Long Non-Coding TP73-AS1: A Potential Biomarker and Therapeutic Target in Cancer
Abstract
1. Introduction
2. Overview of TP73-AS1 in Cancer
2.1. Structure of TP73-AS1
2.2. Dual Role of TP73-AS1 in Cancer
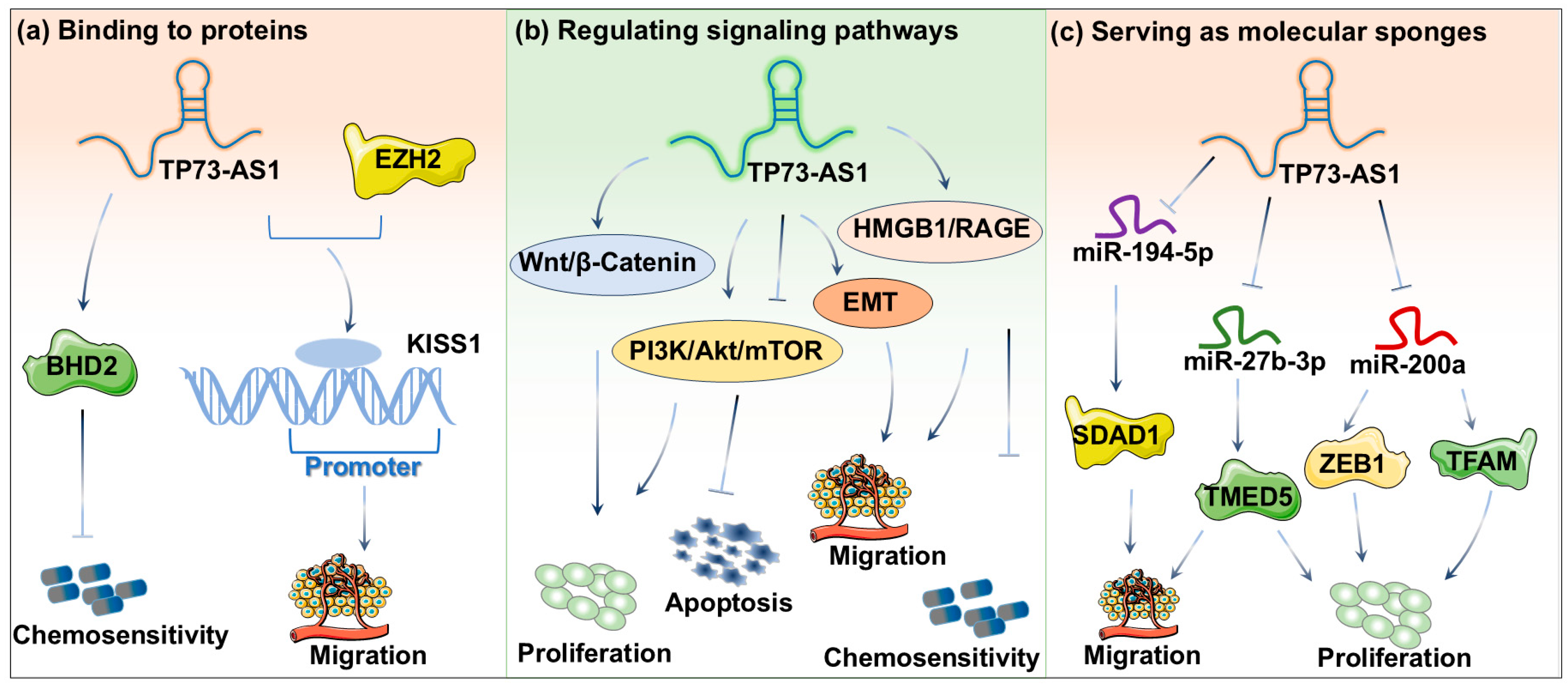
3. Regulatory Mechanisms of TP73-AS1 in Cancer
3.1. Binding to Proteins
3.2. Regulating Signaling Pathways
3.3. Serving as Molecular Sponges
4. Functional Roles of TP73-AS1 in Cancer
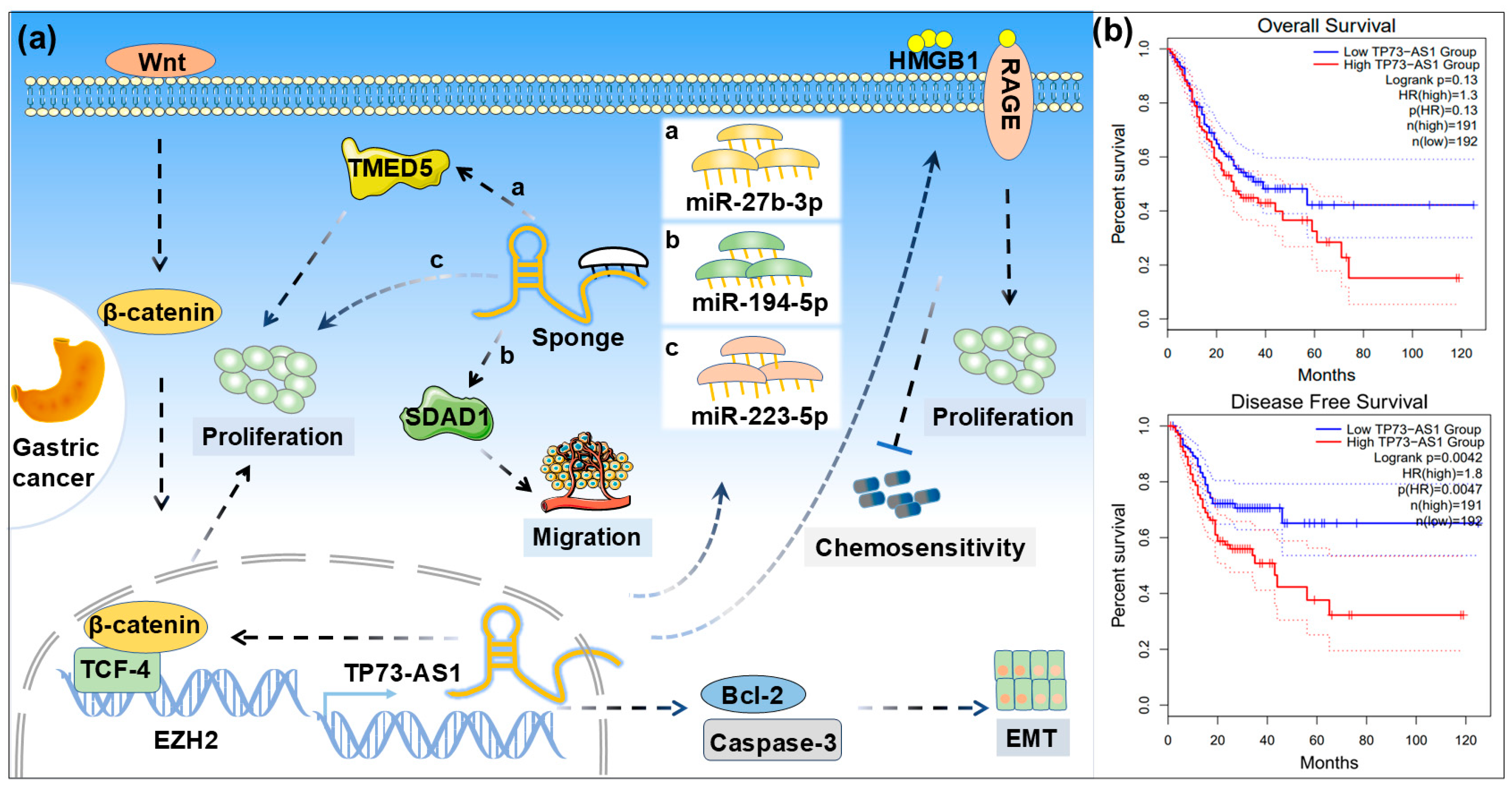
4.1. Gastric Cancer
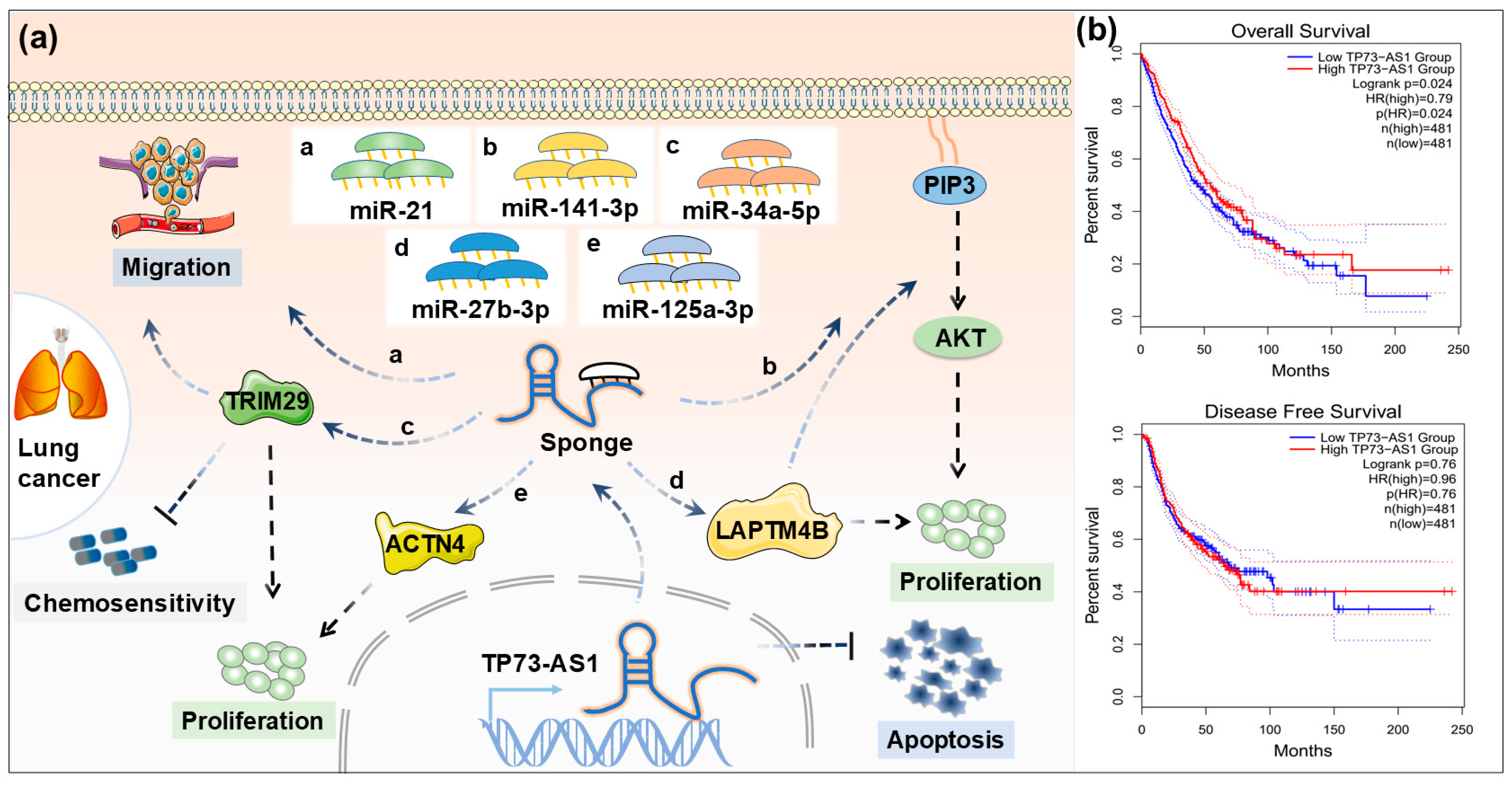
4.2. Lung Cancer
4.3. Colorectal Cancer
4.4. Cervical Cancer
4.5. Hepatocellular Carcinoma
4.6. Medulloblastoma
4.7. Other Cancers
5. Clinical Applications of TP73-AS1 in Cancer
5.1. As a Diagnostic Marker
5.2. As a Therapeutic Target
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, W.; Qin, K.; Li, F.; Chen, W. Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J. Natl. Cancer Cent. 2024, 4, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody-drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, H.; Chen, H.; Zhang, Z.; Xue, Q. LncRNA in tumorigenesis of non-small-cell lung cancer: From bench to bedside. Cell Death Discov. 2022, 8, 359. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu. Rev. Biochem. 2020, 89, 283–308. [Google Scholar] [CrossRef]
- Ferrer, J.; Dimitrova, N. Transcription regulation by long non-coding RNAs: Mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sinica. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Esposito, R.; Bosch, N.; Lanzós, A.; Polidori, T.; Pulido-Quetglas, C.; Johnson, R. Hacking the Cancer Genome: Profiling Therapeutically Actionable Long Non-coding RNAs Using CRISPR-Cas9 Screening. Cancer Cell 2019, 35, 545–557. [Google Scholar] [CrossRef]
- Zhan, F.; Barlogie, B.; Arzoumanian, V.; Huang, Y.; Williams, D.R.; Hollmig, K.; Pineda-Roman, M.; Tricot, G.; van Rhee, F.; Zangari, M.; et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood 2007, 109, 1692–1700. [Google Scholar] [CrossRef]
- Bao, C.; Guo, L. TP73-AS1 promotes gastric cancer proliferation and invasion by regulation miR-27b-3p/TMED5 axis. J. Cancer 2022, 13, 1324–1335. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, S.; Wang, B.; Li, Y.; Chen, Q. Knockdown of lncRNA TP73-AS1 inhibits gastric cancer cell proliferation and invasion via the WNT/β-catenin signaling pathway. Oncol. Lett. 2018, 16, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, J.; Liu, Y.; Du, L.; Zheng, Z.; Qian, X. LncRNA TP73-AS1 is upregulated in non-small cell lung cancer and predicts poor survival. Gene 2019, 710, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, L.; Deng, J.; Wang, S. LncRNA TP73-AS1 promoted the progression of lung adenocarcinoma via PI3K/AKT pathway. Biosci. Rep. 2019, 39, BSR20180999. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yan, P.; Zhang, G.; Yang, W.; Wang, H.; Cheng, X. Long non-coding RNA TP73-AS1 sponges miR-194 to promote colorectal cancer cell proliferation, migration and invasion via up-regulating TGFα. Cancer Biomark. Sect. A Dis. Markers 2018, 23, 145–156. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, B.; Wang, S.; Li, X.; Fan, T. Long non-coding RNA TP73 antisense RNA 1 facilitates the proliferation and migration of cervical cancer cells via regulating microRNA-607/cyclin D2. Mol. Med. Rep. 2019, 20, 3371–3378. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Huang, Y.; Fu, Y.; Tang, D.; Kang, R.; Zhou, R.; Fan, X.-G. The long non-coding RNA TP73-AS1 modulates HCC cell proliferation through miR-200a-dependent HMGB1/RAGE regulation. J. Exp. Clin. Cancer Res. CR 2017, 36, 51. [Google Scholar] [CrossRef]
- Tuo, Z.; Zhang, J.; Xue, W. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem. Biophys. Res. Commun. 2018, 499, 875–881. [Google Scholar] [CrossRef]
- Nair, L.; Chung, H.; Basu, U. Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery. Nat. Rev. Mol. Cell Biol. 2020, 21, 123–136. [Google Scholar] [CrossRef]
- Chen, C.; Shu, L.; Zou, W. Role of long non-coding RNA TP73-AS1 in cancer. Biosci. Rep. 2019, 39, BSR20192274. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.-M.; Hu, Z.; Jiang, X.; Yang, X.; Li, J.; Zhang, Y.; Yu, H.; Khaitovich, P. Recently Evolved Tumor Suppressor Transcript TP73-AS1 Functions as Sponge of Human-Specific miR-941. Mol. Biol. Evol. 2018, 35, 1063–1077. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Feng, Y.; Liang, Y.; Huang, Y.; Zou, W. TP73-AS1 as a predictor of clinicopathological parameters and prognosis in human malignancies: A meta and bioinformatics analysis. BMC Cancer 2022, 22, 581. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, R.; Wu, X.; Liu, W.; Ma, S. The Long Noncoding RNA TP73-AS1 Interacted with miR-124 to Modulate Glioma Growth by Targeting Inhibitor of Apoptosis-Stimulating Protein of p53. DNA Cell Biol. 2018, 37, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-Y.; Tang, R.; Liu, K.-X.; Xiang, G.; Zhang, H.-H. Long non-coding RNA TP73-AS1 in cancers. Clin. Chim. Acta 2020, 503, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Li, Z.; Zhang, X.; Leung, G.K.K.; Chan, G.C.-F.; Chim, C.S. Epigenetic silencing of a long non-coding RNA KIAA0495 in multiple myeloma. Mol. Cancer 2015, 14, 175. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Shi, D.B.; Gao, P. SP-1-activated LINC01016 overexpression promotes gastric cancer invasion and metastasis through inhibiting EIF4A3-mediated MMP9 mRNA decay. Cell Death Dis. 2025, 16, 54. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, L.; Kang, Q.; Li, J.; Chen, K.; Fu, H. Transcription factor HIF1α promotes proliferation, migration, and invasion of cholangiocarcinoma via long noncoding RNA H19/microRNA-612/Bcl-2 axis. Transl. Res. J. Lab. Clin. Med. 2020, 224, 26–39. [Google Scholar] [CrossRef]
- Meng, K.; Li, Y.; Yuan, X.; Shen, H.M.; Hu, L.L.; Liu, D.; Shi, F.; Zheng, D.; Shi, X.; Wen, N.; et al. The cryptic lncRNA-encoded microprotein TPM3P9 drives oncogenic RNA splicing and tumorigenesis. Signal Transduct. Target. Ther. 2025, 10, 43. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Luo, C.; Wei, L.; Qian, F.; Bo, L.; Gao, S.; Yang, G.; Mao, C. LncRNA HOTAIR regulates autophagy and proliferation mechanisms in premature ovarian insufficiency through the miR-148b-3p/ATG14 axis. Cell Death Discov. 2024, 10, 44. [Google Scholar] [CrossRef]
- Harari-Steinfeld, R.; Gefen, M.; Simerzin, A.; Zorde-Khvalevsky, E.; Rivkin, M.; Ella, E.; Friehmann, T.; Gerlic, M.; Zucman-Rossi, J.; Caruso, S.; et al. The lncRNA H19-Derived MicroRNA-675 Promotes Liver Necroptosis by Targeting FADD. Cancers 2021, 13, 411. [Google Scholar] [CrossRef]
- Chen, Y.; Yusenko, M.V.; Kovacs, G. Lack of KISS1R expression is associated with rapid progression of conventional renal cell carcinomas. J. Pathol. 2011, 223, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, X.; Zhou, J.; Cheng, X.; Ye, Z.; Ji, Z. LncRNA TP73-AS1 Promotes Cell Proliferation and Inhibits Cell Apoptosis in Clear Cell Renal Cell Carcinoma Through Repressing KISS1 Expression and Inactivation of PI3K/Akt/mTOR Signaling Pathway. Cell. Physiol. Biochem. 2018, 48, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Wang, T.; Wang, Y.; Chen, X.; Du, Y.; Sun, Q.; Li, M.; Dong, Z.; Zhao, G. Knockdown of long non-coding RNA TP73-AS1 inhibits cell proliferation and induces apoptosis in esophageal squamous cell carcinoma. Oncotarget 2016, 7, 19960–19974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jin, H.; Lou, F. The Long Non-Coding RNA TP73-AS1 Interacted With miR-142 to Modulate Brain Glioma Growth Through HMGB1/RAGE Pathway. J. Cell Biochem. 2018, 119, 3007–3016. [Google Scholar] [CrossRef]
- Peng, J. si-TP73-AS1 suppressed proliferation and increased the chemotherapeutic response of GC cells to cisplatin. Oncol. Lett. 2018, 16, 3706–3714. [Google Scholar] [CrossRef]
- Zhang, W.; Zhai, Y.; Wang, W.; Cao, M.; Ma, C. Enhanced expression of lncRNA TP73-AS1 predicts unfavorable prognosis for gastric cancer and promotes cell migration and invasion by induction of EMT. Gene 2018, 678, 377–383. [Google Scholar] [CrossRef]
- Ding, Z.; Lan, H.; Xu, R.; Zhou, X.; Pan, Y. LncRNA TP73-AS1 accelerates tumor progression in gastric cancer through regulating miR-194-5p/SDAD1 axis. Pathol. Res. Pract. 2018, 214, 1993–1999. [Google Scholar] [CrossRef]
- Yao, J.; Xu, F.; Zhang, D.; Yi, W.; Chen, X.; Chen, G.; Zhou, E. TP73-AS1 promotes breast cancer cell proliferation through miR-200a-mediated TFAM inhibition. J. Cell. Biochem. 2018, 119, 680–690. [Google Scholar] [CrossRef]
- Zou, Q.; Zhou, E.; Xu, F.; Zhang, D.; Yi, W.; Yao, J. A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration. J. Cell Biochem. 2018, 119, 2189–2199. [Google Scholar] [CrossRef]
- Yang, G.; Song, R.; Wang, L.; Wu, X. Knockdown of long non-coding RNA TP73-AS1 inhibits osteosarcoma cell proliferation and invasion through sponging miR-142. Biomed. Pharmacother. 2018, 103, 1238–1245. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Zhu, H.; Zhang, J. Knockdown of long non-coding RNA TP73-AS1 suppresses triple negative breast cancer cell vasculogenic mimicry by targeting miR-490-3p/TWIST1 axis. Biochem. Biophys. Res. Commun. 2018, 504, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, K.; Chen, Y.; Hu, H. LncRNA TP73-AS1 enhances the malignant properties of colorectal cancer by increasing SPP-1 expression through miRNA-539-5p sponging. Pathol. Res. Pract. 2023, 243, 154365. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Liu, S.; Zhou, H.; Qu, L.-H.; Yang, J.-H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, J.; Shi, L.; Lin, L.; Jiang, M.; Ge, Y.; Li, Z.; Fan, H.; Yang, L.; Xu, Z. Association of TP73-AS1 gene polymorphisms with the risk and survival of gastric cancer in a Chinese Han Population. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3814–3822. [Google Scholar] [CrossRef]
- Jing, J.-J.; Wang, Z.-Y.; Li, H.; Sun, L.-P.; Yuan, Y. Key elements involved in Epstein-Barr virus-associated gastric cancer and their network regulation. Cancer Cell Int. 2018, 18, 146. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Liao, X. MicroRNA-223-5p targets long non-coding RNA TP73 antisense RNA1 to promote the invasion of gastric cancer. Hum. Cell 2020, 33, 676–682. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Cui, Y. LncRNA TP73-AS1 interacted with miR-141-3p to promote the proliferation of non-small cell lung cancer. Arch. Med. Sci. AMS 2019, 15, 1547–1554. [Google Scholar] [CrossRef]
- Luo, S.; Shen, M.; Chen, H.; Li, W.; Chen, C. Long non-coding RNA TP73-AS1 accelerates the progression and cisplatin resistance of non-small cell lung cancer by upregulating the expression of TRIM29 via competitively targeting microRNA-34a-5p. Mol. Med. Rep. 2020, 22, 3822–3832. [Google Scholar] [CrossRef]
- Jiang, Q.; Xing, W.; Cheng, J.; Yu, Y. Long Non-Coding RNA TP73-AS1 Promotes the Development of Lung Cancer by Targeting the miR-27b-3p/LAPTM4B Axis. OncoTargets Ther. 2020, 13, 7019–7031. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Feng, Z.; Li, Y.; Yan, C.; He, W.; Chen, X. LncRNA TP73-AS1 Exacerbates the Non-Small-Cell Lung Cancer (NSCLC) Process via Regulating miR-125a-3p-Mediated ACTN4. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 4098271. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, H.; Liu, B.; Jing, F.; He, Q.; Zheng, S.; Shi, H.; Jiao, L.; Fan, C. Association of a novel antisense lncRNA TP73-AS1 polymorphisms and expression with colorectal cancer susceptibility and prognosis. Genes Genom. 2022, 44, 889–897. [Google Scholar] [CrossRef]
- Li, M.; Jin, Y.; Li, Y. LncRNA TP73-AS1 Activates TGF-β1 to Promote the Migration and Invasion of Colorectal Cancer Cell. Cancer Manag. Res. 2019, 11, 10523–10529. [Google Scholar] [CrossRef]
- Jia, Z.; Peng, J.; Yang, Z.; Chen, J.; Liu, L.; Luo, D.; He, P. Long non-coding RNA TP73-AS1 promotes colorectal cancer proliferation by acting as a ceRNA for miR-103 to regulate PTEN expression. Gene 2019, 685, 222–229. [Google Scholar] [CrossRef]
- Song, Z.; Xing, F.; Jiang, H.; He, Y.; Lv, J. Long non-coding RNA TP73-AS1 predicts poor prognosis and regulates cell proliferation and migration in cervical cancer. Arch. Med. Sci. AMS 2022, 18, 523–534. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J. LncRNA TP73-AS1 is a novel regulator in cervical cancer via miR-329-3p/ARF1 axis. J. Cell. Biochem. 2020, 121, 344–352. [Google Scholar] [CrossRef]
- Guan, M.M.; Rao, Q.X.; Huang, M.L.; Wang, L.J.; Lin, S.D.; Chen, Q.; Liu, C.H. Long Noncoding RNA TP73-AS1 Targets MicroRNA-329-3p to Regulate Expression of the SMAD2 Gene in Human Cervical Cancer Tissue and Cell Lines. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8131–8141. [Google Scholar] [CrossRef]
- Amini, M.; Looha, M.A.; Zarean, E.; Pourhoseingholi, M.A. Global pattern of trends in incidence, mortality, and mortality-to-incidence ratio rates related to liver cancer, 1990-2019: A longitudinal analysis based on the global burden of disease study. BMC Public Health 2022, 22, 604. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.-B.; Liao, C.-J.; Hu, X.-W.; Li, S.-L.; Qi, M.; Fan, X.-G.; Huang, Y. LncRNA TP73-AS1/miR-539/MMP-8 axis modulates M2 macrophage polarization in hepatocellular carcinoma via TGF-β1 signaling. Cell. Signal. 2020, 75, 109738. [Google Scholar] [CrossRef]
- Ma, C.-X.; Gao, W.-C.; Tian, L. LncRNA TP73-AS1 promotes malignant progression of hepatoma by regulating microRNA-103. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4713–4722. [Google Scholar] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Varon, M.; Levy, T.; Mazor, G.; Ben David, H.; Marciano, R.; Krelin, Y.; Prasad, M.; Elkabets, M.; Pauck, D.; Ahmadov, U.; et al. The long noncoding RNA TP73-AS1 promotes tumorigenicity of medulloblastoma cells. Int. J. Cancer 2019, 145, 3402–3413. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, M.; Yao, H.; Chen, X.; Xiao, Z. Long Noncoding RNA TP73-AS1 Modulates Medulloblastoma Progression In Vitro And In Vivo By Sponging miR-494-3p And Targeting EIF5A2. OncoTargets Ther. 2019, 12, 9873–9885. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Liu, S.; Zhang, D.; Yang, X.; Zhou, Q.; Song, Y.; Liu, Y. LncRNA TP73-AS1 predicts poor prognosis and functions as oncogenic lncRNA in osteosarcoma. J. Cell. Biochem. 2018, 120, 2569–2575. [Google Scholar] [CrossRef]
- Bozgeyik, E.; Arslan, A.; Temiz, E.; Batar, B.; Koyuncu, I.; Tozkir, H. miR-320a promotes p53-dependent apoptosis of prostate cancer cells by negatively regulating TP73-AS1 invitro. Biochem. Biophys. Res. Commun. 2022, 619, 130–136. [Google Scholar] [CrossRef]
- Wang, B.; Sun, X.; Huang, K.-J.; Zhou, L.-S.; Qiu, Z.-J. Long non-coding RNA TP73-AS1 promotes pancreatic cancer growth and metastasis through miRNA-128-3p/GOLM1 axis. World J. Gastroenterol. 2021, 27, 1993–2014. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Gao, X. TP73-AS1 rs3737589 Polymorphism is Associated with the Clinical Stage of Colorectal Cancer. Evid.-Based Complement. Altern. Med. Ecam 2023, 2023, 3931875. [Google Scholar] [CrossRef]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547–3568. [Google Scholar] [CrossRef]
- Zuccotti, A.; Al-Fatyan, F.; Ferretti, G.D.S.; Bertolini, I.; Long, D.T.; Sahin, O.; Rodriguez-Blanco, J.; Barnoud, T. Molecular Mechanisms and Therapeutic Implications of Long Non-coding RNAs in Cutaneous Biology and Disease. J. Cell. Physiol. 2025, 240, e70006. [Google Scholar] [CrossRef]
- Afroze, N.; Sundaram, M.K.; Haque, S.; Hussain, A. Long non-coding RNA involved in the carcinogenesis of human female cancer—A comprehensive review. Discov. Oncol. 2025, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Akhade, V.S.; Pal, D.; Rao, S.M. Long noncoding RNAs in development and cancer: Potential biomarkers and therapeutic targets. Mol. Cell. Ther. 2015, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.M.; Yekula, A.; Khanna, P.; Hsia, T.; Gamblin, A.S.; Ekanayake, E.; Escobedo, A.K.; You, D.G.; Castro, C.M.; Im, H.; et al. The Liquid Biopsy Consortium: Challenges and opportunities for early cancer detection and monitoring. Cell Rep. Med. 2023, 4, 101198. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, G.; Hao, Y. Mechanism and research progress of lncRNA TP73-AS1 regulating digestive system malignant tumors. China Med. Her. 2024, 21, 191–193. [Google Scholar]
- Zhu, P.W.; Shi, X.J.; Qin, S.J.; Liao, M.; Ma, F. LncRNAs and miRNAs from a ceRNA Network as a Signature Can Improve the Diagnosis and Prognosis of CESC Patients. J. Nanjing Norm. Univ. Nat. Sci. Ed. 2020, 43, 53–61. [Google Scholar]
- Sulewska, A.; Niklinski, J.; Charkiewicz, R.; Karabowicz, P.; Biecek, P.; Baniecki, H.; Kowalczuk, O.; Kozlowski, M.; Modzelewska, P.; Majewski, P.; et al. A Signature of 14 Long Non-Coding RNAs (lncRNAs) as a Step towards Precision Diagnosis for NSCLC. Cancers 2022, 14, 439–460. [Google Scholar] [CrossRef]
- Mazor, G.; Levin, L.; Picard, D.; Ahmadov, U.; Carén, H.; Borkhardt, A.; Reifenberger, G.; Leprivier, G.; Remke, M.; Rotblat, B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019, 10, 246. [Google Scholar] [CrossRef]


| Name | MirAccession | TargetSites | BioComplex | ClipReadNum |
|---|---|---|---|---|
| hsa-miR-200a-3p | MIMAT0000682 | 2 | 4 | 27 |
| hsa-miR-197-3p | MIMAT0000227 | 1 | 2 | 0 |
| hsa-miR-488-3p | MIMAT0004763 | 1 | 1 | 15 |
| hsa-miR-194-5p | MIMAT0000460 | 1 | 2 | 0 |
| hsa-miR-107 | MIMAT0000104 | 1 | 1 | 5 |
| hsa-miR-326 | MIMAT0000756 | 1 | 2 | 0 |
| hsa-miR-141-3p | MIMAT0000432 | 2 | 4 | 27 |
| hsa-miR-329-3p | MIMAT0001629 | 1 | 4 | 28 |
| hsa-miR-494-3p | MIMAT0002816 | 1 | 1 | 3 |
| hsa-miR-539-5p | MIMAT0003163 | 1 | 2 | 40 |
| hsa-miR-485-5p | MIMAT0002175 | 1 | 1 | 12 |
| hsa-miR-154-5p | MIMAT0000452 | 2 | 2 | 0 |
| hsa-miR-193b-3p | MIMAT0002819 | 1 | 2 | 0 |
| hsa-miR-193a-3p | MIMAT0000459 | 1 | 2 | 0 |
| hsa-miR-27a-3p | MIMAT0000084 | 1 | 5 | 24 |
| hsa-miR-330-5p | MIMAT0004693 | 1 | 2 | 0 |
| hsa-miR-125a-3p | MIMAT0004602 | 1 | 2 | 0 |
| hsa-miR-128-3p | MIMAT0000424 | 1 | 5 | 24 |
| hsa-miR-103a-3p | MIMAT0000101 | 1 | 1 | 5 |
| hsa-miR-124-3p | MIMAT0000422 | 1 | 2 | 0 |
| Cancer Types | Expression | Related Gene | Function | Gene Type | Reference |
|---|---|---|---|---|---|
| Gastric cancer | ↑ | MiR-27b-3p/TMED5 | Proliferation | Oncogene | [10] |
| TCF-4/β-catenin | Proliferation, migration | [11] | |||
| HMGB1/RAGE | Chemosensitivity | [35] | |||
| Bcl-2/caspase-3 | Apoptosis, EMT | [36] | |||
| MiR-194-5p/SDAD1 | Migration | [37] | |||
| MiR-223-5p | Proliferation, invasion | [47] | |||
| Lung cancer | ↑ | MiR-21 | Migration, invasion | Oncogene | [12] |
| PI3K/Akt | Proliferation | [13] | |||
| MiR-141-3p | Proliferation | [49] | |||
| MiR-34a-5p/TRIM29 | Proliferation, migration Chemosensitivity | [50] | |||
| MiR-27b-3p/LAPTM4B | Proliferation, migration | [51] | |||
| MiR-125a-3p/ACTN4 | Proliferation, invasion | [52] | |||
| Colorectal cancer | ↑ | MiR-194/TGF-α | Proliferation | Oncogene | [14] |
| MiR-539-5p/SPP-1 | Proliferation, migration | [42] | |||
| TGF-β1 | Proliferation, migration | [54] | |||
| MiR-103/PTEN | Apoptosis | [55] | |||
| Cervical cancer | ↑ | MiR-607/CCND2 | Proliferation | Oncogene | [15] |
| / | Proliferation, migration | [56] | |||
| MiR-329-3p/ARF1 | Proliferation, migration | [57] | |||
| MiR-329-3p/SMAD2 | Proliferation | [58] | |||
| Hepatocellular carcinoma | ↑ | MiR-200a/HMGB1/RAGE | Proliferation | Oncogene | [16] |
| MiR-539 | Proliferation | [60] | |||
| MiR-103 | Apoptosis | [61] | |||
| Glioma | ↑ | MiR-124/iASPP | Proliferation, migration | Oncogene | [22] |
| MiR-142/HMGB1/RAGE | Proliferation, invasion | [34] | |||
| ALDH1A1 | Chemotherapy resistance | [68] | |||
| Clear-cell renal cell carcinoma | ↑ | EZH2/KISS1 | Proliferation, migration | Oncogene | [32] |
| Esophageal cancer | ↑ | BDH2/caspase-3 | Chemosensitivity | Oncogene | [33] |
| Breast cancer | ↑ | MiR-200a/TFAM | Proliferation | Oncogene | [38] |
| MiR-200a/ZEB1 | Migration, invasion | [39] | |||
| MiR-490-3p/TWIST1 | Vasculogenic mimicry | [41] | |||
| Osteosarcoma | ↑ | MiR-142/Rac1 | Migration | Oncogene | [40] |
| / | Migration, invasion | [65] | |||
| Medulloblastoma | ↑ | / | Proliferation, migration | Oncogene | [63] |
| MiR-494-3p/EIF5A2 | Proliferation | [64] | |||
| Prostate cancer | ↑ | MiR-320a | Migration | Oncogene | [66] |
| Pancreatic cancer | ↑ | MiR-128-3p/GOLM1 | Migration | Oncogene | [67] |
| Bladder cancer | ↓ | MMP-2/MMP-9 | Migration, invasion | Anti- oncogene | [17] |
| Multiple myeloma | ↓ | / | / | Anti- oncogene | [9,24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhao, D.; Liu, X.; Cao, Q.; Ruan, L.; Lei, H.; Chen, X.; Jin, X.; Li, Q.; Xie, X.; et al. Long Non-Coding TP73-AS1: A Potential Biomarker and Therapeutic Target in Cancer. Int. J. Mol. Sci. 2025, 26, 3886. https://doi.org/10.3390/ijms26083886
Li K, Zhao D, Liu X, Cao Q, Ruan L, Lei H, Chen X, Jin X, Li Q, Xie X, et al. Long Non-Coding TP73-AS1: A Potential Biomarker and Therapeutic Target in Cancer. International Journal of Molecular Sciences. 2025; 26(8):3886. https://doi.org/10.3390/ijms26083886
Chicago/Turabian StyleLi, Kejing, Dapeng Zhao, Xuena Liu, Qiyou Cao, Longzhu Ruan, Huiwen Lei, Xiaohua Chen, Xiaodong Jin, Qiang Li, Xiaodong Xie, and et al. 2025. "Long Non-Coding TP73-AS1: A Potential Biomarker and Therapeutic Target in Cancer" International Journal of Molecular Sciences 26, no. 8: 3886. https://doi.org/10.3390/ijms26083886
APA StyleLi, K., Zhao, D., Liu, X., Cao, Q., Ruan, L., Lei, H., Chen, X., Jin, X., Li, Q., Xie, X., & Di, C. (2025). Long Non-Coding TP73-AS1: A Potential Biomarker and Therapeutic Target in Cancer. International Journal of Molecular Sciences, 26(8), 3886. https://doi.org/10.3390/ijms26083886






