Abstract
Spinal cord injury (SCI) remains a major clinical challenge, with limited therapeutic options for restoring lost neurological function. While efforts to mitigate secondary damage have improved early-phase management, achieving sustained neurorepair and functional recovery remains elusive. Advances in stem cell engineering and regenerative medicine have opened new avenues for targeted interventions, particularly through the transplantation of neural stem/progenitor cells (NSPCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs). However, patient-specific factors such as cellular senescence, genetic and epigenetic variability, injury microenvironment, and comorbidities influence the efficacy of stem cell therapies by affecting graft survival and differentiation. Overcoming these challenges necessitates cutting-edge technologies, including single-cell transcriptomics, CRISPR-mediated hypoimmunogenic engineering, and biomaterial-based delivery platforms, which enable personalized and precision-driven SCI repair. Leveraging these advancements may help stem cell therapies overcome translational barriers and establish clinically viable regenerative solutions. This review explores the intersection of patient-specific variability, bioengineering innovations, and transcriptomic-guided precision medicine to define the next frontier in SCI therapy.
1. Introduction
Spinal cord injury (SCI) results in irreversible motor, sensory, and autonomic dysfunction, presenting a significant challenge for regenerative medicine [1,2]. Despite decades of research, no curative treatment exists, and current clinical strategies focus primarily on stabilizing the injury site, preventing secondary damage, and maximizing residual function [3,4,5]. Surgical decompression, pharmacological interventions, and rehabilitative therapies provide some neuroprotection but fail to restore lost neural circuits [6,7]. Advanced assistive technologies, such as epidural electrical stimulation (EES) and robotic exoskeletons, have demonstrated promise in improving motor function but remain limited by high variability in efficacy, cost constraints, and their inability to regenerate damaged neural tissue [8,9]. Given these limitations, the future of SCI treatment hinges on regenerative strategies that integrate cell replacement, immune modulation, and biomaterial-based microenvironmental engineering to facilitate functional recovery and neural repair.
Stem cell-based therapies offer a compelling approach for repopulating lost neural populations, restoring connectivity, and modulating the post-injury microenvironment [10,11]. Neural stem/progenitor cells (NSPCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs) have shown promise in remyelination, synaptogenesis, and neuroprotection [11,12]. However, patient-specific variability including age-related cellular senescence, chronic inflammation, genetic and epigenetic diversity, injury severity, and immune response dynamics profoundly influences therapeutic outcomes [12,13,14,15]. These factors could directly impact graft survival, differentiation efficiency, and host integration, highlighting the need to move beyond standardized transplantation protocols. Advances in single-cell transcriptomics, spatial mapping, and CRISPR-based immune engineering now enable a systems-level approach to SCI repair, in which therapies could be tailored to individual molecular landscapes to optimize cellular engraftment and neuroregeneration [16,17]. Clinical studies have highlighted the need for patient-specific approaches in SCI therapy [12,15]. A phase I/II clinical trial evaluating human spinal cord-derived NSPCs in patients with thoracic SCI demonstrated significant variability in cell survival and integration, highlighting the influence of injury-specific microenvironments on graft efficacy [18]. Another preclinical study showed that transplantation of region-specific iPSC-derived neural progenitors enhanced synaptic integration and functional connectivity in a rodent model of SCI, emphasizing the need for lineage-optimized differentiation strategies [19]. Bioengineering innovations have significantly expanded the therapeutic potential of stem cell-based interventions by addressing key structural and functional challenges in neural repair [20,21]. Specifically, hydrogel-based scaffolds, nanofiber-aligned matrices, and 3D-bioprinted constructs provide essential structural support, enhance cellular retention and modulate the inflammatory milieu. Moreover, functionalized hydrogels infused with brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) promote angiogenesis, synaptic plasticity, and neuronal survival, while scaffolds delivering chondroitinase ABC (ChABC) enzymatically degrade glial scar components, thereby facilitating axonal extension and synaptic reconnection [22,23,24]. Additionally, electrically conductive biomaterials hold promise for restoring electrophysiological function by enhancing neural activity and supporting synaptic remodeling, further advancing the integration of bioengineered platforms in precision regenerative medicine [20].
Despite these advancements, the hostile post-injury environment continues to limit functional recovery, with molecular inhibitors impeding axonal regrowth and synaptic reconnection. Emerging interventions aimed at neutralizing these inhibitory cues have demonstrated promise in preclinical models, highlighting the need for targeted strategies to enhance neuroplasticity and circuit reformation [25,26]. Concurrently, hypoimmunogenic stem cell engineering, biomaterial-assisted immune modulation, and patient-specific immune profiling are being developed to reduce graft rejection and enhance the long-term survival of transplanted cells [27]. A multimodal approach, integrating stem cell transplantation, bioengineered scaffolds, adaptive neurostimulation, and immune modulation, offers a promising path toward clinically viable, precision-guided therapies. Machine learning-based transcriptomic analyses now enable patient stratification, identifying individuals most likely to benefit from specific regenerative interventions [28,29]. Additionally, single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics provide a high-resolution molecular blueprint of injury-specific dynamics, allowing for tailored stem cell differentiation and biomaterial customization [17,30]. These developments collectively mark a paradigm shift from conventional one-size-fits-all strategies to personalized regenerative medicine approaches that align biomaterials, transcriptomics, and immune engineering with patient-specific injury profiles. This review explores the intersection of bioengineering, patient-specific variability, and transcriptomics-driven precision medicine in advancing SCI therapeutics. By integrating these cutting-edge approaches, stem cell-based therapies may overcome translational barriers and drive next-generation solutions for restoring neurological function and improving long-term patient outcomes.
2. Stem Cell Therapy for SCI
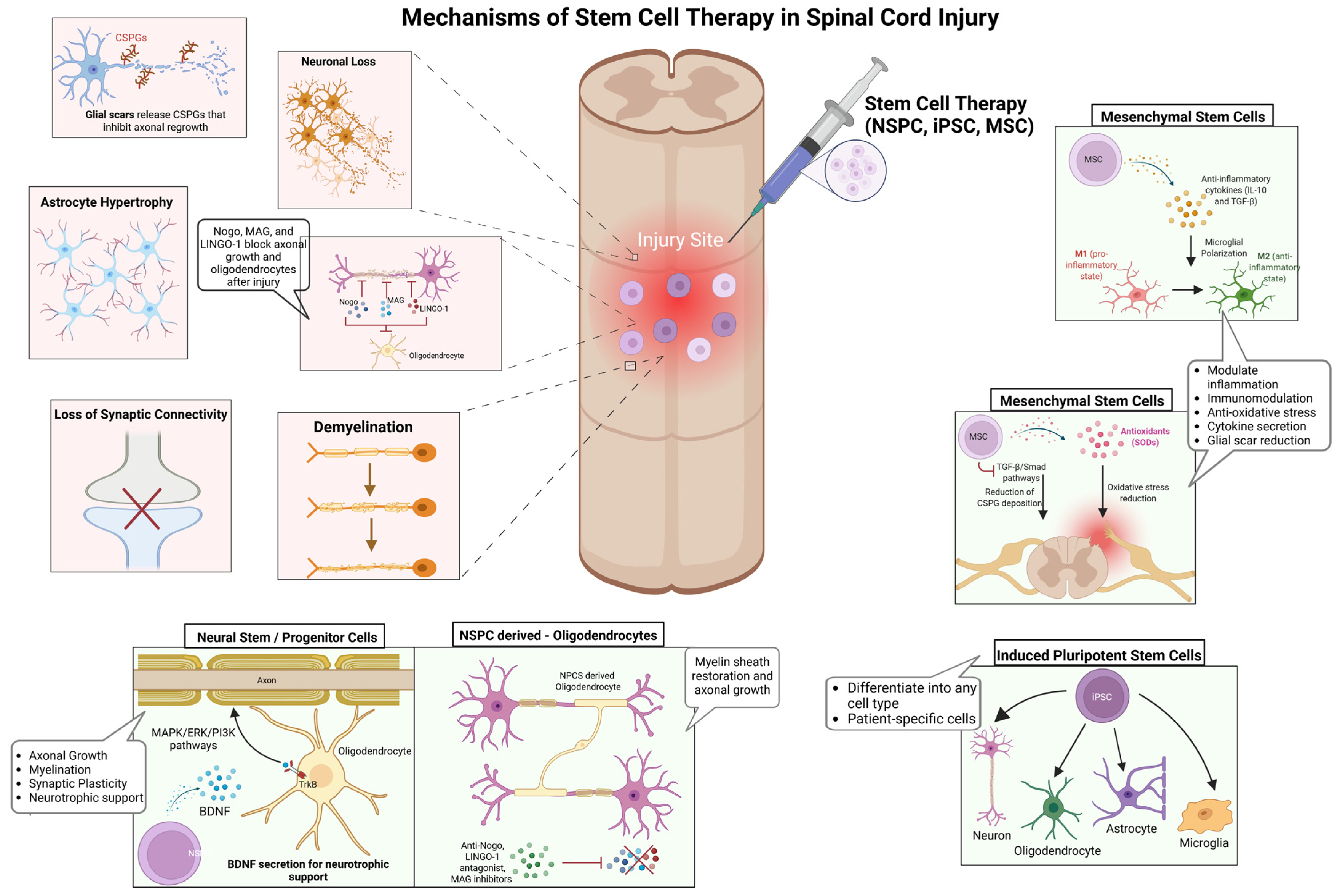
While conventional interventions primarily focus on stabilizing the injury site and mitigating secondary damage, stem cell-based therapies aim to reconstruct neural circuits, promote remyelination, and modulate the inflammatory microenvironment to enhance functional recovery [10,14]. However, the success of these therapies is contingent on several key factors, including graft survival, differentiation potential, and host integration, all of which are dictated by injury-specific and patient-specific variables (Figure 1) [11,12]. Among the most promising stem cell candidates, NSPCs, iPSCs, and MSCs exhibit distinct but complementary regenerative properties. NSPCs integrate into host spinal circuits, differentiating into neurons, oligodendrocytes, and astrocytes, thereby supporting axonal regeneration and remyelination [31,32]. Their secretion of neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF), activates MAPK/ERK and PI3K/Akt signaling pathways, which are critical for neuronal survival, axonal growth, and synaptic plasticity [32,33]. Despite their potential, NSPCs face substantial challenges in transplantation, particularly survival, immune evasion, and efficient integration into the inflammatory injury niche [19,34]. Preclinical studies have demonstrated that hydrogel-based encapsulation enhances NSPC survival by protecting cells from oxidative stress and immune-mediated apoptosis, leading to improved graft retention and functional recovery [35,36]. Similarly, scaffold-supported cell delivery has shown promise in improving NSPC engraftment by mimicking the extracellular matrix and providing biochemical cues that enhance differentiation [24]. On the other hand, iPSC-derived neural progenitors offer a scalable and patient-specific alternative, capable of differentiating into diverse neural and glial lineages by regulating Wnt/β-catenin, Sonic Hedgehog (SHH), and Notch signaling pathways [37,38]. SHH gradients are particularly critical for oligodendrocyte specification, while Wnt signaling influences neuronal differentiation [39]. Despite their therapeutic potential, iPSCs are challenged by tumorigenic risks, susceptibility to oxidative stress, and differentiation inconsistencies, necessitating bioengineered scaffolds and small-molecule modulators to enhance survival, functional maturation, and long-term stability [40,41]. A phase I clinical trial using iPSC-derived neural progenitors in SCI patients demonstrated partial sensory and motor improvements, although variability in integration remained a critical challenge [42].
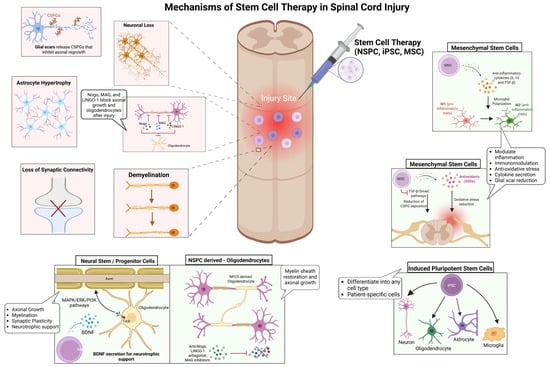
Figure 1.
Mechanisms underlying stem cell therapy in SCI repair. SCI disrupts neural circuits through neuronal loss, demyelination, astrocyte hypertrophy, synaptic disconnection, and glial scar formation, creating significant barriers to regeneration. Stem cell-based therapies aim to restore neural function by addressing these pathological hallmarks. NSPCs enhance axonal growth, synaptic plasticity, and remyelination through neurotrophic support. iPSCs generate patient-specific neural derivatives, reducing immune rejection and supporting neural repair. MSCs regulate the injury microenvironment by shifting microglia from a pro-inflammatory (M1) to an anti-inflammatory (M2) state, mitigating oxidative stress, and secreting neuroprotective cytokines such as IL-10 and TGF-β. By targeting key cellular and molecular deficits, these regenerative strategies offer a multifaceted approach to promoting neuroprotection, remyelination, and functional recovery in SCI. Figure created using BioRender.com (accessed 16 April 2025): https://BioRender.com/411iuf7.
Alternatively, MSCs function primarily through paracrine signaling, exerting immunomodulatory and neuroprotective effects rather than direct neuronal replacement [43]. By secreting anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), MSCs polarize microglia from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype, thereby attenuating neuroinflammation and facilitating tissue repair [44]. Furthermore, MSC-derived vascular endothelial growth factor (VEGF) enhances angiogenesis and vascular remodeling, promoting oxygen and nutrient delivery to injured tissue [45]. A clinical study analyzing MSC-based therapies for SCI highlighted the variability in clinical outcomes due to differences in cell sources, administration routes, and patient selection criteria, emphasizing the need for standardized protocols to enhance therapeutic efficacy [43,46]. Despite significant advances in stem cell transplantation, the inhibitory microenvironment of the injured spinal cord remains a major barrier to functional recovery [7,47]. Myelin-associated inhibitory molecules, including Nogo, myelin-associated glycoprotein (MAG), and leucine-rich repeat and immunoglobulin domain-containing Nogo receptor-interacting protein-1 (LINGO-1), activate the RhoA/ROCK signaling cascade, leading to growth cone collapse and impaired axonal regeneration [25]. Strategies targeting these molecular inhibitors, such as anti-Nogo antibodies, LINGO-1 antagonists, and MAG inhibitors, have shown promise in neutralizing inhibitory cues, enabling neurite outgrowth and enhancing axonal plasticity [48,49]. Collectively, these stem cell-based approaches offer promising avenues for neuroprotection and regeneration; however, overcoming the inhibitory microenvironment remains a critical challenge for achieving sustained functional recovery.
3. Advancements in Bioengineering for Stem Cell-Based SCI Repair
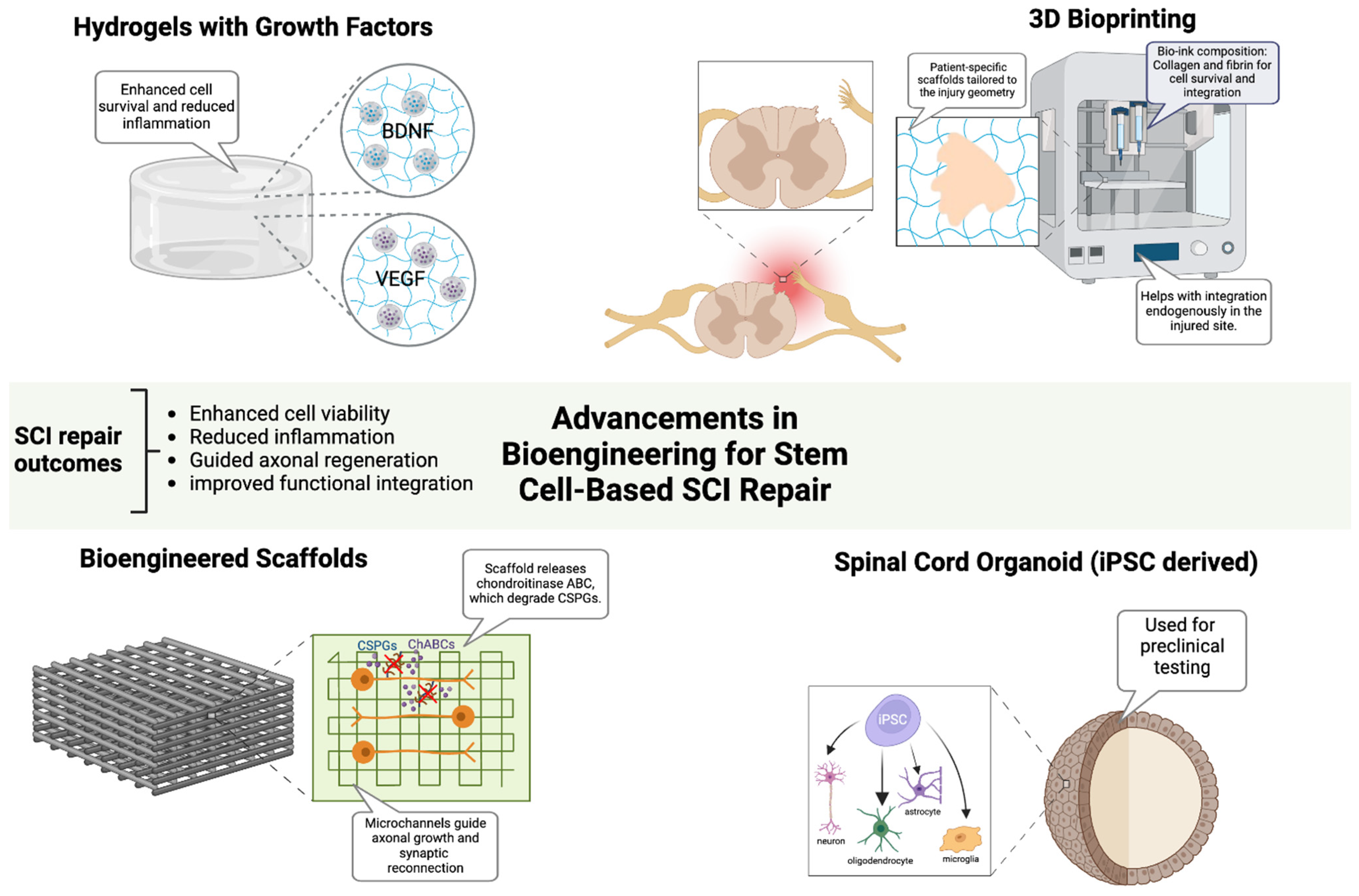
Bioengineering strategies have been developed to enhance the efficacy of stem cell-based therapies by overcoming the inhibitory microenvironment that impedes functional recovery, improving graft survival, optimizing neural integration, and facilitating axonal regeneration in SCI [20,21,36]. Emerging technologies, including biomaterial scaffolds, hydrogel-based delivery platforms, and 3D bioprinting, provide critical tools to optimize stem cell viability, differentiation, and host-circuit integration, while neurotrophic factor-infused hydrogels, enriched with BDNF and VEGF, create a biomimetic niche that enhances neuronal survival, synaptic remodeling, glial support, and stem cell retention (Figure 2) [50,51]. In parallel, the enzymatic modulation of chondroitin sulfate proteoglycans (CSPGs) via chondroitinase ABC (ChABC) continues to show promise in overcoming glial scar-associated barriers, thereby facilitating axonal regeneration [23]. Preclinical studies have demonstrated that aligned nanofiber scaffolds promote axonal regeneration and synaptic reconnection in SCI models, improving functional recovery [50,52]. In a canine L2 SCI model, an aligned fibrin nanofiber hydrogel provided a structured fiber bridge that supported cellular adhesion, facilitated directional axonal regrowth, and successfully reconnected nerve fibers between the rostral and caudal stumps, ultimately enhancing motor function recovery as confirmed by diffusion tensor imaging [53]. To further optimize therapeutic outcomes, electrically conductive biomaterials, including graphene and conductive polymers, have been explored as a means of restoring electrophysiological connectivity and facilitating neural network reactivation [20]. In parallel, 3D bioprinting technologies have revolutionized scaffold fabrication, enabling the construction of patient-specific biomimetic constructs that integrate regionally defined extracellular matrix components, growth factors, and stem cells in precisely controlled spatial arrangements [36]. A recent study using 3D-bioprinted hydrogel scaffolds seeded with iPSC-derived neural progenitors showed enhanced graft survival and axonal extension, demonstrating their potential for personalized regenerative therapies [54]. Additionally, iPSC-derived spinal cord organoids have provided a preclinical model for optimizing patient-matched regenerative strategies. These organoid models faithfully replicated the cytoarchitecture and cellular heterogeneity of the spinal cord, allowing for improved prediction of graft behavior and functional integration [55,56]. Alongside molecular and biomaterial-based interventions, electrical stimulation modalities, including epidural electrical stimulation (EES) and functional electrical stimulation (FES), have emerged as powerful adjuncts to stem cell-based therapies, facilitating circuit reactivation and synaptic plasticity enhancement [8]. EES has been shown to increase the excitability of residual supraspinal pathways, improving voluntary motor function, while FES directly stimulates paralyzed muscle groups, reinforcing neuroplasticity and synaptic remodeling [57]. A clinical study demonstrated that EES in combination with intensive rehabilitation resulted in significant motor recovery in individuals with chronic SCI, reinforcing the potential for neurostimulation-based approaches in regenerative strategies [58]. Furthermore, the development of adaptive neurostimulation devices, capable of the real-time modulation of stimulation parameters based on patient-specific electrophysiological feedback, has opened new avenues for customized rehabilitation strategies that maximize motor circuit activation and recovery potential [57].
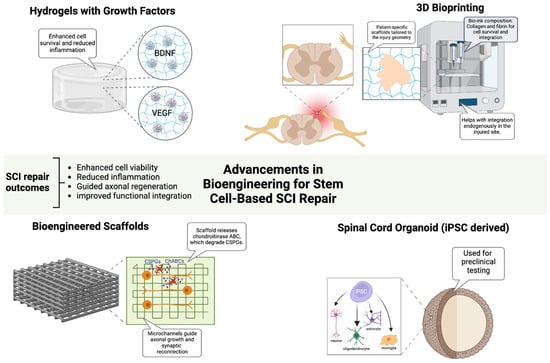
Figure 2.
Advancements in Bioengineering for Stem Cell-Based SCI Repair. Bioengineering innovations enhance stem cell-based therapies for SCI repair by improving cell survival, reducing inflammation, and guiding axonal regeneration. Growth factor-infused hydrogels support neuronal viability, while bioengineered scaffolds with aligned microchannels and ChABC promote axonal growth and synaptic reconnection. Three-dimensional bioprinting enables patient-specific scaffold fabrication to optimize cellular integration. iPSC-derived spinal cord organoids recapitulate native spinal architecture, serving as preclinical models for personalized therapy development. Figure created using BioRender.com (accessed 16 April 2025): https://BioRender.com/jupnwy6.
While these advancements significantly enhance stem cell-based repair strategies, immune rejection remains a formidable barrier to the clinical translation of biomaterial-assisted stem cell therapies, particularly in allogeneic transplantation settings [59,60]. Advances in CRISPR-based hypoimmunogenic engineering have enabled the generation of HLA-null iPSCs, which evade host immune surveillance while retaining regenerative potential [61]. By selectively deleting classical MHC class I and II molecules while preserving non-classical HLA-G, these engineered stem cells achieve immune evasion without compromising differentiation and functional capabilities [61,62]. Additionally, biomaterial scaffolds engineered to deliver localized immunomodulatory agents, such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), have been shown to create immune-privileged niches, preventing T-cell and NK cell-mediated graft rejection while enhancing transplanted cell survival [63]. Unlike systemic immunosuppression, which carries significant risks of infection, metabolic complications, and immune dysregulation, biomaterial-based localized immunosuppression minimizes adverse effects while optimizing host–graft interactions [59]. Preclinical models have shown that localized IL-10 delivery significantly improves stem cell survival and functional outcomes in SCI, providing a promising immunomodulatory strategy [64]. The ability to customize biomaterials for patient-specific needs, combined with transcriptomic insights into injury-specific microenvironments, could enable highly individualized regenerative strategies that optimize cellular engraftment, circuit reconstruction, and functional recovery.
4. Patient-Specific Variables Impacting Stem Cell Therapy for SCI
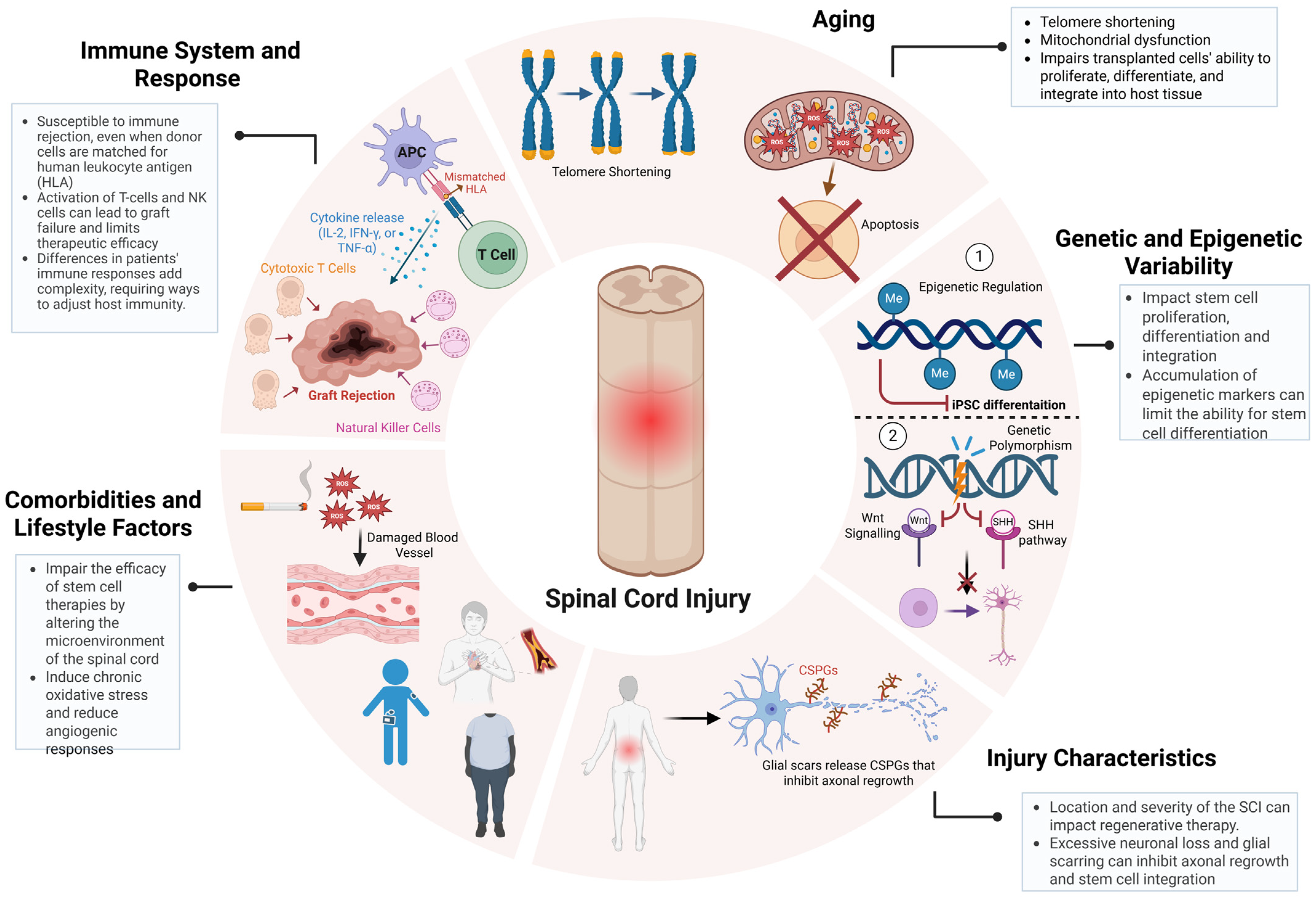
Another critical determinant of stem cell therapy outcomes in SCI repair is patient-specific variability, encompassing age-related cellular senescence, genetic and epigenetic diversity, injury microenvironmental constraints, and systemic comorbidities [47,65,66,67]. These factors not only modulate the host response to transplantation but also govern the long-term integration of grafted cells into existing neural circuits, ultimately influencing therapeutic efficacy (Figure 3) [65,68]. Aging profoundly alters the cellular and systemic landscape of SCI, introducing biochemical and molecular factors that disrupt neuroregeneration and compromise stem cell function [69]. Hallmarks of age-related dysfunction, including telomere attrition, mitochondrial impairment, and chronic low-grade inflammation (inflammaging), create a hostile post-injury microenvironment characterized by elevated oxidative stress and sustained neuroinflammatory signaling. Increased reactive oxygen species (ROS) accumulation exacerbates neuronal loss, while heightened levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) intensify glial scarring, further restricting axonal regrowth [66,69,70]. These factors collectively diminish the regenerative potential of stem cells, including NSPCs and iPSCs, exhibiting declined proliferative capacity, impaired lineage commitment, and epigenetic silencing of neurogenic genes with age [71]. Addressing these deficits requires preconditioning strategies, such as antioxidant-based interventions such as N-acetylcysteine to mitigate oxidative stress or the transient expression of Yamanaka factors to rejuvenate aged NSPCs, restoring their proliferative and neurogenic potential [72]. Recent studies had demonstrated that transient Yamanaka factors (Oct4, Sox2, Klf4, and c-Myc) expression enhances neuronal differentiation and synaptic integration in aged iPSC-derived progenitors, highlighting its potential for improving SCI repair in older patients [73,74]. As SCI incidence continues to rise in aging populations, the development of age-adapted regenerative protocols is becoming increasingly critical for maximizing stem cell therapy outcomes [1,5].
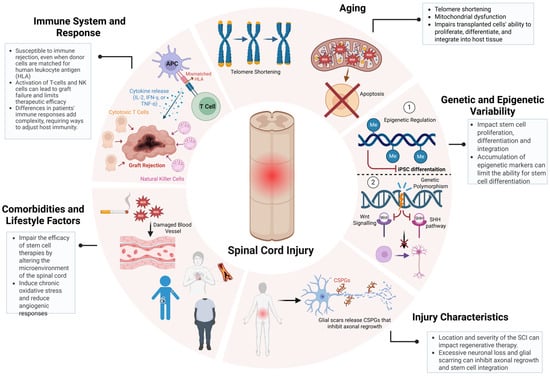
Figure 3.
Patient-specific variables impacting the efficacy of stem cell-based therapies for SCI. Key patient-specific factors influence SCI treatment outcomes. Aging-driven telomere shortening and mitochondrial dysfunction impair stem cell proliferation and differentiation. Genetic and epigenetic variability modulate neural integration and regenerative potential. Injury severity, glial scarring, and chronic inflammation create barriers to axonal regrowth. Comorbidities such as diabetes and obesity exacerbate oxidative stress and inflammation, further limiting therapeutic efficacy. Immune rejection by T-cells and NK cells remains a major challenge. Addressing these factors through precision regenerative strategies is crucial for optimizing SCI repair. Figure created using BioRender.com (accessed 16 April 2025): https://BioRender.com/wcuh6xo.
Additionally, genetic and epigenetic variability further dictates the regenerative potential of transplanted cells, influencing molecular pathways governing neurorepair [71]. Polymorphisms within key signaling cascades, such as Wnt/β-catenin and Sonic Hedgehog (SHH), modulate neural differentiation efficiency, while VEGFA variants regulate angiogenesis and graft vascularization [42,71,75]. Similarly, epigenetic modifications, such as DNA methylation and histone acetylation serve as transcriptional regulators of neurogenic genes, with hypermethylation of NEUROD1 and ASCL1 linked to impaired neuronal differentiation [71,75]. A recent study using scRNA-seq in rats revealed distinct inflammatory and neurogenic subpopulations that correlate with differential recovery trajectories, highlighting the necessity of patient-specific interventions [76]. The injury microenvironment is another key determinant of stem cell therapy outcomes, as the anatomical level, severity, and chronicity of SCI create biophysical and biochemical barriers to neurorepair [35,77]. Cervical injuries, which disrupt extensive descending motor and autonomic pathways, present greater regenerative challenges than thoracic or lumbar injuries, where residual circuitry is more intact [13]. Severe SCI cases are characterized by widespread neuronal loss, demyelination, and glial scarring, all of which contribute to a neuroinhibitory environment enriched in chondroitin sulfate proteoglycans (CSPGs) that restrict axonal regrowth and limit stem cell integration [23]. A recent preclinical trial demonstrated that region-specific NSPCs, derived based on spatial transcriptomics data, exhibited significantly improved survival and myelination in a rodent SCI model [77]. Further compounding the complexity of SCI repair, systemic comorbidities such as diabetes, obesity, and cardiovascular disease could exacerbate SCI pathophysiology by amplifying chronic inflammation, oxidative stress, and vascular dysfunction; however, limited research has been conducted on the precise mechanisms underlying these interactions and their impact on regenerative therapies [78,79]. Hyperglycemia in diabetic patients has been shown to induce chronic inflammation, impair endothelial function, and restrict blood supply to the injury site, compromising stem cell engraftment and survival [80]. Similarly, obesity-driven metabolic dysregulation disrupts the secretion of neuroprotective factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1), reducing neuronal viability and limiting neurorepair capacity [81,82]. A clinical study evaluating MSC transplantation in diabetic SCI patients reported lower graft survival and integration rates compared to non-diabetic cohorts, highlighting the need for metabolic intervention strategies alongside cell therapy [82]. These findings highlight the critical need for metabolic regulation in conjunction with stem cell therapy, reinforcing the necessity of a precision-medicine approach that accounts for patient-specific physiological and molecular factors to enhance therapeutic efficacy.
5. Transcriptomics as a Gateway to Precision Medicine for SCI
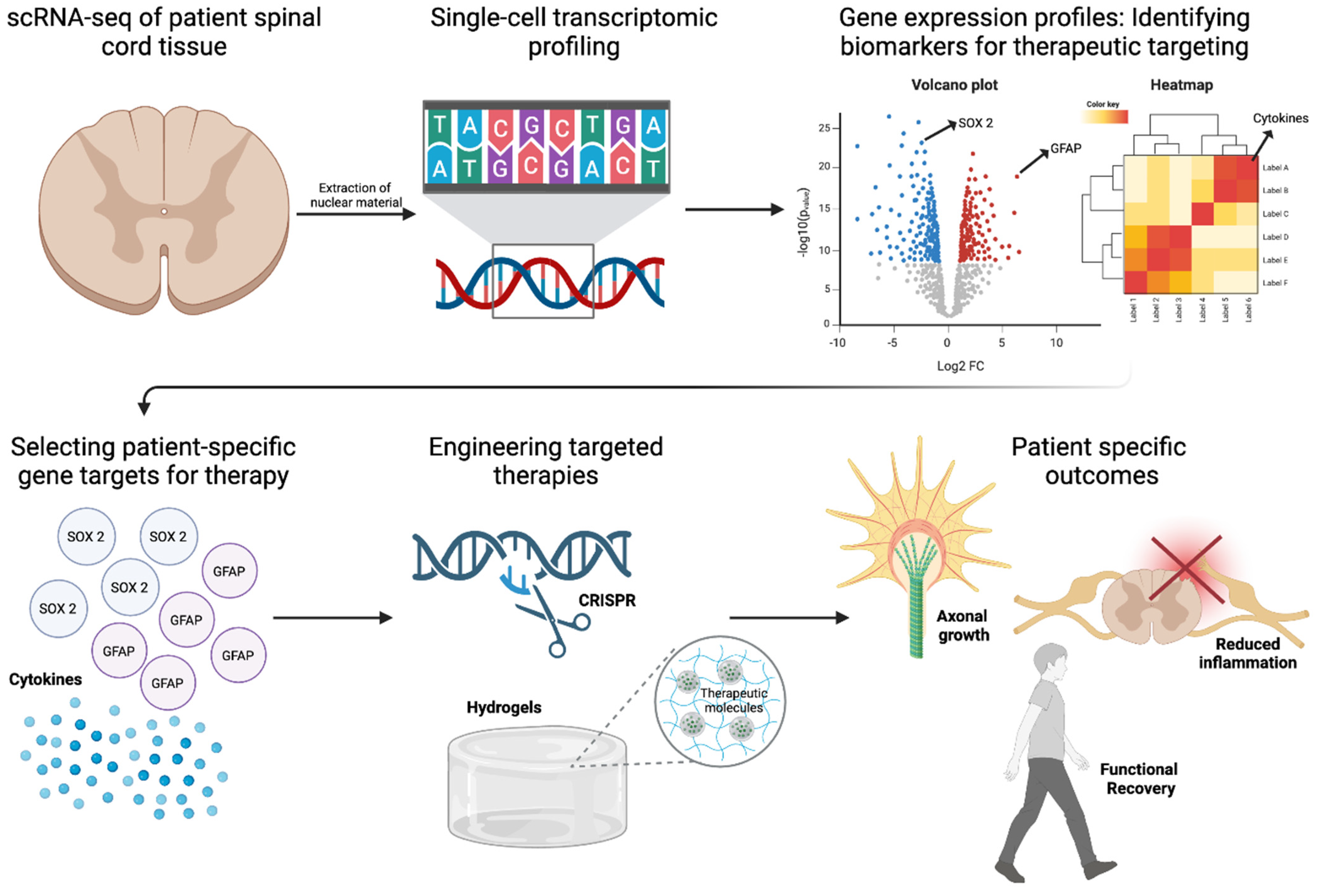
Single-cell RNA sequencing (scRNA-seq), spatial transcriptomics, and epigenomic profiling provide an in-depth characterization of injury and patient-specific molecular landscapes, enabling precision-driven approaches to stem cell differentiation, immune evasion, and functional restoration [16,17,77,83]. The resolution afforded by single-cell transcriptomics has illuminated the cellular complexity of SCI, uncovering distinct astrocytic, microglial, and neuronal subpopulations that orchestrate injury progression and repair (Figure 4). Recent scRNA-seq studies have revealed functionally divergent astrocyte phenotypes, including A1 astrocytes, which drive neuroinflammation through IL-1β and TNF-α secretion, and A2 astrocytes, which promote neuroprotection and axonal regeneration via BDNF and GDNF release [84,85]. Similarly, microglial heterogeneity has been dissected at the transcriptional level, demonstrating that M1 microglia perpetuate inflammatory damage, whereas M2 microglia exhibit pro-reparative properties by upregulating Arg1 and IL-10 [86]. In addition, scRNA-seq enables the real-time tracking of transplanted stem cells, revealing transcriptional signatures that distinguish successfully integrated neurons and oligodendrocytes from those undergoing apoptosis [16]. Furthermore, scRNA-seq could provide insight into the molecular mechanisms governing lineage specification by identifying how tightly regulated pathways, including Sonic Hedgehog (SHH), Wnt/β-catenin, and Notch, influence the fate of transplanted stem cells, while also revealing how region-specific SHH gradients drive caudalization, facilitating spinal cord-specific oligodendrocyte differentiation, remyelination, and improved axonal conduction [87,88]. Additionally, the spatial transcriptomic mapping of the spinal cord has identified regionally distinct transcriptional programs, facilitating the development of cervical, thoracic, and lumbar NSPC subtypes that are molecularly optimized for site-specific integration [89]. This spatially refined approach extends to growth factor-mediated lineage modulation, as transcriptomic-guided differentiation has identified BDNF and GDNF as key drivers of neuronal maturation, while PDGF-AA supports oligodendrocyte lineage commitment. These insights are now being leveraged in bioengineered hydrogel-based delivery systems, where precisely timed release of trophic factors enhances survival and lineage fidelity of transplanted stem cells [22,64].
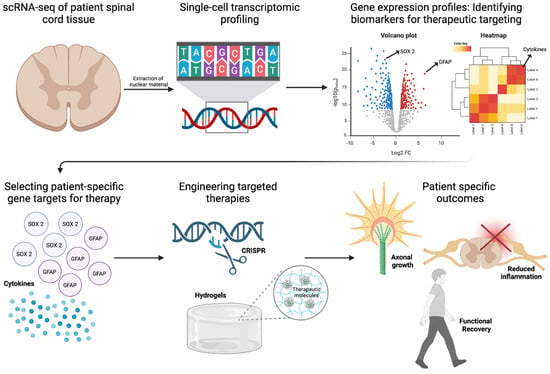
Figure 4.
Role of Transcriptomics in SCI Repair. Single-cell RNA sequencing (scRNA-seq) of patient spinal cord tissue enables transcriptional profiling to identify biomarkers for therapeutic targeting. Differential gene expression analysis highlights key regulators such as SOX2 and GFAP, guiding the selection of patient-specific gene targets. Red and blue dots represent significantly upregulated and downregulated genes, respectively, identified via single-cell RNA sequencing. Engineered therapies, including CRISPR-based gene editing and hydrogel-based delivery of therapeutic molecules, are designed to promote axonal growth and reduce inflammation. These precision approaches aim to enhance functional recovery in SCI repair. Figure created using BioRender.com (accessed 16 April 2025): https://BioRender.com/74iqv1j.
The growing application of patient-specific transcriptomic profiling reinforces the shift toward individualized regenerative strategies in SCI. Molecular polymorphisms, such as VEGFA variants influencing angiogenesis and SHH mutations affecting neural differentiation, have been identified as key determinants of stem cell therapy responsiveness [90]. By integrating scRNA-seq with machine learning-based predictive modeling, transcriptomic datasets could now enable patient stratification for clinical trials, optimizing patient selection for iPSC-derived NSPC transplantation based on gene expression biomarkers, including BDNF, NT-3, and VEGF [91]. Additionally, CRISPR-based transcriptomic-guided interventions offer novel avenues for modulating patient-specific gene targets to enhance stem cell resilience [61,92]. Targeted silencing of pro-apoptotic genes and upregulation of neurotrophic factors have demonstrated efficacy in promoting graft survival and functional recovery [14,93]. In a rat model of traumatic brain injury, transplantation of bone marrow MSCs with silenced Rac1 enhanced survival and improved neurological function by inhibiting NADPH oxidase subunits, thereby reducing oxidative stress and apoptosis [94]. Additionally, the administration of neurotrophic factors has been shown to support neuronal survival and function [95]. While these technologies have refined our understanding of cellular heterogeneity, their application in clinical decision-making remains underdeveloped. Translating transcriptomic insights into personalized therapeutic strategies necessitates an approach that aligns biomarker discovery with real-time patient stratification. Incorporating transcriptomic profiling into clinical workflows would facilitate precision-driven interventions, enabling the selection of patient-specific stem cell populations optimized for engraftment and functional recovery. Moreover, dynamic monitoring of cerebrospinal fluid (CSF) biomarkers, coupled with predictive modeling, could potentially refine immunomodulatory strategies, rehabilitation protocols, and neurostimulation parameters. Future directions could include, establishing a framework that integrates transcriptomics with clinical algorithms, that has the potential to transform SCI treatment paradigms, shifting regenerative medicine toward individualized therapeutic regimens tailored to molecularly defined patient subgroups.
6. Conclusions
SCI is a complex, multifactorial condition that cannot be addressed by targeting a single gene or pathway. Unlike monogenic disorders that can be corrected through single-gene modifications, SCI involves a dynamic interplay of injury severity, immune response, extracellular matrix remodeling, and neuroinflammation. Advances in region-specific NSPCs and iPSC-derived neural progenitors have demonstrated promise in preclinical and early-phase clinical studies, with lineage-optimized differentiation enhancing synaptic integration and functional recovery [4,15,18]. Meanwhile, biomaterial-based scaffolds incorporating electrically conductive polymers and growth factor-releasing hydrogels have enhanced cell survival and host integration, accelerating axonal regeneration in preclinical models [21,51,60]. Despite these advances, challenges persist in optimizing immune compatibility, as evidenced by variability in graft survival across patient cohorts [63,68,96]. Strategies such as CRISPR-mediated hypoimmunogenic engineering and localized biomaterial-assisted immune modulation are now emerging as viable solutions for overcoming rejection and improving long-term outcomes [27,61].
Single-cell transcriptomics and machine learning-driven patient stratification are further transforming SCI treatment by enabling personalized regenerative strategies [16,29,91]. Spatially refined transcriptomic profiling has facilitated the development of region-specific NSPCs that optimize site-specific engraftment and functional recovery, with preclinical models demonstrating enhanced myelination and neuronal survival [39,77]. The application of adaptive neurostimulation, in conjunction with stem cell transplantation, has also shown clinical promise, with studies demonstrating improved motor function recovery in SCI patients [8,57]. As these technologies converge, the field is shifting toward precision regenerative medicine, integrating stem cell engineering, immune modulation, bioengineering, and transcriptomics to develop individualized therapies. Among the key stem cell candidates for SCI repair, neural stem/progenitor cells (NSPCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs) each present distinct advantages and limitations. NSPCs exhibit strong neurogenic capacity and regional specificity, promoting circuit reconstruction and myelination, but face scalability and ethical sourcing challenges [97]. iPSCs offer patient-specific, pluripotent versatility with scalable expansion, though clinical application is hampered by high production costs, tumorigenicity risks, and complex regulatory oversight [38,98]. MSCs, in contrast, are widely accessible and inexpensive to manufacture, exert potent immunomodulatory and trophic effects, but have limited neuronal differentiation capacity and integration potential [44,46]. Clinically, MSCs currently represent the most accessible option, while iPSC- and NSPC-based therapies are advancing in early trials, driven by improvements in manufacturing and immune engineering. Emerging technologies such as CRISPR/Cas9 offer the ability to generate hypoimmunogenic stem cell lines with improved graft survival and reduced rejection, especially for iPSCs and NSPCs [61,92]. Concurrently, machine learning models trained on single-cell transcriptomic data are revolutionizing patient stratification, enabling predictive modeling of treatment response and personalized therapeutic design in SCI repair [28].
In parallel, five core technological platforms—bioengineered scaffolds, growth factor-infused hydrogels, adaptive neurostimulation, transcriptomics, and CRISPR-based gene editing—are catalyzing the next generation of personalized regenerative interventions. Bioengineered scaffolds and 3D-bioprinted matrices provide essential architectural support for stem cell engraftment and axonal regeneration; while materials are scalable and relatively affordable, their customization for individual patient demands advanced fabrication infrastructure and rigorous quality assurance. Growth factor-infused hydrogels are among the most clinically translatable innovations, offering low-cost and modular delivery of neurotrophic signals, although achieving consistent in vivo performance remains a challenge [22,64]. Adaptive neurostimulation devices like epidural electrical stimulation (EES) and functional electrical stimulation (FES) show robust clinical efficacy, but their accessibility is limited by the need for surgical expertise, physical rehabilitation infrastructure, and significant upfront cost [8,58]. Transcriptomic profiling, especially when combined with spatial resolution and machine learning, enables precision-matched stem cell therapies, yet its clinical integration is currently hindered by the cost of high-throughput sequencing, data complexity, and the need for cross-disciplinary workflows [76]. Finally, CRISPR technologies hold transformative potential for creating universal, immune-evasive cell products, but face translational delays due to regulatory and ethical hurdles. For these advanced platforms to be effectively implemented in clinical care, translational strategies must prioritize cost containment, manufacturing automation, cross-sector collaboration, and equitable deployment models, particularly for underserved populations. Synergizing these innovations with stem cell-based therapies is key to realizing scalable, accessible, and individualized solutions for spinal cord injury repair. Future directions will focus on translating these advancements into large-scale clinical applications, ensuring their efficacy and scalability. Translating these advances into clinical solutions will be transformative in restoring function and promoting long-term neurorepair in SCI patients.
Author Contributions
Conceptualization: S.K.J. and E.C.T.; Writing and Original Draft Preparation: S.K.J.; Review and Editing: S.K.J., S.M. and R.V.S.; Visualization: S.M. and R.V.S.; Supervision: E.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Josette Robertson and Joan Johnston Family Foundation Fund (Grant No. JRJJ-2024-001), the Michael T. Richard Clinical Research Fellowship in Neurosurgery at the University of Ottawa Brain and Mind Research Institute (Grant No. MTRF-23-567), the Department of Surgery at the University of Ottawa (Grant No. DOS-2024-045), and the Society of Neurological Surgeons, RUNN Course Resident Award (Grant No. RUNN-2024-678).
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
This review was made possible through the collaborative efforts of clinical and academic professionals at the Ottawa Hospital and the University of Ottawa. The authors would like to thank Rebecca Porteous and Maitreya Patel for their support in coordinating organ and tissue donation efforts that informed the perspectives outlined in this manuscript. We also acknowledge the contributions of Maryam Mashayekhi, Ahmed Alfawaz, Vatsal Trivedi, Alick Wang, and Ragulojan, for their surgical expertise in spinal cord and muscle tissue harvests.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| SCI | Spinal Cord Injury |
| NSPCs | Neural Stem/Progenitor Cells |
| iPSCs | Induced Pluripotent Stem Cells |
| MSCs | Mesenchymal Stem Cells |
| BDNF | Brain-Derived Neurotrophic Factor |
| VEGF | Vascular Endothelial Growth Factor |
| ChABC | Chondroitinase ABC |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| scRNA-seq | Single-cell RNA Sequencing |
| EES | Epidural Electrical Stimulation |
| FES | Functional Electrical Stimulation |
| SHH | Sonic Hedgehog |
| MAG | Myelin-Associated Glycoprotein |
| OPCs | Oligodendrocyte Precursor Cells |
| BBB | Blood–Brain Barrier |
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef] [PubMed]
- Saremi, J.; Mahmoodi, N.; Rasouli, M.; Ranjbar, F.E.; Mazaheri, E.L.; Akbari, M.; Hasanzadeh, E.; Azami, M. Advanced approaches to regenerate spinal cord injury: The development of cell and tissue engineering therapy and combinational treatments. Biomed. Pharmacother. 2022, 146, 112529. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 3994. [Google Scholar] [CrossRef]
- Krueger, H.; Noonan, V.K.; Trenaman, L.M.; Joshi, P.; Rivers, C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis. Inj. Can. 2013, 33, 113–122. [Google Scholar] [CrossRef]
- Flack, J.; Sharma, K.; Xie, J. Delving into the recent advancements of spinal cord injury treatment: A review of recent progress. Neural Regen. Res. 2022, 17, 283. [Google Scholar] [PubMed]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Hachmann, J.T.; Yousak, A.; Wallner, J.J.; Gad, P.N.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, 126, 1843–1859. [Google Scholar] [CrossRef]
- Gorgey, A.S. Robotic exoskeletons: The current pros and cons. World J. Orthop. 2018, 9, 112–119. [Google Scholar] [CrossRef]
- Zeng, C.-W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Damianakis, E.I.; Benetos, I.S.; Evangelopoulos, D.S.; Kotroni, A.; Vlamis, J.; Pneumaticos, S.G. Stem Cell Therapy for Spinal Cord Injury: A Review of Recent Clinical Trials. Cureus 2022, 14, e24575. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Wang, M.; Zhang, B.; Wang, X.; Wanyan, P. Clinical translation of stem cell therapy for spinal cord injury still premature: Results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022, 20, 284. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Chen, Y.; Aarabi, B.; Ahmad, F.; Anderson, K.D.; Dumont, T.; Fourney, D.R.; Harrop, J.S.; Kim, K.D.; Kwon, B.K.; et al. A randomized controlled trial of local delivery of a Rho inhibitor (VX-210) in patients with acute traumatic cervical spinal cord injury. J. Neurotrauma 2021, 38, 2065–2072. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transpl. 2021, 30, 0963689721989266. [Google Scholar] [CrossRef]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Coskun, V.; Liang, A.; Yu, J.; Cheng, L.; Ge, W.; Shi, Z.; Zhang, K.; Li, C.; Cui, Y.; et al. Single-Cell Transcriptome Analyses Reveal Signals to Activate Dormant Neural Stem Cells. Cell 2015, 161, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Sanosaka, T.; Lundin, A.; Imaizumi, K.; Etal, D.; Karlsson, F.H.; Clausen, M.; Cairns, J.; Hicks, R.; Kohyama, J.; et al. Single-cell study of neural stem cells derived from human iPSCs reveals distinct progenitor populations with neurogenic and gliogenic potential. Genes. Cells 2019, 24, 836–847. [Google Scholar] [CrossRef]
- Martin, J.R.; Cleary, D.; Abraham, M.E.; Mendoza, M.; Cabrera, B.; Jamieson, C.; Marsala, M.; Ciacci, J.D. Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury. Cell Rep. Med. 2024, 5, 101841. [Google Scholar] [CrossRef]
- Zheng, Y.; Gallegos, C.M.; Xue, H.; Li, S.; Kim, D.H.; Zhou, H.; Xia, X.; Liu, Y.; Cao, Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells 2022, 11, 2765. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative Therapies for Central Nervous System Diseases: A Biomaterials Approach. Neuropsychopharmacology 2014, 39, 169–188. [Google Scholar] [CrossRef]
- Katoh, H.; Yokota, K.; Fehlings, M.G. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front. Cell Neurosci. 2019, 13, 248. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Liu, C.; Zhang, S.; Gao, F.; Guo, W.; Sun, X.; Zhang, C.; Li, H.; Rao, Z.; et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials 2021, 268, 120596. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Schut, D.; Wang, J.; Fehlings, M.G. Chondroitinase and Growth Factors Enhance Activation and Oligodendrocyte Differentiation of Endogenous Neural Precursor Cells after Spinal Cord Injury. PLoS ONE 2012, 7, e37589. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Geoffroy, C.C.; Zheng, B. Myelin-Associated Inhibitors in Axonal Growth after Central Nervous System Injury. In Neural Regeneration; Elsevier: Amsterdam, The Netherlands, 2015; pp. 153–170. [Google Scholar] [CrossRef]
- Rashidbenam, Z.; Ozturk, E.; Pagnin, M.; Theotokis, P.; Grigoriadis, N.; Petratos, S. How does Nogo receptor influence demyelination and remyelination in the context of multiple sclerosis? Front. Cell Neurosci. 2023, 17, 1197492. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, N.; Hosoya, T.; Takeno, Y.; Saitoh, K.; Murai, H.; Amimoto, N.; Tatsumi, R.; Watanabe, S.; Hasegawa, Y.; Kikkawa, E.; et al. Hypoimmunogenic human iPSCs expressing HLA-G, PD-L1, and PD-L2 evade innate and adaptive immunity. Stem Cell Res. Ther. 2024, 15, 193. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Tzelves, L.; Paxinou, E.; Hitas, C.; Vamvakou, G.; Verykios, V.S.; Nikolaou, M. Integrating Machine Learning with Multi-Omics Technologies in Geroscience: Towards Personalized Medicine. J. Pers. Med. 2024, 14, 931. [Google Scholar] [CrossRef] [PubMed]
- Garuffo, L.; Leoni, A.; Gatta, R.; Bernardi, S. The Applications of Machine Learning in the Management of Patients Undergoing Stem Cell Transplantation: Are We Ready? Cancers 2025, 17, 395. [Google Scholar] [CrossRef]
- Song, B.; Xiong, G.; Luo, H.; Zuo, Z.; Zhou, Z.; Chang, X. Single-cell RNA sequencing of mouse neural stem cell differentiation reveals adverse effects of cadmium on neurogenesis. Food Chem. Toxicol. 2021, 148, 111936. [Google Scholar] [CrossRef]
- Finkel, Z.; Esteban, F.; Rodriguez, B.; Fu, T.; Ai, X.; Cai, L. Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease. Cells 2021, 10, 2045. [Google Scholar] [CrossRef]
- Xu, B.; Yin, M.; Yang, Y.; Zou, Y.; Liu, W.; Qiao, L.; Zhang, J.; Wang, Z.; Wu, Y.; Shen, H.; et al. Transplantation of neural stem progenitor cells from different sources for severe spinal cord injury repair in rat. Bioact. Mater. 2023, 23, 300–313. [Google Scholar] [CrossRef]
- Deleyrolle, L.P.; Reynolds, B.A. Isolation, Expansion, and Differentiation of Adult Mammalian Neural Stem and Progenitor Cells Using the Neurosphere Assay. Neural Cell Transplant. Methods Protoc. 2009, 549, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, W.; Xiao, C.; Zhao, J.; Xiang, C.; Liu, W.; Gu, R. Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair. Theranostics 2023, 13, 3966–3987. [Google Scholar] [CrossRef]
- Mata, R.; Yao, Y.; Cao, W.; Ding, J.; Zhou, T.; Zhai, Z.; Gao, C. The Dynamic Inflammatory Tissue Microenvironment: Signality and Disease Therapy by Biomaterials. Research 2021, 2021, 4189516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yao, C.; Wei, B.; Xu, C.; Huang, X.; Liu, Y.; He, J.; Zhang, J.; Li, D. 3D printing of functional bioengineered constructs for neural regeneration: A review. Int. J. Extrem. Manuf. 2023, 5, 042004. [Google Scholar] [CrossRef]
- Silva, M.C.; Haggarty, S.J. Human pluripotent stem cell–derived models and drug screening in CNS precision medicine. Ann. New York Acad. Sci. 2020, 1471, 18–56. [Google Scholar] [CrossRef]
- Jagadeesan, S.K.; Galuta, A.; Sandarage, R.V.; Tsai, E.C. Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells. Cells 2025, 14, 64. [Google Scholar] [CrossRef]
- Solomon, E.; Davis-Anderson, K.; Hovde, B.; Micheva-Viteva, S.; Harris, J.F.; Twary, S.; Iyer, R. Global transcriptome profile of the developmental principles of in vitro iPSC-to-motor neuron differentiation. BMC Mol. Cell Biol. 2021, 22, 13. [Google Scholar] [CrossRef]
- Yasuda, S.; Kusakawa, S.; Kuroda, T.; Miura, T.; Tano, K.; Takada, N.; Matsuyama, S.; Matsuyama, A.; Nasu, M.; Umezawa, A.; et al. Tumorigenicity-associated characteristics of human iPS cell lines. PLoS ONE 2018, 13, e0205022. [Google Scholar] [CrossRef]
- Itakura, G.; Kawabata, S.; Ando, M.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Ookubo, T.; Kojima, K.; Kashiwagi, R.; et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Rep. 2017, 8, 673–684. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, B.; Chen, J.; Liu, D.; Ma, J.; Li, B.; Hao, J.; Zhou, X. Epigenetic regulation and factors that influence the effect of iPSCs-derived neural stem/progenitor cells (NS/PCs) in the treatment of spinal cord injury. Clin. Epigenetics 2024, 16, 30. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.-M.; Chen, S.-Y.; Fu, S.-P.; Zhou, H.; Zhang, Q.; Ao, J.; Luo, X.-P.; Zhang, T. Regulatory Role of Mesenchymal Stem Cells on Secondary Inflammation in Spinal Cord Injury. J Inflamm Res 2022, 15, 573–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Gao, T.; Wang, W.; Wang, L.; Xie, Y.; Tai, C.; Liu, S.; Cui, Y.; Wang, B. Engineered basic fibroblast growth factor-overexpressing human umbilical cord-derived mesenchymal stem cells improve the proliferation and neuronal differentiation of endogenous neural stem cells and functional recovery of spinal cord injury by activating the PI3K-Akt-GSK-3β signaling pathway. Stem Cell Res. Ther. 2021, 12, 468. [Google Scholar]
- de Teixeira de Araújo, L.; Thé Macêdo, C.; Fonseca Damasceno, P.K.; Costa das Neves, Í.G.; Souza de Lima, C.; Café Santos, G.; Alves de Santana, T.; de Almeida Sampaio, G.L.; Nascimento Silva, D.; Villarreal, C.F.; et al. Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence. Cells 2022, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, Z.; Feng, S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022, 10, 35. [Google Scholar] [CrossRef]
- Wu, H.; Ding, L.; Wang, Y.; Zou, T.-B.; Wang, T.; Fu, W.; Lin, Y.; Zhang, X.; Chen, K.; Lei, Y.; et al. MiR-615 Regulates NSC Differentiation In Vitro and Contributes to Spinal Cord Injury Repair by Targeting LINGO-1. Mol. Neurobiol. 2020, 57, 3057–3074. [Google Scholar] [CrossRef]
- Sartori, A.M.; Hofer, A.-S.; Schwab, M.E. Recovery after spinal cord injury is enhanced by anti-Nogo-A antibody therapy—from animal models to clinical trials. Curr. Opin. Physiol. 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Ortega, J.A.; Soares de Aguiar, G.P.; Chandravanshi, P.; Levy, N.; Engel, E.; Álvarez, Z. Exploring the properties and potential of the neural extracellular matrix for next-generation regenerative therapies. WIREs Nanomed. Nanobiotechnology 2024, 16, e1962. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, X.; Lu, X.; Liao, Q.; Luo, H.; Tian, Y.; Cheng, X.; Jiang, Y.; Liu, G.; Chen, J. Biomaterials and tissue engineering in traumatic brain injury: Novel perspectives on promoting neural regeneration. Neural Regen. Res. 2024, 19, 2157–2174. [Google Scholar] [CrossRef]
- Puhl, D.L.; Funnell, J.L.; Nelson, D.W.; Gottipati, M.K.; Gilbert, R.J. Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration. Bioengineering 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yao, S.; Xiong, Y.; Zhang, Z.; Yang, Y.; He, F.; Zhao, H.; Guo, Y.; Wang, G.; Xie, S.; et al. Directional axonal regrowth induced by an aligned fibrin nanofiber hydrogel contributes to improved motor function recovery in canine L2 spinal cord injury. J. Mater. Sci. Mater. Med. 2020, 31, 40. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Han, Y.; King, M.; Tikhomirov, E.; Barasa, P.; Dos Santos Souza, C.; Lindh, J.; Baltriukiene, D.; Ferraiuolo, L.; Azzouz, M.; Gullo, M.R.; et al. Towards 3D Bioprinted Spinal Cord Organoids. Int. J. Mol. Sci. 2022, 23, 5788. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell Neurosci. 2023, 17, 1095259. [Google Scholar] [CrossRef]
- Balbinot, G.; Li, G.; Gauthier, C.; Musselman, K.E.; Kalsi-Ryan, S.; Zariffa, J. Functional electrical stimulation therapy for upper extremity rehabilitation following spinal cord injury: A pilot study. Spinal Cord. Ser. Cases 2023, 9, 11. [Google Scholar] [CrossRef]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Nosrati-Siahmazgi, V.; Musaie, K.; Rezaei, S.; Qahremani, M.; Xiao, B.; Santos, H.A.; Shahbazi, M.-A. Emerging strategies to bypass transplant rejection via biomaterial-assisted immunoengineering: Insights from islets and beyond. Adv. Drug Deliv. Rev. 2023, 200, 115050. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef]
- Fortress, A.M.; Miyagishima, K.J.; Reed, A.A.; Temple, S.; Clegg, D.O.; Tucker, B.A.; Blenkinsop, T.A.; Harb, G.; Greenwell, T.N.; Ludwig, T.E.; et al. Stem cell sources and characterization in the development of cell-based products for treating retinal disease: An NEI Town Hall report. Stem Cell Res. Ther. 2023, 14, 53. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Jiang, A.Y.; Eufrásio-da-Silva, T.; Dolatshahi-Pirouz, A.; Langer, R.; Orive, G.; Grattoni, A. Emerging immunomodulatory strategies for cell therapeutics. Trends Biotechnol. 2023, 41, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xu, B.; Yang, C.; Xue, W.; You, Z.; Wu, X.; Ma, D.; Shao, D.; Leong, K.; Dai, J. A DAMP-scavenging, IL-10-releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials 2022, 280, 121279. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaleh, Z.M. The use of patient-specific stem cells in different autoimmune diseases. Saudi J. Biol. Sci. 2022, 29, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Spehar, K.; Pan, A.; Beerman, I. Restoring aged stem cell functionality: Current progress and future directions. Stem Cells 2020, 38, 1060. [Google Scholar] [CrossRef]
- Hsieh, J.; Zhao, X. Genetics and Epigenetics in Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018911. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Forsythe, S.; Sivakumar, H.; Mazzocchi, A.; Aleman, J.; Miller, L.; Levine, E.; Triozzi, P.; Skardal, A. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol. 2020, 27, 1956–1967. [Google Scholar] [CrossRef]
- Neves, J.; Sousa-Victor, P.; Jasper, H. Rejuvenating Strategies for Stem Cell-based Therapies in Aging. Cell Stem Cell 2017, 20, 161. [Google Scholar] [CrossRef]
- Wan, Y.; Finkel, T. The mitochondria regulation of stem cell aging. Mech. Ageing Dev. 2020, 191, 111334. [Google Scholar] [CrossRef]
- Li, M.; Guo, H.; Carey, M.; Huang, C. Transcriptional and epigenetic dysregulation impairs generation of proliferative neural stem and progenitor cells during brain aging. Nat. Aging 2024, 4, 62–79. [Google Scholar] [CrossRef]
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget 2018, 9, 4223–4238. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.E.; Lee, S.; Seo, J.H.; Kang, S.-W.; Choi, W.A.; Cho, S.-R. In Vivo Reprogramming Using Yamanaka Factors in the CNS: A Scoping Review. Cells 2024, 13, 343. [Google Scholar] [CrossRef]
- Antón-Fernández, A.; Roldán-Lázaro, M.; Vallés-Saiz, L.; Ávila, J.; Hernández, F. In vivo cyclic overexpression of Yamanaka factors restricted to neurons reverses age-associated phenotypes and enhances memory performance. Commun. Biol. 2024, 7, 631. [Google Scholar] [CrossRef]
- Kramer, A.S.; Harvey, A.R.; Plant, G.W.; Hodgetts, S.I. Systematic Review of Induced Pluripotent Stem Cell Technology as a Potential Clinical Therapy for Spinal Cord Injury. Cell Transplant. 2013, 22, 571–617. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Yan, R.; Yan, K.; Zhang, R.; Zhang, Q.; Zou, P.; Wang, H.; Qiao, H.; Li, S.; Ma, Q.; et al. Single-cell RNA sequencing reveals the role of immune-related autophagy in spinal cord injury in rats. Front. Immunol. 2022, 13, 987344. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, L.; Xie, Y.; Guo, S.; Cao, X.; Yang, M. Spatial multi-omics analysis of the microenvironment in traumatic spinal cord injury: A narrative review. Front. Immunol. 2024, 15, 1432841. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Schroth, S.L.; Haywood, C.; Heinemann, A.; Kessler, A.; Morse, L.; Khan, S.S.; Kershaw, K.N.; Nash, M.S. Diagnosis and Management of Cardiovascular Risk in Individuals With Spinal Cord Injury: A Narrative Review. Circulation 2023, 148, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Gater, D.R.; Farkas, G.J.; Tiozzo, E. Pathophysiology of Neurogenic Obesity After Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Hartge, M.M.; Unger, T.; Kintscher, U. The endothelium and vascular inflammation in diabetes. Diab Vasc. Dis. Res. 2007, 4, 84–88. [Google Scholar] [CrossRef]
- Bowers, E.; Singer, K. Obesity-induced inflammation: The impact of the hematopoietic stem cell niche. JCI Insight 2021, 6, e145295. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Barati, S. Effects of mesenchymal stem cell transplantation on spinal cord injury patients. Cell Tissue Res. 2022, 389, 373–384. [Google Scholar] [CrossRef]
- Yadav, A.; Matson, K.J.E.; Li, L.; Hua, I.; Petrescu, J.; Kang, K.; Alkaslasi, M.R.; Lee, D.I.; Hasan, S.; Galuta, A.; et al. A cellular taxonomy of the adult human spinal cord. Neuron 2023, 111, 328–344.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ren, Y.; Zhu, Y.; Huang, R.; Zhu, R.; Cheng, L.; Xie, N. The origins and dynamic changes of C3- and S100A10-positive reactive astrocytes after spinal cord injury. Front. Cell Neurosci. 2023, 17, 1276506. [Google Scholar] [CrossRef]
- Moulson, A.J.; Squair, J.W.; Franklin RJ, M.; Tetzlaff, W.; Assinck, P. Diversity of Reactive Astrogliosis in CNS Pathology: Heterogeneity or Plasticity? Front. Cell Neurosci. 2021, 15, 703810. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shui, X.; Sun, R.; Wan, L.; Zhang, B.; Xiao, B.; Luo, Z. Microglial Phenotypic Transition: Signaling Pathways and Influencing Modulators Involved in Regulation in Central Nervous System Diseases. Front. Cell Neurosci. 2021, 15, 736310. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.-J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef]
- Wu, J.; Li, W.; Guo, L.; Zhao, L.; Sun, S.; Li, H. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 2021, 386, 281–296. [Google Scholar] [CrossRef]
- Li, X.; Andrusivova, Z.; Czarnewski, P.; Langseth, C.M.; Andersson, A.; Liu, Y.; Gyllborg, D.; Braun, E.; Larsson, L.; Hu, L.; et al. Profiling spatiotemporal gene expression of the developing human spinal cord and implications for ependymoma origin. Nat. Neurosci. 2023, 26, 891–901. [Google Scholar] [CrossRef]
- Mackenzie, F.; Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 2012, 139, 1371–1380. [Google Scholar] [CrossRef]
- Huang, S.; Tu, T. Integrating single cell analysis and machine learning methods reveals stem cell-related gene S100A10 as an important target for prediction of liver cancer diagnosis and immunotherapy. Front. Immunol. 2025, 15, 1534723. [Google Scholar] [CrossRef]
- Pulecio, J.; Verma, N.; Mejía-Ramírez, E.; Huangfu, D.; Raya, A. CRISPR/Cas9-Based Engineering of the Epigenome. Cell Stem Cell 2017, 21, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Yin, Q.; Liu, H.; He, Y.; Wang, Y. Deep learning-based predictive identification of neural stem cell differentiation. Nat. Commun. 2021, 12, 2614. [Google Scholar] [CrossRef]
- Huang, D.; Siaw-Debrah, F.; Wang, H.; Ye, S.; Wang, K.; Wu, K.; Zhang, Y.; Wang, H.; Yao, C.; Chen, J.; et al. Transplanting Rac1-silenced bone marrow mesenchymal stem cells promote neurological function recovery in TBI mice. Aging 2021, 13, 2822–2850. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.; Balzamino, B.; Micera, A. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef]
- Kajikawa, K.; Imaizumi, K.; Shinozaki, M.; Shibata, S.; Shindo, T.; Kitagawa, T.; Shibata, R.; Kamata, Y.; Kojima, K.; Nagoshi, N.; et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol. Brain 2020, 13, 120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).