Behavioral Effects of Stimulated Dopamine Release and D2-like Receptor Displacement in Parkinson’s Patients with Impulse-Control Disorder
Abstract
:1. Introduction
2. Results
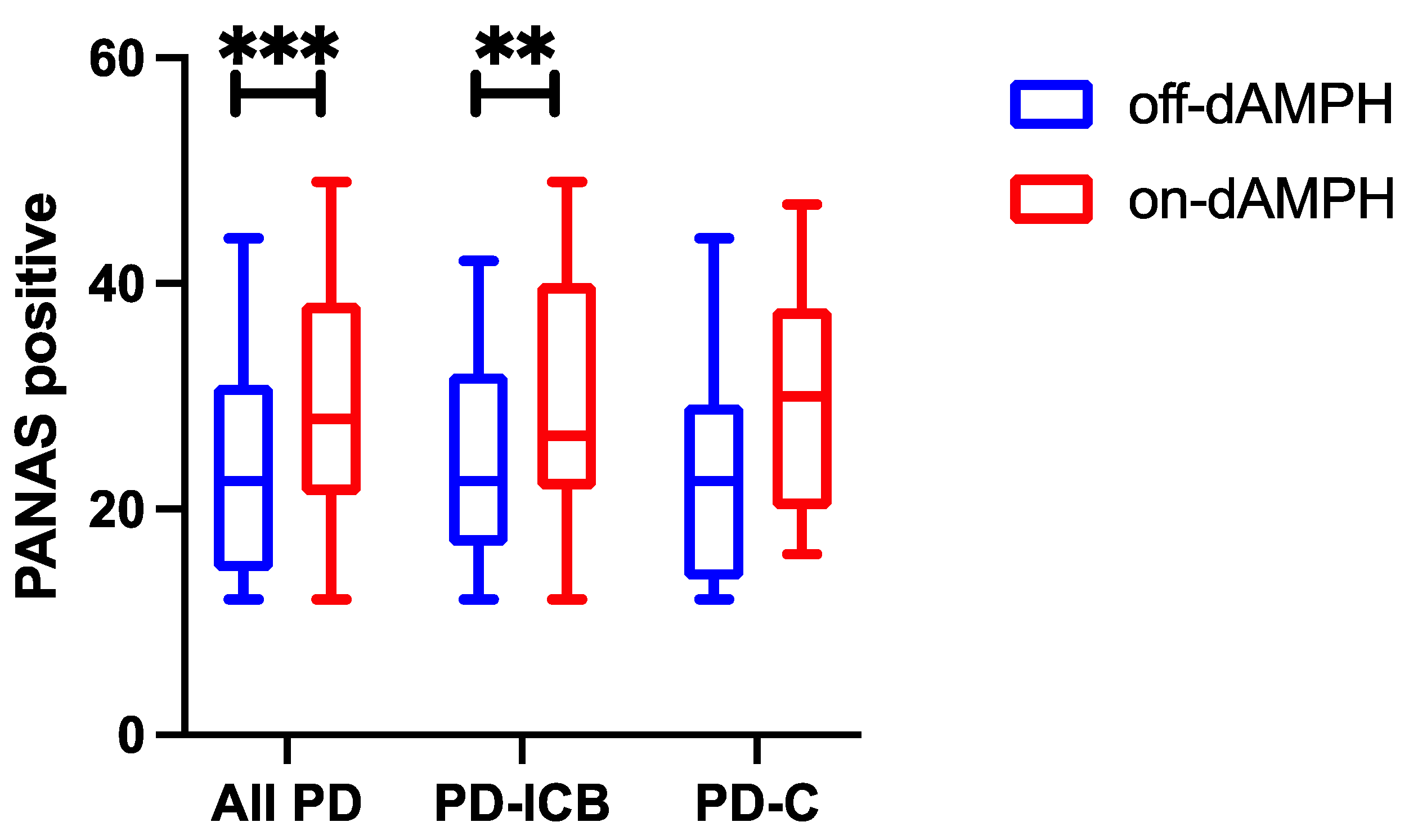
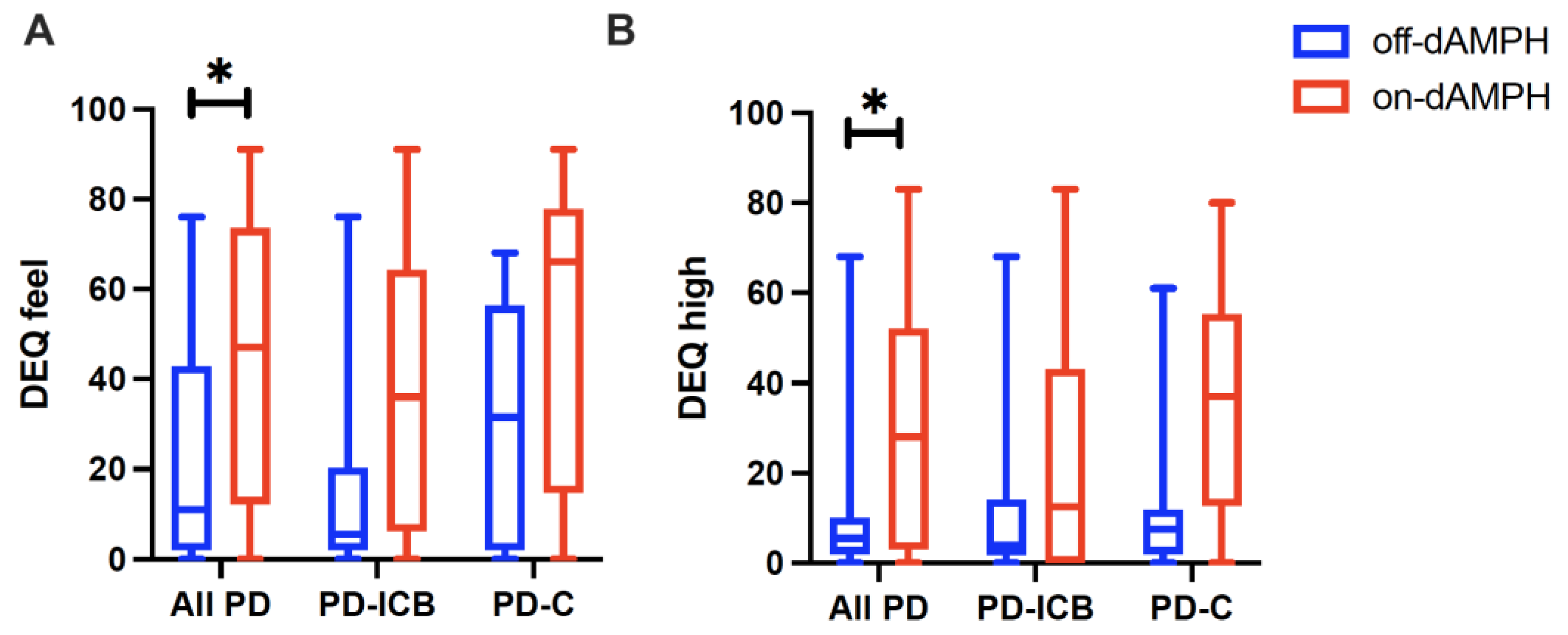
2.1. dAMPH Effects on Mood
2.2. Localization of DA Release Associations with Subjective Experiences
2.3. Baseline D2-R Availability as a Predictor of Amphetamine Effects
3. Discussion
3.1. Localization of dAMPH Effects
3.2. Impulsivity and Mood in PD
3.3. Baseline BPND as Predictors of Mood Effects
4. Materials and Methods
4.1. Population
4.2. Trait Impulsivity, Subjective Measures
4.3. MRI Acquisition
4.4. PET Imaging, Data Processing
4.5. Experimental Design
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| AIRS | Amphetamine Interview Rating Scale |
| BPND | Non-displaceable binding potential |
| cmOFC | Caudo-medial orbitofrontal cortex |
| DA | Dopamine |
| DAA | Dopamine agonist |
| dAMPH | Dextro-amphetamine |
| DAT | Dopamine transporter |
| DA-R | Dopamine receptor |
| DEQ | Drug Effects Questionnaire |
| FDR | False discovery rate |
| GP | Globus pallidus |
| MD-UPDRS | Movement Disorders Society—Unified Parkinson’s Disease Rating Scale |
| MRI | Magnetic resonance imaging |
| MoCA | Montreal Cognitive Assessment |
| QUIP-RS | Questionnaire for Impulsive–Compulsive Disorders in Parkinson’s Disease Rating Scale |
| PANAS | Positive and Negative Affect Scale |
| PC | Principal component |
| PD | Parkinson’s disease |
| PD-ICB | Parkinson’s disease with impulsive–compulsive behaviors |
| PD-C | Parkinson’s disease control |
| PET | Positron emission tomography |
| ROI | Region of interest |
| SRTM | Simplified reference tissue model |
| VS | Ventral striatum |
References
- Ahearn, D.J.; McDonald, K.; Barraclough, M.; Leroi, I. An exploration of apathy and impulsivity in Parkinson disease. Curr. Gerontol. Geriatr. Res. 2012, 2012, 390701. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.C.; Chan, L.L.; Tan, E.K. Depression, anxiety, and apathy in Parkinson’s disease: Insights from neuroimaging studies. Eur. J. Neurol. 2016, 23, 1001–1019. [Google Scholar] [CrossRef] [PubMed]
- Menza, M.A.; Sage, J.; Marshall, E.; Cody, R.; Duvoisin, R. Mood changes and ‘on-off’ phenomena in Parkinson’s disease. Mov. Disord. 1990, 5, 148–151. [Google Scholar] [CrossRef]
- Weiss, H.D.; Marsh, L. Impulse control disorders and compulsive behaviors associated with dopaminergic therapies in Parkinson disease. Neurol. Clin. Pract. 2012, 2, 267–274. [Google Scholar] [CrossRef]
- Ambermoon, P.; Carter, A.; Hall, W.D.; Dissanayaka, N.N.W.; O’Sullivan, J.D. Impulse control disorders in patients with Parkinson’s disease receiving dopamine replacement therapy: Evidence and implications for the addictions field. Addiction 2011, 106, 283–293. [Google Scholar] [CrossRef]
- Garcia-Ruiz, P.J.; Castrillo, J.C.M.; Alonso-Canovas, A.; Barcenas, A.H.; Vela, L.; Alonso, P.S.; Mata, M.; Gonzalez, N.O.; Fernandez, I.M. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 840–844. [Google Scholar] [CrossRef]
- Park, A.; Stacy, M. Dopamine-induced nonmotor symptoms of Parkinson’s disease. Park. Dis. 2011, 2011, 485063. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Liu, L.; Yang, J.; Huang, J.; Xiong, N.; Wang, T. Impulsive and compulsive behaviors in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 318. [Google Scholar] [CrossRef]
- Mestre, T.A.; Strafella, A.P.; Thomsen, T.; Voon, V.; Miyasaki, J. Diagnosis and treatment of impulse control disorders in patients with movement disorders. Ther. Adv. Neurol. Disord. 2013, 6, 175–188. [Google Scholar] [CrossRef]
- Song, A.K.; Hay, K.R.; Trujillo, P.; Aumann, M.; Stark, A.J.; Yan, Y.; Kang, H.; Donahue, M.J.; Zald, D.H.; Claassen, D.O. Amphetamine-induced dopamine release and impulsivity in Parkinson disease. Brain 2021, 145, 3488–3499. [Google Scholar] [CrossRef]
- Stark, A.J.; Smith, C.T.; Lin, Y.-C.; Petersen, K.J.; Trujillo, P.; van Wouwe, N.C.; Kang, H.; Donahue, M.J.; Kessler, R.M.; Zald, D.H.; et al. Nigrostriatal and Mesolimbic D2/3 Receptor Expression in Parkinson’s Disease Patients with Compulsive Reward-Driven Behaviors. J. Neurosci. 2018, 38, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; Smith, C.E.; et al. Dopaminergic network differences in human impulsivity. Science 2010, 329, 532. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.C.; van Eimeren, T. The functional anatomy of impulse control disorders. Curr. Neurol. Neurosci. Rep. 2013, 13, 386. [Google Scholar] [CrossRef]
- Evans, A.H.; Pavese, N.; Lawrence, A.D.; Tai, Y.F.; Appel, S.; Doder, M.; Brooks, D.J.; Lees, A.J.; Piccini, P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann. Neurol. 2006, 59, 852–858. [Google Scholar] [CrossRef]
- Steeves, T.D.L.; Miyasaki, J.; Zurowski, M.; Lang, A.E.; Pellecchia, G.; Van Eimeren, T.; Rusjan, P.; Houle, S.; Strafella, A.P. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: A [11C] raclopride PET study. Brain 2009, 132 Pt 5, 1376–1385. [Google Scholar] [CrossRef]
- Trifilieff, P.; Martinez, D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 2014, 76 Pt B, 498–509. [Google Scholar] [CrossRef]
- Voon, V.; Rizos, A.; Chakravartty, R.; Mulholland, N.; Robinson, S.; Howell, N.A.; Harrison, N.; Vivian, G.; Chaudhuri, K.R. Impulse control disorders in Parkinson’s disease: Decreased striatal dopamine transporter levels. J. Neurol. Neurosurg. Psychiatry 2014, 85, 148–152. [Google Scholar] [CrossRef]
- Smith, C.T.; Juan, M.D.S.; Dang, L.C.; Katz, D.T.; Perkins, S.F.; Burgess, L.L.; Cowan, R.L.; Manning, H.C.; Nickels, M.L.; Claassen, D.O.; et al. Ventral striatal dopamine transporter availability is associated with lower trait motor impulsivity in healthy adults. Transl. Psychiatry 2018, 8, 269. [Google Scholar] [CrossRef]
- Cilia, R.; Siri, C.; Marotta, G.; Isaias, I.U.; De Gaspari, D.; Canesi, M.; Pezzoli, G.; Antonini, A. Functional abnormalities underlying pathological gambling in Parkinson disease. Arch. Neurol. 2008, 65, 1604–1611. [Google Scholar] [CrossRef]
- Cilia, R.; van Eimeren, T. Impulse control disorders in Parkinson’s disease: Seeking a roadmap toward a better understanding. Brain Struct. Funct. 2011, 216, 289–299. [Google Scholar] [CrossRef]
- Carriere, N.; Lopes, R.; Defebvre, L.; Delmaire, C.; Dujardin, K. Impaired corticostriatal connectivity in impulse control disorders in Parkinson disease. Neurology 2015, 84, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.J.; Demers, C.H.; Braud, J.; Briggs, R.; Adinoff, B.; Stein, E.A. Striatal-insula circuits in cocaine addiction: Implications for impulsivity and relapse risk. Am. J. Drug Alcohol. Abuse 2013, 39, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.T.; Dang, L.C.; Cowan, R.L.; Kessler, R.M.; Zald, D.H. Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacology 2016, 108, 394–402. [Google Scholar] [CrossRef]
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef]
- Johanson, C.E.; Uhlenhuth, E.H. Drug preference and mood in humans: Repeated assessment of d-amphetamine. Pharmacol. Biochem. Behav. 1981, 14, 159–163. [Google Scholar] [CrossRef]
- Kelly, B.C.; Parsons, J.T.; Wells, B.E. Prevalence and predictors of club drug use among club-going young adults in New York City. J. Urban Health 2006, 83, 884–895. [Google Scholar] [CrossRef]
- Ashby, F.G.; Isen, A.M.; Turken, A.U. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999, 106, 529–550. [Google Scholar] [CrossRef]
- Riccardi, P.; Park, S.; Anderson, S.; Doop, M.; Ansari, M.S.; Schmidt, D.; Baldwin, R. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F]fallypride. Synapse 2011, 65, 99–102. [Google Scholar] [CrossRef]
- Riccardi, P.; Li, R.; Ansari, M.S.; Zald, D.; Park, S.; Dawant, B.; Anderson, S.; Doop, M.; Woodward, N.; Schoenberg, E.; et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 2006, 31, 1016–1026. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Wang, S.-M.; Tickle-Degnen, L. Emotional cues from expressive behavior of women and men with Parkinson’s disease. PLoS ONE 2018, 13, e0199886. [Google Scholar] [CrossRef] [PubMed]
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Msc, D.K.M.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. 2019, 34, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Morean, M.E.; de Wit, H.; King, A.C.; Sofuoglu, M.; Rueger, S.Y.; O’Malley, S.S. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology 2013, 227, 177–192. [Google Scholar] [CrossRef]
- Fischman, M.W.; Foltin, R.W. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br. J. Addict. 1991, 86, 1563–1570. [Google Scholar] [CrossRef]
- Van Kammen, D.P.; Murphy, D.L. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975, 44, 215–224. [Google Scholar] [CrossRef]
- Schneier, F.R.; Abi-Dargham, A.; Martinez, D.; Slifstein, M.; Hwang, D.-R.; Liebowitz, M.R.; Laruelle, M. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress. Anxiety 2009, 26, 411–418. [Google Scholar] [CrossRef]
- Yoo, S.W.; Oh, Y.-S.; Hwang, E.-J.; Ryu, D.-W.; Lee, K.-S.; Lyoo, C.H.; Kim, J.-S. ‘Depressed’ caudate and ventral striatum dopamine transporter availability in de novo Depressed Parkinson’s disease. Neurobiol. Dis. 2019, 132, 104563. [Google Scholar] [CrossRef]
- Harro, J. Neuropsychiatric Adverse Effects of Amphetamine and Methamphetamine. Int. Rev. Neurobiol. 2015, 120, 179–204. [Google Scholar] [CrossRef]
- Wardle, M.C.; De Wit, H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology 2012, 220, 143–153. [Google Scholar] [CrossRef]
- Wardle, M.C.; Garner, M.J.; Munafò, M.R.; de Wit, H. Amphetamine as a social drug: Effects of d-amphetamine on social processing and behavior. Psychopharmacology 2012, 223, 199–210. [Google Scholar] [CrossRef]
- Kirkpatrick, M.G.; Goldenson, N.I.; Kapadia, N.; Kahler, C.W.; de Wit, H.; Swift, R.M.; McGeary, J.E.; Sussman, S.; Leventhal, A.M. Emotional traits predict individual differences in amphetamine-induced positive mood in healthy volunteers. Psychopharmacology 2016, 233, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Parkinson, J.A.; Olmstead, M.C.; Arroyo, M.; Robledo, P.; Robbins, T.W. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. N. Y. Acad. Sci. 1999, 877, 412–438. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Pollmann, S. A universal role of the ventral striatum in reward-based learning: Evidence from human studies. Neurobiol. Learn. Mem. 2014, 114, 90–100. [Google Scholar] [CrossRef]
- Yin, H.H.; Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006, 7, 464–476. [Google Scholar] [CrossRef]
- Zald, D.H.; Boileau, I.; El-Dearedy, W.; Gunn, R.; McGlone, F.; Dichter, G.S.; Dagher, A. Dopamine transmission in the human striatum during monetary reward tasks. J. Neurosci. 2004, 24, 4105–4112. [Google Scholar] [CrossRef]
- Rempel-Clower, N.L. Role of orbitofrontal cortex connections in emotion. Ann. N. Y. Acad. Sci. 2007, 1121, 72–86. [Google Scholar] [CrossRef]
- O’Doherty, J.P. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr. Opin. Neurobiol. 2004, 14, 769–776. [Google Scholar] [CrossRef]
- Bonnet, L.; Comte, A.; Tatu, L.; Millot, J.L.; Moulin, T.; De Bustos, E.M. The role of the amygdala in the perception of positive emotions: An ‘intensity detector’. Front. Behav. Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef]
- Fotros, A.; Casey, K.F.; Larcher, K.; Verhaeghe, J.A.; Cox, S.M.; Gravel, P.; Reader, A.J.; Dagher, A.; Benkelfat, C.; Leyton, M. Cocaine cue-induced dopamine release in amygdala and hippocampus: A high-resolution PET [18F]fallypride study in cocaine dependent participants. Neuropsychopharmacology 2013, 38, 1780–1788. [Google Scholar] [CrossRef]
- Goursaud, A.P.S.; Bachevalier, J. Altered face scanning and arousal after orbitofrontal cortex lesion in adult rhesus monkeys. Behavioral neuroscience 2020, 134, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Trifilieff, P.; Feng, B.; Urizar, E.; Winiger, V.; Ward, R.D.; Taylor, K.M.; Martinez, D.; Moore, H.; Balsam, P.D.; Simpson, E.H.; et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 2013, 18, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Nakamura, K.; Daw, N.D. Serotonin and dopamine: Unifying affective, activational, decision functions. Neuropsychopharmacology 2011, 36, 98–113. [Google Scholar] [CrossRef]
- Scott, B.M.; Eisinger, R.S.; Burns, M.R.; Lopes, J.; Okun, M.S.; Gunduz, A.; Bowers, D. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology 2020, 95, e2769–e2780. [Google Scholar] [CrossRef]
- Aumann, M.A.; Stark, A.J.; Hughes, S.B.; Lin, Y.; Kang, H.; Bradley, E.; Zald, D.H.; Claassen, D.O. Self-reported rates of impulsivity in Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 437–448. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113-25. [Google Scholar] [CrossRef]
- Hisahara, S.; Shimohama, S. Dopamine receptors and Parkinson’s disease. Int. J. Med. Chem. 2011, 2011, 403039. [Google Scholar] [CrossRef]
- Stark, A.J.; Smith, C.T.; Petersen, K.J.; Trujillo, P.; van Wouwe, N.C.; Donahue, M.J.; Kessler, R.M.; Deutch, A.Y.; Zald, D.H.; Claassen, D.O. [18F]fallypride characterization of striatal and extrastriatal D2/3 receptors in Parkinson’s disease. Neuroimage Clin. 2018, 18, 433–442. [Google Scholar] [CrossRef]
- American Psychiatric Publishing. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Ebersbach, G.; Baas, H.; Csoti, I.; Müngersdorf, M.; Deuschl, G. Scales in Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. S4), IV32–IV35. [Google Scholar] [CrossRef]
- Poewe, W.; Mahlknecht, P. The clinical progression of Parkinson’s disease. Park. Relat. Disord. 2009, 15 (Suppl. S4), S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Hoops, S.; Shea, J.A.; Lyons, K.E.; Pahwa, R.; Driver-Dunckley, E.D.; Adler, C.H.; Potenza, M.N.; Miyasaki, J.; Siderowf, A.D.; et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov. Disord. 2009, 24, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Mamikonyan, E.; Papay, K.; Shea, J.A.; Xie, S.X.; Siderowf, A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale. Mov. Disord. 2012, 27, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Mann, L.G.; Hay, K.R.; Song, A.K.; Errington, S.P.; Trujillo, P.; Zald, D.H.; Yan, Y.; Kang, H.; Logan, G.D.; Claassen, D.O. D2-Like Receptor Expression in the Hippocampus and Amygdala Informs Performance on the Stop-Signal Task in Parkinson’s Disease. J. Neurosci. 2021, 41, 10023–10030. [Google Scholar] [CrossRef]
- Klomp, A.; Koolschijn, P.C.M.P.; Pol, H.E.H.; Kahn, R.S.; Van Haren, N.E.M. Hypothalamus and pituitary volume in schizophrenia: A structural MRI study. Int. J. Neuropsychopharmacol. 2012, 15, 281–288. [Google Scholar] [CrossRef]
- Ongür, D.; Price, J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 2000, 10, 206–219. [Google Scholar] [CrossRef]



| BPND (Regression Coefficient, p-Value Uncorrected, p-Value Corrected) | ||||
|---|---|---|---|---|
| Questionnaire | Caudate Head | Ventral Striatum | Amygdala | cmOFC |
| PANAS positive | −0.019, 0.32, 0.48 | −0.055, 0.03 *, 0.28 | −0.004, 0.33, 0.48 | −0.027, 0.11, 0.28 |
| DEQ feel | −0.009, 0.02 *, 0.12 | −0.012, 0.03 *, 0.16 | −0.001, 0.27, 0.41 | −0.006, 0.07, 0.23 |
| DEQ high | −0.008, 0.05 *, 0.47 | −0.010, 0.11, 0.47 | −0.001, 0.24, 0.47 | −0.004, 0.28, 0.47 |
| AIRS total | −0.005, 0.062, 0.16 | −0.011, 0.003, 0.03 | −0.001, 0.024, 0.07 | −0.006, 0.014, 0.07 |
| Variables | All PD | ICB+ (PD-ICB) | ICB− (PD-C) | Test Statistic, p (PD-ICB vs. PD-C) |
|---|---|---|---|---|
| N | 20 | 10 | 10 | - |
| Sex (M/F) | 12/8 | 7/3 | 5/5 | 0.833, 0.361 |
| Age (yrs) | 64.1 ± 5.78 | 65.8 ± 6.60 | 62.4 ± 4.53 | 2.12, 0.198 |
| Disease duration (yrs) | 6.43 ± 3.07 | 6.10 ± 2.28 | 6.75 ± 3.81 | 2.13, 0.650 |
| MDS-UPDRS-III (off-dAMPH) | 28.7 ± 13.1 | 27.6 ± 12.4 | 29.7 ± 14.3 | 2.10, 0.730 |
| Total LEDD (mg/day) | 671 ± 302 | 671 ± 314 | 672 ± 306 | 2.10, 0.994 |
| QUIP-RS | 26.0 ± 13.9 | 30.0 ± 12.0 | 19.9 ± 12.1 | 1.88, 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aumann, M.A.; Lee, S.J.; Song, A.K.; O’Rourke, K.R.; Trujillo, P.; Yan, Y.; Kang, H.; Claassen, D.O. Behavioral Effects of Stimulated Dopamine Release and D2-like Receptor Displacement in Parkinson’s Patients with Impulse-Control Disorder. Int. J. Mol. Sci. 2025, 26, 3866. https://doi.org/10.3390/ijms26083866
Aumann MA, Lee SJ, Song AK, O’Rourke KR, Trujillo P, Yan Y, Kang H, Claassen DO. Behavioral Effects of Stimulated Dopamine Release and D2-like Receptor Displacement in Parkinson’s Patients with Impulse-Control Disorder. International Journal of Molecular Sciences. 2025; 26(8):3866. https://doi.org/10.3390/ijms26083866
Chicago/Turabian StyleAumann, Megan A., Sean J. Lee, Alexander K. Song, Kaitlyn R. O’Rourke, Paula Trujillo, Yan Yan, Hakmook Kang, and Daniel O. Claassen. 2025. "Behavioral Effects of Stimulated Dopamine Release and D2-like Receptor Displacement in Parkinson’s Patients with Impulse-Control Disorder" International Journal of Molecular Sciences 26, no. 8: 3866. https://doi.org/10.3390/ijms26083866
APA StyleAumann, M. A., Lee, S. J., Song, A. K., O’Rourke, K. R., Trujillo, P., Yan, Y., Kang, H., & Claassen, D. O. (2025). Behavioral Effects of Stimulated Dopamine Release and D2-like Receptor Displacement in Parkinson’s Patients with Impulse-Control Disorder. International Journal of Molecular Sciences, 26(8), 3866. https://doi.org/10.3390/ijms26083866






