Transnasal PLGA Nanoparticles with Terpene Permeation Enhancers: Membrane Remodeling and Tight Junction Modulation for Enhanced Brain Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
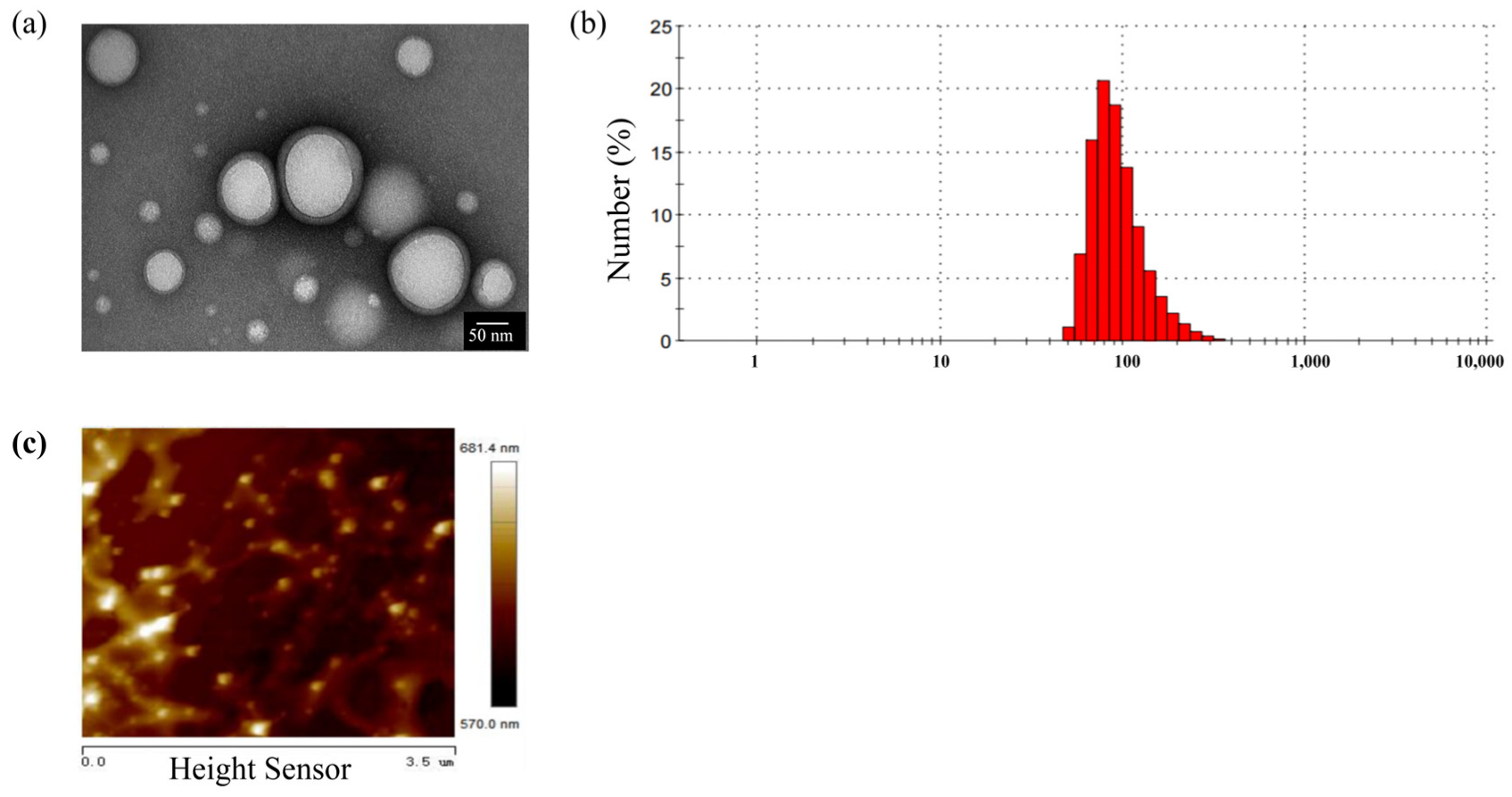
2.1. Characterization of PLGA-NPs
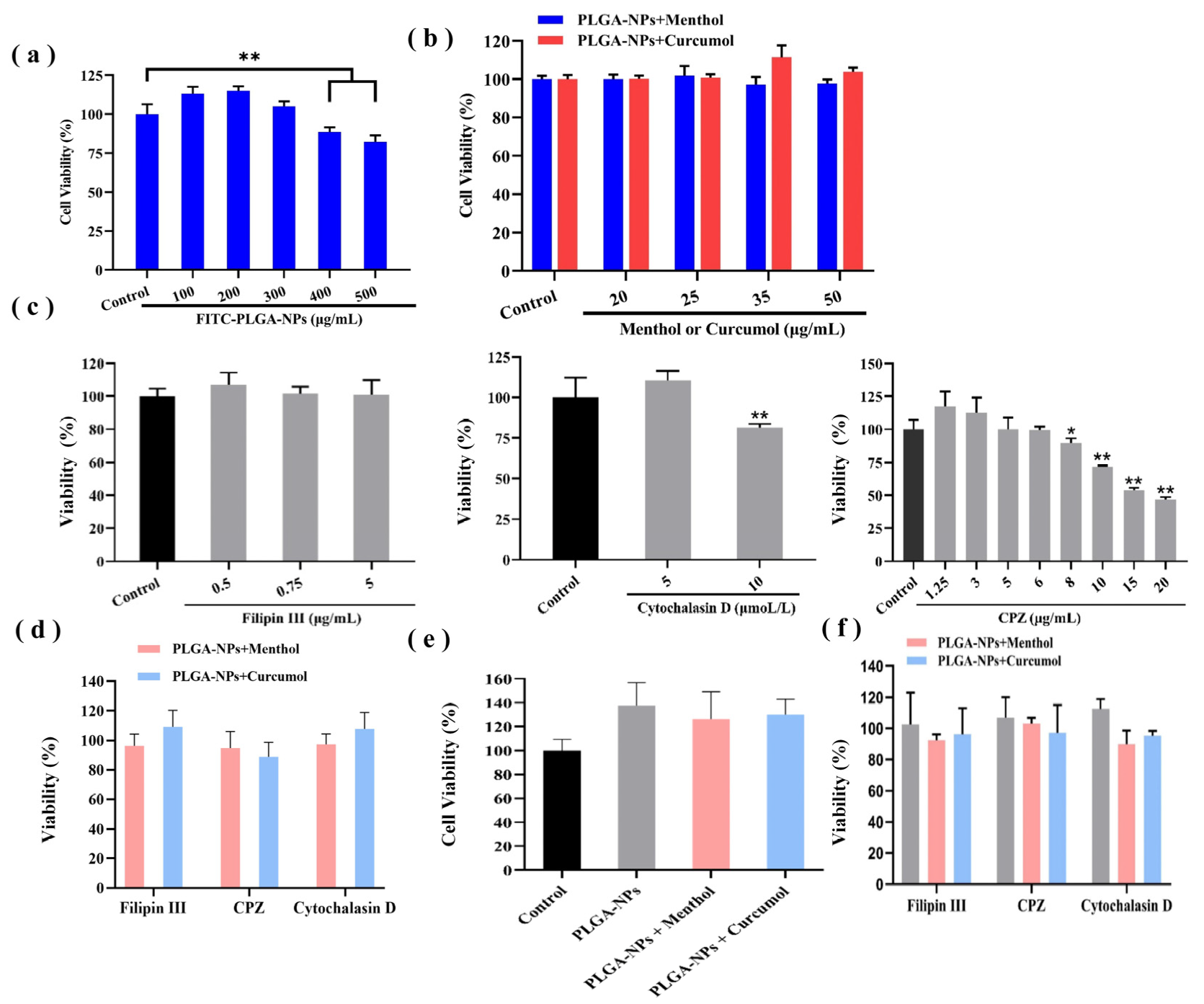
2.2. Biocompatibility Studies of PLGA-NPs
2.3. Study on the Transport Behavior of PLGA-NPs Combined with Aromatic Components
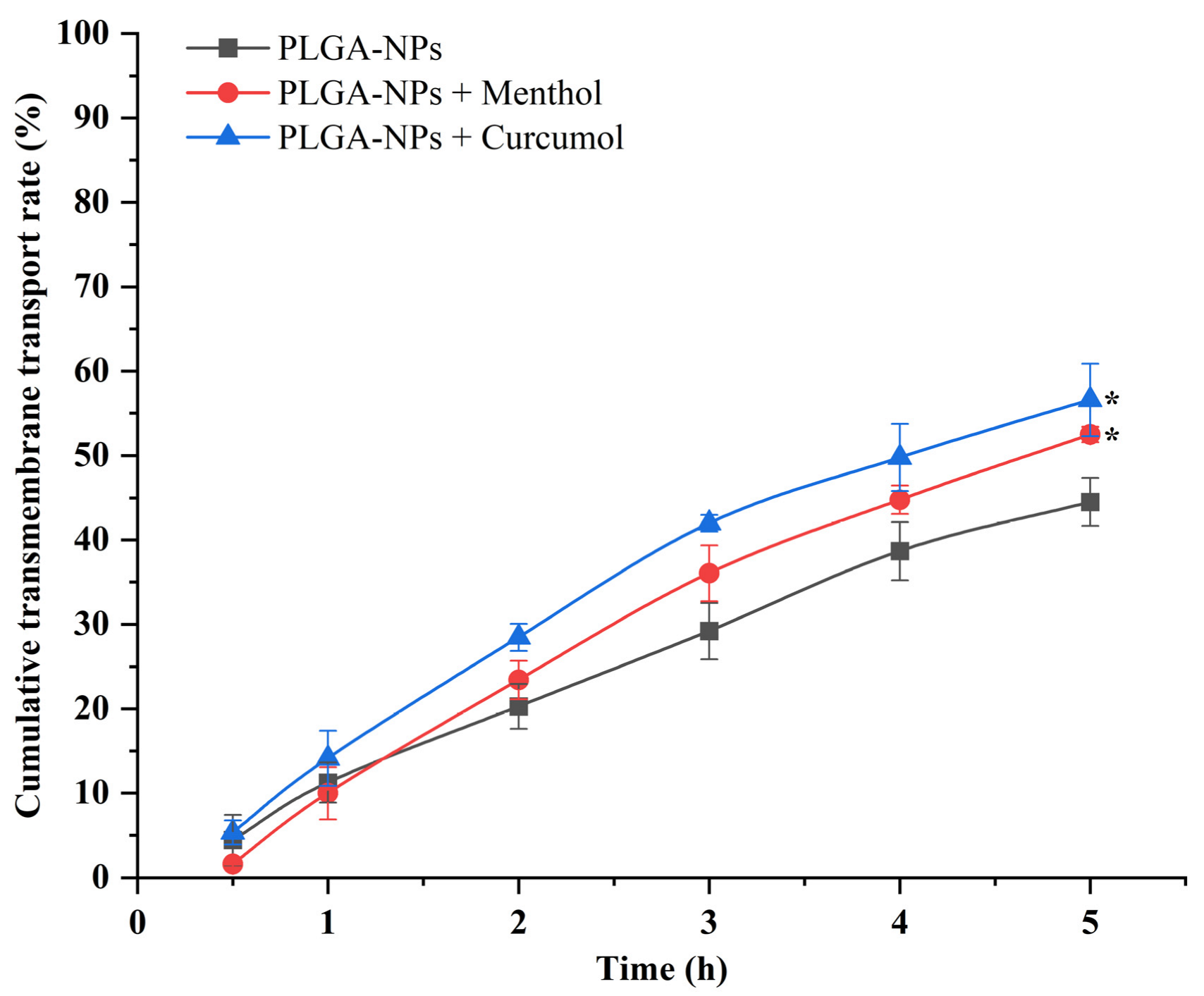
2.3.1. PLGA-NPs Transport Across the BBB Monolayer
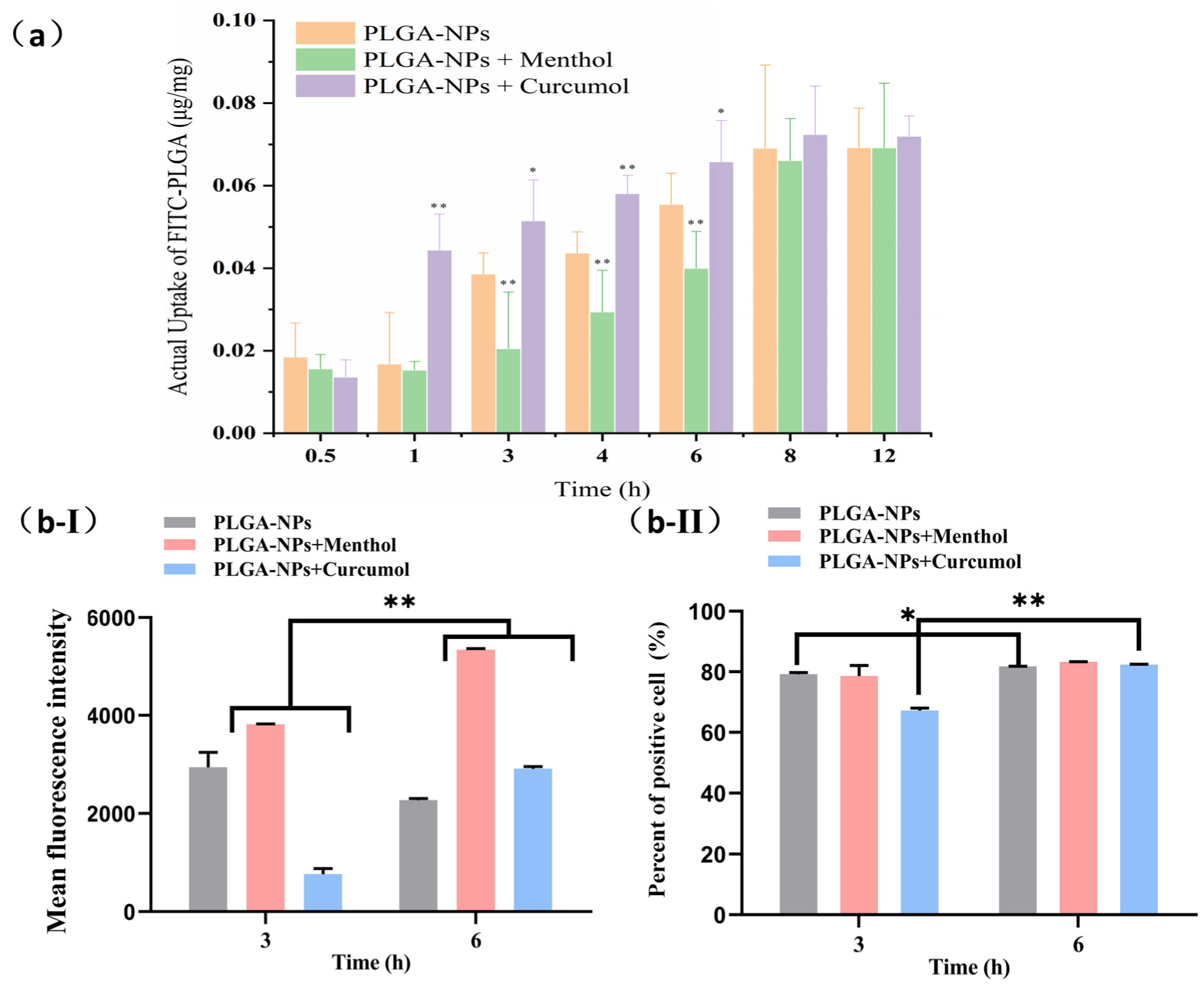
2.3.2. The Cellular Uptake Behavior of PLGA-NPs in bEnd.3 Cells and OECs
2.4. Mechanism Study of PLGA-NPs Transport Combined with Aromatic Components
2.4.1. Expression of the TJ-Associated Proteins
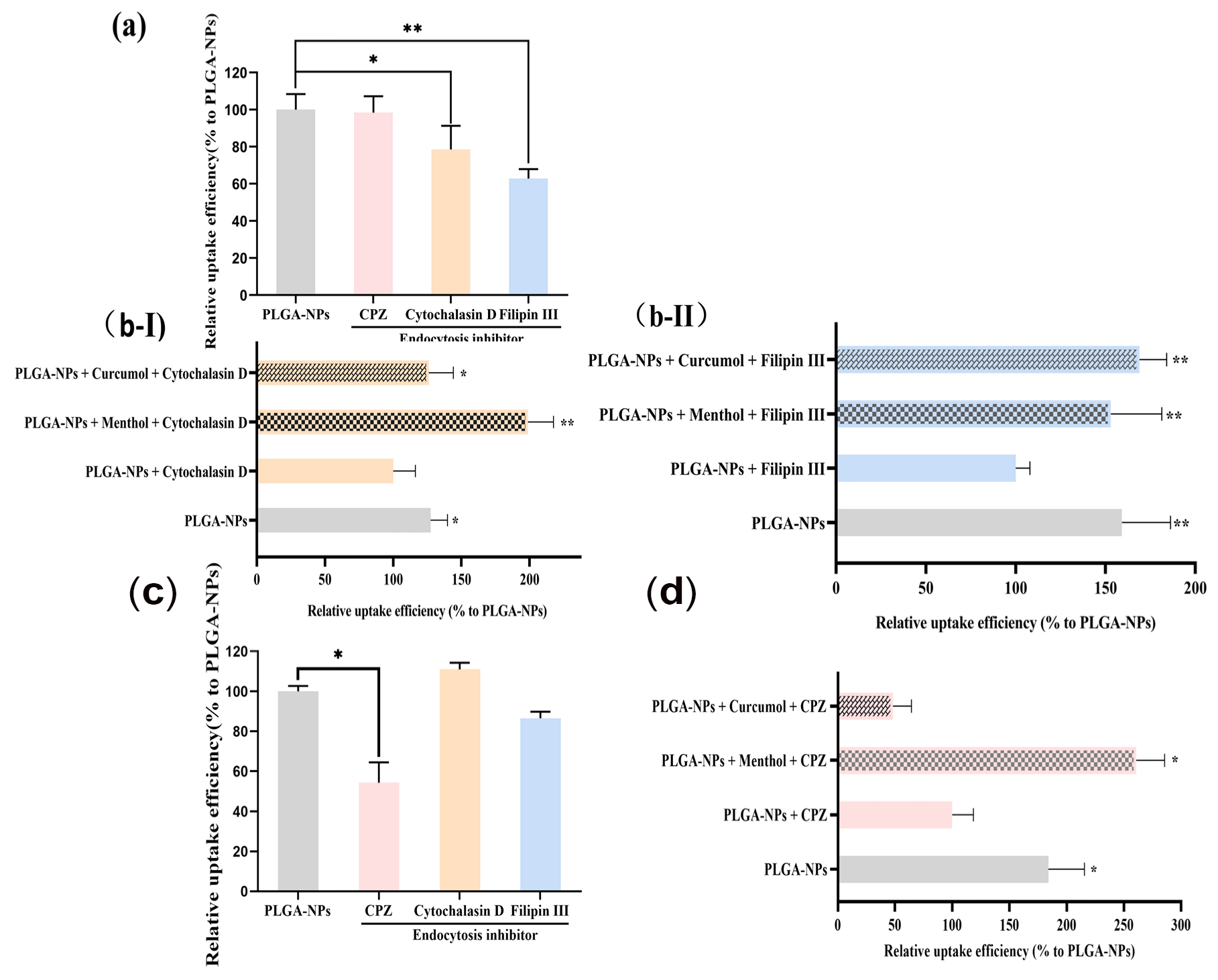
2.4.2. Studies on Interventions for Endocytic Pathways
2.4.3. Biomechanical Properties Assessment of bEnd.3 Cells and OEC
3. Discussion
Prospects
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation and Characterization of Formulations
4.2.2. Cell Culture of bEnd.3 Cells and OECs
4.2.3. Cytoxicity Study
4.2.4. Transport Across bEnd.3 Cell Monolayers
4.2.5. Accumulation of PLGA-NPs with Aromatic Components in bEnd.3 Cells and OECs
4.2.6. Immunostaining of bEnd.3 Cells
4.2.7. Endocytic Mechanism of PLGA-NPs with Aromatic Components in bEnd.3 Cells and OECs
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full name in English |
| AFM | Atomic force microscope |
| ALog P | Oil–water partition coefficient |
| BBB | Blood brain barrier |
| BCA | Bicinchonininc acid |
| bEnd.3 cells | Brain-derived endothelial cells.3 |
| BPE | Bovine Pituitary Extract |
| CNS | Central nervous system |
| CPZ | Chlorpromazine |
| DAPI | 4,6-diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbeccos modified eagle medium |
| EMA | European Medicine Agency |
| FAK | Focal adhesion kinase |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| FITC | Fluorescein isothiocyanate |
| HBSS | Hanks’ Balanced Salt Solution |
| IN | Intranasa |
| MRPs | Multidrugresistance-associated proteins |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide |
| NPs | Nanoparticles |
| OECs | Olfactory ensheathing cells |
| Papp | Apparent permeability coefficients |
| PBS | Phosphate buffer saline |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLGA-NPs | Poly(lactic-co-glycolicacid)-Nanoparticles |
| PLGA-FITC | Poly(lactic-co-glycolicacid)-Fluorescein isothiocyanate |
| PDI | Polydispersity index |
| PVA | Polyvinyl alcohol |
| Ra | Roughness average |
| Rq | RMS roughness |
| TCM | Traditional Chinese Medicine |
| TEER | Transepithellal electric resistance |
| TEM | Transmission electron microscope |
| TJs | Tight junctions |
| US | The United States of America |
| WHO | World Health Organization |
| ZO-1 | Zonula occludens 1 |
References
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Sahm, K.; Weiss, T. Immunotherapy against gliomas. Der Nervenarzt 2024, 95, 111–116. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Langeskov-Christensen, M.; Franzén, E.; Grøndahl Hvid, L.; Dalgas, U. Exercise as medicine in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2024, 95, 1077–1088. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. 2023, 9, e12385. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Giunchedi, P.; Gavini, E.; Bonferoni, M.C. Nose-to-Brain Delivery. Pharmaceutics 2020, 12, 138. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Pernet, V.; Joly, S.; Spiegel, S.; Meli, I.; Idriss, S.; Maigler, F.; Mdzomba, J.B.; Roenneke, A.K.; Franceschini, A.; Silvestri, L.; et al. Nogo-A antibody delivery through the olfactory mucosa mitigates experimental autoimmune encephalomyelitis in the mouse CNS. Cell Death Discov. 2023, 9, 290. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the CNS: Strategies to overcome the BBB. Int. J. Pharm. 2023, 636, 122759. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sinica B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021, 23, 92. [Google Scholar] [CrossRef]
- Rahmani, F.; Atabaki, R.; Behrouzi, S.; Mohamadpour, F.; Kamali, H. The recent advancement in the PLGA-based thermo-sensitive hydrogel for smart drug delivery. Int. J. Pharm. 2023, 631, 122484. [Google Scholar] [CrossRef]
- Alghareeb, S.; Asare-Addo, K.; Conway, B.R.; Adebisi, A.O. PLGA nanoparticles for nasal drug delivery. J. Drug Deliv. Sci. Technol. 2024, 95, 105564. [Google Scholar] [CrossRef]
- Aghaei Delche, N.; Kheiri, R.; Ghorbani Nejad, B.; Sheikhi, M.; Razavi, M.S.; Rahimzadegan, M.; Salmasi, Z. Recent progress in the intranasal PLGA-based drug delivery for neurodegenerative diseases treatment. Iran. J. Basic Med. Sci. 2023, 26, 1107–1119. [Google Scholar] [CrossRef]
- Jia, L.; Nie, X.Q.; Ji, H.M.; Yuan, Z.X.; Li, R.S. Multiple-Coated PLGA Nanoparticles Loading Triptolide Attenuate Injury of a Cellular Model of Alzheimer’s Disease. BioMed Res. Int. 2021, 2021, 8825640. [Google Scholar] [CrossRef]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Bae, S.; Li, Y.; Kim, C.; Choi, B.; Nam, H.Y.; Kim, S.H.; Yun, C.O.; Lee, K.Y.; et al. Nose-to-Brain Delivery of Cancer-Targeting Paclitaxel-Loaded Nanoparticles Potentiates Antitumor Effects in Malignant Glioblastoma. Mol. Pharm. 2020, 17, 1193–1204. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Le, Q.V.; Oh, Y.K. Nanotherapeutics for immune network modulation in tumor microenvironments. Semin. Cancer Biol. 2022, 86, 1066–1087. [Google Scholar] [CrossRef]
- Kang, S.; Duan, W.; Zhang, S.; Chen, D.; Feng, J.; Qi, N. Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics 2020, 10, 4308–4322. [Google Scholar] [CrossRef]
- Cai, Z.; Lei, X.; Lin, Z.; Zhao, J.; Wu, F.; Yang, Z.; Pu, J.; Liu, Z. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with borneol as an oral brain-targeting enhancer. Acta Pharm. Sinica B 2014, 4, 86–93. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Han, L.; Qin, J.; Lu, W.; Wang, J. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the brain. Pharm. Res. 2013, 30, 2560–2572. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Caldwell, J.P.; Farmer, P.B. Metabolic fate of [3H]-l-menthol in the rat. Drug Metab. Dispos. Biol. Fate Chem. 1994, 22, 616–624. [Google Scholar] [CrossRef]
- Shojaei, A.H.; Khan, M.A.; Lim, G.W.; Khosravan, R. Transbuccal permeation of a nucleoside analog, dideoxycytidine: Effects of menthol as a permeation enhancer. Int. J. Pharm. 1999, 192, 139–146. [Google Scholar] [CrossRef]
- Zhang, L.; Du, S.Y.; Lu, Y.; Liu, C.; Tian, Z.H.; Yang, C.; Wu, H.C.; Wang, Z. Puerarin transport across a Calu-3 cell monolayer—An in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol. Drug Des. Dev. Ther. 2016, 10, 2227–2237. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Gao, C.; Ling, C.; Qin, J.; Wang, Q.; Huang, Y.; Lu, W.; Wang, J. Menthol-modified BSA nanoparticles for glioma targeting therapy using an energy restriction strategy. NPG Asia Mater. 2019, 11, 38. [Google Scholar] [CrossRef]
- Wu, L.; Huang, W.; Peng, K.; Wang, Y.; Chen, Q.; Lu, B. Enhancing the stability, BBB permeability and neuroprotective activity of verbascoside in vitro using lipid nanocapsules in combination with menthol. Food Chem. 2023, 414, 135682. [Google Scholar] [CrossRef]
- Zhang, L.; Du, S.; Lu, Y.; Liu, C.; Wu, H.; Yang, B.; Bai, J.; Li, P. Influence of puerarin, paeoniflorin, and menthol on structure and barrier function of tight junctions in mdck and mdck-Mdr1 Cells. J. Tradit. Chin. Med. Sci. 2015, 2, 111–119. [Google Scholar] [CrossRef]
- Wei, W.; Rasul, A.; Sadiqa, A.; Sarfraz, I.; Hussain, G.; Nageen, B.; Liu, X.; Watanabe, N.; Selamoglu, Z.; Ali, M.; et al. Curcumol: From Plant Roots to Cancer Roots. Int. J. Biol. Sci. 2019, 15, 1600–1609. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Hong, Y.; Gai, R.; Hong, W.; Tang, B.; Lin, C.; Wang, X.; Wang, Q.; Chen, C.; et al. Safety Evaluation of Curcumol by a Repeated Dose 28-Day Oral Exposure Toxicity Study in Rats. Toxics 2023, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Han, M.; Li, X.; Zhou, L.; Dou, T.; Liu, Y.; HuangFu, M.; Guan, X.; Wang, Y.; et al. RNA-binding domain 2 of nucleolin is important for the autophagy induction of curcumol in nasopharyngeal carcinoma cells. Phytomed. Int. J. Phytother. Phytopharm. 2023, 115, 154833. [Google Scholar] [CrossRef]
- Jin, H.; Liang, Q.; Chen, T.; Wang, X. Resveratrol protects chondrocytes from apoptosis via altering the ultrastructural and biomechanical properties: An AFM study. PLoS ONE 2014, 9, e91611. [Google Scholar] [CrossRef]
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness via integrin β1, FAK, and ERK pathways. Cell Biochem. Biophys. 2013, 65, 455–462. [Google Scholar] [CrossRef]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release Off. J. Control. Release Soc. 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Angeloni, L.; Reggente, M.; Passeri, D.; Natali, M.; Rossi, M. Identification of nanoparticles and nanosystems in biological matrices with scanning probe microscopy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1521. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Otake, H.; Yamaguchi, M.; Ogata, F.; Deguchi, S.; Yamamoto, N.; Sasaki, H.; Kawasaki, N.; Nagai, N. Energy-Dependent Endocytosis Is Responsible for Skin Penetration of Formulations Based on a Combination of Indomethacin Nanoparticles and l-Menthol in Rat and Göttingen Minipig. Int. J. Mol. Sci. 2021, 22, 5137. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.; Kumar, M.S.; Raju, V.; Lakshmi, M.; Rama, B. Penetration-enhancing effect of ethanolic solution of menthol on transdermal permeation of ondansetron hydrochloride across rat epidermis. Drug Deliv. 2008, 15, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.L.; Piccirillo, C.A.; Tabrizian, M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V 2008, 68, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Abbruscato, T.J.; Egleton, R.D.; Brown, R.C.; Huber, J.D.; Campos, C.R.; Davis, T.P. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004, 1027, 48–58. [Google Scholar] [CrossRef]
- Huber, J.D.; Egleton, R.D.; Davis, T.P. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001, 24, 719–725. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Yang, B. Studies on Absorption and Transport Characteristics and Mechanism of Tongqiao Sanyu Nasal-Microemulsion Based on “Nose-to-Brain” Multi-Pathway Cell Models. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. (In Chinese). [Google Scholar]
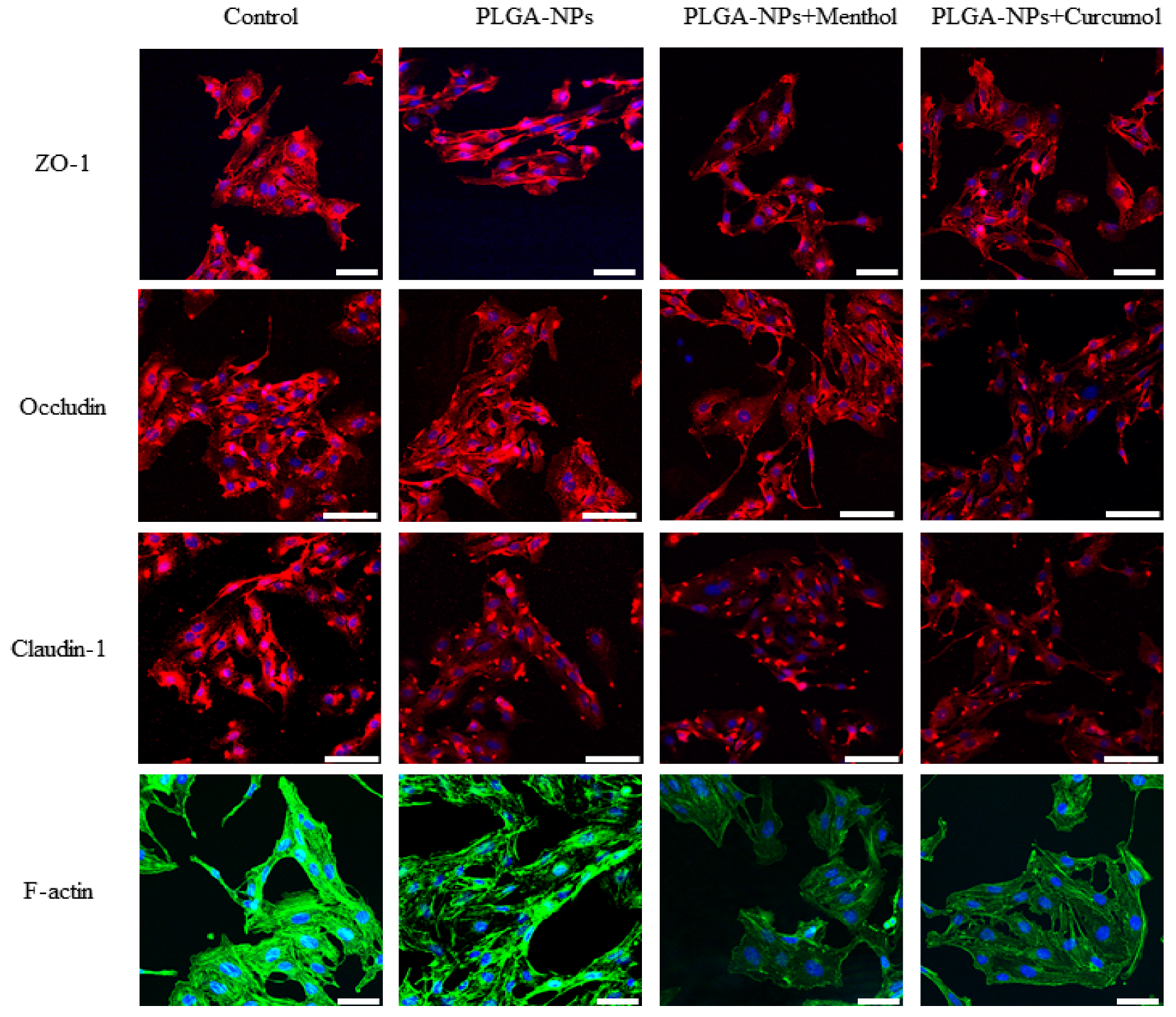

| Group | Average Fluorescence Intensity (%) | |||
|---|---|---|---|---|
| ZO-1 | Occludin | Claudin-1 | F-Actin | |
| Control | 185.4 ± 4.3 | 167.7 ± 1.2 | 164.7 ± 1.9 | 182.6 ± 5.4 |
| PLGA-NPs | 182.7 ± 4.2 | 162.9 ± 6.4 | 143.7 ± 3.8 ** | 141.1 ± 4.4 ** |
| PLGA-NPs + Curcumol | 143.5 ± 0.5 **, ## | 129.5 ± 18.4 # | 113.5 ± 14.6 *, # | 142.2 ± 2.0 ** |
| PLGA-NPs + Menthol | 135.9 ± 2.5 **, ## | 132.9 ± 13.4 *, # | 115.9 ± 10.9 **, # | 90.1 ± 0.7 **, ## |
| Cell Type | Group | Rq (nm) | Ra (nm) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| OECs | Control | 23.5 ± 1.5 | 16.7 ± 1.3 | 15.9 ± 0.2 |
| PLGA-NPs | 34.2 ± 1.9 ** | 26.7 ± 1.5 ** | 18.7 ± 0.6 ** | |
| PLGA-NPs + Menthol | 45.8 ± 2.6 ## | 36.3 ± 1.5 ## | 8.2 ± 0.7 ## | |
| PLGA-NPs + Curcumol | 62.6 ± 2.6 ## | 51.6 ± 2.1 ## | 8.3 ± 1.0 ## | |
| bEnd.3 cells | Control | 20.7 ± 0.6 | 16.1 ± 0.7 | 17.6 ± 0.6 |
| PLGA-NPs | 33.8 ± 3.3 ** | 26.5 ± 3.0 ** | 21.5 ± 0.6 ** | |
| PLGA-NPs + Menthol | 92.2 ± 2.5 ## | 74.7 ± 1.9 ## | 4.3 ± 1.3 ## | |
| PLGA-NPs + Curcumol | 50.8 ± 8.6 ## | 39.9 ± 6.7 ## | 11.4 ± 1.0 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, Z.; Zhang, H.; Wei, H.; Wang, T.; Du, S.; Li, P. Transnasal PLGA Nanoparticles with Terpene Permeation Enhancers: Membrane Remodeling and Tight Junction Modulation for Enhanced Brain Drug Delivery. Int. J. Mol. Sci. 2025, 26, 3861. https://doi.org/10.3390/ijms26083861
Zhang Y, Guo Z, Zhang H, Wei H, Wang T, Du S, Li P. Transnasal PLGA Nanoparticles with Terpene Permeation Enhancers: Membrane Remodeling and Tight Junction Modulation for Enhanced Brain Drug Delivery. International Journal of Molecular Sciences. 2025; 26(8):3861. https://doi.org/10.3390/ijms26083861
Chicago/Turabian StyleZhang, Yi, Zishuo Guo, Haitong Zhang, Hongmei Wei, Tieshan Wang, Shouying Du, and Pengyue Li. 2025. "Transnasal PLGA Nanoparticles with Terpene Permeation Enhancers: Membrane Remodeling and Tight Junction Modulation for Enhanced Brain Drug Delivery" International Journal of Molecular Sciences 26, no. 8: 3861. https://doi.org/10.3390/ijms26083861
APA StyleZhang, Y., Guo, Z., Zhang, H., Wei, H., Wang, T., Du, S., & Li, P. (2025). Transnasal PLGA Nanoparticles with Terpene Permeation Enhancers: Membrane Remodeling and Tight Junction Modulation for Enhanced Brain Drug Delivery. International Journal of Molecular Sciences, 26(8), 3861. https://doi.org/10.3390/ijms26083861





