Plasma Levels of Matrilysins (MMP-7 and MMP-26) and Stromelysins (MMP-3 and MMP-10) in Diagnosis of Endometrial Cancer Patients
Abstract
1. Introduction
2. Results
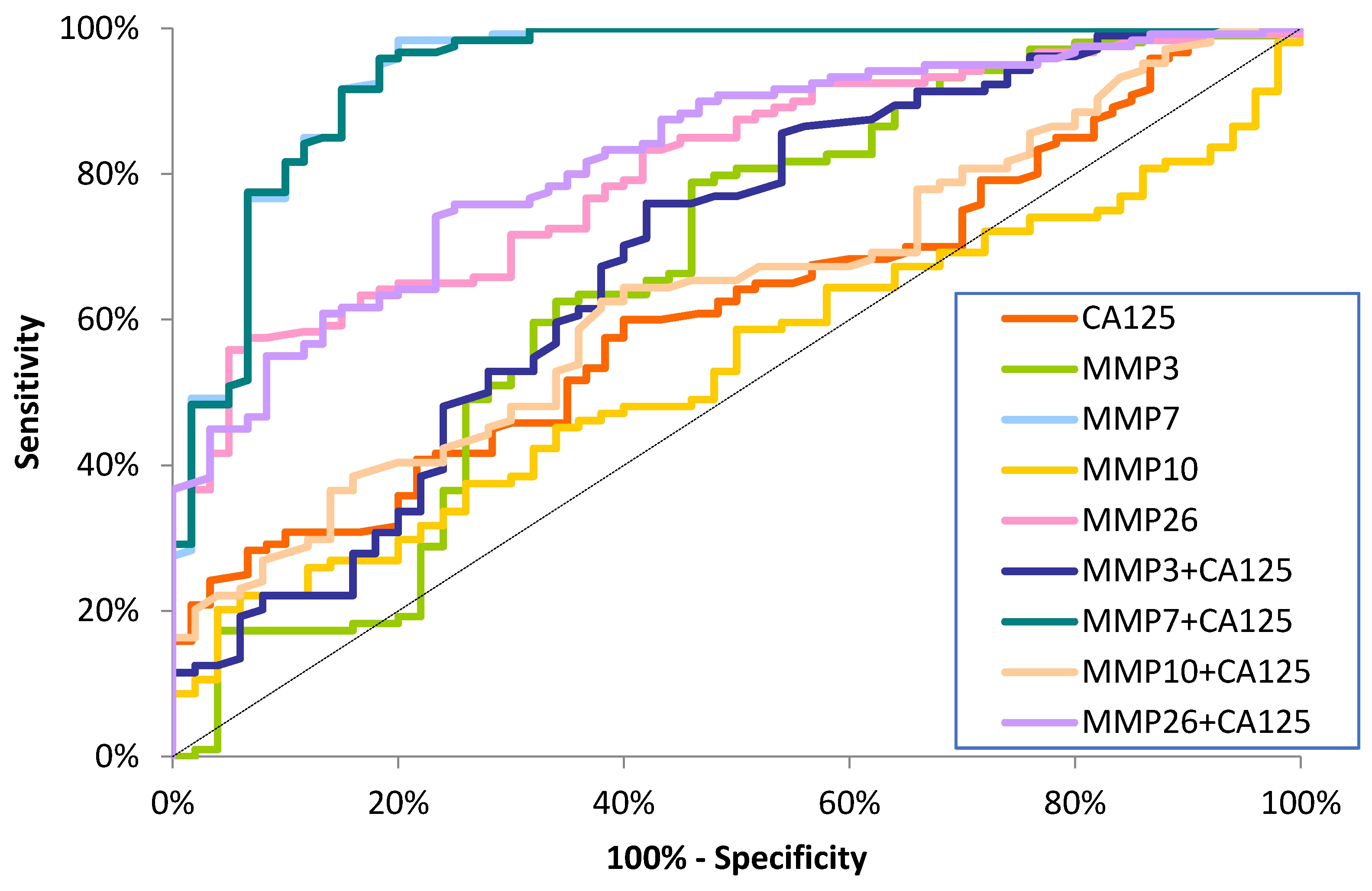
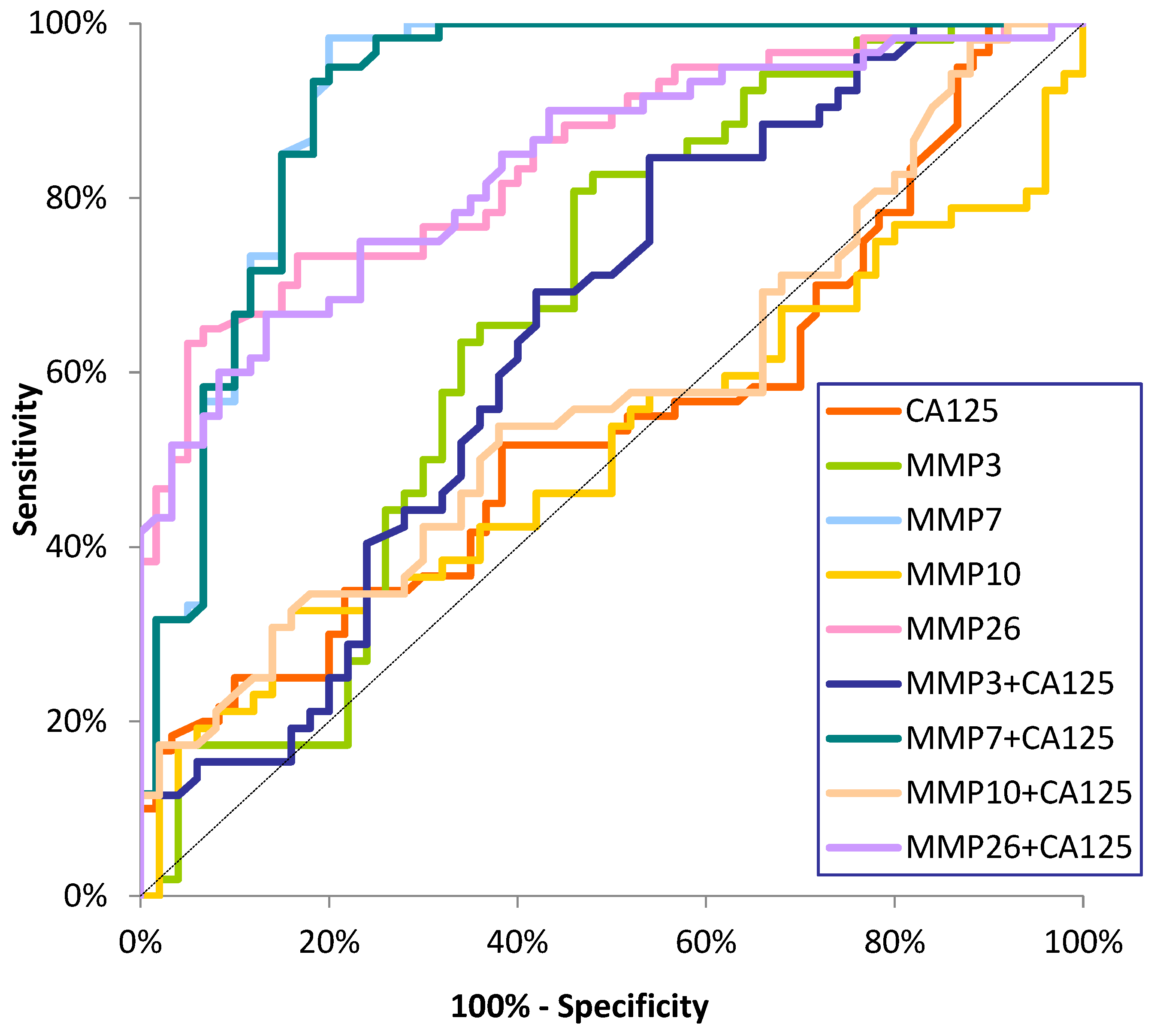
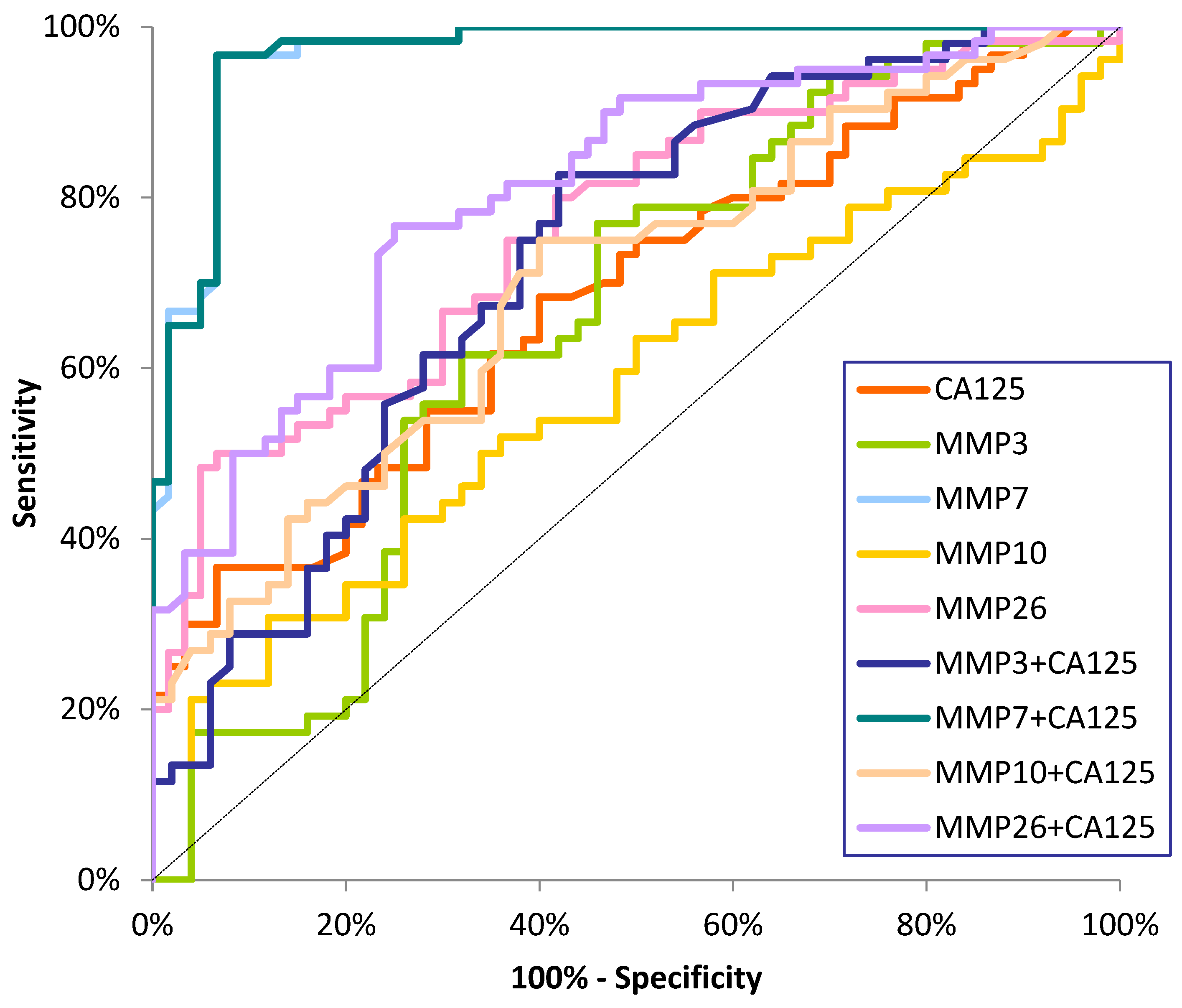
Diagnostic Criteria of Studied MMPs and CA125
3. Discussion
4. Materials and Methods
4.1. Patient Eligibility
4.2. Biochemical Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, L.; Zhang, S. Preoperative Serum CA125: A Useful Marker for Surgical Management of Endometrial Cancer. BMC Cancer 2015, 15, 396. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Qu, W.; Wang, J.; Jiang, S.-W. Comparison of Serum Human Epididymis Protein 4 and CA125 on Endometrial Cancer Detection: A Meta-Analysis. Clin. Chim. Acta 2019, 488, 215–220. [Google Scholar] [CrossRef]
- Bian, J.; Sun, X.; Li, B.; Ming, L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients with Endometrial Cancer. Technol. Cancer Res. Treat. 2017, 16, 435–439. [Google Scholar] [CrossRef]
- Rižner, T.L. Discovery of Biomarkers for Endometrial Cancer: Current Status and Prospects. Expert Rev. Mol. Diagn. 2016, 16, 1315–1336. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Ii, M.; Yamamoto, H.; Adachi, Y.; Maruyama, Y.; Shinomura, Y. Role of Matrix Metalloproteinase-7 (Matrilysin) in Human Cancer Invasion, Apoptosis, Growth, and Angiogenesis. Exp. Biol. Med. 2006, 231, 20–27. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Da, C.-M.; Liao, B.; Zhang, H.-H. Roles of Matrix Metalloproteinase-7 (MMP-7) in Cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma Concentrations of Matrilysins MMP-7 and MMP-26 as Diagnostic Biomarkers in Breast Cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Mylona, E.; Kapranou, A.; Mavrommatis, J.; Markaki, S.; Keramopoulos, A.; Nakopoulou, L. The Multifunctional Role of the Immunohistochemical Expression of MMP-7 in Invasive Breast Cancer. APMIS 2005, 113, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.L.; Wang, K.; Wang, C.B.; Li, X.F.; Yang, Z.; Wang, H.Y. Expressions of FOXC1 and MMP-7 in molecular subtypes of breast cancer and their association with clinicopathological characteristics. Zhejiang Da Xue Xue Bao Yi Xue Ban 2014, 43, 406–412. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Osada, J.; Szmitkowski, M.; Chrostek, L.; Dąbrowska, M.; Ławicki, S. Plasma Levels of MMP-7 and TIMP-1 in Laboratory Diagnostics and Differentiation of Selected Histological Types of Epithelial Ovarian Cancers. J. Ovarian Res. 2017, 10, 39. [Google Scholar] [CrossRef]
- Davidson, B.; Stavnes, H.T.; Hellesylt, E.; Hager, T.; Zeppa, P.; Pinamonti, M.; Wohlschlaeger, J. MMP-7 Is a Highly Specific Negative Marker for Benign and Malignant Mesothelial Cells in Serous Effusions. Hum. Pathol. 2016, 47, 104–108. [Google Scholar] [CrossRef]
- Simmons, A.R.; Fourkala, E.O.; Gentry-Maharaj, A.; Ryan, A.; Sutton, M.N.; Baggerly, K.; Zheng, H.; Lu, K.H.; Jacobs, I.; Skates, S.; et al. Complementary Longitudinal Serum Biomarkers to CA125 for Early Detection of Ovarian Cancer. Cancer Prev. Res. 2019, 12, 391–400. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Piskór, B.; Gacuta, E.; Zajkowska, M.; Osada, J.; Szmitkowski, M.; Dąbrowska, M.; Ławicki, S. Diagnostic Power of Selected Cytokines, MMPs and TIMPs in Ovarian Cancer Patients–ROC Analysis. Anticancer Res. 2019, 39, 2575–2582. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, X.; Du, Y.; Xing, Y.; Xu, K.; Cui, L. Matrix metalloproteinase-7 may serve as a novel biomarker for cervical cancer. Onco Targets Ther. 2018, 11, 4207–4220. [Google Scholar] [CrossRef]
- Chen, W.; Huang, S.; Shi, K.; Yi, L.; Liu, Y.; Liu, W. Prognostic Role of Matrix Metalloproteinases in Cervical Cancer: A Meta-Analysis. Cancer Control 2021, 28, 10732748211033743. [Google Scholar] [CrossRef]
- Guo, H.; Dai, Y.; Wang, A.; Wang, C.; Sun, L.; Wang, Z. Association between Expression of MMP-7 and MMP-9 and Pelvic Lymph Node and Para-aortic Lymph Node Metastasis in Early Cervical Cancer. J. Obs. Gynaecol. 2018, 44, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Gershtein, E.S.; Mushtenko, S.V.; Ermilova, V.D.; Levchenko, N.E.; Kushlinskii, N.E. Matrix Metalloproteinases and Their Tissue Inhibitors in Blood Serum of Patients with Endometrial Cancer: Clinical and Morphological Correlations. Bull. Exp. Biol. Med. 2018, 165, 75–79. [Google Scholar] [CrossRef]
- Misugi, F.; Sumi, T.; Okamoto, E.; Nobeyama, H.; Hattori, K.; Yoshida, H.; Matsumoto, Y.; Yasui, T.; Honda, K.; Ishiko, O. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in uterine endometrial carcinoma and a correlation between expression of matrix metalloproteinase-7 and prognosis. Int. J. Mol. Med. 2005, 16, 541–546. [Google Scholar]
- Ueno, H.; Yamashita, K.; Azumano, I.; Inoue, M.; Okada, Y. Enhanced Production and Activation of Matrix Metalloproteinase-7 (Matrilysin) in Human Endometrial Carcinomas. Int. J. Cancer 1999, 84, 470–477. [Google Scholar] [CrossRef]
- Graesslin, O.; Cortez, A.; Fauvet, R.; Lorenzato, M.; Birembaut, P.; Daraï, E. Metalloproteinase-2, -7 and -9 and Tissue Inhibitor of Metalloproteinase-1 and -2 Expression in Normal, Hyperplastic and Neoplastic Endometrium: A Clinical-Pathological Correlation Study. Ann. Oncol. 2006, 17, 637–645. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Maleysson, E.; Darcha, C. Analysis of Matrix Metalloproteinase-7 Expression in Eutopic and Ectopic Endometrium Samples from Patients with Different Forms of Endometriosis. Hum. Reprod. 2010, 25, 742–750. [Google Scholar] [CrossRef]
- Grzechocinska, B.; Dabrowski, F.A.; Cyganek, A.; Chlebus, M.; Kobierzycki, C.; Michalowski, L.; Gornicka, B.; Wielgos, M. Matrix Metalloproteinases-2, -7 and Tissue Metalloproteinase Inhibitor-1 Expression in Human Endometrium. Folia Histochem. Cytobiol. 2018, 56, 133–140. [Google Scholar] [CrossRef]
- Isaka, K.; Nishi, H.; Nakai, H.; Nakada, T.; Feng Li, Y.; Ebihara, Y.; Takayama, M. Matrix Metalloproteinase-26 Is Expressed in Human Endometrium but Not in Endometrial Carcinoma. Cancer 2003, 97, 79–89. [Google Scholar] [CrossRef]
- Pilka, R.; Norata, G.D.; Domanski, H.; Andersson, C.; Hansson, S.; Eriksson, P.; Casslén, B. Matrix Metalloproteinase-26 (Matrilysin-2) Expression Is High in Endometrial Hyperplasia and Decreases with Loss of Histological Differentiation in Endometrial Cancer. Gynecol. Oncol. 2004, 94, 661–670. [Google Scholar] [CrossRef]
- Nishi, H.; Kuroda, M.; Isaka, K. Estrogen and Estrogen Receptor Induce Matrix Metalloproteinase-26 Expression in Endometrial Carcinoma Cells. Oncol. Rep. 2013, 30, 751–756. [Google Scholar] [CrossRef]
- Liokumovich, P.; Goldberg, I.; Davidson, B.; Gotlieb, W.H.; Zahavi, T.; Ben-Baruch, G.; Reder, I.; Kopolovic, J. Expression of Metalloproteinases Endometrial Stromal Sarcoma: Immunohistochemical Study Using Image Analysis. J. Clin. Pathol. 1999, 52, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Pilka, R.; Marková, I.; Dusková, M.; Zapletalová, J.; Tozzi, M.; Kudela, M. Porovnání exprese imunohistochemických markerů ve vzorcích z hysteroskopie a hysterektomie u pacientek s karcinomem endometria [Immunohistochemical markers expression in hysteroscopy and hysterectomy specimens from endometrial cancer patients: Comparison]. Ceska Gynekol. 2010, 75, 165–170. (In Czech) [Google Scholar] [PubMed]
- Tunuguntla, R.; Ripley, D.; Sang, Q.-X.A.; Chegini, N. Expression of Matrix Metalloproteinase-26 and Tissue Inhibitors of Metalloproteinases TIMP-3 and -4 in Benign Endometrium and Endometrial Cancer. Gynecol. Oncol. 2003, 89, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Satohisa, S.; Kohno, T.; Konno, T.; Takano, K.; Takahashi, S.; Hatakeyama, T.; Arimoto, C.; Saito, T.; Kojima, T. Downregulation of Lipolysis-stimulated Lipoprotein Receptor Promotes Cell Invasion via Claudin-1-mediated Matrix Metalloproteinases in Human Endometrial Cancer. Oncol. Lett. 2017, 14, 6776–6782. [Google Scholar] [CrossRef][Green Version]
- Pilka, R.; Marková, I.; Dusková, M.; Procházka, M.; Tozzi, M.; Kudela, M. Immunohistochemical evaluation and lymph node metastasis in surgically staged endometrial carcinoma. Eur. J. Gynaecol. Oncol. 2010, 31, 530. [Google Scholar]
- Costas, L.; Frias-Gomez, J.; Guardiola, M.; Benavente, Y.; Pineda, M.; Pavón, M.Á.; Martínez, J.M.; Climent, M.; Barahona, M.; Canet, J.; et al. New perspectives on screening and early detection of endometrial cancer. Int. J. Cancer 2019, 145, 3194–3206. [Google Scholar] [CrossRef]
- Hanley, J.A.; Hajian-Tilaki, K.O. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: An update. Acad. Radiol. 1997, 4, 49–58. [Google Scholar] [CrossRef]
| Tested Parameters | Endometrial Cancer (EC) | Control Groups | ||||
|---|---|---|---|---|---|---|
| Total EC n = 120 | Stage I n = 60 | Stage II n = 60 | Myoma Uteri n = 60 | Healthy Women n = 60 | ||
| MMP-7 (pg/mL) | *1, 2 | *1 | *1, 2, 4 | *3 | ||
| Median | 4.26 | 2.96 | 4.93 | 2.82 | 1.23 | |
| IQR | 2.89–5.56 | 2.5–4.54 | 4.03–6.48 | 2.33–3.84 | 0.68–2.01 | |
| Range | 1.92–22.55 | 1.98–7.74 | 1.92–22.55 | 1.66–28.14 | 0.04–5.23 | |
| MMP-26 (pg/mL) | *1 | *1 | *1 | *3 | ||
| Median | 11.69 | 13.62 | 8.77 | 15.98 | 3.28 | |
| IQR | 5.05–19.94 | 6.03–23.02 | 4.38–15.86 | 4.47–36.71 | 1.91–6.695 | |
| Range | 0.26–104.82 | 0.71–104.82 | 0.26–65.78 | 0.59–129.02 | 0.42–17.62 | |
| MMP-3 (pg/mL) | *1 | *1 | *1 | |||
| Median | 10.31 | 10.10 | 10.82 | 8.73 | 7.26 | |
| IQR | 8.08–13.60 | 8.13–13.57 | 7.69–14.08 | 5.93–13.37 | 4.97–11.81 | |
| Range | 2.02–41.29 | 4.08–41.29 | 2.02–29.75 | 2.34–22.61 | 1.81–44.80 | |
| MMP-10 (pg/mL) | *2 | |||||
| Median | 789.52 | 731.6 | 916.3 | 654.73 | 725.22 | |
| IQR | 349.69–1606.61 | 265.86–1408.0 | 481.68–1659.74 | 330.0–870.40 | 454.76–1202.03 | |
| Range | 30.2–6665.42 | 69.6–4512.6 | 30.2–6665.4 | 20.56–9421.2 | 65.37–3006.1 | |
| CA125 (IU/mL) | *1 | *1 | ||||
| Median | 20.45 | 19.30 | 22.70 | 20.55 | 17.55 | |
| IQR | 14.2–29.85 | 11.46–27.66 | 17.0–33.5 | 13.33–29.73 | 12.88–23.70 | |
| Range | 7.25–374.5 | 8.04–358.9 | 7.25–374.5 | 3.94–154.1 | 4.24–39.94 | |
| Group | CA125 | MMP-3 | MMP-7 | MMP-10 | MMP-26 | ||
|---|---|---|---|---|---|---|---|
| Endometrial Cancer (EC) | CA125 | r | 1.00 | 0.07 | 0.20 | 0.19 | −0.04 |
| p ** | 0.499 | 0.027 | 0.059 | 0.640 | |||
| MMP-3 | r | 1.00 | 0.03 | 0.17 | 0.09 | ||
| p ** | 0.738 | 0.080 | 0.380 | ||||
| MMP-7 | r | 1.00 | 0.23 | −0.18 | |||
| p ** | 0.017 | 0.049 | |||||
| MMP-10 | r | 1.00 | 0.08 | ||||
| p ** | 0.414 | ||||||
| MMP-26 | r | 1.00 | |||||
| p ** | |||||||
| Myoma Uteri | CA125 | r | 1.00 | 0.15 | 0.08 | −0.11 | −0.02 |
| p ** | 0.283 | 0.527 | 0.439 | 0.891 | |||
| MMP-3 | r | 1.00 | 0.01 | 0.17 | 0.29 | ||
| p ** | 0.964 | 0.233 | 0.038 | ||||
| MMP-7 | r | 1.00 | 0.02 | 0.35 | |||
| p ** | 0.876 | 0.006 | |||||
| MMP-10 | r | 1.00 | 0.13 | ||||
| p ** | 0.366 | ||||||
| MMP-26 | r | 1.00 | |||||
| p ** | |||||||
| Healthy Women | CA125 | r | 1.00 | 0.29 | 0.23 | 0.07 | 0.12 |
| p ** | 0.041 | 0.072 | 0.628 | 0.363 | |||
| MMP-3 | r | 1.00 | 0.47 | 0.09 | −0.04 | ||
| p ** | 0.001 | 0.553 | 0.791 | ||||
| MMP-7 | r | 1.00 | 0.09 | 0.05 | |||
| p ** | 0.512 | 0.719 | |||||
| MMP-10 | r | 1.00 | 0.08 | ||||
| p ** | 0.590 | ||||||
| MMP-26 | r | 1.00 | |||||
| p ** | |||||||
| Tested Parameter | Diagnostic Criteria | Endometrial Cancer (EC) | ||
|---|---|---|---|---|
| Stage I | Stage II | Total | ||
| MMP-7 | SE (%) | 86 | 96 | 94 |
| SP (%) | 84 | 84 | 84 | |
| PPV (%) | 86 | 88 | 92 | |
| NPV (%) | 92 | 96 | 90 | |
| AUC | 0.9121 | 0.9689 | 0.9410 | |
| SEAUC | 0.0275 | 0.0143 | 0.0199 | |
| 95% CI | 0.861–0.969 | 0.941–0.997 | 0.903–0.981 | |
| p (AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | |
| MMP-26 | SE (%) | 76 | 80 | 78 |
| SP (%) | 46 | 46 | 46 | |
| PPV (%) | 60 | 58 | 74 | |
| NPV (%) | 70 | 66 | 51 | |
| AUC | 0.8458 | 0.8542 | 0.8050 | |
| SEAUC | 0.0351 | 0.0431 | 0.0322 | |
| 95% CI | 0.777–0.915 | 0.680–0.849 | 0.742–0.868 | |
| p (AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | |
| MMP-3 | SE (%) | 76 | 80 | 78 |
| SP (%) | 54 | 54 | 54 | |
| PPV (%) | 63 | 62 | 77 | |
| NPV (%) | 73 | 69 | 55 | |
| AUC | 0.6650 | 0.6542 | 0.6596 | |
| SEAUC | 0.0552 | 0.0553 | 0.0508 | |
| 95% CI | 0.557–0.773 | 0.564–0.783 | 0.560–0.759 | |
| p (AUC = 0.5) | 0.0028 | 0.0053 | 0.0017 | |
| MMP-10 | SE (%) | 20 | 24 | 22 |
| SP (%) | 94 | 94 | 94 | |
| PPV (%) | 77 | 80 | 88 | |
| NPV (%) | 54 | 55 | 38 | |
| AUC | 0.5112 | 0.5804 | 0.5346 | |
| SEAUC | 0.0585 | 0.0572 | 0.0469 | |
| 95% CI | 0.396–0.626 | 0.468–0.692 | 0.443–0.627 | |
| p (AUC = 0.5) | 0.8489 | 0.1598 | 0.4603 | |
| CA125 | SE (%) | 34 | 46 | 40 |
| SP (%) | 84 | 84 | 84 | |
| PPV (%) | 68 | 74 | 83 | |
| NPV (%) | 56 | 61 | 41 | |
| AUC | 0.5428 | 0.6831 | 0.6129 | |
| SEAUC | 0.0535 | 0.0484 | 0.0199 | |
| 95% CI | 0.438–0.648 | 0.588–0.778 | 0.9031–0.9811 | |
| p (AUC = 0.5) | 0.4241 | 0.0002 | 0.0080 | |
| MMP-3+CA125 | SE (%) | 84 | 86 | 85 |
| SP (%) | 48 | 48 | 48 | |
| PPV (%) | 62 | 62 | 77 | |
| NPV (%) | 75 | 77 | 62 | |
| AUC | 0.6460 | 0.7260 | 0.6860 | |
| SEAUC | 0.0552 | 0.0502 | 0.0478 | |
| 95% CI | 0.538–0.754 | 0.627–0.824 | 0.592–0.780 | |
| p (AUC = 0.5) | 0.0082 | <0.0001 | <0.0001 | |
| MMP-7+CA125 | SE (%) | 92 | 96 | 96 |
| SP (%) | 68 | 68 | 68 | |
| PPV (%) | 75 | 75 | 86 | |
| NPV (%) | 96 | 98 | 95 | |
| AUC | 0.9158 | 0.9693 | 0.9420 | |
| SEAUC | 0.0277 | 0.0142 | 0.0199 | |
| 95% CI | 0.859–0.967 | 0.942–0.997 | 0.902–0.980 | |
| p (AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | |
| MMP-10+CA125 | SE (%) | 44 | 56 | 50 |
| SP (%) | 78 | 78 | 78 | |
| PPV (%) | 67 | 72 | 82 | |
| NPV (%) | 58 | 64 | 44 | |
| AUC | 0.5702 | 0.5702 | 0.6345 | |
| SEAUC | 0.0574 | 0.0574 | 0.0459 | |
| 95% CI | 0.458–0.683 | 0.458–0.683 | 0.545–0.724 | |
| p (AUC = 0.5) | 0.2210 | 0.2210 | 0.0034 | |
| MMP-26+CA125 | SE(%) | 86 | 86 | 86 |
| SP (%) | 38 | 38 | 38 | |
| PPV (%) | 58 | 58 | 74 | |
| NPV (%) | 73 | 73 | 58 | |
| AUC | 0.8086 | 0.8353 | 0.8219 | |
| SEAUC | 0.0363 | 0.0363 | 0.0311 | |
| 95% CI | 0.764–0.906 | 0.764–0.906 | 0.761–0.883 | |
| p (AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | |
| Study Group | Group Size | Age (Median) Range (Min.–Max.) |
|---|---|---|
| Preoperative EC Total: | 120 | 59 (51–71) |
| Stage I | 60 | 58 (51–71) |
| Stage II | 60 | 61 (58–70) |
| Benign Endometrial Lesions (Myoma Uteri) | 60 | 56 (49–70) |
| Healthy Women | 60 | 55 (48–69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacuta, E.; Ławicki, M.; Kulesza, M.; Ławicki, P.; Kicman, A.; Łubińska, E.; Ławicki, S. Plasma Levels of Matrilysins (MMP-7 and MMP-26) and Stromelysins (MMP-3 and MMP-10) in Diagnosis of Endometrial Cancer Patients. Int. J. Mol. Sci. 2025, 26, 3824. https://doi.org/10.3390/ijms26083824
Gacuta E, Ławicki M, Kulesza M, Ławicki P, Kicman A, Łubińska E, Ławicki S. Plasma Levels of Matrilysins (MMP-7 and MMP-26) and Stromelysins (MMP-3 and MMP-10) in Diagnosis of Endometrial Cancer Patients. International Journal of Molecular Sciences. 2025; 26(8):3824. https://doi.org/10.3390/ijms26083824
Chicago/Turabian StyleGacuta, Ewa, Michał Ławicki, Monika Kulesza, Paweł Ławicki, Aleksandra Kicman, Emilia Łubińska, and Sławomir Ławicki. 2025. "Plasma Levels of Matrilysins (MMP-7 and MMP-26) and Stromelysins (MMP-3 and MMP-10) in Diagnosis of Endometrial Cancer Patients" International Journal of Molecular Sciences 26, no. 8: 3824. https://doi.org/10.3390/ijms26083824
APA StyleGacuta, E., Ławicki, M., Kulesza, M., Ławicki, P., Kicman, A., Łubińska, E., & Ławicki, S. (2025). Plasma Levels of Matrilysins (MMP-7 and MMP-26) and Stromelysins (MMP-3 and MMP-10) in Diagnosis of Endometrial Cancer Patients. International Journal of Molecular Sciences, 26(8), 3824. https://doi.org/10.3390/ijms26083824





