Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower
Abstract
1. Introduction
2. The Combined Effect of Genetic Regulation and Environmental Factors on Secondary Metabolites
2.1. Composition and Function of Major Secondary Metabolites in Plants
2.2. Genetic Regulation of Major Secondary Metabolites in Plants
2.3. The Impact of Environmental Factors on Secondary Metabolites
3. Omics Application in Plant Secondary Metabolites
3.1. Genomics
3.2. Metabolomics
3.3. Integration of Multi-Omics
4. A Case Study of Multi-Omics Application in Safflower Flavonoids
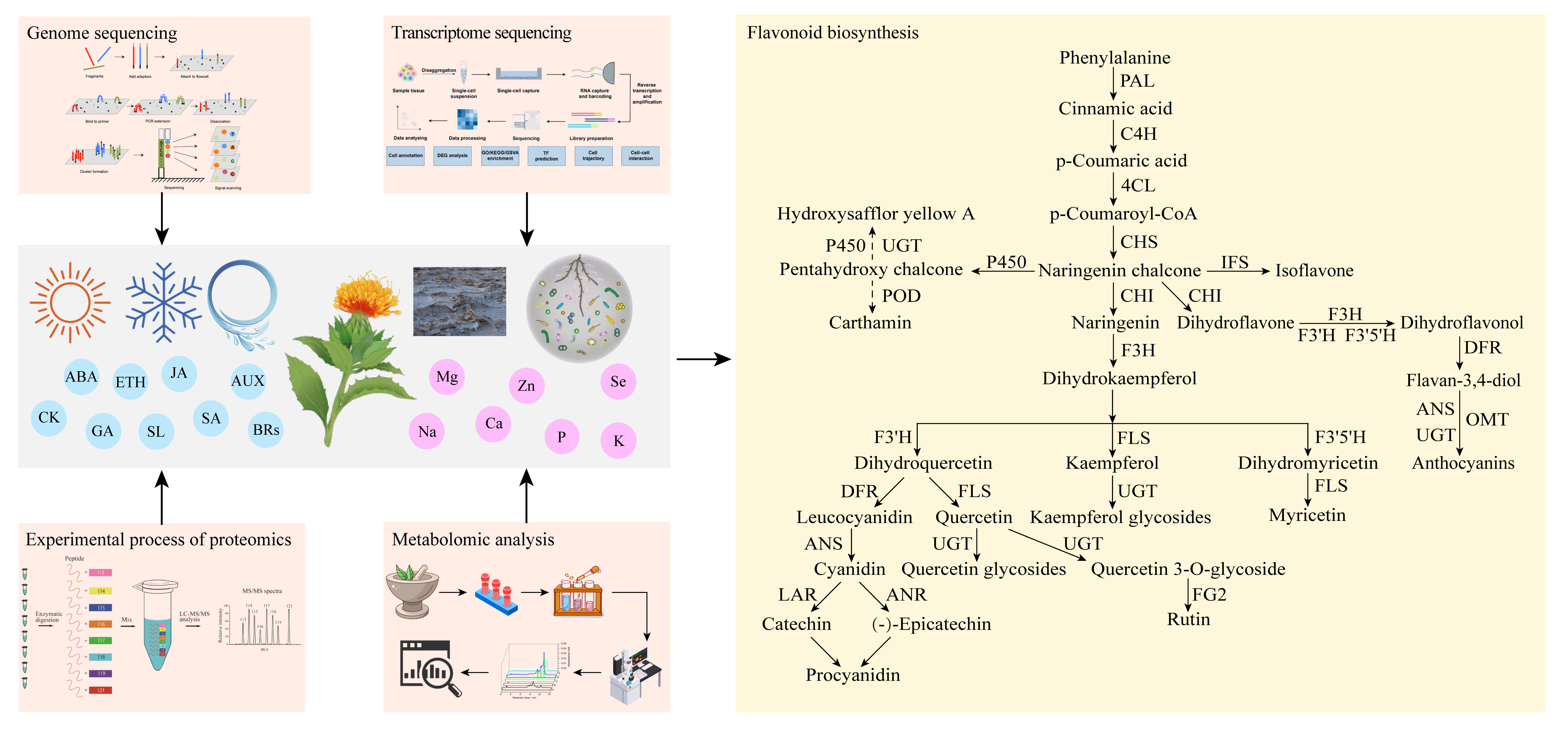
5. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pei, Y.; Leng, L.; Sun, W.; Liu, B.; Feng, X.; Li, X.; Chen, S. Whole-genome sequencing in medicinal plants: Current progress and prospect. Sci. China Life Sci. 2024, 67, 258–273. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. PPB 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Wu, Z.; Li, R.; Sun, M.; Hu, X.; Xiao, M.; Hu, Z.; Jiao, P.; Pu, S.; Zhai, J.; Zhang, J. Current advances of Carthamus tinctorius L.: A review of its application and molecular regulation of flavonoid biosynthesis. Med. Plant Biol. 2024, 3, e004. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, Y.Y.; Wang, J.; Bai, W.J.; Miao, N.J.; Wang, J. Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes anti-tumor immune response. J. Immunother. Cancer 2023, 11, e007253. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, P.; Zhu, M.; Sui, D.; Wang, S.; Hu, Z.; Wang, Y.; Jing, L.; Zheng, J. Synthesis and biological activity assay of novel camptothecin-peptidic conjugates based on PEPT1. Bioorganic Med. Chem. Lett. 2023, 96, 129502. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dou, W.; Zhao, Y.; Hu, J. A comparison of the effects of topical treatment of calcipotriol, camptothecin, clobetasol and tazarotene on an imiquimod-induced psoriasis-like mouse model. Immunopharmacol. Immunotoxicol. 2014, 36, 17–24. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 173–184. [Google Scholar] [CrossRef]
- Wu, Z.; Spencer, L.G.; Banya, W.; Westoby, J.; Tudor, V.A.; Rivera-Ortega, P.; Chaudhuri, N.; Jakupovic, I.; Patel, B.; Thillai, M.; et al. Morphine for treatment of cough in idiopathic pulmonary fibrosis (PACIFY COUGH): A prospective, multicentre, randomised, double-blind, placebo-controlled, two-way crossover trial. Lancet Respir. Med. 2024, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lai, W.; Liu, X.; Shen, Y.; Hong, K. The safety of morphine in patients with acute heart failure: A systematic review and meta-analysis. Clin. Cardiol. 2021, 44, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Węgrzyn, P.; Lis, G.; Rudzinski, P.; Piatek, J.; Pyka-Fosciak, G.; Korbut, R.; Kapelak, B.; Bartus, K.; Litwinowicz, R. Vasodilatory efficacy and impact of papaverine on endothelium in radial artery predilatation for CABG surgery: In search for optimal concentration. Braz. J. Cardiovasc. Surg. 2018, 33, 553–558. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Sultana, A.; Raj, A.; Emon, N.U.; Richi, F.T.; Sharmin, T.; Moon, M.; Park, M.N.; Kim, B. Papaverine: A miraculous alkaloid from opium and its multimedicinal application. Molecules 2023, 28, 3149. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.X.; Sun, Y.L.; Han, Z.F.; Zhou, Y.T.; Liu, Y.; Fan, T.H.; Li, Z.G.; Qi, X.M.; Luo, Y.; et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages. Acta Pharmacol. Sin. 2022, 43, 1274–1284. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, L.; Shen, J.; Ma, Y.; Wu, Z.; Li, H.; He, Y. Tetrandrine inhibits the occurrence and development of frozen shoulder by inhibiting inflammation, angiogenesis, and fibrosis. Biomed. Pharmacother. 2021, 140, 111700. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular mechanisms of sanguinarine in cancer prevention and treatment. Anti-Cancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef]
- Huang, L.J.; Lan, J.X.; Wang, J.H.; Huang, H.; Lu, K.; Zhou, Z.N.; Xin, S.Y.; Zhang, Z.Y.; Wang, J.Y.; Dai, P.; et al. Bioactivity and mechanism of action of sanguinarine and its derivatives in the past 10 years. Biomed. Pharmacother. 2024, 173, 116406. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Gasmi, A.; Asghar, F.; Zafar, S.; Oliinyk, P.; Khavrona, O.; Lysiuk, R.; Peana, M.; Piscopo, S.; Antonyak, H.; Pen, J.J.; et al. Berberine: Pharmacological features in health, disease and aging. Curr. Med. Chem. 2024, 31, 1214–1234. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.J.; Du, S.Y.; Mu, L.S.; Fan, J.J.; Hu, J.C.; Ye, Y.; Ding, M.; Zhou, W.Y.; Yu, Q.H.; et al. Artemisinins ameliorate polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction. Science 2024, 384, eadk5382. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A.; et al. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des. Dev. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Gao, F.; Jia, W.; Li, C. Salvia miltiorrhiza and tanshinone IIA reduce endothelial inflammation and atherosclerotic plaque formation through inhibiting COX-2. Biomed. Pharmacother. 2023, 167, 115501. [Google Scholar] [CrossRef]
- Guan, W.; Qi, W. Ginsenoside Rh2: A shining and potential natural product in the treatment of human nonmalignant and malignant diseases in the near future. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 118, 154938. [Google Scholar] [CrossRef]
- Li, K.; Li, Z.; Men, L.; Li, W.; Gong, X. Potential of ginsenoside Rh(2) and its derivatives as anti-cancer agents. Chin. J. Nat. Med. 2022, 20, 881–901. [Google Scholar] [CrossRef]
- Hoang, D.; Wong, A.; Olympia, R.P. Looking back to move forward: The current state of research on the clinical applications of camphor- and menthol-containing agents. Cureus 2023, 15, e41426. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, R.; Mazumder, A.; Salahuddin; Yadav, R.K.; Chauhan, B.; Abdulah, M.M. Camphor and menthol as anticancer agents: Synthesis, structure-activity relationship and interaction with cancer cell lines. Anti-Cancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [CrossRef]
- Timalsina, B.; Haque, M.N.; Choi, H.J.; Dash, R.; Moon, I.S. Thymol in Trachyspermum ammi seed extract exhibits neuroprotection, learning, and memory enhancement in scopolamine-induced Alzheimer’s disease mouse model. Phytother. Res. PTR 2023, 37, 2811–2826. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Mares, J. Lutein and zeaxanthin isomers in eye health and disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Wang, C.; Hou, J.; Zhang, M.; Zheng, Y.; Ye, H.; Qi, Y.; Guo, L.; Hu, Y. Effects of HSYA on serum and brain cholesterol levels in AD rats based on quantitative proteomics. Int. J. Neurosci. 2023, 133, 1411–1423. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Wen, J.; Pan, X.; Deng, Z.; Chen, J.; Chen, G.; Yu, L.; Tang, Y.; Li, G.; et al. Pharmacological activities of safflower yellow and its clinical applications. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 2108557. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, K.; Ren, Y.; Liu, Y.; Xu, Z.; Wang, S.; Zhang, Y. Scutellaria baicalensis ameliorates allergic airway inflammation through agonism and transcriptional regulation of TAS2Rs. J. Ethnopharmacol. 2024, 337, 118881. [Google Scholar] [CrossRef]
- Zhu, Q.J. Research advances on baicalin and baicalein as potential therapeutic agents for fibrotic disease. China J. Chin. Mater. Medica 2017, 42, 1271–1276. [Google Scholar] [CrossRef]
- Song, Q.; Peng, S.; Zhu, X. Baicalein protects against MPP(+)/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson’s disease. Neurotoxicology 2021, 87, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Fang, Y.; Zhao, Q. Comparative analysis of flavones from six commonly used Scutellaria species. Med. Plant Biol. 2023, 2, 12. [Google Scholar] [CrossRef]
- Baqer, S.H.; Al-Shawi, S.G.; Al-Younis, Z.K. Quercetin, the potential powerful flavonoid for human and food: A review. Front. Biosci. (Elite Ed.) 2024, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Wang, J.; Liu, Y.; Yan, P.; Meng, Q.; Yin, Y.; Wang, S. SIRT5-related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance. Oxidative Med. Cell. Longev. 2021, 2021, 5876841. [Google Scholar] [CrossRef]
- Yao, Y.X.; Yu, Y.J.; Dai, S.; Zhang, C.Y.; Xue, X.Y.; Zhou, M.L.; Yao, C.H.; Li, Y.X. Kaempferol efficacy in metabolic diseases: Molecular mechanisms of action in diabetes mellitus, obesity, non-alcoholic fatty liver disease, steatohepatitis, and atherosclerosis. Biomed. Pharmacother. 2024, 175, 116694. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Ellis, L.R.; Boesch, C.; Dye, L. Effects of anthocyanins on cognition and vascular function: A systematic review. Mol. Nutr. Food Res. 2024, 68, e2300502. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Lu, J.; Sun, Y.; Cui, Y. Research progress of proanthocyanidins and anthocyanidins. Phytother. Res. PTR 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Ali, F.; Siddique, Y.H. Bioavailability and pharmaco-therapeutic potential of luteolin in overcoming Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2019, 18, 352–365. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Pandima Devi, K.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Y.; Wang, W.; Gao, Q.; Gong, T.; Feng, Y.; Wu, D.; Zheng, X.; Zhang, G.; Wang, H. Aloe-emodin inhibits African swine fever virus replication by promoting apoptosis via regulating NF-κB signaling pathway. Virol. J. 2023, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Rat, A.; Koletti, A.E.; Rodić, N.; Papageorgiou, V.P.; Willems, A.; Assimopoulou, A.N. Bacterial responses to plant antimicrobials: The case of alkannin and shikonin derivatives. Front. Pharmacol. 2023, 14, 1244270. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Lei, Y.; Zhang, X.F.; Li, J.X.; Lin, Q.; Wu, X.D.; Jiang, Y.G.; Zhang, W.; Qian, R.; Xiong, S.Y.; et al. Design of CoQ(10) crops based on evolutionary history. Cell 2025, 188, 1941–1954.e1915. [Google Scholar] [CrossRef] [PubMed]
- Dongmo Zeukang, R.; Kalinski, J.-C.; Tembeni, B.; Goosen, E.D.; Tembu, J.; Tabopda Kuiate, T.; Ngono Bikobo, D.S.; Tagatsing Fotsing, M.; Atchadé, A.d.T.; Siwe-Noundou, X. Quinones from Cordia species from 1972 to 2023: Isolation, structural diversity and pharmacological activities. Nat. Prod. Bioprospecting 2023, 13, 52. [Google Scholar] [CrossRef]
- Jahan, S.; Ikram, M.; Siraj, S.; Ullah, S.; Zakria, M.; Ahmad, N. Emodin, a potent anthraquinone mitigates MPTP-induced Parkinsons’ disease pathology by regulating Nrf2 and its downstream targets: In silico and in vivo approach. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic role of alkaloids and alkaloid derivatives in cancer management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef]
- Kamle, M.; Pandhi, S.; Mishra, S.; Barua, S.; Kurian, A.; Mahato, D.K.; Rasane, P.; Büsselberg, D.; Kumar, P.; Calina, D.; et al. Camptothecin and its derivatives: Advancements, mechanisms and clinical potential in cancer therapy. Med. Oncol. 2024, 41, 263. [Google Scholar] [CrossRef]
- Chang, Y.L.; Hou, N.C.; Fei, J.L.; Qin, Z.; Niu, Y.L.; Zhang, Z.F.; Wang, R.; Qin, Z.; Liu, H.M. Uncovering phenolic profiles of different forms in safflower seeds and their antioxidant capacity, and biological activity. J. Food Sci. 2025, 90, e70025. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Lv, Y.; Fu, L. The potential mechanism for Hydroxysafflor yellow A attenuating blood–brain barrier dysfunction via tight junction signaling pathways excavated by an integrated serial affinity chromatography and shotgun proteomics analysis approach. Neurochem. Int. 2018, 112, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, G.; Xu, S.; Song, Y. Recent advances of quinones as a privileged structure in drug discovery. Eur. J. Med. Chem. 2021, 223, 113632. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Acharya, P.C.; De, U.C. Redefining anthraquinone-based anticancer drug design through subtle chemical modifications. Anti-Cancer Agents Med. Chem. 2025, 25, 1–14. [Google Scholar] [CrossRef]
- Kishimoto, S.; Sato, M.; Tsunematsu, Y.; Watanabe, K. Evaluation of biosynthetic pathway and engineered biosynthesis of alkaloids. Molecules 2016, 21, 1078. [Google Scholar] [CrossRef]
- Singh, D.; Singh, N.; Dwivedi, S.; Trivedi, P.K. Transcriptional regulation of secondary plant product biosynthesis: Insights into flavonoid, alkaloid, and terpenoid pathways. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 160, 6. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, T.; Shao, C.; Li, X.; Zhou, B.; Peng, L.; Jin, Q.; Jin, H.; Xie, S.; Shang, F.; et al. Cloning and functional characterization of the caffeine oxidase gene CsCDH from Camellia sinensis. Int. J. Biol. Macromol. 2025, 302, 140429. [Google Scholar] [CrossRef]
- Bergman, M.E.; Kortbeek, R.W.J.; Gutensohn, M.; Dudareva, N. Plant terpenoid biosynthetic network and its multiple layers of regulation. Prog. Lipid Res. 2024, 95, 101287. [Google Scholar] [CrossRef]
- Michael, R.; Ranjan, A.; Kumar, R.S.; Pathak, P.K.; Trivedi, P.K. Light-regulated expression of terpene synthase gene, AtTPS03, is controlled by the bZIP transcription factor, HY5, in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 529, 437–443. [Google Scholar] [CrossRef]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Ren, H.; Shi, Y.; Dong, D.; Li, Z.; Cui, G.; Shen, Y.; Mou, Z.; Kennelly, E.J.; et al. Multi-omics analysis reveals the evolutionary origin of diterpenoid alkaloid biosynthesis pathways in Aconitum. J. Integr. Plant Biol. 2023, 65, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ren, C.; Hu, J.; Chen, J.; Wang, J.; Wang, R.; Wu, Q.; Liao, W.; Pei, J. Cloning, expression and activity analysises of chalcone synthase genes in Carthamus tinctorius. Chin. Herb. Med. 2023, 15, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lv, Y.; Zhang, J.; Ahmad, N.; Li, X.; Yao, N.; Liu, X.; Li, H. The safflower MBW complex regulates HYSA accumulation through degradation by the E3 ligase CtBB1. J. Integr. Plant Biol. 2023, 65, 1277–1296. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Xia, D.-N.; Xu, J.; Guo, D.; Li, H.-L.; Wang, Y.; Mei, W.-L.; Peng, S.-Q. Identification of the bHLH gene family in Dracaena cambodiana reveals candidate genes involved in flavonoid biosynthesis. Ind. Crops Prod. 2020, 150, 112407. [Google Scholar] [CrossRef]
- Yang, K.; Han, H.; Li, Y.; Ye, J.; Xu, F. Significance of miRNA in enhancement of flavonoid biosynthesis. Plant Biol. 2022, 24, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Zhang, T.; Duan, A.; Zhang, J.; He, C. Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2018, 25, 465–476. [Google Scholar] [CrossRef]
- Nallakaruppan, N.; Thiagarajan, K. In vitro elicitation of anthraquinones—A review. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 70. [Google Scholar] [CrossRef]
- Lian, C.; Liu, X.; Guo, K.; Yang, H.; Yang, J.; Lan, J.; Chen, S. Dynamic analysis of growth characteristics, secondary metabolites accumulation, and an in-depth understanding of anthraquinones biosynthesis in Rubia cordifolia Linn. Front. Plant Sci. 2024, 15, 1504863. [Google Scholar] [CrossRef]
- Zhang, H.; He, Q.; Xing, L.; Wang, R.; Wang, Y.; Liu, Y.; Zhou, Q.; Li, X.; Jia, Z.; Liu, Z.; et al. The haplotype-resolved genome assembly of autotetraploid rhubarb Rheum officinale provides insights into its genome evolution and massive accumulation of anthraquinones. Plant Commun. 2024, 5, 100677. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Hua, H.; Zhang, Y.; Zhang, X.; Fang, Q.; Li, Q.; Zhang, Y.; Tan, P.; Yang, A.; et al. Research progress on quality assurance of genuine Chinese medicinal in Sichuan. Chin. Med. 2021, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Yu, S.; Zhang, Y.; Huang, Y.; He, X.; Chen, W. Effects of geographical, soil and climatic factors on the two marker secondary metabolites contents in the roots of Rubia cordifolia L. Front. Plant Sci. 2024, 15, 1419392. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Bai, M.; Peng, T.; Li, H.; Xu, H.-J.; Guo, G.; Bai, H.; Rong, N.; Sahu, S.K.; et al. Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of Citrus reticulata ‘Chachi’. Microbiome 2023, 11, 61. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Ahluwalia, A.S.; Saxena, A.K. Minerals solubilizing and mobilizing microbiomes: A sustainable approach for managing minerals’ deficiency in agricultural soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research progress on the effects of selenium on the growth and quality of tea plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Siadjeu, C.; Pucker, B. Medicinal plant genomics. BMC Genom. 2023, 24, 429. [Google Scholar] [CrossRef]
- Alami, M.M.; Ouyang, Z.; Zhang, Y.; Shu, S.; Yang, G.; Mei, Z.; Wang, X. The current developments in medicinal plant genomics enabled the diversification of secondary metabolites’ biosynthesis. Int. J. Mol. Sci. 2022, 23, 15932. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Zhan, W.; Yu, Z.; Qin, E.; Liu, S.; Yang, T.; Xiang, N.; Kudrna, D.; Chen, Y.; et al. The chromosome-scale reference genome of safflower (Carthamus tinctorius) provides insights into linoleic acid and flavonoid biosynthesis. Plant Biotechnol. J. 2021, 19, 1725–1742. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, T.; Qin, R.; Liu, H. Complete mitogenome and phylogenetic analysis of the Carthamus tinctorius L. Genes 2023, 14, 979. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liao, R.; Dong, X.; Qin, R.; Liu, H. Complete chloroplast genome sequence of Carthamus tinctorius L. from PacBio Sequel Platform. Mitochondrial DNA. Part B 2019, 4, 2635–2636. [Google Scholar] [CrossRef]
- Guo, L.; Yao, H.; Chen, W.; Wang, X.; Ye, P.; Xu, Z.; Zhang, S.; Wu, H. Natural products of medicinal plants: Biosynthesis and bioengineering in post-genomic era. Hortic. Res. 2022, 9, uhac223. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Zhang, C.; Liang, T.; Tian, Y.; Ma, G.; Courdavault, V.; Sun, S.; Ma, B.; Li, Z.; Li, R.; et al. Insights into dammarane-type triterpenoid saponin biosynthesis from the telomere-to-telomere genome of Gynostemma pentaphyllum. Plant Commun. 2024, 5, 100932. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Zhu, S.; Liao, W.; Fang, Y.; Liu, J.; Kong, Y.; Yan, M.; Cui, M.; Zhao, Q. Gap-free genome assembly and CYP450 gene family analysis reveal the biosynthesis of anthocyanins in Scutellaria baicalensis. Hortic. Res. 2023, 10, uhad235. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, S.; Xiao, Z.; Wang, J.; Mei, Y.; Hu, H.; Li, J.; Liu, J.; Hou, Z.; Zhao, J.; et al. Gap-free genome assembly and comparative analysis reveal the evolution and anthocyanin accumulation mechanism of Rhodomyrtus tomentosa. Hortic. Res. 2023, 10, uhad005. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhang, H.; Liu, Z.; Wang, Y.; Xing, L.; He, Q.; Du, H. Plant pan-genomics: Recent advances, new challenges, and roads ahead. J. Genet. Genom. 2022, 49, 833–846. [Google Scholar] [CrossRef]
- Zhang, K.; He, Y.; Lu, X.; Shi, Y.; Zhao, H.; Li, X.; Li, J.; Liu, Y.; Ouyang, Y.; Tang, Y.; et al. Comparative and population genomics of buckwheat species reveal key determinants of flavor and fertility. Mol. Plant 2023, 16, 1427–1444. [Google Scholar] [CrossRef]
- Guo, L.; Winzer, T.; Yang, X.; Li, Y.; Ning, Z.; He, Z.; Teodor, R.; Lu, Y.; Bowser, T.A.; Graham, I.A.; et al. The opium poppy genome and morphinan production. Science 2018, 362, 343–347. [Google Scholar] [CrossRef]
- Mao, L.; Kawaide, H.; Higuchi, T.; Chen, M.; Miyamoto, K.; Hirata, Y.; Kimura, H.; Miyazaki, S.; Teruya, M.; Fujiwara, K.; et al. Genomic evidence for convergent evolution of gene clusters for momilactone biosynthesis in land plants. Proc. Natl. Acad. Sci. USA 2020, 117, 12472–12480. [Google Scholar] [CrossRef]
- Liao, X.; Xie, D.; Bao, T.; Hou, M.; Li, C.; Nie, B.; Sun, S.; Peng, D.; Hu, H.; Wang, H.; et al. Inversions encounter relaxed genetic constraints and balance birth and death of TPS genes in Curcuma. Nat. Commun. 2024, 15, 9349. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef]
- Garcia, E.; Hayden, A.; Birts, C.; Britton, E.; Cowie, A.; Pickard, K.; Mellone, M.; Choh, C.; Derouet, M.; Duriez, P.; et al. Authentication and characterisation of a new oesophageal adenocarcinoma cell line: MFD-1. Sci. Rep. 2016, 6, 32417. [Google Scholar] [CrossRef]
- He, S.; Yang, L.; Ye, S.; Lin, Y.; Li, X.; Wang, Y.; Chen, G.; Liu, G.; Zhao, M.; Zhao, X.; et al. MPOD: Applications of integrated multi-omics database for medicinal plants. Plant Biotechnol. J. 2022, 20, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Tang, Q.; Chu, T.; Li, X.; Lin, Y.; Song, X.; Chen, W. TCMPG: An integrative database for traditional Chinese medicine plant genomes. Hortic. Res. 2022, 9, uhac060. [Google Scholar] [CrossRef]
- Li, G.; Cai, L.; Chang, H.; Hong, P.; Zhou, Q.; Kulakova, E.V.; Kolchanov, N.A.; Ruan, Y. Chromatin Interaction Analysis with Paired-End Tag (ChIA-PET) sequencing technology and application. BMC Genom. 2014, 15 (Suppl. 12), S11. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luo, C.; Dai, R.; Huang, X.; Chen, X.; He, L.; Mao, H.; Li, J.; Zhang, L.; Yang, Q.Y.; et al. AMIR: A multi-omics data platform for Asteraceae plants genetics and breeding research. Nucleic Acids Res. 2024, 53, D1563–D1575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Q.; Qiu, S.; Dai, J.; Gao, X. DNA barcoding: An efficient technology to authenticate plant species of traditional Chinese medicine and recent advances. Chin. Med. 2022, 17, 112. [Google Scholar] [CrossRef]
- He, D.; Rao, X.; Deng, J.; Damaris, R.N.; Yang, P. Integration of metabolomics and transcriptomics analyses investigates the accumulation of secondary metabolites in maturing seed plumule of sacred lotus (Nelumbo nucifera). Food Res. Int. 2023, 163, 112172. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Hawkins, C.; Xue, B.; Yasmin, F.; Wyatt, G.; Zerbe, P.; Rhee, S.Y. Plant Metabolic Network 16: Expansion of underrepresented plant groups and experimentally supported enzyme data. Nucleic Acids Res. 2025, 53, D1606–D1613. [Google Scholar] [CrossRef]
- Hu, Y.; Ruan, Y.; Zhao, X.L.; Jiang, F.; Liu, D.; Zhu, Q.; Zhang, Q.Y.; Yang, Q.Y. PCMD: A multilevel comparison database of intra- and cross-species metabolic profiling in 530 plant species. Plant Commun. 2024, 5, 101038. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Jeong, E.; Son, S.; Lee, E.; Lee, S.; Choi, S.Y.; Kim, H.W.; Yang, H.; Shim, S.H. Mass spectrometry data on specialized metabolome of medicinal plants used in East Asian traditional medicine. Sci. Data 2022, 9, 528. [Google Scholar] [CrossRef]
- Ren, C.; Wang, J.; Xian, B.; Tang, X.; Liu, X.; Hu, X.; Hu, Z.; Wu, Y.; Chen, C.; Wu, Q.; et al. Transcriptome analysis of flavonoid biosynthesis in safflower flowers grown under different light intensities. PeerJ 2020, 8, e8671. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, F.; Guo, D.; Fan, L.; Zhu, Z.; Xue, Y.; Gao, Y.; Guo, M. Molecular characterization of flavanone 3-hydroxylase gene and flavonoid accumulation in two chemotyped safflower lines in response to methyl jasmonate stimulation. BMC Plant Biol. 2016, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Orešič, M. Deep learning meets metabolomics: A methodological perspective. Brief. Bioinform. 2021, 22, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Sun, S.; Shen, X.; Li, Y.; Li, Y.; Wang, S.; Li, R.; Zhang, H.; Shen, G.; Guo, B.; Wei, J.; et al. Single-cell RNA sequencing provides a high-resolution roadmap for understanding the multicellular compartmentation of specialized metabolism. Nat. Plants 2023, 9, 179–190. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Z.; Jiao, Y.; Yu, K.; Bhatara, S.; Yang, X.; Natarajan, S.; Zhang, J.; Pan, Q.; Easton, J.; et al. Spotiphy enables single-cell spatial whole transcriptomics across an entire section. Nat. Methods 2025, 22, 724–736. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.; Luo, E.; Yang, J.; Wang, S. Plant metabolomics: Applications and challenges in the era of multi-omics big data. aBIOTECH 2025, 6, 116–132. [Google Scholar] [CrossRef]
- Li, P.; Yan, M.X.; Liu, P.; Yang, D.J.; He, Z.K.; Gao, Y.; Jiang, Y.; Kong, Y.; Zhong, X.; Wu, S.; et al. Multiomics analyses of two Leonurus species illuminate leonurine biosynthesis and its evolution. Mol. Plant 2024, 17, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chen, C.; Gao, X.; Tan, C.; Bai, H.; Ning, K. Multi-omics profiling reveals comprehensive microbe-plant-metabolite regulation patterns for medicinal plant Glycyrrhiza uralensis Fisch. Plant Biotechnol. J. 2022, 20, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.K.; Craft, D.; Weng, J.K. Toward an integrated omics approach for plant biosynthetic pathway discovery in the age of AI. Trends Biochem. Sci. 2025, 50, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Cembrowska-Lech, D.; Krzemińska, A.; Miller, T.; Nowakowska, A.; Adamski, C.; Radaczyńska, M.; Mikiciuk, G.; Mikiciuk, M. An integrated multi-omics and artificial intelligence framework for advance plant phenotyping in horticulture. Biology 2023, 12, 1298. [Google Scholar] [CrossRef]
- Chen, J.; Guo, S.; Hu, X.; Wang, R.; Jia, D.; Li, Q.; Yin, X.; Liao, X.; Hu, Z.; Wang, P.; et al. Whole-genome and genome-wide association studies improve key agricultural traits of safflower for industrial and medicinal use. Hortic. Res. 2023, 10, uhad197. [Google Scholar] [CrossRef]
- Tan, Z.; Lu, D.; Yu, Y.; Li, L.; Dong, W.; Xu, L.; Yang, Q.; Wan, X.; Liang, H. Genome-wide identification and characterization of the bHLH gene family and its response to abiotic stresses in Carthamus tinctorius. Plants 2023, 12, 3764. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Xu, X.; Yu, J.; Chen, Z.; Wei, B.; Wu, W. Temporal transcriptome profiling of developing seeds reveals candidate genes involved in oil accumulation in safflower (Carthamus tinctorius L.). BMC Plant Biol. 2021, 21, 181. [Google Scholar] [CrossRef]
- Ren, C.; Chen, C.; Dong, S.; Wang, R.; Xian, B.; Liu, T.; Xi, Z.; Pei, J.; Chen, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in flowers of safflower (Carthamus tinctorius L.) during colour-transition. PeerJ 2022, 10, e13591. [Google Scholar] [CrossRef]
- Vincent, D.; Reddy, P.; Isenegger, D. Integrated proteomics and metabolomics of safflower petal wilting and seed development. Biomolecules 2024, 14, 414. [Google Scholar] [CrossRef]
- Liu, X.; Ahmad, N.; Yang, L.; Fu, T.; Kong, J.; Yao, N.; Dong, Y.; Wang, N.; Li, X.; Wang, F.; et al. Molecular cloning and functional characterization of chalcone isomerase from Carthamus tinctorius. AMB Express 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Pearl, S.A.; Burke, J.M. Genetic mapping of millions of SNPs in safflower (Carthamus tinctorius L.) via whole-genome resequencing. G3 (Bethesda Md.) 2016, 6, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ahmad, N.; Meng, W.; Zhao, S.; Chang, Y.; Wang, N.; Zhang, M.; Yao, N.; Liu, X.; Zhang, J. Integrated metabolomics and transcriptomics provide key molecular insights into floral stage-driven flavonoid pathway in safflower. Int. J. Mol. Sci. 2024, 25, 11903. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zou, Y.; Jiang, Z.; Tu, L.; Wu, X.; Li, D.; Wang, J.; Huang, L.; Xu, C.; Gao, W. Multiomics driven identification of glycosyltransferases in flavonoid glycoside biosynthesis in safflower. Hortic. Plant J. 2024. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q.; Pei, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Chen, J.; Tang, X.; Ren, C.; Wei, B.; Wu, Y.; Wu, Q.; Pei, J. Full-length transcriptome sequences and the identification of putative genes for flavonoid biosynthesis in safflower. BMC Genom. 2018, 19, 548. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Yao, N.; Zhang, Y.; Wang, N.; Cui, X.; Li, X.; Wang, Y.; Wang, F.; Yang, J.; et al. De novo sequencing and analysis of the safflower transcriptome to discover putative genes associated with Safflor yellow in Carthamus tinctorius L. Int. J. Mol. Sci. 2015, 16, 25657–25677. [Google Scholar] [CrossRef]
- Xian, B.; Zhou, Y.; Hu, Y.; Peng, Y.; Song, X.; Xi, Z.; Li, Y.; Yan, J.; Ren, C.; Pei, J.; et al. Genome-wide screen and multi-omics analysis reveal OGT1 participate in the biosynthesis of safflower flavonoid glycosides. Hortic. Res. 2024, 11, uhae261. [Google Scholar] [CrossRef]
- Guo, D.D.; Liu, F.; Tu, Y.H.; He, B.X.; Gao, Y.; Guo, M.L. Expression patterns of three UGT genes in different chemotype safflower lines and under MeJA stimulus revealed their potential role in flavonoid biosynthesis. PLoS ONE 2016, 11, e0158159. [Google Scholar] [CrossRef]
- Gharabli, H.; Della Gala, V.; Welner, D.H. The function of UDP-glycosyltransferases in plants and their possible use in crop protection. Biotechnol. Adv. 2023, 67, 108182. [Google Scholar] [CrossRef]
- Xie, K.; Chen, R.; Li, J.; Wang, R.; Chen, D.; Dou, X.; Dai, J. Exploring the catalytic promiscuity of a new glycosyltransferase from Carthamus tinctorius. Org. Lett. 2014, 16, 4874–4877. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ren, C.; Dong, S.; Chen, C.; Xian, B.; Wu, Q.; Wang, J.; Pei, J.; Chen, J. Integrated metabolomics and transcriptome analysis of flavonoid biosynthesis in safflower (Carthamus tinctorius L.) with different colors. Front. Plant Sci. 2021, 12, 712038. [Google Scholar] [CrossRef]
- Ren, C.; Xi, Z.; Xian, B.; Chen, C.; Huang, X.; Jiang, H.; Chen, J.; Peng, C.; Pei, J. Identification and characterization of CtUGT3 as the key player of astragalin biosynthesis in Carthamus tinctorius L. J. Agric. Food Chem. 2023, 71, 16221–16232. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, X.; Cheng, J.; Zhang, L.; Chu, H.; Wang, R.; Li, H.; Chang, H.; Ahmed, N.; et al. pUGTdb: A comprehensive database of plant UDP-dependent glycosyltransferases. Mol. Plant 2023, 16, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Alasvandyari, F.; Mahdavi, B. Effect of glycine betaine and salinity on photosynthetic pigments and ion concentration of safflower. Alasvandyari Mahdavi Desert 2018, 23, 265–271. [Google Scholar]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. Impact of silicon foliar application on the growth and physiological traits of Carthamus tinctorius L. Expo. Salt Stress. Silicon 2023, 15, 1235–1245. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Salt, D.E. Plant ionomics: From elemental profiling to environmental adaptation. Mol. Plant 2016, 9, 787–797. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Dai, Y.; Zhao, Z.; Zhu, J.; Guo, H.; Yang, R. Single-cell sequencing to multi-omics: Technologies and applications. Biomark. Res. 2024, 12, 110. [Google Scholar] [CrossRef]
- Cheng, L.-T.; Wang, Z.-L.; Zhu, Q.-H.; Ye, M.; Ye, C.-Y. A long road ahead to reliable and complete medicinal plant genomes. Nat. Commun. 2025, 16, 2150. [Google Scholar] [CrossRef]
- Shi, J.; Tian, Z.; Lai, J.; Huang, X. Plant pan-genomics and its applications. Mol. Plant 2023, 16, 168–186. [Google Scholar] [CrossRef]
- Shen, S.; Zhan, C.; Yang, C.; Fernie, A.R.; Luo, J. Metabolomics-centered mining of plant metabolic diversity and function: Past decade and future perspectives. Mol. Plant 2023, 16, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, H.; Chen, F.; Wang, Y.; Gao, Q.; Yang, F.; He, C.; Zhang, L.; Wan, Y. The genome of Areca catechu provides insights into sex determination of monoecious plants. New Phytol. 2022, 236, 2327–2343. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Sun, S.; Ji, J.; Nie, B.; Maker, G.; Ren, Y.; Wang, L. The chromosome-level genome of female ginseng (Angelica sinensis) provides insights into molecular mechanisms and evolution of coumarin biosynthesis. Plant J. Cell Mol. Biol. 2022, 112, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Cao, Z.; Yang, C.; Ran, D.; Wu, P.; Gao, H.; He, N.; Liu, G.; Chen, Z. A R2R3-MYB transcriptional activator LmMYB15 regulates chlorogenic acid biosynthesis and phenylpropanoid metabolism in Lonicera macranthoides. Plant Sci. Int. J. Exp. Plant Biol. 2021, 308, 110924. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Yue, M. Spatial multi-omics in medicinal plants: From biosynthesis pathways to industrial applications. Trends Plant Sci. 2024, 29, 510–513. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, F.; Wu, C.; Chang, X.; Cheng, W.; Wang, Q.; Deng, Z.; Liu, T.; Lu, L. Structure-based virtual screening aids the identification of glycosyltransferases in the biosynthesis of salidroside. Plant Biotechnol. J. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, X.; Deng, Z.; Liu, T. Innovative approaches in the discovery of terpenoid natural products. Curr. Opin. Microbiol. 2025, 83, 102575. [Google Scholar] [CrossRef]
- Chen, Z.; Ain, N.U.; Zhao, Q.; Zhang, X. From tradition to innovation: Conventional and deep learning frameworks in genome annotation. Brief. Bioinform. 2024, 25, bbae138. [Google Scholar] [CrossRef]
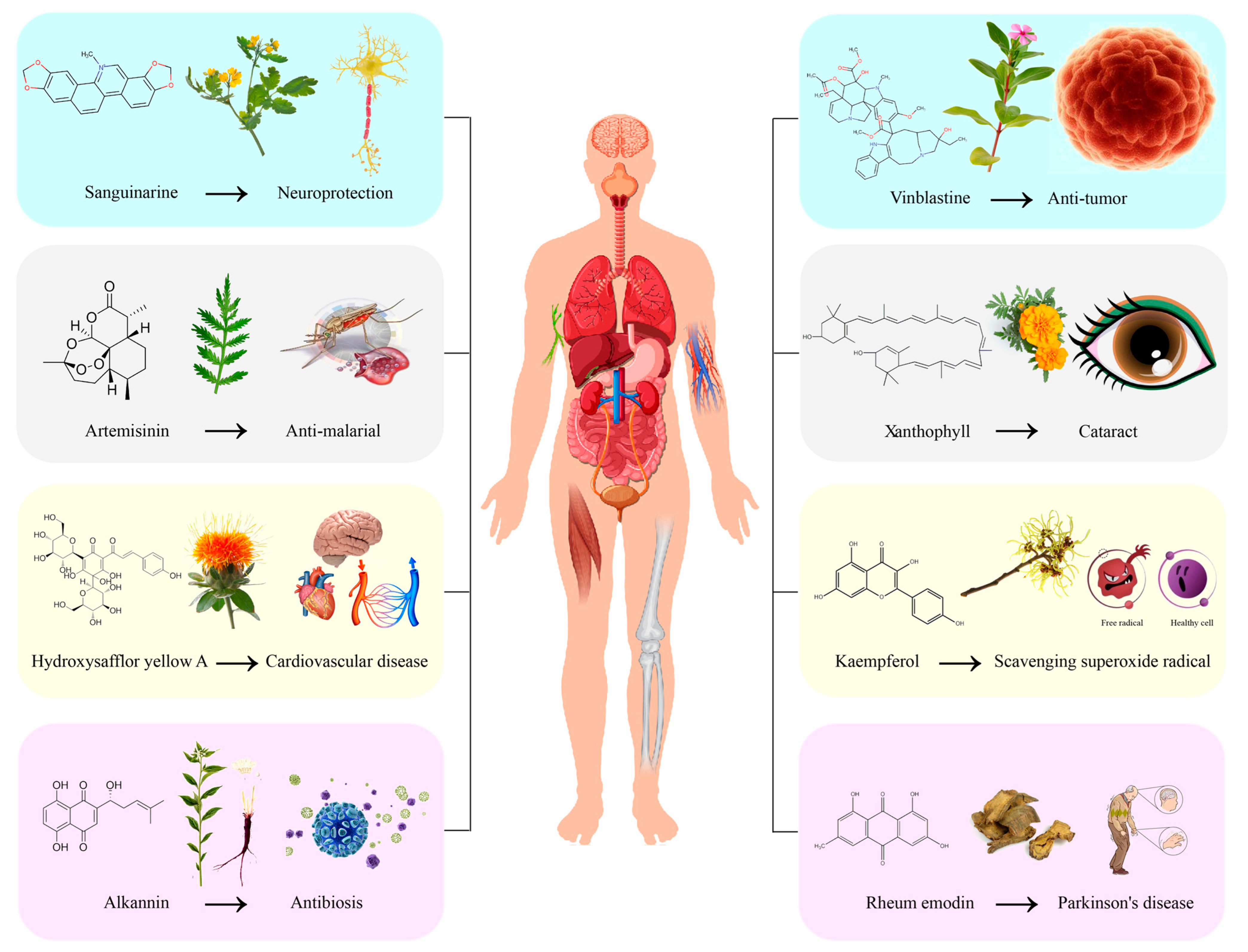
| Secondary Metabolites | Plant Sources | Pharmacological Activity | References | |
|---|---|---|---|---|
| Alkaloid | Vincristine | Catharanthus roseus | Anti-tumor | [10] |
| Camptothecin | Camptotheca acuminata Decne. | Anti-tumor, treatment of psoriasis | [11,12] | |
| Piperine | Piper nigrum L. Piper longum L. | Reduce insulin resistance, anti-inflammatory, anti-liver steatosis, improve bioavailability | [13] | |
| Morphine | Papaver somniferum L. | Pain relief, cough suppression, treatment of cardiovascular diseases | [14,15] | |
| Papaverine | Papaver somniferum L. | Anti-tumor, analgesic, treatment of cardiovascular diseases | [16,17] | |
| Tetrandrine | Stephania tetrandra S. Moore | Anti-inflammatory, cardiovascular diseases, treatment of silicosis | [18,19] | |
| Bloodroot alkaloid | Chelidonium majus Corydalis edulis Maxim. Macleaya cordata (Willd.) R. Br. | Anti-tumor, antibacterial, anti-osteoporosis, neuroprotection | [20,21] | |
| Berberine | Coptis chinensis Franch Phellodendri Cortex Berberidis Radix | Anti-tumor, treatment of cardiovascular diseases and diabetes | [22,23] | |
| Terpenoids | Artemisinin | Artemisia carvifolia | Anti-malarial, anti-tumor, treatment of cardiovascular diseases and polycystic ovary syndrome | [24,25] |
| Paclitaxel | Taxus chinensis | Anti-tumor | [26] | |
| Tanshinon | Salvia miltiorrhiza Bunge | Antibacterial, anti-inflammatory, treatment of cardiovascular diseases | [27,28] | |
| Ginsenoside | Panax ginseng C. A. Mey. | Anti-tumor, anti-inflammatory, anti-allergic reaction, antidepressant | [29,30] | |
| Menthol | Mentha canadensis L. | Anti-tumor, antibacterial, analgesic | [31,32] | |
| Camphor | Camphora officinarum Nees ex Wall. | Anti-tumor, antibacterial, analgesic | [31,32] | |
| Thymol | Tachyspermum ammi Origanum vulgare L. | Anti-tumor, anti-inflammatory, neuroprotective | [33,34] | |
| Lutein | Tagetes erecta L. Calendula officinalis L. Brassica oleracea var. capitata Linnaeus | Treatment of neurodegenerative diseases and eye diseases | [35,36] | |
| Phenols | HSYA | Carthamus tinctorius L. | Anti-tumor, neuroprotection, treatment of cardiovascular diseases | [9,37,38] |
| Baicalein | Scutellaria baicalensis Georgi Oroxylum indicum Plantago major L. | Anti-tumor, antibacterial, antiviral, treatment of cardiovascular diseases | [39,40,41,42] | |
| Quercetin | Flos Sophorae Immaturus Notoginseng Radix Ginkgo biloba L. | Anti-tumor, antibacterial, antiviral, treatment of cardiovascular diseases | [43,44] | |
| Kaempferol | Kaempferia galanga L. Forsythia suspensa (Thunb.) Vahl Ginkgo biloba L. | Anti-tumor, antibacterial, anti-inflammatory, diabetes, treatment of cardiovascular diseases | [45,46] | |
| Anthocyanidin | Vaccinium spp. Lycium ruthenicum Brassica oleracea var. capitata Linnaeus | Anti-tumor, treatment of cardiovascular diseases, vision protection | [47,48] | |
| Luteolin | Dracocephalum integrifolium Bge. Lonicera japonica Thunb. Perilla frutescens (L.) Britt. | Anti-tumor, anti-inflammatory, antioxidant | [49,50] | |
| Genistein | Sophora japonica Linn Euchresta japonica Benth. ex Oliv. | Anti-tumor, anti-inflammatory, antibacterial, treatment of cardiovascular diseases | [51] | |
| Catechin | Camellia sinensis (L.) O. Ktze. | Anti-tumor, treatment of diabetes and cardiovascular diseases | [52,53] | |
| Quinones | Aloe-emodin | Cassia occidentalis Rheum palmatum L. Polygonum multiflorum Thunb | Anti-tumor, antiviral, anti-inflammatory, immunological modulation | [54] |
| Alkannin | Lithospermum erythrorhizon Alkanna tinctoria (L.) | Anti-tumor, wound healing, antibacterial | [55] | |
| Coenzyme Q10 | Widely present in organisms | Antioxidant, improve heart health | [56] | |
| Oncocalyxone A | Cordia oncocalyx | Anti-inflammatory, analgesic, neuroinhibitory | [57] | |
| Emodin | Rheum palmatum (Chinese rhubarb) | Neurodegenerative diseases, Parkinson’s disease | [58] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Hu, Y.; Hao, R.; Li, R.; Lu, X.; Itale, M.W.; Yuan, Y.; Zhu, X.; Zhang, J.; Wang, L.; et al. Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower. Int. J. Mol. Sci. 2025, 26, 3867. https://doi.org/10.3390/ijms26083867
Wu Z, Hu Y, Hao R, Li R, Lu X, Itale MW, Yuan Y, Zhu X, Zhang J, Wang L, et al. Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower. International Journal of Molecular Sciences. 2025; 26(8):3867. https://doi.org/10.3390/ijms26083867
Chicago/Turabian StyleWu, Zhihua, Yan Hu, Ruru Hao, Ruting Li, Xiaona Lu, Mdachi Winfrida Itale, Yang Yuan, Xiaoxian Zhu, Jiaqiang Zhang, Longxiang Wang, and et al. 2025. "Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower" International Journal of Molecular Sciences 26, no. 8: 3867. https://doi.org/10.3390/ijms26083867
APA StyleWu, Z., Hu, Y., Hao, R., Li, R., Lu, X., Itale, M. W., Yuan, Y., Zhu, X., Zhang, J., Wang, L., Sun, M., & Hou, X. (2025). Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower. International Journal of Molecular Sciences, 26(8), 3867. https://doi.org/10.3390/ijms26083867








