AMX0035 Mitigates Oligodendrocyte Apoptosis and Ameliorates Demyelination in MCAO Rats by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction
Abstract
1. Introduction
2. Results
2.1. Successful Establishment of the MCAO Rat Model
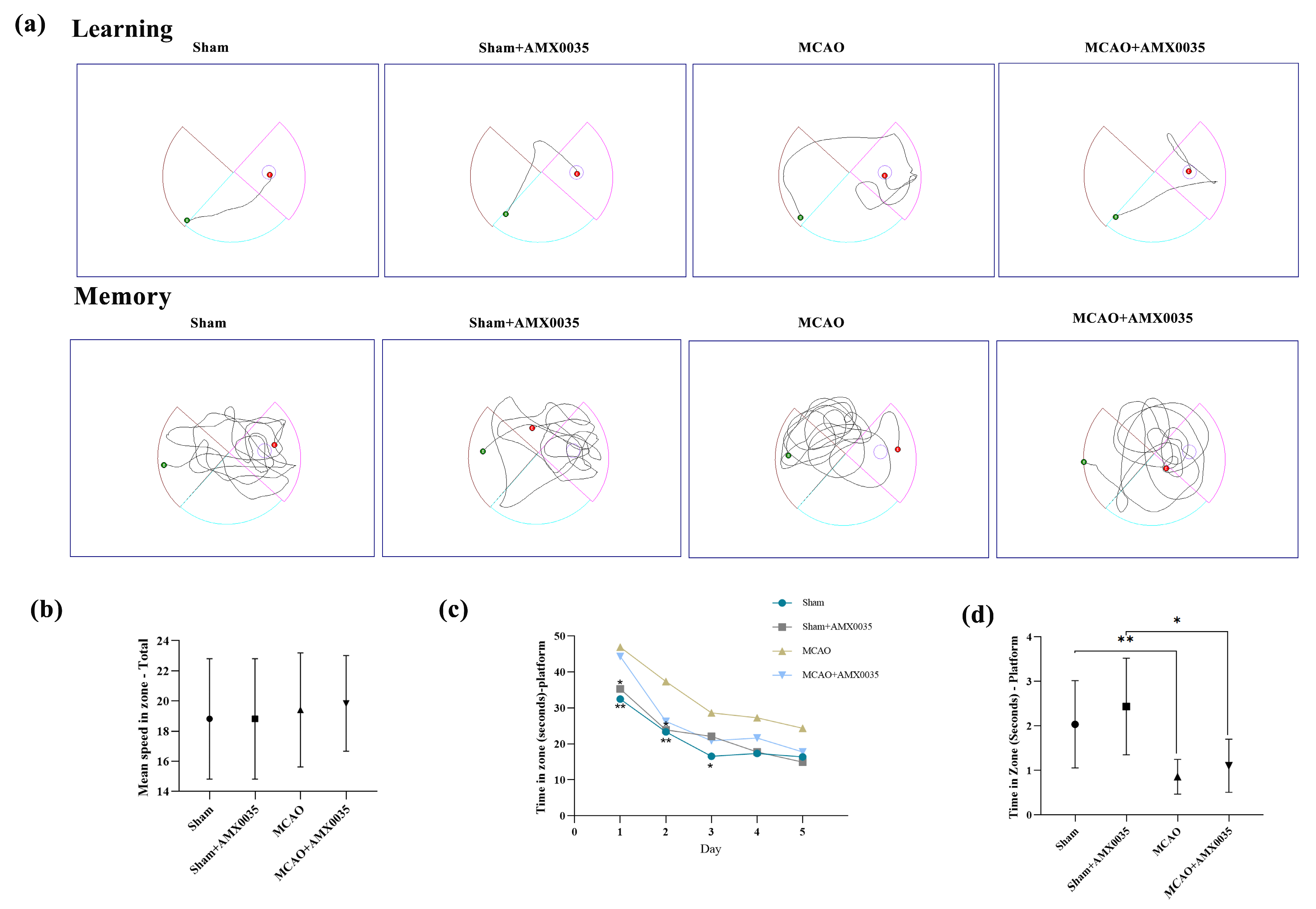
2.2. AMX0035 Improves Cognitive Function in MCAO Rats
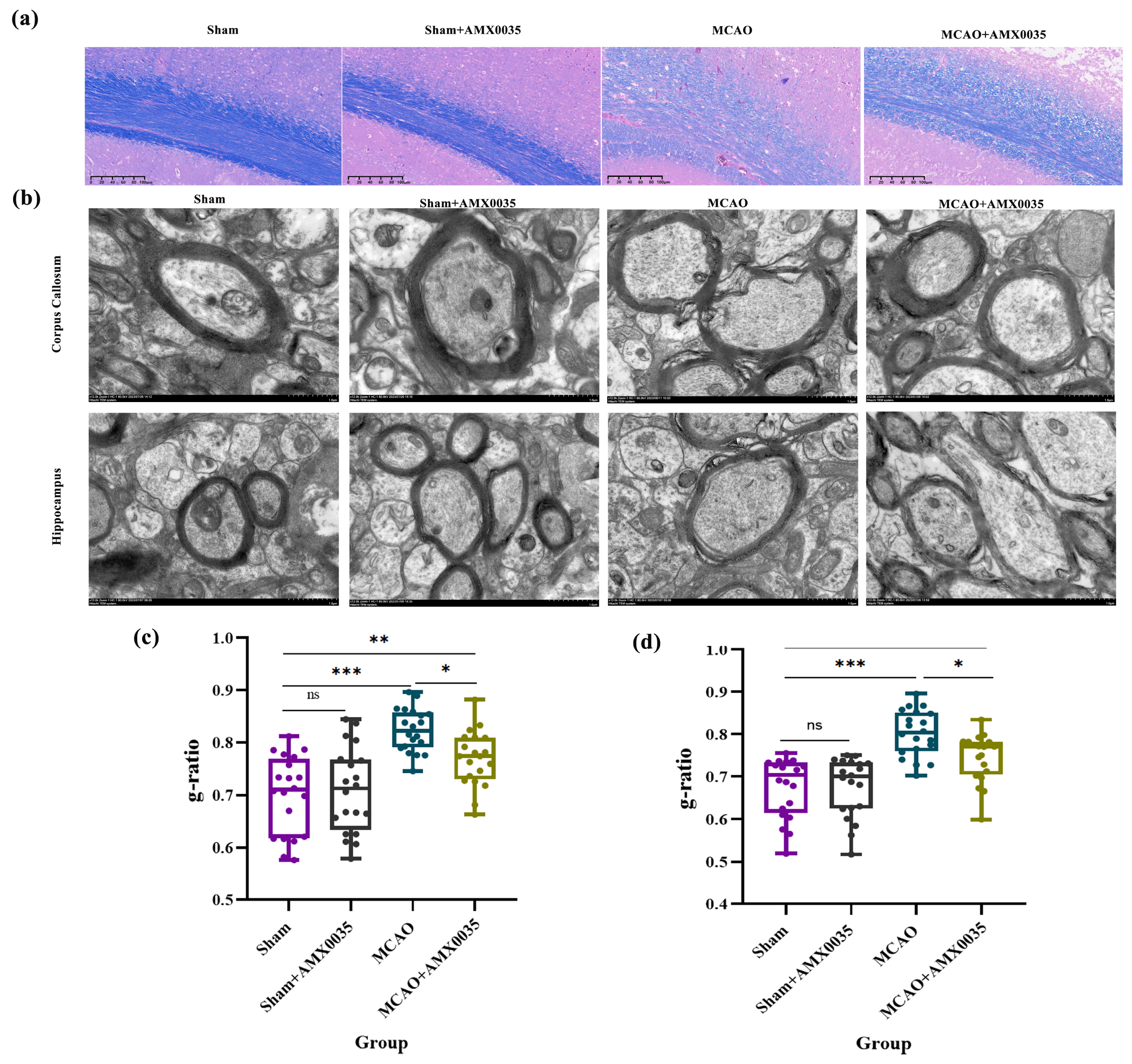
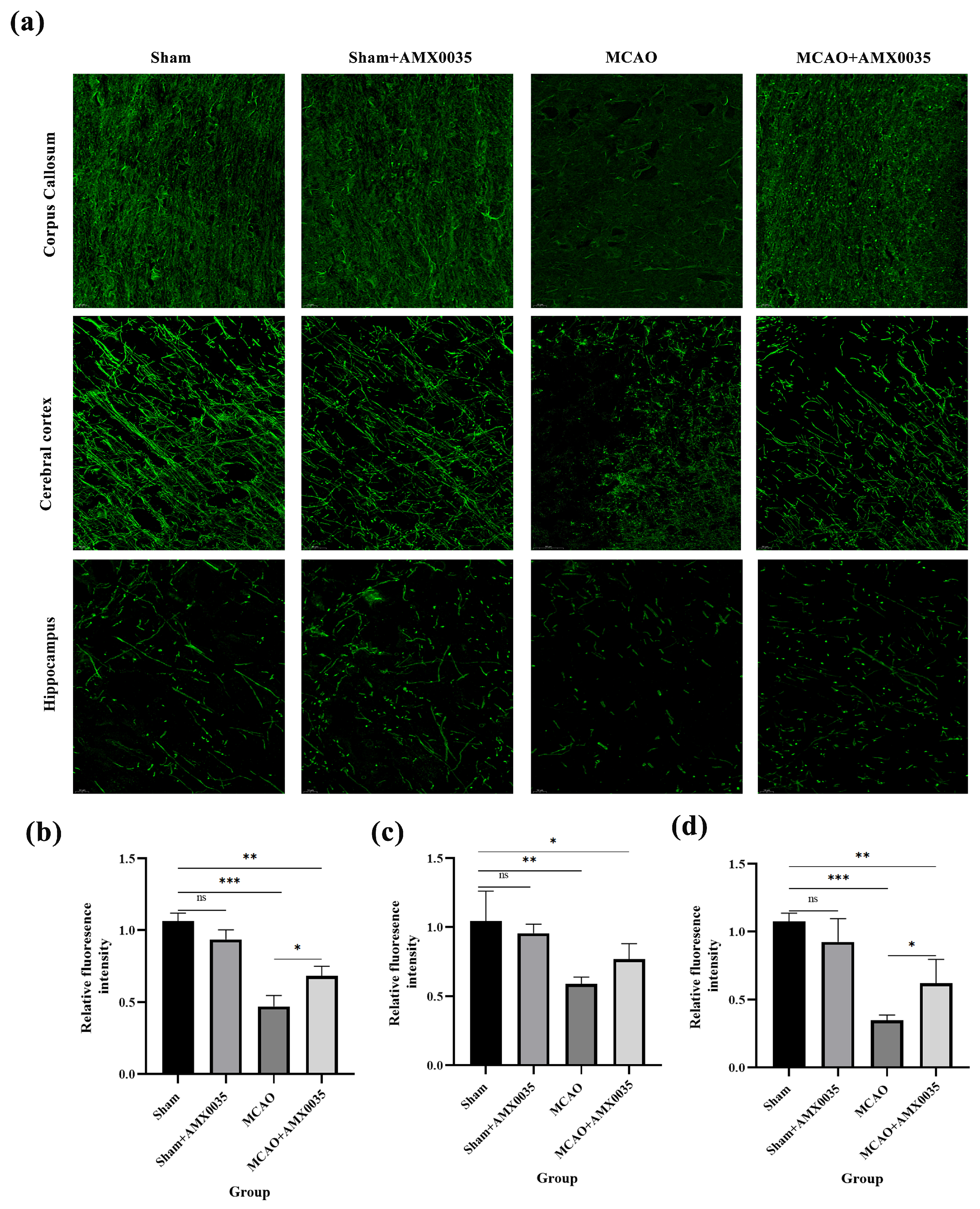
2.3. AMX0035 Alleviates Demyelination in the Brain Tissue of MCAO Rats
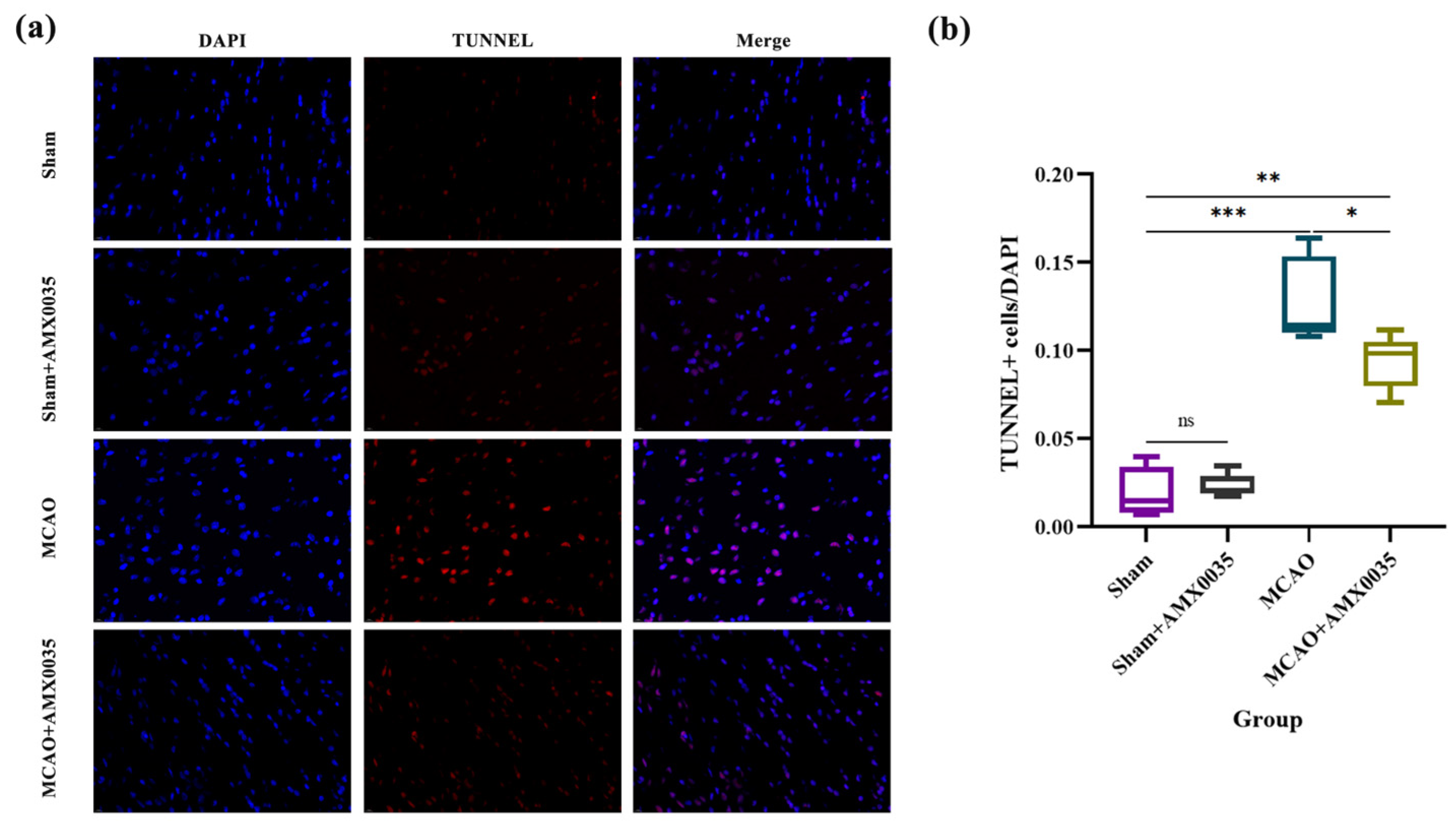
2.4. AMX0035 Inhibits Cell Apoptosis in the Brain Tissue of MCAO Rats
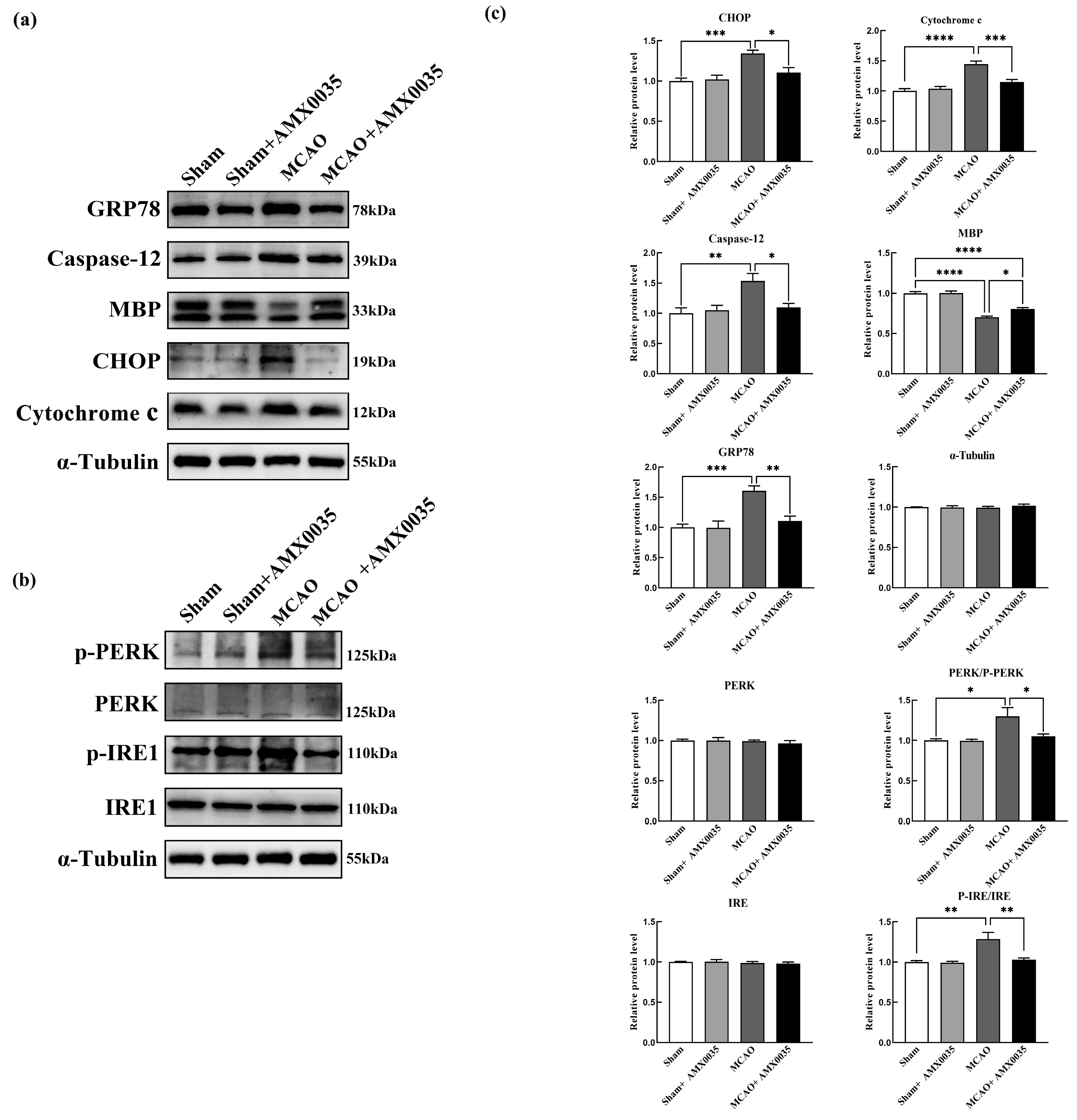
2.5. AMX0035 Reduces Endoplasmic Reticulum Stress in the Oligodendrocyte of MCAO Rats
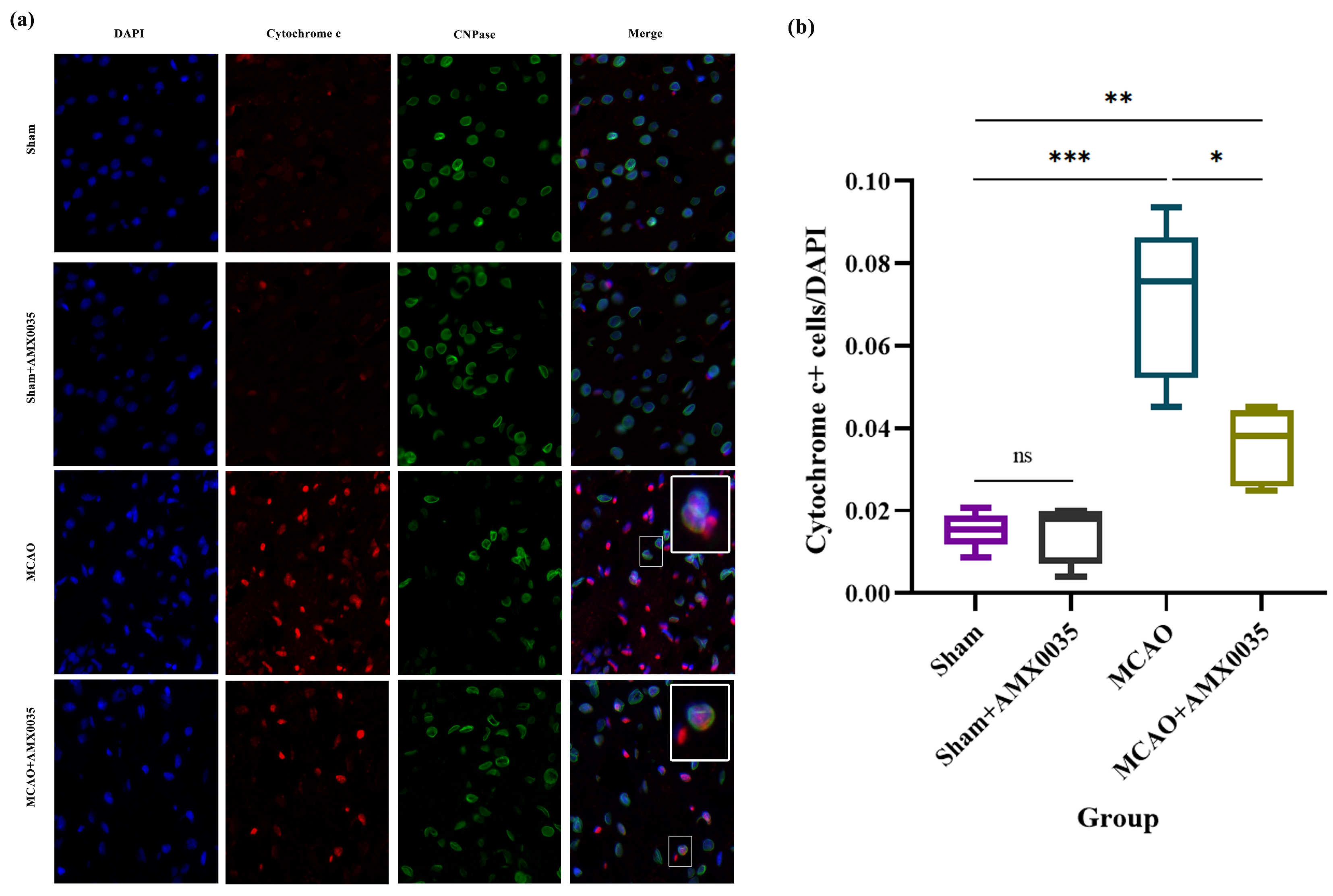
2.6. AMX0035 Inhibits Mitochondrial Dysfunction in OLs of MCAO Rats
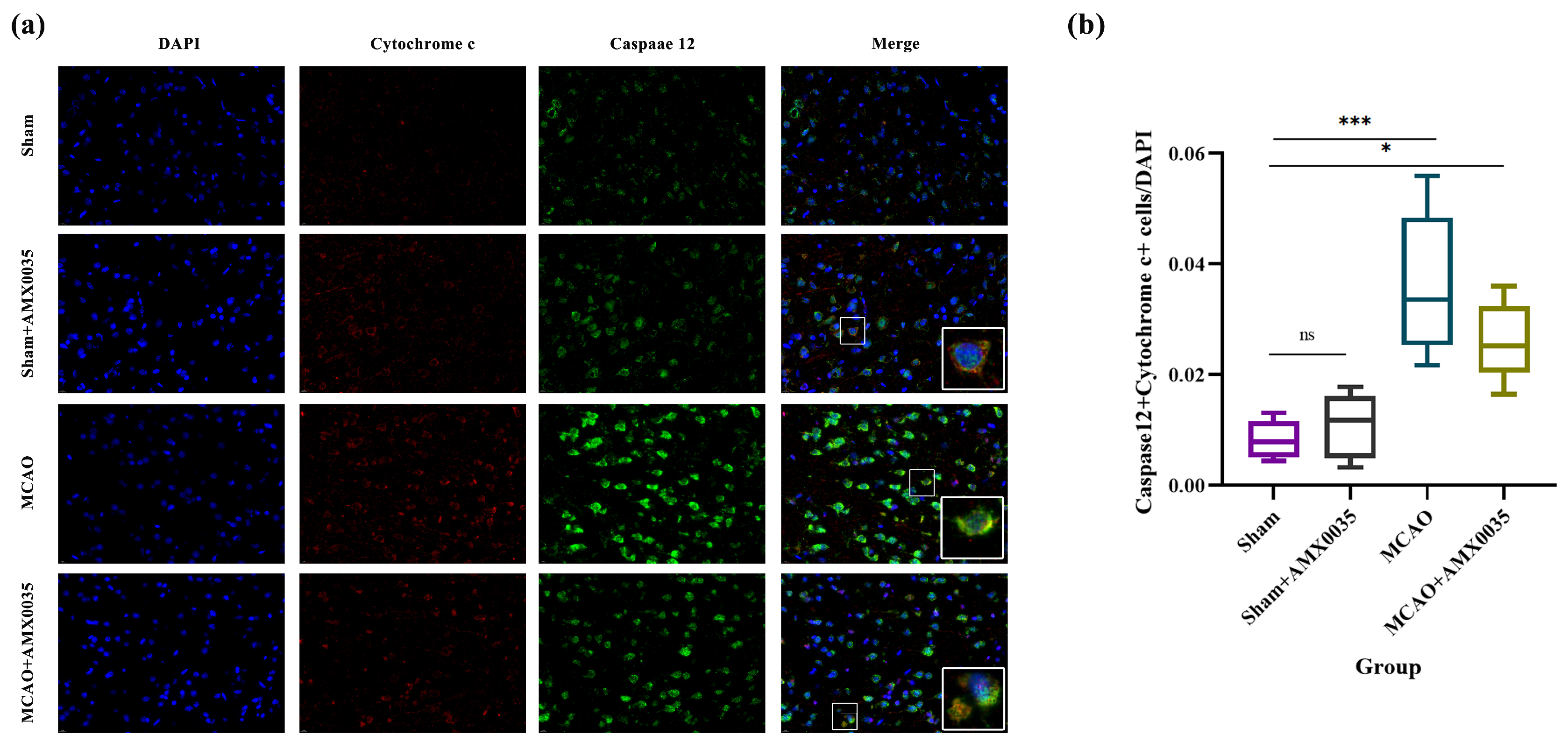
2.7. AMX0035 Inhibits the Interaction Between ER Stress and Mitochondrial Dysfunction in the Brain Tissue of MCAO Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. MCAO Model Establishment
4.3. Neurological Function Assessment
4.4. TTC Staining
4.5. Screening of PSCI Rats and AMX0035 Intervention
4.6. Behavioral Experiments
4.7. Transmission Electron Microscopy (TEM)
4.8. Luxol Fast Blue (LFB) Staining
4.9. Immunofluorescence Staining
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSCI | Post-stroke cognitive impairment |
| MCAO | Middle cerebral artery occlusion |
| TEM | Transmission electron microscopy |
| LFB | Luxol fast blue |
| MBP | Myelin basic protein |
| CNS | Central nervous system |
| ER | Endoplasmic reticulum |
| PBA | Sodium phenylbutyrate |
| TUDCA | Taurursodiol |
| SPF | Specific pathogen-free (rats) |
| SD | Sprague Dawley |
| MCA | Middle cerebral artery |
| CCA | Common carotid artery |
| ECA | External carotid artery |
| ICA | Internal carotid artery |
| BCA | Bicinchoninic acid |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| PVDF | Polyvinylidene fluoride |
| SEM | Standard error of the mean |
| ANOVA | Analysis of variance |
| ROS | Reactive oxygen species |
| ICH | Intracerebral hemorrhage |
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, Z.X.; Gu, H.Q.; Zhai, Y.; Zhou, Q.; Jiang, Y.; Zhao, X.Q.; Wang, Y.L.; Yang, X.; Wang, C.J.; et al. China Stroke Statistics: An update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc. Neurol. 2022, 7, e001374. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Feng, J.; Guo, Y. Identifying the pattern of immune related cells and genes in the peripheral blood of ischemic stroke. J. Transl. Med. 2020, 18, 296. [Google Scholar] [CrossRef]
- Health, O. Continual Long-Term Physiotherapy After Stroke: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2020, 20, 1–70. [Google Scholar]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Yuan, J.; Feng, L.; Hu, W.; Zhang, Y. Use of Multimodal Magnetic Resonance Imaging Techniques to Explore Cognitive Impairment in Leukoaraiosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8910–8915. [Google Scholar] [CrossRef]
- Li, Q.; Fadoul, G.; Ikonomovic, M.; Yang, T.; Zhang, F. Sulforaphane promotes white matter plasticity and improves long-term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic. Biol. Med. 2022, 193, 292–303. [Google Scholar] [CrossRef]
- Huang, S.Q.; Tang, C.L.; Sun, S.Q.; Yang, C.; Xu, J.; Wang, K.J.; Lu, W.T.; Huang, J.; Zhuo, F.; Qiu, G.P.; et al. Demyelination initiated by oligodendrocyte apoptosis through enhancing endoplasmic reticulum-mitochondria interactions and Id2 expression after compressed spinal cord injury in rats. CNS Neurosci. Ther. 2013, 20, 20–31. [Google Scholar] [CrossRef]
- Xiao, Y.; Geng, F.; Wang, G.; Li, X.; Zhu, J.; Zhu, W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J. Cell. Biochem. 2018, 120, 2109–2118. [Google Scholar] [CrossRef]
- Li, B.; Xu, Y.; Quan, Y.; Cai, Q.; Le, Y.; Ma, T.; Liu, Z.; Wu, G.; Wang, F.; Bao, C.; et al. Inhibition of RhoA/ROCK Pathway in the Early Stage of Hypoxia Ameliorates Depression in Mice via Protecting Myelin Sheath. ACS Chem. Neurosci. 2020, 11, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, X.; Wang, Z.G.; Shi, H.X.; Wu, F.Z.; Lin, B.B.; Xu, X.L.; Wang, X.J.; Fu, X.B.; Li, Z.Y.; et al. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci. Ther. 2012, 19, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Junichi, H.; Taiichi, K.; Manabu, T.; Akiko, H.; Kazunori, I.; Masaya, T. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci. Lett. 2004, 357, 127–130. [Google Scholar]
- Rasheva, V.I.; Domingos, P.M. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis Int. J. Program. Cell Death 2009, 14, 996–1007. [Google Scholar] [CrossRef]
- Lin, H.; Peng, Y.; Li, J.; Wang, Z.; Chen, S.; Qing, X.; Pu, F.; Lei, M.; Shao, Z. Reactive Oxygen Species Regulate Endoplasmic Reticulum Stress and ER-Mitochondrial Ca(2+) Crosstalk to Promote Programmed Necrosis of Rat Nucleus Pulposus Cells under Compression. Oxidative Med. Cell. Longev. 2021, 2021, 8810698. [Google Scholar] [CrossRef]
- Ma, L.; Yu, H.J.; Gan, S.W.; Gong, R.; Mou, K.J.; Xue, J.; Sun, S.Q. p53-Mediated oligodendrocyte apoptosis initiates demyelination after compressed spinal cord injury by enhancing ER-mitochondria interaction and E2F1 expression. Neurosci. Lett. 2017, 644, 55–61. [Google Scholar] [CrossRef]
- Wu, Z.; Niu, J.; Xue, H.; Wang, S.; Zhao, P. Sodium 4-Phenylbutyrate Protects Hypoxic-Ischemic Brain Injury via Attenuating Endoplasmic Reticulum Stress in Neonatal Rats. Front. Behav. Neurosci. 2021, 15, 632143. [Google Scholar] [CrossRef]
- Shen, H.; Kim, K.; Oh, Y.; Yoon, K.S.; Baik, H.H.; Kim, S.S.; Ha, J.; Kang, I.; Choe, W. Neurotoxin β-N-methylamino-L-alanine induces endoplasmic reticulum stress-mediated neuronal apoptosis. Mol. Med. Rep. 2016, 14, 4873–4880. [Google Scholar] [CrossRef]
- Lim, S.C.; Choi, J.E.; Kang, H.S.; Si, H. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int. J. Cancer 2009, 126, 1582–1595. [Google Scholar] [CrossRef]
- Pooja, K.; Mubashshir, A.; Mohd, S.; Heena, T.; Suhel, P. Harnessing the mitochondrial integrity for neuroprotection: Therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef] [PubMed]
- Barbarese, E.; Pfeiffer, S.E. Developmental regulation of myelin basic protein in dispersed cultures. Proc. Natl. Acad. Sci. USA 1981, 78, 1953–1957. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–443. [Google Scholar] [CrossRef]
- Ham, P.B., 3rd.; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2016, 157, 92–116. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Kaufman, R.J. Stress signaling from the lumen of the endoplasmic reticulum: Coordination of gene transcriptional and translational controls. Genes Dev. 1999, 13, 1211–1233. [Google Scholar] [CrossRef]
- Lin, W.; Stone, S. Unfolded protein response in myelin disorders. Neural Regen. Res. 2019, 15, 636–645. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Groenendyk, J.; Agellon, L.B.; Michalak, M. Calcium signaling and endoplasmic reticulum stress. Int. Rev. Cell Mol. Biol. 2021, 363, 1–20. [Google Scholar] [PubMed]
- Ma, Y.; Hendershot, L.M. The unfolding tale of the unfolded protein response. Cell 2002, 107, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The developing oligodendrocyte: Key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhou, G.; Zhuang, J.; Xu, C.; Zhou, H.; Peng, Y.; Cao, Y.; Zeng, H.; Li, J.; Yan, F.; et al. White Matter Injury After Intracerebral Hemorrhage. Front. Neurol. 2021, 12, 562090. [Google Scholar] [CrossRef]
- Darbinian, N.; Selzer, M.E. Oligodendrocyte pathology in fetal alcohol spectrum disorders. Neural Regen. Res. 2021, 17, 497–502. [Google Scholar]
- Zhuo, F.; Qiu, G.; Xu, J.; Yang, M.; Wang, K.; Liu, H.; Huang, J.; Lu, W.; Liu, Q.; Xu, S.; et al. Both endoplasmic reticulum and mitochondrial pathways are involved in oligodendrocyte apoptosis induced by capsular hemorrhage. Mol. Cell. Neurosci. 2016, 72, 64–71. [Google Scholar] [CrossRef]
- Mohammed Thangameeran, S.I.; Tsai, S.T.; Hung, H.Y.; Hu, W.F.; Pang, C.Y.; Chen, S.Y.; Liew, H.K. A Role for Endoplasmic Reticulum Stress in Intracerebral Hemorrhage. Cells 2020, 9, 750. [Google Scholar] [CrossRef]
- Wilding, A.S.; Patte-Mensah, C.; Taleb, O.; Brun, S.; Kemmel, V.; Mensah-Nyagan, A.G. Protective effect of 4-Phenylbutyrate against proteolipid protein mutation-induced endoplasmic reticulum stress and oligodendroglial cell death. Neurochem. Int. 2018, 118, 185–194. [Google Scholar] [CrossRef]
- Ohri, S.S.; Hetman, M.; Whittemore, S.R. Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol. Dis. 2013, 58, 29–37. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Y.; Ren, Y.; Yin, S.; Yu, L.; Huang, R.; Song, S.; Hu, X.; Zhu, R.; Cheng, L.; et al. Neurogenesis potential of oligodendrocyte precursor cells from oligospheres and injured spinal cord. Front. Cell. Neurosci. 2023, 16, 1049562. [Google Scholar] [CrossRef]
- Davis, C.K.; Bathula, S.; Hsu, M.; Morris-Blanco, K.C.; Chokkalla, A.K.; Jeong, S.; Choi, J.; Subramanian, S.; Park, J.S.; Fabry, Z.; et al. An Antioxidant and Anti-ER Stress Combo Therapy Decreases Inflammation, Secondary Brain Damage and Promotes Neurological Recovery following Traumatic Brain Injury in Mice. J. Neurosci. 2022, 42, 6810–6821. [Google Scholar] [CrossRef] [PubMed]
- Corbett, G.T.; Roy, A.; Pahan, K. Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): Implications for Alzheimer disease therapy. J. Biol. Chem. 2013, 288, 8299–8312. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Y.; Lu, X.; Shi, J.; Gong, Q. Icariin improves the cognitive function of APP/PS1 mice via suppressing endoplasmic reticulum stress. Life Sci. 2019, 234, 116739. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Huang, T.C.; Chen, S.H.; Ramasamy, T.S.; Hsueh, Y.Y.; Lin, S.P.; Lu, F.I.; Liu, Y.H.; Wu, C.C. Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination. J. Neuroinflam. 2021, 18, 238. [Google Scholar] [CrossRef]
- Lupachyk, S.; Watcho, P.; Obrosov, A.A.; Stavniichuk, R.; Obrosova, I.G. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Exp. Neurol. 2012, 247, 342–348. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Chen, L.; Du, Y.F.; Hu, M.; Reed, M.N.; Long, Y.; Suppiramaniam, V.; Hong, H.; Tang, S.S. Protective effects of tauroursodeoxycholic acid on lipopolysaccharide-induced cognitive impairment and neurotoxicity in mice. Int. Immunopharmacol. 2019, 72, 166–175. [Google Scholar] [CrossRef]
- Kumar, S.; Chowdhury, S.; Razdan, A.; Kumari, D.; Purty, R.S.; Ram, H.; Kumar, P.; Nayak, P.; Shukla, S.D. Downregulation of Candidate Gene Expression and Neuroprotection by Piperine in Streptozotocin-Induced Hyperglycemia and Memory Impairment in Rats. Front. Pharmacol. 2021, 11, 595471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Bian, C.; Wang, Y.; Wei, L.; Sun, S.; Liu, Q. AMX0035 Mitigates Oligodendrocyte Apoptosis and Ameliorates Demyelination in MCAO Rats by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 3865. https://doi.org/10.3390/ijms26083865
Zhang L, Bian C, Wang Y, Wei L, Sun S, Liu Q. AMX0035 Mitigates Oligodendrocyte Apoptosis and Ameliorates Demyelination in MCAO Rats by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. International Journal of Molecular Sciences. 2025; 26(8):3865. https://doi.org/10.3390/ijms26083865
Chicago/Turabian StyleZhang, Li, Cunhao Bian, Yusen Wang, Ling Wei, Shanquan Sun, and Qian Liu. 2025. "AMX0035 Mitigates Oligodendrocyte Apoptosis and Ameliorates Demyelination in MCAO Rats by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction" International Journal of Molecular Sciences 26, no. 8: 3865. https://doi.org/10.3390/ijms26083865
APA StyleZhang, L., Bian, C., Wang, Y., Wei, L., Sun, S., & Liu, Q. (2025). AMX0035 Mitigates Oligodendrocyte Apoptosis and Ameliorates Demyelination in MCAO Rats by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. International Journal of Molecular Sciences, 26(8), 3865. https://doi.org/10.3390/ijms26083865






