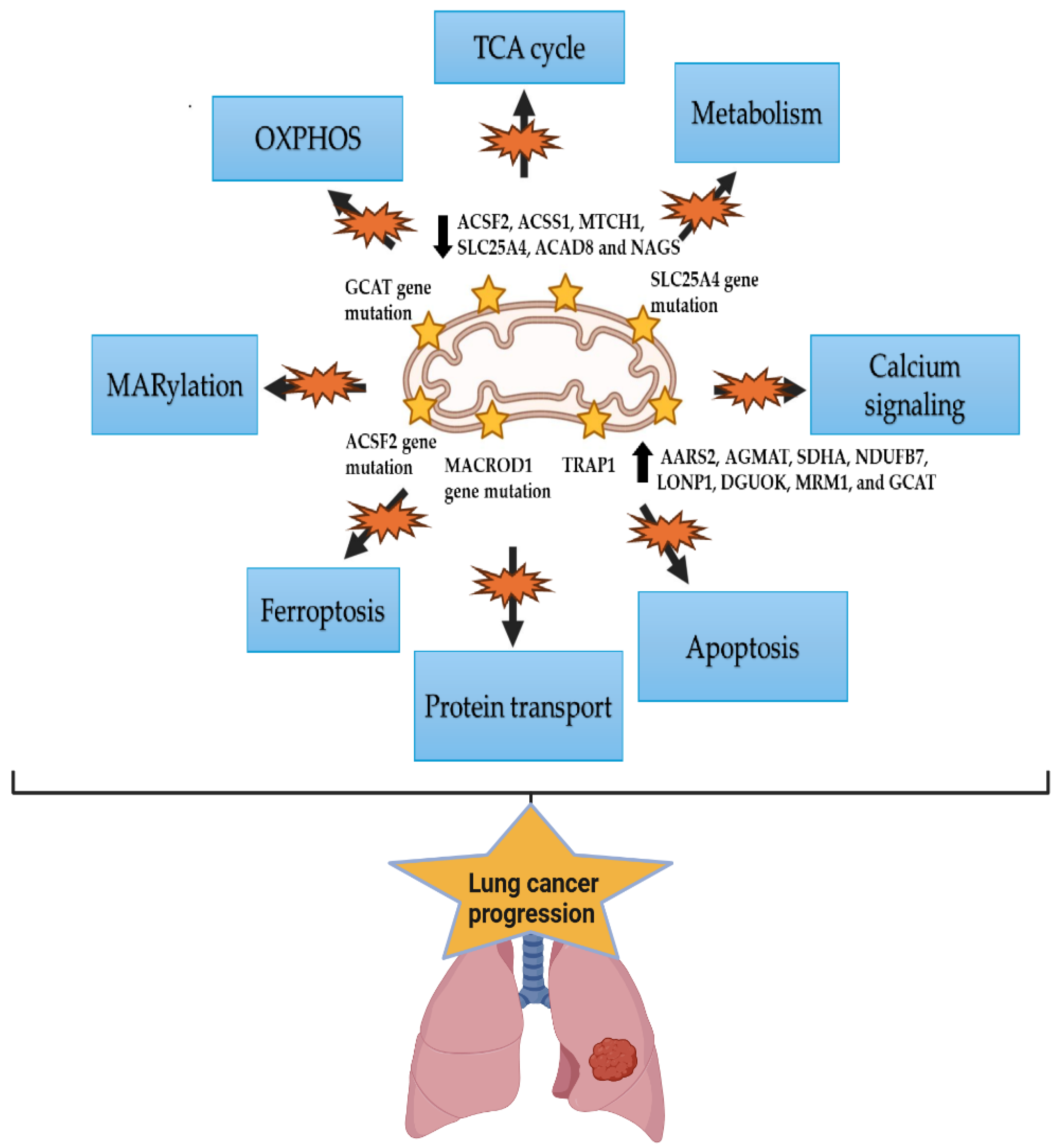
6. The Mitochondrial Alterations in Lung Cancer and Racial Disparity
7. The Epigenetic Alteration Patterns in Lung Cancer Patients with Various Ethnic and Racial Backgrounds
8. The Microbiome Signature in Lung Cancer Subtypes and Racial Differences
| Microbiome | Significance | Citations |
|---|---|---|
| Actinobacteria phylum, Corynebacteriaceae, Halomonadaceae families, Corynebacterium, Lachnoanaerobaculum, and Halomonas genera | Decreased in lung cancer patients compared to control people | [153] |
| Enterococcus, Lactobacillus, Escherichia, Phylum TM7, Capnocytophaga, Blautia, Streptococcus, Neisseria, and Prevotella | Bacterial markers in lung cancer | [155,156] |
| Acidovorax, Clostridioides, Succinimonas, and Shewanella | Prediction of recurrence or metastasis (RM) lung cancer tissue | [157] |
| Actinomyces graevenitzii | Abundant in LUSC | [161] |
| Haemophilus parainfluenza, Neisseria subflava, Porphyromonas endodontics, Fusobacterium nucleatum, and Pseudomonas | Abundant in LUAD | [161] |
| Acidovorax and Veillonella | Differentiating between LUSC and LUAD | [162] |
| Streptococcus and Neisseria | Most prevalent in LUAD | [156] |
| Streptococcus | Most prevalent in LUSC | [156] |
| Bacillus and Castellaniella | Enriched in LUAD | [163] |
| Brucella | Enriched in LUSC | [163] |
| Proteobacteria | Discriminated in LUAD and LUSC | [164] |
| Thermus and Gram-positive bacteria | The prevalence is higher in LUAD than in LUSC | [165,166] |
| Leptotrichia sp._oral_taxon_225 | Reducing lung cancer risk in African Americans (AA) | [150] |
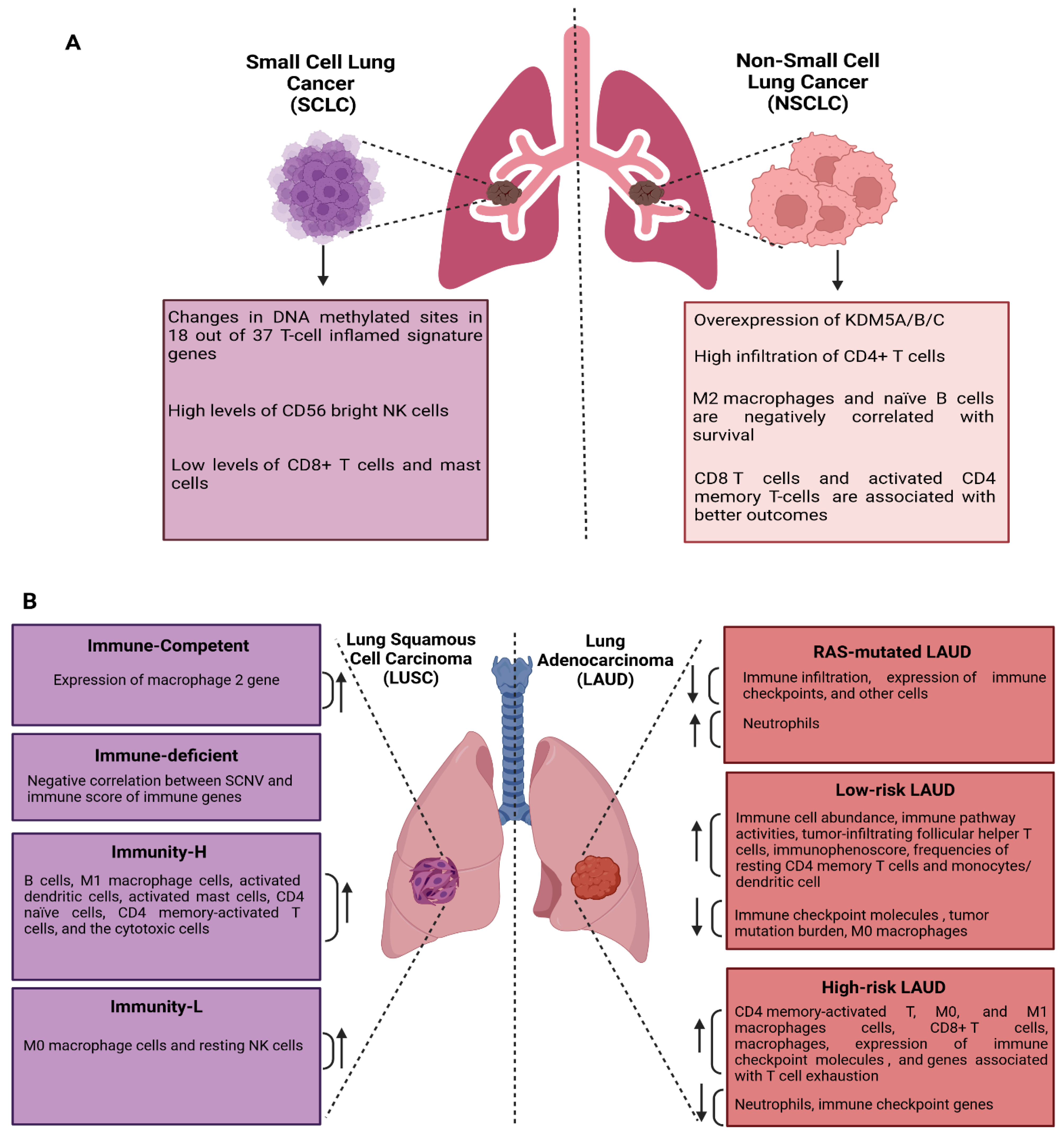
9. The Immune Alteration Signature in Lung Cancer
9.1. Immune Alteration Signatures in NSCLC and SCLC
9.2. Immune Profiles of LUAD and LUSC
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, J.; Chen, Y.; Liu, Z.; Xia, H.; Xu, H. Gender disparities in lung cancer incidence in the United States during 2001–2019. Sci. Rep. 2023, 13, 12581. [Google Scholar] [CrossRef]
- Nolen, L. Lung Cancer Incidence in Young & Middle-Aged U.S. Women. Oncol. Times 2023, 45, 11–12. [Google Scholar]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef]
- Cranford, H.; Koru-Sengul, T.; Lopes, G.; Pinheiro, P. Lung Cancer Incidence by Detailed Race–Ethnicity. Cancers 2023, 15, 2164. [Google Scholar] [CrossRef]
- World Health Organization. Lung Cancer. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 11 August 2018).
- Wéber, A.; Morgan, E.; Vignat, J.; Laversanne, M.; Pizzato, M.; Rumgay, H.; Singh, D.; Nagy, P.; Kenessey, I.; Soerjomataram, I.; et al. Lung cancer mortality in the wake of the changing smoking epidemic: A descriptive study of the global burden in 2020 and 2040. BMJ Open 2023, 13, e065303. [Google Scholar] [CrossRef]
- Sharma, R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int. J. Clin. Oncol. 2022, 27, 665–675. [Google Scholar] [CrossRef]
- Patil, S.; Tandel, N.; Bhangdiya, O. Case report: Small cell lung cancer presenting as the “sunray sign” in the chest radiograph and recurrent hemoptysis. BOHR Int. J. Cancer Res. 2022, 1, 26–31. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Lung Cancer Types; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/united-states-cancer-statistics/publications/lung-cancer-types.html (accessed on 1 December 2024).
- American Cancer Society. Key Statistics for Lung Cancer; American Cancer Society: Atlanta, GA, USA, 2024; Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 1 December 2024).
- Hasan, M.T.; Uddin, J.M.; Yasmin, M.T.; Chatterjee, M. Effect of Smoking Duration and Pack-Year History on Histological Subtypes of Lung Cancer. SAS J. Med. 2024, 10, 1338–1343. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Lindsey, A.M.; Estrada, C.A.; Martinez, C.C.; Cusnir, M.; Schwartz, M.; Sriganeshan, V.; Poppiti, R. Genomic landscape of squamous cell carcinoma-Different genetic pathways culminating in a common phenotype. Cancer Treat. Res. Commun. 2020, 25, 100238. [Google Scholar] [CrossRef] [PubMed]
- Marant Micallef, C.; Shield, K.D.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guénel, P.; Olsson, A.; Rushton, L.; Hutchings, S.J.; et al. Occupational exposures and cancer: A review of agents and relative risk estimates. Occup. Environ. Med. 2018, 75, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Bunjaku, J.; Lama, A.; Pesanayi, T.; Shatri, J.; Chamberlin, M.; Hoxha, I. Lung Cancer and Lifestyle Factors. Hematol. Oncol. Clin. N. Am. 2024, 38, 171–184. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.H.; Kim, S.K.; Ha, S.J.; Mok, T.S.; Mitsudomi, T.; Cho, B.C. Lung cancer in never smokers: Change of a mindset in the molecular era. Lung Cancer 2011, 72, 9–15. [Google Scholar] [CrossRef]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung Cancer in Never Smokers: Clinical Epidemiology and Environmental Risk Factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Wen, Y.W.; Tsai, C.R.; Tsai, T.I. Peer Pressure, Psychological Distress and the Urge to Smoke. Int. J. Environ. Res. Public Health 2009, 6, 1799–1811. [Google Scholar] [CrossRef]
- Rozi, S.; Mahmud, S.; Lancaster, G.; Zahid, N. Peer Pressure and Family Smoking Habits Influence Smoking Uptake in Teenage Boys Attending School: Multilevel Modeling of Survey Data. Open J. Epidemiol. 2016, 6, 167–172. [Google Scholar] [CrossRef]
- Harrell, J.S.; Bangdiwala, S.I.; Deng, S.; Webb, J.P.; Bradley, C. Smoking initiation in youth. J. Adolesc. Health 1998, 23, 271–279. [Google Scholar] [CrossRef]
- Sharma, N.; Agarwal, A.K.; Eastwood, P.; Gupta, T.; Singh, A.P. (Eds.) Air Pollution and Control; Energy, Environment, and Sustainability; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Sakoda, L.C.; Alabaster, A.; Sumner, E.T.; Gordon, N.P.; Quesenberry, C.P.; Velotta, J.B. Trends in Smoking-Specific Lung Cancer Incidence Rates Within a US Integrated Health System, 2007–2018. Chest 2023, 164, 785–795. [Google Scholar] [CrossRef]
- Borg, M.; Tønnesen, H.; Ibsen, R.; Hilberg, O.; Løkke, A. Lung cancer: A nationwide analysis of sex and age incidence trends from 1980 to 2022. Acta Oncol. 2024, 63, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cui, X.; Sidorenkov, G.; Groen, H.J.M.; Vliegenthart, R.; Heuvelmans, M.A.; Liu, S.; Oudkerk, M.; De Bock, G.H. Lung cancer occurrence attributable to passive smoking among never smokers in China: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2020, 9, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Zakkouri, F.A.Z.; Saloua, O.; Halima, A.; Rachid, R.; Hind, M.; Hassan, E. Smoking, passive smoking and lung cancer cell types among women in Morocco: Analysis of epidemiological profiling of 101 cases. BMC Res. Notes 2015, 8, 530. [Google Scholar] [CrossRef]
- Zhu, J.; Smith-Warner, S.A.; Yu, D.; Zhang, X.; Blot, W.J.; Xiang, Y.; Sinha, R.; Park, Y.; Tsugane, S.; White, E.; et al. Associations of coffee and tea consumption with lung cancer risk. Int. J. Cancer 2021, 148, 2457–2470. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef]
- Li, L.; Shao, M.; He, X.; Ren, S.; Tian, T. Risk of lung cancer due to external environmental factor and epidemiological data analysis. Math. Biosci. Eng. 2021, 18, 6079–6094. [Google Scholar] [CrossRef]
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of lung cancer. Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Li, Y.; Wei, H. Identifying the Environmental Determinants of Lung Cancer: A Case Study of Henan, China. GeoHealth 2023, 7, e2023GH000794. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Cao, M.; Sun, D.; Lei, L.; Li, N.; Peng, J.; Chen, W. Trends and risk factors of lung cancer in China. Chin. J. Cancer Res. 2020, 32, 683–694. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, X.; Zhu, Y.; Li, D.; Jing, D.; Su, X.; Pan, P.; Liu, H.; Zhang, Y. Association of outdoor air pollution, lifestyle, genetic factors with the risk of lung cancer: A prospective cohort study. Environ. Res. 2023, 218, 114996. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Lung Cancer. Continuous Update Project Expert Report 2018. Available online: https://www.wcrf.org/wp-content/uploads/2024/10/lung-cancer-report.pdf (accessed on 11 June 2024).
- Huang, Y.; Zhu, M.; Ji, M.; Fan, J.; Xie, J.; Wei, X.; Jiang, X.; Xu, J.; Chen, L.; Yin, R.; et al. Air Pollution, Genetic Factors, and the Risk of Lung Cancer: A Prospective Study in the UK Biobank. Am. J. Respir. Crit. Care Med. 2021, 204, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Ruwali, M.; Shukla, R. Interactions of Environmental Risk Factors and Genetic Variations: Association with Susceptibility to Cancer. In Environmental Microbiology and Biotechnology; Singh, A., Srivastava, S., Rathore, D., Pant, D., Eds.; Springer: Singapore, 2021; pp. 211–234. [Google Scholar] [CrossRef]
- Pettit, R.W.; Byun, J.; Han, Y.; Ostrom, Q.T.; Edelson, J.; Walsh, K.M.; Bondy, M.L.; Hung, R.J.; McKay, J.D.; Amos, C.I. The shared genetic architecture between epidemiological and behavioral traits with lung cancer. Sci. Rep. 2021, 11, 17559. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Evans, N.; Mitchell, E. Disparities in lung cancer. J. Natl. Med. Assoc. 2023, 115 (Suppl. S2), S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Judd, J.; Chin, S.; Ragin, C. Disparities in Lung Cancer Treatment. Curr. Oncol. Rep. 2022, 24, 241–248. [Google Scholar] [CrossRef]
- Dator, R.; Villalta, P.W.; Thomson, N.; Jensen, J.; Hatsukami, D.K.; Stepanov, I.; Warth, B.; Balbo, S. Metabolomics Profiles of Smokers from Two Ethnic Groups with Differing Lung Cancer Risk. Chem. Res. Toxicol. 2020, 33, 2087–2098. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Didier, A.J.; Roof, L.; Stevenson, J. Demographic Disparities in Lung Cancer Mortality and Trends in the United States From 1999 Through 2020: A Population-Based CDC Database Analysis. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, e247004. [Google Scholar] [CrossRef]
- Primm, K.M.; Zhao, H.; Hernandez, D.C.; Chang, S. Racial and Ethnic Trends and Disparities in NSCLC. JTO Clin. Res. Rep. 2022, 3, 100374. [Google Scholar] [CrossRef]
- Zhu, F.; Shogan, J.; Wang, H.; Ashamalla, H. Racial Disparities in Treatment and Outcome of Non-Small Cell Lung Cancer (NSCLC) Patients Across Different Facility Types. Int. J. Radiat. Oncol. 2022, 112, e16. [Google Scholar] [CrossRef]
- Sinha, S.; Mitchell, K.A.; Zingone, A.; Bowman, E.; Sinha, N.; Schäffer, A.A.; Lee, J.S.; Ruppin, E.; Ryan, B.M. Higher prevalence of homologous recombination deficiency in tumors from African Americans versus European Americans. Nat. Cancer 2020, 1, 112–121. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Nichols, N.; Tang, W.; Walling, J.; Stevenson, H.; Pineda, M.; Stefanescu, R.; Edelman, D.C.; Girvin, A.T.; Zingone, A.; et al. Recurrent PTPRT/JAK2 mutations in lung adenocarcinoma among African Americans. Nat. Commun. 2019, 10, 5735. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Nie, W.; Lu, J.; Zhang, L.; Zhang, Y.; Zhang, B.; Wang, S.; Hu, M.; Xu, J.; Lou, Y.; et al. Racial disparities in characteristics and prognosis in Asian versus white patients receiving atezolizumab: An ancillary analysis of POPLAR and OAK studies. Ann. Oncol. 2019, 30, ii48. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Mack, P.C. Ethnic difference in lung cancer: An important issue in a globalized society. J. Thorac. Dis. 2020, 12, 3774–3775. [Google Scholar] [CrossRef]
- Kohan, A.; Kulanthaivelu, R.; Hinzpeter, R.; Liu, Z.A.; Ortega, C.; Leighl, N.; Metser, U.; Veit-Haibach, P. Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database. Cancers 2023, 15, 2096. [Google Scholar] [CrossRef]
- Dwyer, L.L.; Vadagam, P.; Vanderpoel, J.; Cohen, C.; Lewing, B.; Tkacz, J. Disparities in Lung Cancer: A Targeted Literature Review Examining Lung Cancer Screening, Diagnosis, Treatment, and Survival Outcomes in the United States. J. Racial Ethn. Health Disparities 2024, 11, 1489–1500. [Google Scholar] [CrossRef]
- Zhu, M.; Lv, J.; Huang, Y.; Ma, H.; Li, N.; Wei, X.; Ji, M.; Ma, Z.; Song, C.; Wang, C.; et al. Ethnic differences of genetic risk and smoking in lung cancer: Two prospective cohort studies. Int. J. Epidemiol. 2023, 52, 1815–1825. [Google Scholar] [CrossRef]
- Izumi, M.; Suzumura, T.; Ogawa, K.; Matsumoto, Y.; Sawa, K.; Yoshimoto, N.; Tani, Y.; Watanabe, T.; Kaneda, H.; Mitsuoka, S.; et al. Differences in molecular epidemiology of lung cancer among ethnicities (Asian vs. Caucasian). J. Thorac. Dis. 2020, 12, 3776–3784. [Google Scholar] [CrossRef]
- Weiner, G.J.; Winn, R.A. Disparate groups share cancer disparities. Trends Cancer 2022, 8, 283–285. [Google Scholar] [CrossRef]
- Brouwer, A.F.; Engle, J.M.; Jeon, J.; Meza, R. Sociodemographic Survival Disparities for Lung Cancer in the United States, 2000-2016. J. Natl. Cancer Inst. 2022, 114, 1492–1500. [Google Scholar] [CrossRef]
- Zeng, H.; Yuan, Z.; Zhang, G.; Li, W.; Guo, L.; Li, N.; Xue, Q.; Tan, F. Racial disparities in histological subtype, stage, tumor grade and cancer-specific survival in lung cancer. Transl. Lung Cancer Res. 2022, 11, 1348–1358. [Google Scholar] [CrossRef]
- National Cancer Institute. Non-Small Cell Lung Cancer Treatment (PDQ®)–Patient Version. Available online: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq (accessed on 25 August 2024).
- Islam, M.S.; Ahasan, M.N. Role of Immunotherapy in Non-Small Cell Lung Cancer (NSCLC). Sch. J. Appl. Med. Sci. 2024, 12, 1302–1308. [Google Scholar] [CrossRef]
- Zhang, R.; Zou, C.; Zeng, L.; Zhang, Y. Perioperative immunotherapy in nonsmall cell lung cancer. Curr. Opin. Oncol. 2024, 37, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yang, D.; Cao, D.; Gong, Z.; He, F.; Hou, Y.; Lin, S. Effect of smoking status on immunotherapy for lung cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1422160. [Google Scholar] [CrossRef] [PubMed]
- Dessai, A.; Nayak, U.Y.; Nayak, Y. Precision nanomedicine to treat non-small cell lung cancer. Life Sci. 2024, 346, 122614. [Google Scholar] [CrossRef]
- Felten, M.K.; Knoll, L.; Schikowsky, C.; Das, M.; Feldhaus, C.; Hering, K.G.; Böcking, A.; Kraus, T. Is it useful to combine sputum cytology and low-dose spiral computed tomography for early detection of lung cancer in formerly asbestos-exposed power industry workers? J. Occup. Med. Toxicol. 2014, 9, 14. [Google Scholar] [CrossRef]
- Qin, T. Application of CRISPR Technology for Early Detection of Lung Cancer. Highlights Sci. Eng. Technol. 2024, 102, 227–231. [Google Scholar] [CrossRef]
- Zhu, J. Targeted therapies for non-small cell lung cancer based on CRISPR-Cas9 technology. Theor. Nat. Sci. 2023, 17, 229–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, C.; Fu, F.; Ma, Z.; Wen, Z.; Ma, X.; Wang, S.; Li, Y.; Chen, H. Excellent Prognosis of Patients With Invasive Lung Adenocarcinomas During Surgery Misdiagnosed as Atypical Adenomatous Hyperplasia, Adenocarcinoma In Situ, or Minimally Invasive Adenocarcinoma by Frozen Section. Chest 2021, 159, 1265–1272. [Google Scholar] [CrossRef]
- Weichert, W.; Warth, A. Early lung cancer with lepidic pattern: Adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant adenocarcinoma. Curr. Opin. Pulm. Med. 2014, 20, 309–316. [Google Scholar] [CrossRef]
- Colby, T.V.; Wistuba, I.I.; Gazdar, A. Precursors to pulmonary neoplasia. Adv. Anat. Pathol. 1998, 5, 205–215. [Google Scholar] [CrossRef]
- Kadara, H.; Scheet, P.; Wistuba, I.I.; Spira, A.E. Early Events in the Molecular Pathogenesis of Lung Cancer. Cancer Prev. Res. 2016, 9, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Lucas, F.A.S.; McDowell, T.L.; Lang, W.; Xu, L.; Fujimoto, J.; Zhang, J.; Futreal, P.A.; Fukuoka, J.; Yatabe, Y.; et al. Genomic Landscape of Atypical Adenomatous Hyperplasia Reveals Divergent Modes to Lung Adenocarcinoma. Cancer Res. 2017, 77, 6119–6130. [Google Scholar] [CrossRef]
- Qian, J.; Zhao, S.; Zou, Y.; Rahman, S.M.J.; Senosain, M.F.; Stricker, T.; Chen, H.; Powell, C.A.; Borczuk, A.C.; Massion, P.P. Genomic Underpinnings of Tumor Behavior in In Situ and Early Lung Adenocarcinoma. Am. J. Respir. Crit. Care Med. 2020, 201, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yu, S.; Cao, L.; Sun, P.L.; Gao, H. Clinicopathologic Features and Genetic Alterations in Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma of the Lung: Long-Term Follow-Up Study of 121 Asian Patients. Ann. Surg. Oncol. 2020, 27, 3052–3063. [Google Scholar] [CrossRef]
- Hu, X.; Fujimoto, J.; Ying, L.; Fukuoka, J.; Ashizawa, K.; Sun, W.; Reuben, A.; Chow, C.-W.; McGranahan, N.; Chen, R.; et al. Multi-region exome sequencing reveals genomic evolution from preneoplasia to lung adenocarcinoma. Nat. Commun. 2019, 10, 2978. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Xiong, Y.; Xu, S.; Chen, J.; Wen, M.; Zhao, Y.; Lei, J.; Jiang, T. Evolution of lung adenocarcinoma from preneoplasia to invasive adenocarcinoma. Cancer Med. 2023, 12, 5545–5557. [Google Scholar] [CrossRef]
- Haga, Y.; Sakamoto, Y.; Kajiya, K.; Kawai, H.; Oka, M.; Motoi, N.; Shirasawa, M.; Yotsukura, M.; Watanabe, S.-I.; Arai, M.; et al. Whole-genome sequencing reveals the molecular implications of the stepwise progression of lung adenocarcinoma. Nat. Commun. 2023, 14, 8375. [Google Scholar] [CrossRef]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef]
- Sheikine, Y.; Pavlick, D.; Klempner, S.J.; Trabucco, S.E.; Chung, J.H.; Rosenzweig, M.; Wang, K.; Velcheti, V.; Frampton, G.M.; Peled, N.; et al. BRAF in Lung Cancers: Analysis of Patient Cases Reveals Recurrent BRAF Mutations, Fusions, Kinase Duplications, and Concurrent Alterations. JCO Precis. Oncol. 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Ou, S.H.I.; Schrock, A.B.; Bocharov, E.V.; Klempner, S.J.; Haddad, C.K.; Steinecker, G.; Johnson, M.; Gitlitz, B.J.; Chung, J.; Campregher, P.V.; et al. HER2 Transmembrane Domain (TMD) Mutations (V659/G660) That Stabilize Homo- and Heterodimerization Are Rare Oncogenic Drivers in Lung Adenocarcinoma That Respond to Afatinib. J. Thorac. Oncol. 2017, 12, 446–457. [Google Scholar] [CrossRef]
- Arcila, M.E.; Drilon, A.; Sylvester, B.E.; Lovly, C.M.; Borsu, L.; Reva, B.; Kris, M.G.; Solit, D.B.; Ladanyi, M. MAP2K1 (MEK1) Mutations Define a Distinct Subset of Lung Adenocarcinoma Associated with Smoking. Clin. Cancer Res. 2015, 21, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Song, J.; Xu, Y.; Yang, Z.; Liu, Y.; Zhang, P.; Wang, X.; Sun, C.; Guo, Y.; Qiu, S.; Shao, G.; et al. Coexistence of atypical adenomatous hyperplasia, minimally invasive adenocarcinoma and invasive adenocarcinoma: Gene mutation analysis. Thorac. Cancer 2021, 12, 693–698. [Google Scholar] [CrossRef]
- Licchesi, J.D.F.; Westra, W.H.; Hooker, C.M.; Herman, J.G. Promoter Hypermethylation of Hallmark Cancer Genes in Atypical Adenomatous Hyperplasia of the Lung. Clin. Cancer Res. 2008, 14, 2570–2578. [Google Scholar] [CrossRef]
- Husni, R.E.; Shiba-Ishii, A.; Nakagawa, T.; Dai, T.; Kim, Y.; Hong, J.; Sakashita, S.; Sakamoto, N.; Sato, Y.; Noguchi, M. DNA hypomethylation-related overexpression of SFN, GORASP2 and ZYG11A is a novel prognostic biomarker for early stage lung adenocarcinoma. Oncotarget 2019, 10, 1625–1636. [Google Scholar] [CrossRef]
- Hu, X.; Estecio, M.R.; Chen, R.; Reuben, A.; Wang, L.; Fujimoto, J.; Carrot-Zhang, J.; McGranahan, N.; Ying, L.; Fukuoka, J.; et al. Evolution of DNA Methylome from Precancerous Lesions to Invasive Lung Adenocarcinomas. Nat. Commun. 2021, 12, 687. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, P.; Zhang, X.; Guo, X.; Gao, Q.; Ma, L.; Mei, W.; Zhang, J.; Zheng, J. Metabolic phenotypes, serum tumor markers, and histopathological subtypes in predicting bone metastasis: Analysis of 695 patients with lung cancer in China. Quant. Imaging Med. Surg. 2023, 13, 1642–1654. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Z.W.; Ni, N.; Li, L.; Kong, X.Y. Similarity and difference of pathogenesis among lung cancer subtypes suggested by expression profile data. Pathol.-Res. Pract. 2021, 220, 153365. [Google Scholar] [CrossRef]
- Wang, L.; Pei, Y.; Li, S.; Zhang, S.; Yang, Y. Distinct Molecular Mechanisms Analysis of Three Lung Cancer Subtypes Based on Gene Expression Profiles. J. Comput. Biol. 2019, 26, 1140–1155. [Google Scholar] [CrossRef]
- Singh, G.; Singh, A.; Dave, R. An Update on WHO Classification of Thoracic Tumours 2021- Newly Described Entities and Terminologies. J. Clin. Diagn. Res. 2023. [Google Scholar] [CrossRef]
- Lutfi, W.; Martinez-Meehan, D.; Sultan, I.; Evans, N.; Dhupar, R.; Luketich, J.D.; Christie, N.A.; Okusanya, O.T. Racial disparities in local therapy for early stage non-small-cell lung cancer. J. Surg. Oncol. 2020, 122, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Vikramdeo, K.S.; Singh, S.; Singh, A.P.; Dasgupta, S. Racial disparities in the genetic landscape of lung cancer. Cancer Health Disparities 2022, 6, 210. [Google Scholar] [PubMed]
- Bhatia, A.; Sobti, R.C.; Sharma, S. Tumor Inflammatory Microenvironment in Lung Cancer: Heterogeneity and Implications. In Handbook of Oncobiology: From Basic to Clinical Sciences; Sobti, R.C., Ganguly, N.K., Kumar, R., Eds.; Springer Nature: Singapore, 2023; pp. 1–19. [Google Scholar] [CrossRef]
- Miura, K.; Shukuya, T.; Greenstein, R.; Kaplan, B.; Wakelee, H.; Kurokawa, K.; Furuta, K.; Kato, S.; Suh, J.; Sivakumar, S.; et al. Ancestry-, Sex-, and Age-Based Differences of Gene Alterations in NSCLC: From the Real-World Data of Cancer Genomic Profiling Tests. J. Natl. Compr. Cancer Netw. 2024, 22, e247021. [Google Scholar] [CrossRef] [PubMed]
- James, B.A.; Williams, J.L.; Nemesure, B. A systematic review of genetic ancestry as a risk factor for incidence of non-small cell lung cancer in the US. Front. Genet. 2023, 14, 1141058. [Google Scholar] [CrossRef]
- Theik, N.W.Y.; Uribe, C.C.; Alvarez, A.; Muminovic, M.; Raez, L.E. Diversity and Disparities in Lung Cancer Outcomes Among Minorities. Cancer J. 2023, 29, 323–327. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, G.; Yu, Y.; Chen, H.; Liu, H. Identification of pathological subtypes of early lung adenocarcinoma based on artificial intelligence parameters and CT signs. Biosci. Rep. 2022, 42, BSR20212416. [Google Scholar] [CrossRef]
- Hu, F.; Zhou, Y.; Wang, Q.; Yang, Z.; Shi, Y.; Chi, Q. Gene Expression Classification of Lung Adenocarcinoma into Molecular Subtypes. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 17, 1187–1197. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Guo, R.; Gong, J.; Zhong, X.; Liu, Y.; Wang, D.; Hao, Y.; Hu, B. Identification of molecular subtypes in lung adenocarcinoma based on DNA methylation and gene expression profiling—A bioinformatic analysis. Ann. Transl. Med. 2022, 10, 882. [Google Scholar] [CrossRef]
- Tlemsani, C.; Pécuchet, N.; Gruber, A.; Laurendeau, I.; Danel, C.; Riquet, M.; Le Pimpec-Barthes, F.; Fabre, E.; Mansuet-Lupo, A.; Damotte, D.; et al. NF1 mutations identify molecular and clinical subtypes of lung adenocarcinomas. Cancer Med. 2019, 8, 4330–4337. [Google Scholar] [CrossRef]
- Cai, M.; Chen, M.; Ma, P.; Wu, J.; Lu, H.; Zhang, S.; Liu, J.; Zhao, X.; Zhuang, G.; Yu, Z.; et al. Clinicopathological, microenvironmental and genetic determinants of molecular subtypes in KEAP1/NRF2-mutant lung cancer. Int. J. Cancer 2019, 144, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Shi, H.; Seegobin, K.; Heng, F.; Zhou, K.; Chen, R.; Qin, H.; Manochakian, R.; Zhao, Y.; Lou, Y. Genomic landscape of lung adenocarcinomas in different races. Front. Oncol. 2022, 12, 946625. [Google Scholar] [CrossRef]
- Adib, E.; Nassar, A.H.; Abou Alaiwi, S.; Groha, S.; Akl, E.W.; Sholl, L.M.; Michael, K.S.; Awad, M.M.; Jänne, P.A.; Gusev, A.; et al. Variation in targetable genomic alterations in non-small cell lung cancer by genetic ancestry, sex, smoking history, and histology. Genome Med. 2022, 14, 39. [Google Scholar] [CrossRef]
- Ji, X.; Mukherjee, S.; Landi, M.T.; Bosse, Y.; Joubert, P.; Zhu, D.; Gorlov, I.; Xiao, X.; Han, Y.; Gorlova, O.; et al. Protein-altering germline mutations implicate novel genes related to lung cancer development. Nat. Commun. 2020, 11, 2220. [Google Scholar] [CrossRef]
- Carrot-Zhang, J.; Soca-Chafre, G.; Patterson, N.; Thorner, A.R.; Nag, A.; Watson, J.; Genovese, G.; Rodriguez, J.; Gelbard, M.K.; Corrales-Rodriguez, L.; et al. Genetic Ancestry Contributes to Somatic Mutations in Lung Cancers from Admixed Latin American Populations. Cancer Discov. 2021, 11, 591–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Li, Y.; Shen, X.; Yu, Y.; Pan, Y.; Zhang, Y.; Zheng, D.; Zhao, Y.; Ye, T.; et al. Comparative analysis of co-occurring mutations of specific tumor suppressor genes in lung adenocarcinoma between Asian and Caucasian populations. J. Cancer Res. Clin. Oncol. 2019, 145, 747–757. [Google Scholar] [CrossRef]
- Dhieb, D.; Belguith, I.; Capelli, L.; Chiadini, E.; Canale, M.; Bravaccini, S.; Yangui, I.; Boudawara, O.; Jlidi, R.; Boudawara, T.; et al. Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma. Cells 2019, 8, 514. [Google Scholar] [CrossRef]
- Distefano, R.; Nigita, G.; Le, P.; Romano, G.; Acunzo, M.; Nana-Sinkam, P. Disparities in Lung Cancer: miRNA Isoform Characterization in Lung Adenocarcinoma. Cancers 2022, 14, 773. [Google Scholar] [CrossRef]
- Davidson, M.R.; Gazdar, A.F.; Clarke, B.E. The pivotal role of pathology in the management of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S463–S478. [Google Scholar]
- Bozinovski, S.; Vlahos, R.; Anthony, D.; McQualter, J.; Anderson, G.; Irving, L.; Steinfort, D. COPD and squamous cell lung cancer: Aberrant inflammation and immunity is the common link. Br. J. Pharmacol. 2016, 173, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Mishra, R.; Desai, S.; Chandrani, P.; Kore, H.; Sunder, R.; Hait, S.; Iyer, P.; Trivedi, V.; Choughule, A.; et al. Molecular characterization of lung squamous cell carcinoma tumors reveals therapeutically relevant alterations. Oncotarget 2021, 12, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, H. Adenosquamous Carcinoma of the Lung. OncoTargets Ther. 2018, 11, 4829–4835. [Google Scholar] [CrossRef]
- Vassella, E.; Langsch, S.; Dettmer, M.S.; Schlup, C.; Neuenschwander, M.; Frattini, M.; Gugger, M.; Schäfer, S.C. Molecular profiling of lung adenosquamous carcinoma: Hybrid or genuine type? Oncotarget 2015, 6, 23905–23916. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Zhu, S.; Miao, K.; Li, Z.; Qi, X.; Huang, L.; Guo, L.; Wang, Y.; Cai, Y. Comprehensive analyses of genomic features and mutational signatures in adenosquamous carcinoma of the lung. Front. Oncol. 2022, 12, 945843. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, Y.; Chen, Y.; Zhang, J.; Teng, F.; Liao, S.; Chen, S.; Wu, Q.; Xiang, C.; Pang, J.; et al. Clonal dynamics and Stereo-seq resolve origin and phenotypic plasticity of adenosquamous carcinoma. NPJ Precis. Oncol. 2023, 7, 80. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Zamora, I.; Freeman, M.R.; Encío, I.J.; Rotinen, M. Actionable Driver Events in Small Cell Lung Cancer. Int. J. Mol. Sci. 2023, 25, 105. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Ding, X.L.; Su, Y.G.; Yu, L.; Bai, Z.L.; Bai, X.H.; Chen, X.Z.; Yang, X.; Zhao, R.; He, J.-X.; Wang, Y.-Y. Clinical characteristics and patient outcomes of molecular subtypes of small cell lung cancer (SCLC). World J. Surg. Oncol. 2022, 20, 54. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Park, S.Y.; Lee, G.K.; Lim, K.Y.; Kim, J.Y.; Hwang, J.-A.; Yu, N.; Kang, E.H.; Hwang, M.; et al. Molecular Subtypes and Tumor Microenvironment Characteristics of Small-Cell Lung Cancer Associated with Platinum-Resistance. Cancers 2023, 15, 3568. [Google Scholar] [CrossRef]
- Miyakawa, K.; Miyashita, N.; Horie, M.; Terasaki, Y.; Tanaka, H.; Urushiyama, H.; Fukuda, K.; Okabe, Y.; Ishii, T.; Kuwahara, N.; et al. ASCL1 regulates super-enhancer-associated miRNAs to define molecular subtypes of small cell lung cancer. Cancer Sci. 2022, 113, 3932–3946. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets. Onco 2022, 2, 186–244. [Google Scholar] [CrossRef]
- Pongor, L.S.; Schultz, C.W.; Rinaldi, L.; Wangsa, D.; Redon, C.E.; Takahashi, N.; Fialkoff, G.; Desai, P.; Zhang, Y.; Burkett, S.; et al. Extrachromosomal DNA Amplification Contributes to Small Cell Lung Cancer Heterogeneity and Is Associated with Worse Outcomes. Cancer Discov. 2023, 13, 928–949. [Google Scholar] [CrossRef]
- Sivakumar, S.; Moore, J.A.; Montesion, M.; Sharaf, R.; Lin, D.I.; Colón, C.I.; Fleishmann, Z.; Ebot, E.M.; Newberg, J.Y.; Mills, J.M.; et al. Integrative Analysis of a Large Real-World Cohort of Small Cell Lung Cancer Identifies Distinct Genetic Subtypes and Insights into Histologic Transformation. Cancer Discov. 2023, 13, 1572–1591. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, X.; Wang, D.; Fu, H.; Xia, Q. Genetic Alteration and Their Significance on Clinical Events in Small Cell Lung Cancer. Cancer Manag. Res. 2022, 14, 1493–1505. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Wang, X.; Sheng, W.; Chen, Z.; Shu, W.; Han, J.; Zhao, S.; Dai, Y.; Wang, K.; et al. Genomic based analyses reveal unique mutational profiling and identify prognostic biomarker for overall survival in Chinese small-cell lung cancer. Jpn. J. Clin. Oncol. 2019, 49, 1143–1150. [Google Scholar] [CrossRef]
- Jin, W.; Lei, Z.; Xu, S.; Fachen, Z.; Yixiang, Z.; Shilei, Z.; Tao, G.; Zhe, S.; Fengzhou, L.; Su, W.-H.; et al. Genetic Mutation Analysis in Small Cell Lung Cancer by a Novel NGS-Based Targeted Resequencing Gene Panel and Relation with Clinical Features. BioMed Res. Int. 2021, 2021, 3609028. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, J.; Li, Z.; Li, P.; Sun, Y.; Yang, Y.; Zhou, X.; Wang, J.; Wang, Y.; Qi, H.; et al. Prognostic impact of tumor mutation burden and the mutation in KIAA1211 in small cell lung cancer. Respir. Res. 2019, 20, 248. [Google Scholar] [CrossRef]
- Tang, M.; Abbas, H.A.; Negrao, M.V.; Ramineni, M.; Hu, X.; Hubert, S.M.; Fujimoto, J.; Reuben, A.; Varghese, S.; Zhang, J.; et al. The Histologic Phenotype of Lung Cancers Is Associated with Transcriptomic Features Rather than Genomic Characteristics. Nat. Commun. 2021, 12, 7081. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Zhang, Y.; Yu, Y.; Chen, H.; Liu, K.; Yao, M.; Wang, K.; Gu, W.; Shou, T. Comprehensive genomic profiling of small cell lung cancer in Chinese patients and the implications for therapeutic potential. Cancer Med. 2019, 8, 4338–4347. [Google Scholar] [CrossRef]
- O’Malley, J.; Kumar, R.; Inigo, J.; Yadava, N.; Chandra, D. Mitochondrial Stress Response and Cancer. Trends Cancer 2020, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, K.L.; Vikramdeo, K.S.; Galeas, J.N.; Marbut, S.M.; Pramanik, P.; Yunus, F.; Singh, S.; Singh, A.P.; Dasgupta, S. Clinicopathological Significance of Unraveling Mitochondrial Pathway Alterations in Non-small-cell Lung Cancer. FASEB J. 2023, 37, e23018. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, X.; Shen, D.; Sun, Y.; Guan, N.; Qi, C. Ectopic expression of LAG-3 in non–small-cell lung cancer cells and its clinical significance. J. Clin. Lab. Anal. 2020, 34, e23244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ju, Y.S.; Kim, Y.; Li, J.; Wang, Y.; Yoon, C.J.; Yang, Y.; Martincorena, I.; Creighton, C.J.; Weinstein, J.N.; et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat. Genet. 2020, 52, 342–352. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Gao, M.; Hao, M.; Ren, H.; Guo, L.; Guo, H. Knockdown of TRAP1 promotes cisplatin-induced apoptosis by promoting the ROS-dependent mitochondrial dysfunction in lung cancer cells. Mol. Cell. Biochem. 2021, 476, 1075–1082. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Nagashio, R.; Igawa, S.; Kusuhara, S.; Tsuchiya, B.; Ichinoe, M.; Satoh, Y.; Naoki, K.; Murakumo, Y.; Saegusa, M.; et al. TRAP1 Is a Predictive Biomarker of Platinum-Based Adjuvant Chemotherapy Benefits in Patients with Resected Lung Adenocarcinoma. Biomed. Res. 2020, 41, 53–65. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, S.; Wu, Y.; Liu, Y.; Hu, T.; Huang, J.; Yu, J.; Pei, Z.; Zeng, T.; et al. TRAP1 Shows Clinical Significance in the Early Diagnosis of Small Cell Lung Cancer. J. Inflamm. Res. 2021, 14, 2507–2514. [Google Scholar] [CrossRef]
- Chuang, C.H.; Dorsch, M.; Dujardin, P.; Silas, S.; Ueffing, K.; Hölken, J.M.; Yang, D.; Winslow, M.M.; Grüner, B.M. Altered Mitochondria Functionality Defines a Metastatic Cell State in Lung Cancer and Creates an Exploitable Vulnerability. Cancer Res. 2021, 81, 567–579. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, H.; Sung, J.A.; Koh, J.; Cho, S.; Chung, D.H.; Jeon, Y.K.; Lee, S.D. Whole Mitochondrial Genome Analysis in Non–Small Cell Lung Carcinoma Reveals Unique Tumor-Specific Somatic Mutations. Arch. Pathol. Lab. Med. 2023, 147, 1268–1277. [Google Scholar] [CrossRef]
- Raghav, L.; Chang, Y.H.; Hsu, Y.C.; Li, Y.C.; Chen, C.Y.; Yang, T.Y.; Chen, K.-C.; Hsu, K.-H.; Tseng, J.-S.; Chuang, C.-Y.; et al. Landscape of Mitochondria Genome and Clinical Outcomes in Stage 1 Lung Adenocarcinoma. Cancers 2020, 12, 755. [Google Scholar] [CrossRef]
- Kazdal, D.; Harms, A.; Endris, V.; Penzel, R.; Kriegsmann, M.; Eichhorn, F.; Muley, T.; Stenzinger, A.; Pfarr, N.; Weichert, W.; et al. Prevalence of somatic mitochondrial mutations and spatial distribution of mitochondria in non-small cell lung cancer. Br. J. Cancer 2017, 117, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Gao, Y.; Ding, K.; Wang, N.; Zhou, D.; Jen, J.; Cheng, S. Relationship between mitochondrial DNA mutations and clinical characteristics in human lung cancer. Mitochondrion 2007, 7, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Soudry, E.; Mukhopadhyay, N.; Shao, C.; Yee, J.; Lam, S.; Lam, W.; Zhang, W.; Gazdar, A.F.; Fisher, P.B.; et al. Mitochondrial DNA mutations in respiratory complex-I in never-smoker lung cancer patients contribute to lung cancer progression and associated with EGFR gene mutation. J. Cell. Physiol. 2012, 227, 2451–2460. [Google Scholar] [CrossRef]
- Dasgupta, S.; Yung, R.C.; Westra, W.H.; Rini, D.A.; Brandes, J.; Sidransky, D. Following Mitochondrial Footprints through a Long Mucosal Path to Lung Cancer. Toland, A.E.; editor. PLoS ONE 2009, 4, e6533. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhao, Q. Epigenetic Alterations and Inflammation as Emerging Use for the Advancement of Treatment in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 878740. [Google Scholar] [CrossRef]
- Saldanha, S. (Ed.) Epigenetic Mechanisms in Cancer; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://linkinghub.elsevier.com/retrieve/pii/C20150062074 (accessed on 15 September 2024).
- Vaissière, T.; Hung, R.J.; Zaridze, D.; Moukeria, A.; Cuenin, C.; Fasolo, V.; Ferro, G.; Paliwal, A.; Hainaut, P.; Brennan, P.; et al. Quantitative Analysis of DNA Methylation Profiles in Lung Cancer Identifies Aberrant DNA Methylation of Specific Genes and Its Association with Gender and Cancer Risk Factors. Cancer Res. 2009, 69, 243–252. [Google Scholar] [CrossRef]
- Chao, Y.L.; Pecot, C.V. Targeting Epigenetics in Lung Cancer. Cold Spring Harb. Perspect. Med. 2021, 11, a038000. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Romashin, D.; Zborovskaya, I.B.; Davydov, M.M.; Shogenov, M.S.; Gratchev, A. Human Lung Microbiome on the Way to Cancer. J. Immunol. Res. 2019, 2019, 1394191. [Google Scholar] [CrossRef]
- Czarnecka-Chrebelska, K.H.; Kordiak, J.; Brzeziańska-Lasota, E.; Pastuszak-Lewandoska, D. Respiratory Tract Oncobiome in Lung Carcinogenesis: Where Are We Now? Cancers 2023, 15, 4935. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, T. Human microbiota: A crucial gatekeeper in lung cancer initiation, progression, and treatment. Med. Microecol. 2022, 13, 100055. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Xie, H.; Wang, X.; Wu, J.; Long, J.; Courtney, R.; Shu, X.-O.; Zheng, W.; Blot, W.J.; et al. Association of oral microbiota with lung cancer risk in a low-income population in the Southeastern USA. Cancer Causes Control 2021, 32, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Hayes, R.B.; Goparaju, C.; Reid, C.; Pass, H.I.; Ahn, J. The Microbiome in Lung Cancer Tissue and Recurrence-Free Survival. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Suo, L.; Zhang, W.; Cao, H.; Wang, R.; Luan, J.; Yu, X.; Dong, L.; Wang, W.; et al. Characterizing microbiota and metabolomics analysis to identify candidate biomarkers in lung cancer. Front. Oncol. 2022, 12, 1058436. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Abedini, F.; Azimzadeh Jamalkandi, S.; Shariati, P.; Ahmadi, A.; Gholami Fesharaki, M. The composition of lung microbiome in lung cancer: A systematic review and meta-analysis. BMC Microbiol. 2021, 21, 315. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Z.; Wang, J.; Ding, C.; Sun, C.; Liu, P.; Xu, X.; Liu, Y.; Chen, B.; Gu, B. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl. Lung Cancer Res. 2020, 9, 693–704. [Google Scholar] [CrossRef]
- Han, W.; Wang, N.; Han, M.; Liu, X.; Sun, T.; Xu, J. Identification of microbial markers associated with lung cancer based on multi-cohort 16 s rRNA analyses: A systematic review and meta-analysis. Cancer Med. 2023, 12, 19301–19319. [Google Scholar] [CrossRef]
- Ran, Z.; Liu, J.; Wang, F.; Xin, C.; Shen, X.; Zeng, S.; Song, Z.; Xiong, B. Analysis of Pulmonary Microbial Diversity in Patients with Advanced Lung Cancer Based on High-throughput Sequencing Technology. Zhongguo Fei Ai Za Zhi Chin. J. Lung Cancer 2020, 23, 1031–1038. [Google Scholar]
- Yuan, X.; Wang, Z.; Li, C.; Lv, K.; Tian, G.; Tang, M.; Ji, L.; Yang, J. Bacterial biomarkers capable of identifying recurrence or metastasis carry disease severity information for lung cancer. Front. Microbiol. 2022, 13, 1007831. [Google Scholar] [CrossRef]
- Liu, N.N.; Ma, Q.; Ge, Y.; Yi, C.X.; Wei, L.Q.; Tan, J.C.; Chu, Q.; Li, J.-Q.; Zhang, P.; Wang, H. Microbiome dysbiosis in lung cancer: From composition to therapy. NPJ Precis. Oncol. 2020, 4, 33. [Google Scholar] [CrossRef]
- Goto, T. Airway Microbiota as a Modulator of Lung Cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, X.; Hu, Y. Distinct respiratory microbiota associates with lung cancer clinicopathological characteristics. Front. Oncol. 2023, 13, 847182. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, E.; Cho, Y.J.; Lee, S.H. Subtype-Based Microbial Analysis in Non-small Cell Lung Cancer. Tuberc. Respir. Dis. 2023, 86, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Holden, V.K.; Deepak, J.; Todd, N.W.; Jiang, F. Microbiota Biomarkers for Lung Cancer. Diagnostics 2021, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Li, J.; Tan, Y.; An, T.; Zhuo, M.; Pan, Z.; Ma, M.; Jia, B.; Zhang, H.; et al. Characterization of Lung and Oral Microbiomes in Lung Cancer Patients Using Culturomics and 16S rRNA Gene Sequencing. Li, D.; editor. Microbiol. Spectr. 2023, 11, e00314-23. [Google Scholar] [CrossRef]
- Gomes, S.; Cavadas, B.; Ferreira, J.C.; Marques, P.I.; Monteiro, C.; Sucena, M.; Sousa, C.; Vaz Rodrigues, L.; Teixeira, G.; Pinto, P.; et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci. Rep. 2019, 9, 12838. [Google Scholar] [CrossRef]
- Kovaleva, O.; Podlesnaya, P.; Rashidova, M.; Samoilova, D.; Petrenko, A.; Zborovskaya, I.; Mochalnikova, V.; Kataev, V.; Khlopko, Y.; Plotnikov, A.; et al. Lung Microbiome Differentially Impacts Survival of Patients with Non-Small Cell Lung Cancer Depending on Tumor Stroma Phenotype. Biomedicines 2020, 8, 349. [Google Scholar] [CrossRef]
- Huang, D.; Su, X.; Yuan, M.; Zhang, S.; He, J.; Deng, Q.; Qiu, W.; Dong, H.; Cai, S. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am. J. Cancer Res. 2019, 9, 2047–2063. [Google Scholar]
- Su, Y.; Li, S.; Sang, D.; Zhang, Y. The characteristics of intratumoral microbial community reflect the development of lung adenocarcinoma. Front. Microbiol. 2024, 15, 1353940. [Google Scholar] [CrossRef]
- Fortunato, O.; Huber, V.; Segale, M.; Cova, A.; Vallacchi, V.; Squarcina, P.; Rivoltini, L.; Suatoni, P.; Sozzi, G.; Pastorino, U.; et al. Development of a Molecular Blood-Based Immune Signature Classifier as Biomarker for Risks Assessment in Lung Cancer Screening. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 2020–2029. [Google Scholar] [CrossRef]
- Hao, F. Systemic Profiling of KDM5 Subfamily Signature in Non-Small-Cell Lung Cancer. Int. J. Gen. Med. 2021, 14, 7259–7275. [Google Scholar] [CrossRef]
- Han, S.; Jiang, D.; Zhang, F.; Li, K.; Jiao, K.; Hu, J.; Song, H.; Ma, Q.-Y.; Wang, J. A new immune signature for survival prediction and immune checkpoint molecules in non-small cell lung cancer. Front. Oncol. 2023, 13, 1095313. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Park, S.; Jo, A.; Eum, H.H.; Kim, H.K.; Lee, K.; Cho, J.H.; Ku, B.M.; Jung, H.A.; Sun, J.-M.; et al. Unveiling the Influence of Tumor and Immune Signatures on Immune Checkpoint Therapy in Advanced Lung Cancer. eLife 2024, 13, RP98366. [Google Scholar] [CrossRef] [PubMed]
- Almotawa, M.Z.; Albasha, S.A.; Alsaleh, A.A.; Alibrahim, N.N.; Radwan, Z.H.; Alhashem, Y.N.; Almatouq, J.; Alamoudi, M.K. Targeting Epigenetic Modification of T-Cell Inflamed Signature to Enhance Immune Checkpoint Inhibitors Response in Small Cell Lung Cancer. J. Pharmacol. Exp. Ther. 2023, 385, 435. [Google Scholar] [CrossRef]
- Xie, Q.; Chu, H.; Yi, J.; Yu, H.; Gu, T.; Guan, Y.; Liu, X.; Liang, J.; Li, Y.; Wang, J. Identification of a prognostic immune-related signature for small cell lung cancer. Cancer Med. 2021, 10, 9115–9128. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, J.W.; Kim, A.; Shin, J.Y.; Jung, Y.J.; Lee, S.B.; Kim, Y.H.; Park, S.; Lee, H.J.; Park, I.-K.; et al. Whole Exome and Transcriptome Analyses Integrated with Microenvironmental Immune Signatures of Lung Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 848–859. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Zhan, X. Identification of Immune-Related Gene Signatures in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 752643. [Google Scholar] [CrossRef]
- Chen, R.L.; Zhou, J.X.; Cao, Y.; Sun, L.L.; Su, S.; Deng, X.J.; Lin, J.-T.; Xiao, Z.-W.; Chen, Z.-Z.; Wang, S.-Y.; et al. Construction of a Prognostic Immune Signature for Squamous-Cell Lung Cancer to Predict Survival. Front. Immunol. 2020, 11, 1933. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Chen, J.; Lin, L.; Chen, Y. Immune signature of T follicular helper cells predicts clinical prognostic and therapeutic impact in lung squamous cell carcinoma. Int. Immunopharmacol. 2020, 81, 105932. [Google Scholar] [CrossRef]
- Wu, J.; Xu, C.; Guan, X.; Ni, D.; Yang, X.; Yang, Z.; Wang, M. Comprehensive analysis of tumor microenvironment and identification of an immune signature to predict the prognosis and immunotherapeutic response in lung squamous cell carcinoma. Ann. Transl. Med. 2021, 9, 569. [Google Scholar] [CrossRef]
- Ma, C. A Novel Gene Signature based on Immune Cell Infiltration Landscape Predicts Prognosis in Lung Adenocarcinoma Patients. Curr. Med. Chem. 2024, 31, 6319–6335. [Google Scholar] [CrossRef]
- Wang, L.; Luo, X.; Cheng, C.; Amos, C.I.; Cai, G.; Xiao, F. A gene expression-based immune signature for lung adenocarcinoma prognosis. Cancer Immunol. Immunother. 2020, 69, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, M.; Hu, D. Identification of Prognostic Immune-Related Genes by Integrating mRNA Expression and Methylation in Lung Adenocarcinoma. Int. J. Genom. 2020, 2020, 9548632. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Guo, Z.; Yu, D.; Wang, Y.; Wang, Q.; Dong, Z.; Hu, W. A Prognostic Nomogram Combining Immune-Related Gene Signature and Clinical Factors Predicts Survival in Patients With Lung Adenocarcinoma. Front. Oncol. 2020, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xia, Z.; Guo, Y.; Li, Y. Immune landscape and prognostic immune-related signature in KRAS-mutated lung adenocarcinoma. Aging 2023, 15, 4889–4905. [Google Scholar] [CrossRef]
- Chen, H.; Shen, W.; Ni, S.; Sang, M.; Wu, S.; Mu, Y.; Liu, K.; Li, N.; Zhu, L.; Xu, G. Construction of an immune-related lncRNA signature as a novel prognosis biomarker for LUAD. Aging 2021, 13, 20684–20697. [Google Scholar] [CrossRef]
- Chen, H.; Lin, R.; Lin, W.; Chen, Q.; Ye, D.; Li, J.; Feng, J.; Cheng, W.; Zhang, M.; Qi, Y. An immune gene signature to predict prognosis and immunotherapeutic response in lung adenocarcinoma. Sci. Rep. 2022, 12, 8230. [Google Scholar] [CrossRef]
- Yi, M.; Li, A.; Zhou, L.; Chu, Q.; Luo, S.; Wu, K. Immune signature-based risk stratification and prediction of immune checkpoint inhibitor’s efficacy for lung adenocarcinoma. Cancer Immunol. Immunother. 2021, 70, 1705–1719. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.; Kota, V.; Rojiani, M.V.; Rojiani, A.M.; Kolhe, R. Immunogenomic Gene Signature of Cell-Death Associated Genes with Prognostic Implications in Lung Cancer. Cancers 2021, 13, 155. [Google Scholar] [CrossRef]
- Huang, Z.; Li, B.; Guo, Y.; Wu, L.; Kou, F.; Yang, L. Signatures of Multi-Omics Reveal Distinct Tumor Immune Microenvironment Contributing to Immunotherapy in Lung Adenocarcinoma. Front. Immunol. 2021, 12, 723172. [Google Scholar] [CrossRef]
- Xu, J.Z.; Gong, C.; Xie, Z.F.; Zhao, H. Development of an Oncogenic Driver Alteration Associated Immune-Related Prognostic Model for Stage I-II Lung Adenocarcinoma. Front. Oncol. 2021, 10, 593022. [Google Scholar] [CrossRef]
- Hua, L.; Wu, J.; Ge, J.; Li, X.; You, B.; Wang, W.; Hu, B. Identification of lung adenocarcinoma subtypes and predictive signature for prognosis, immune features, and immunotherapy based on immune checkpoint genes. Front. Cell Dev. Biol. 2023, 11, 1060086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, X.; Wang, Q.; Song, X.; Xia, W.; Mao, Q.; Chen, B.; Liang, Y.; Zhang, T.; Xu, L.; et al. A Novel Gene Expression Signature-Based on B-Cell Proportion to Predict Prognosis of Patients with Lung Adenocarcinoma. BMC Cancer 2021, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, M.; Chen, Z.; Bian, Y.; Zheng, Y.; Hu, Z.; Liang, J.; Huang, Y.; Yin, J.; Zhan, C.; et al. Identification of immune-related gene signature predicting survival in the tumor microenvironment of lung adenocarcinoma. Immunogenetics 2020, 72, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Raza, F.; He, N. Nanoscale Extracellular Vesicle-Enabled Liquid Biopsy: Advances and Challenges for Lung Cancer Detection. Micromachines 2024, 15, 1181. [Google Scholar] [CrossRef]
- Ren, F.; Fei, Q.; Qiu, K.; Zhang, Y.; Zhang, H.; Sun, L. Liquid biopsy techniques and lung cancer: Diagnosis, monitoring and evaluation. J. Exp. Clin. Cancer Res. 2024, 43, 96. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, F. AI-Enhanced CAD in Low-Dose CT: Balancing Accuracy, Efficiency, and Overdiagnosis in Lung Cancer Screening. Thorac. Cancer 2025, 16, e15499. [Google Scholar] [CrossRef]
- Bharmjeet; Das, A. Racial disparities in cancer care, an eyeopener for developing better global cancer management strategies. Cancer Rep. 2023, 6 (Suppl. S1), e1807. [Google Scholar] [CrossRef]
| Lung Cancer Types | Race Information | Nuclear Genetic Alterations |
|---|---|---|
| Small-cell lung cancer (SCLC) | Chinese | TP53 and RB1 gene mutations are the most prevalent LRP1B, FAM135B, SPTA1, KMT2D, FAT1, and NOTCH3 |
| EA | Co-mutation of TP53 and RB1 Wnt and Notch signaling pathways mutations | |
| Squamous cell carcinoma (NSCLC) | EA | TP53, PIK3CA, KEAP1, and NFE2L2 mutations |
| Indian | EGFR mutations | |
| AA | Increased homologous recombination deficiency (HRD) Higher rates of PTEN deletion and KRAS amplification | |
| Adenosquamous carcinoma (NSCLC) | EA | Less prevalent KRAS mutation |
| Adenocarcinoma (NSCLC) | EA | Positively associated with KRAS G12C mutation Negatively associated with EGFR mutation STK11 mutations The common driver is KRAS, and the second is EGFR TP53, BRAF, PIK3CA, KEAP1, NF1, STK11, RBM10, and MET mutations |
| East Asian, Hispanic/Latino, and American Indigenous (AMR) | Negatively associated with KRAS G12C mutation Positively associated with EGFR mutation | |
| Never-smoker non-Hispanic Asian, specifically East Asian ancestry | CTNNB1 driver mutations | |
| Asian | EGFR exon 21 L858R mutation RET rearrangements ERBB2 amplifications | |
| AA | STK11 mutations | |
| LA | EGFR and KRAS mutations | |
| EA and AA | Specific miRNA isoforms | |
| Ashkenazi Jewish | ATM L2307F mutation | |
| Tunisian | Reduced frequency of EGFR and KRAS mutations and ALK rearrangement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsatari, E.S.; Smith, K.R.; Galappaththi, S.P.L.; Turbat-Herrera, E.A.; Dasgupta, S. The Current Roadmap of Lung Cancer Biology, Genomics and Racial Disparity. Int. J. Mol. Sci. 2025, 26, 3818. https://doi.org/10.3390/ijms26083818
Alsatari ES, Smith KR, Galappaththi SPL, Turbat-Herrera EA, Dasgupta S. The Current Roadmap of Lung Cancer Biology, Genomics and Racial Disparity. International Journal of Molecular Sciences. 2025; 26(8):3818. https://doi.org/10.3390/ijms26083818
Chicago/Turabian StyleAlsatari, Enas S., Kelly R. Smith, Sapthala P. Loku Galappaththi, Elba A. Turbat-Herrera, and Santanu Dasgupta. 2025. "The Current Roadmap of Lung Cancer Biology, Genomics and Racial Disparity" International Journal of Molecular Sciences 26, no. 8: 3818. https://doi.org/10.3390/ijms26083818
APA StyleAlsatari, E. S., Smith, K. R., Galappaththi, S. P. L., Turbat-Herrera, E. A., & Dasgupta, S. (2025). The Current Roadmap of Lung Cancer Biology, Genomics and Racial Disparity. International Journal of Molecular Sciences, 26(8), 3818. https://doi.org/10.3390/ijms26083818







