Upregulation of GZMK, TREM2, and OR4D10 as Prognostic Biomarkers in Thyroid Cancer: A Pan-Cancer and TCGA Data Analysis
Abstract
:1. Introduction
2. Results
2.1. Pan-Cancer GZMK, TREM2, and OR4D10 Expression Analysis
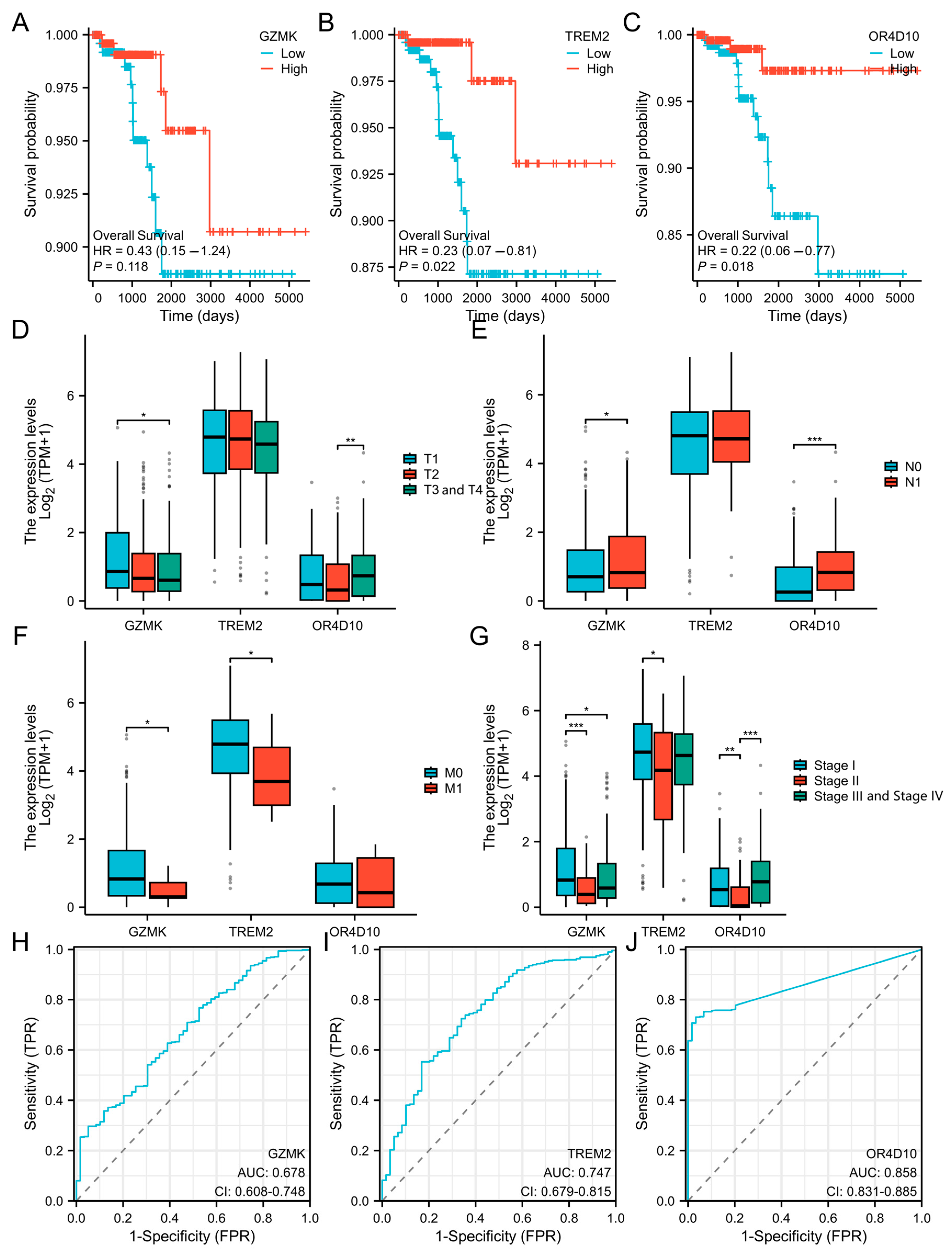
2.2. Association Between GZMK, TREM2, and OR4D10 Expression and Cancer Patient Prognosis
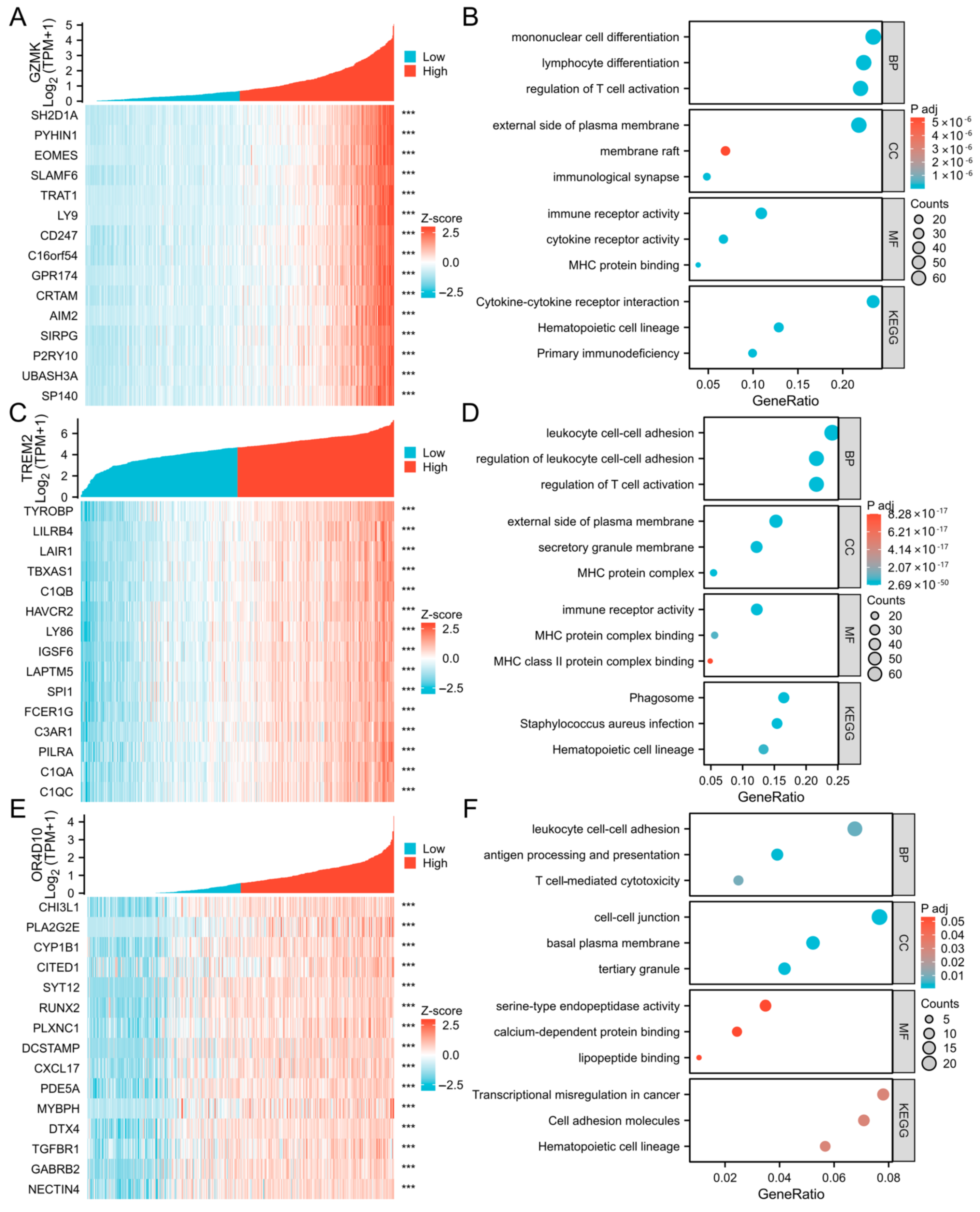
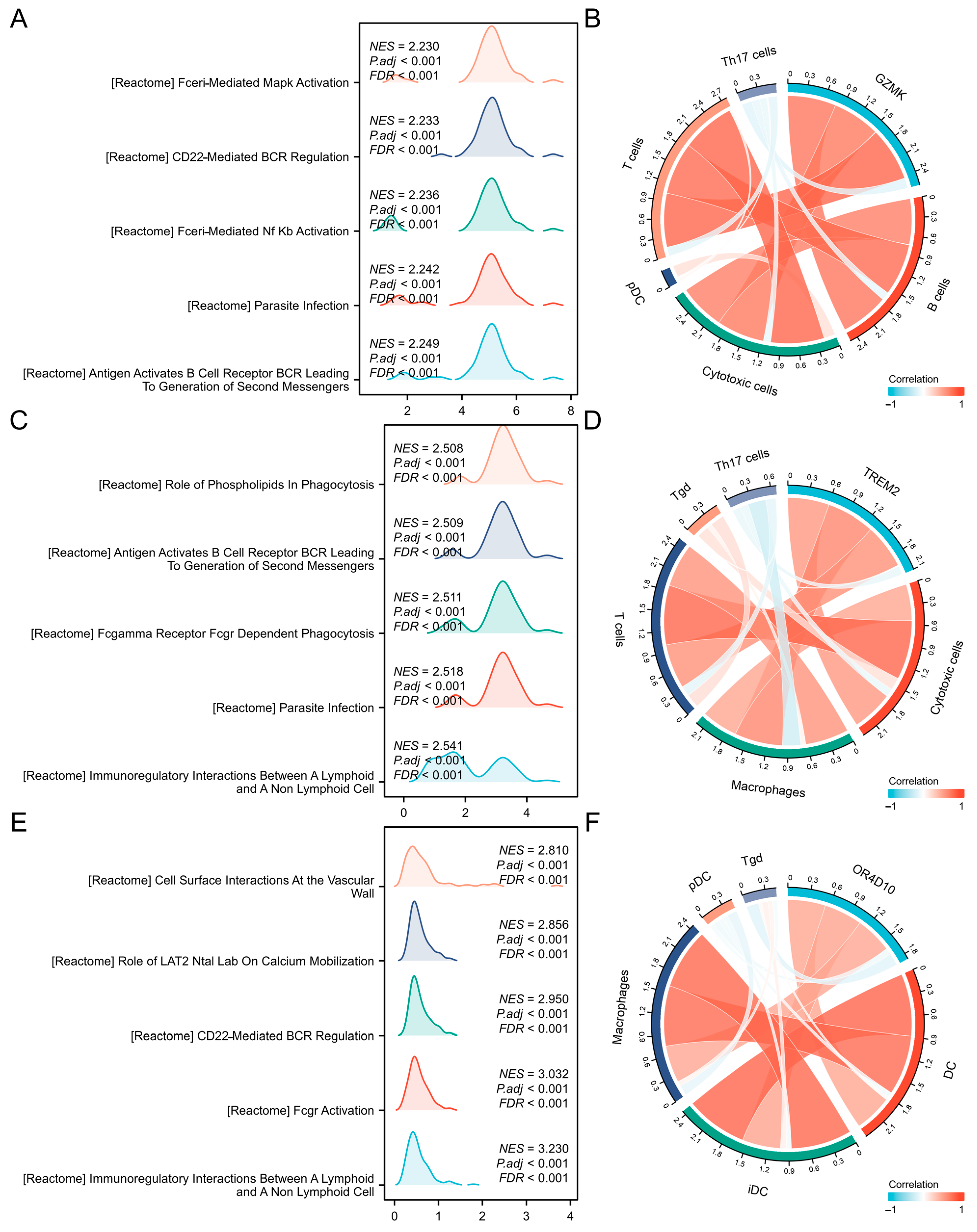
2.3. Correlation and Enrichment Analyses
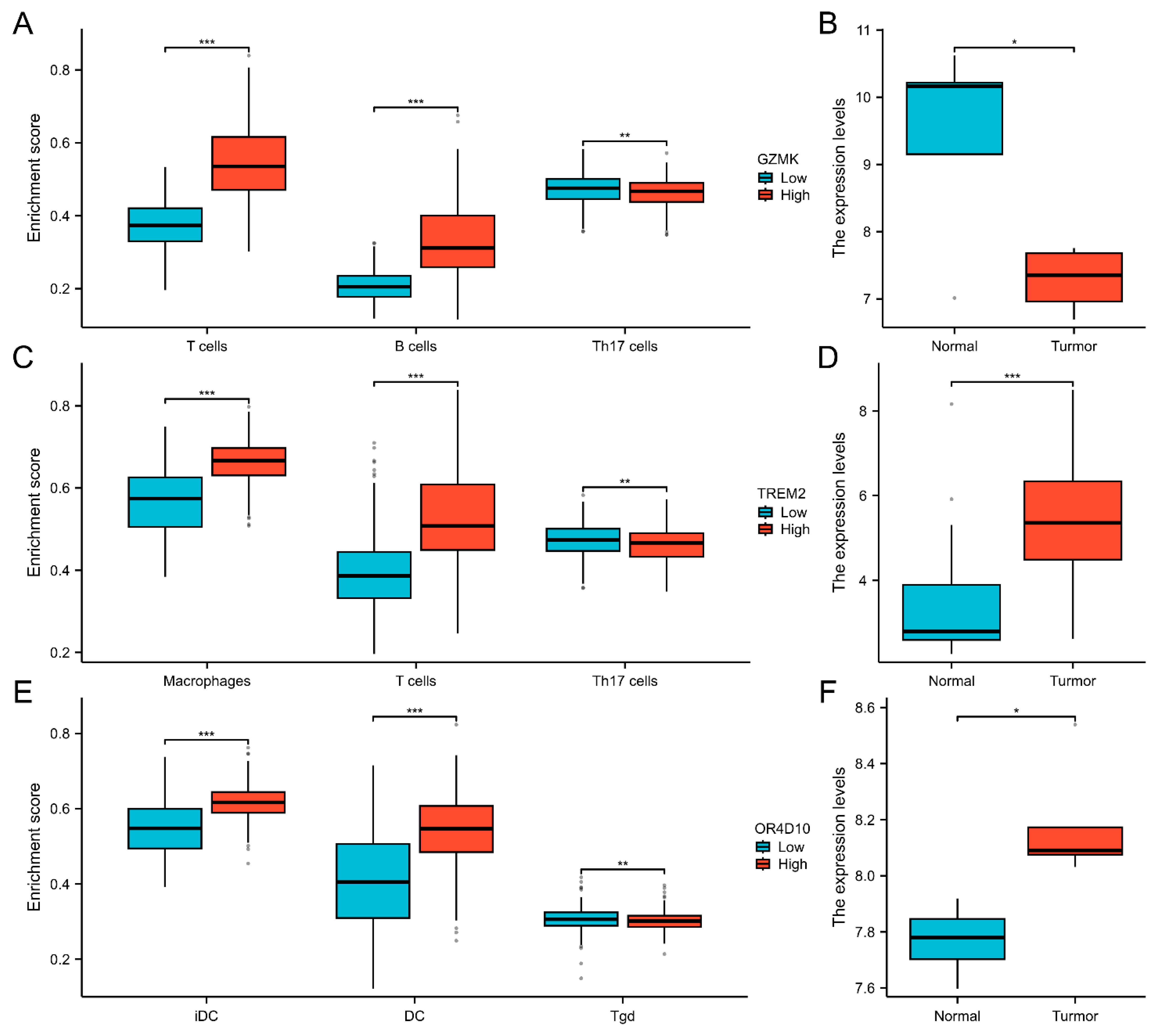
2.4. Correlation Between Immune Cell Infiltration and GZMK, TREM2, OR4D10 Expression
2.5. Validation of Gene Expression in GEO Database
3. Discussion
4. Materials and Methods
4.1. Data Collection and Analysis
4.2. Correlation and Enrichment Analyses
4.3. Immune Cell Infiltration
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, F.; Dedhia, P.H.; Ringel, M.D. Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 2021, 149, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Araque, K.A.; Gubbi, S.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Shan, Y.; Muzza, M.; Nucci, N.; Sponziello, M. Role of the granzyme family in rheumatoid arthritis: Current Insights and future perspectives. Front. Immunol. 2023, 14, 1137918. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Li, J.; Miao, W.; Sun, F.; Duan, S.; Song, Y.; Yao, J.; Wang, X.; Wang, C.; Liu, X.; et al. GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases. Nature 2025, 638, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Teng, Z.; Liu, C.; Li, Q.; Yin, Y.; Tang, Y. TREM2, microglia, and Alzheimer’s disease. Mech. Ageing. Dev. 2021, 195, 111438. [Google Scholar] [CrossRef] [PubMed]
- Di Luccia, B.; Molgora, M.; Khantakova, D.; Jaeger, N.; Chang, H.-W.; Czepielewski, R.S.; Helmink, B.A.; Onufer, E.J.; Fachi, J.L.; Bhattarai, B.; et al. TREM2 deficiency reprograms intestinal macrophages and microbiota to enhance anti-PD-1 tumor immunotherapy. Sci. Immunol. 2024, 9, eadi5374. [Google Scholar] [CrossRef] [PubMed]
- Kogelman, L.J.; Pant, S.D.; Fredholm, M.; Kadarmideen, H.N. Systems genetics of obesity in an F2 pig model by genome-wide association, genetic network, and pathway analyses. Front. Genet. 2014, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Tituaña, M.; Arias, M.A.; Sierra-Monzón, J.L.; Morte-Romea, E.; Santiago, L.; Ramirez-Labrada, A.; Martinez-Lostao, L.; Paño-Pardo, J.R.; Galvez, E.M.; Pardo, J. The Multifaceted Function of Granzymes in Sepsis: Some Facts and a Lot to Discover. Front. Immunol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Molgora, M.; Liu, Y.A.; Colonna, M.; Cella, M. TREM2: A new player in the tumor microenvironment. Semin. Immunol. 2023, 67, 101739. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Bruna, J.; Bosch-Barrera, J.; Valiente, M.; Nadal, E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat. Rev. 2020, 89, 102067. [Google Scholar] [CrossRef] [PubMed]
- Sahin Ozkan, H.; Ugurlu, M.U.; Yumuk, P.F.; Kaya, H. Prognostic Role of Immune Markers in Triple Negative Breast Carcinoma. Pathol. Oncol. Res. 2020, 26, 2733–2745. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer. 2020, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 2020, 53, 100715. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Cai, L.; Qian, L. Upregulation of GZMK, TREM2, and OR4D10 as Prognostic Biomarkers in Thyroid Cancer: A Pan-Cancer and TCGA Data Analysis. Int. J. Mol. Sci. 2025, 26, 3887. https://doi.org/10.3390/ijms26083887
Zhu N, Cai L, Qian L. Upregulation of GZMK, TREM2, and OR4D10 as Prognostic Biomarkers in Thyroid Cancer: A Pan-Cancer and TCGA Data Analysis. International Journal of Molecular Sciences. 2025; 26(8):3887. https://doi.org/10.3390/ijms26083887
Chicago/Turabian StyleZhu, Nuoyan, Liangliang Cai, and Li Qian. 2025. "Upregulation of GZMK, TREM2, and OR4D10 as Prognostic Biomarkers in Thyroid Cancer: A Pan-Cancer and TCGA Data Analysis" International Journal of Molecular Sciences 26, no. 8: 3887. https://doi.org/10.3390/ijms26083887
APA StyleZhu, N., Cai, L., & Qian, L. (2025). Upregulation of GZMK, TREM2, and OR4D10 as Prognostic Biomarkers in Thyroid Cancer: A Pan-Cancer and TCGA Data Analysis. International Journal of Molecular Sciences, 26(8), 3887. https://doi.org/10.3390/ijms26083887





