Lack of asmt1 or asmt2 Yields Different Phenotypes and Malformations in Larvae to Adult Zebrafish
Abstract
1. Introduction
2. Results
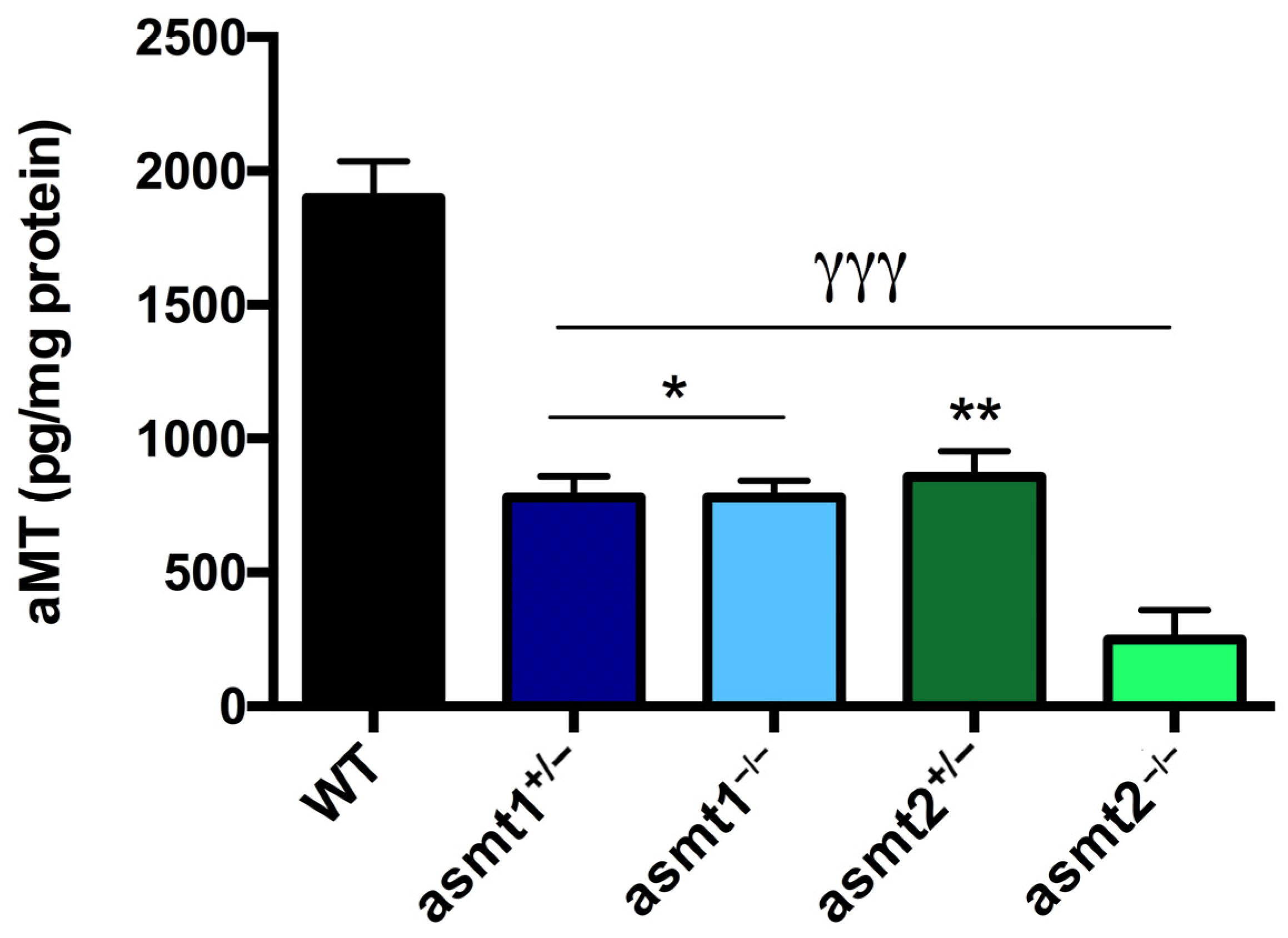
2.1. Drop in Melatonin Level Is Higher in asmt2 than in asmt1 Mutants
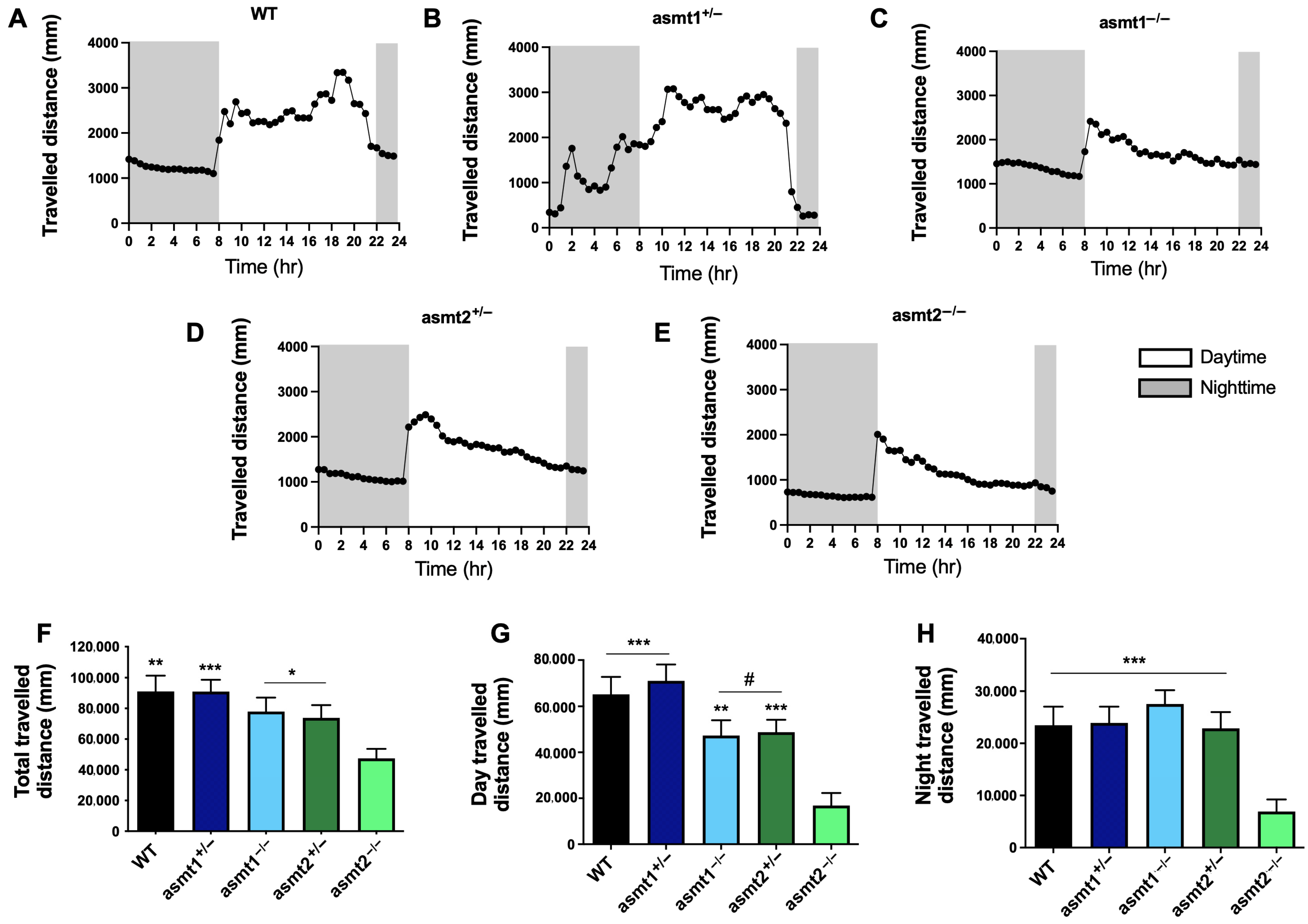
2.2. Sleep/Wake Rhythm Is Disrupted by Mutations in asmt1 and asmt2
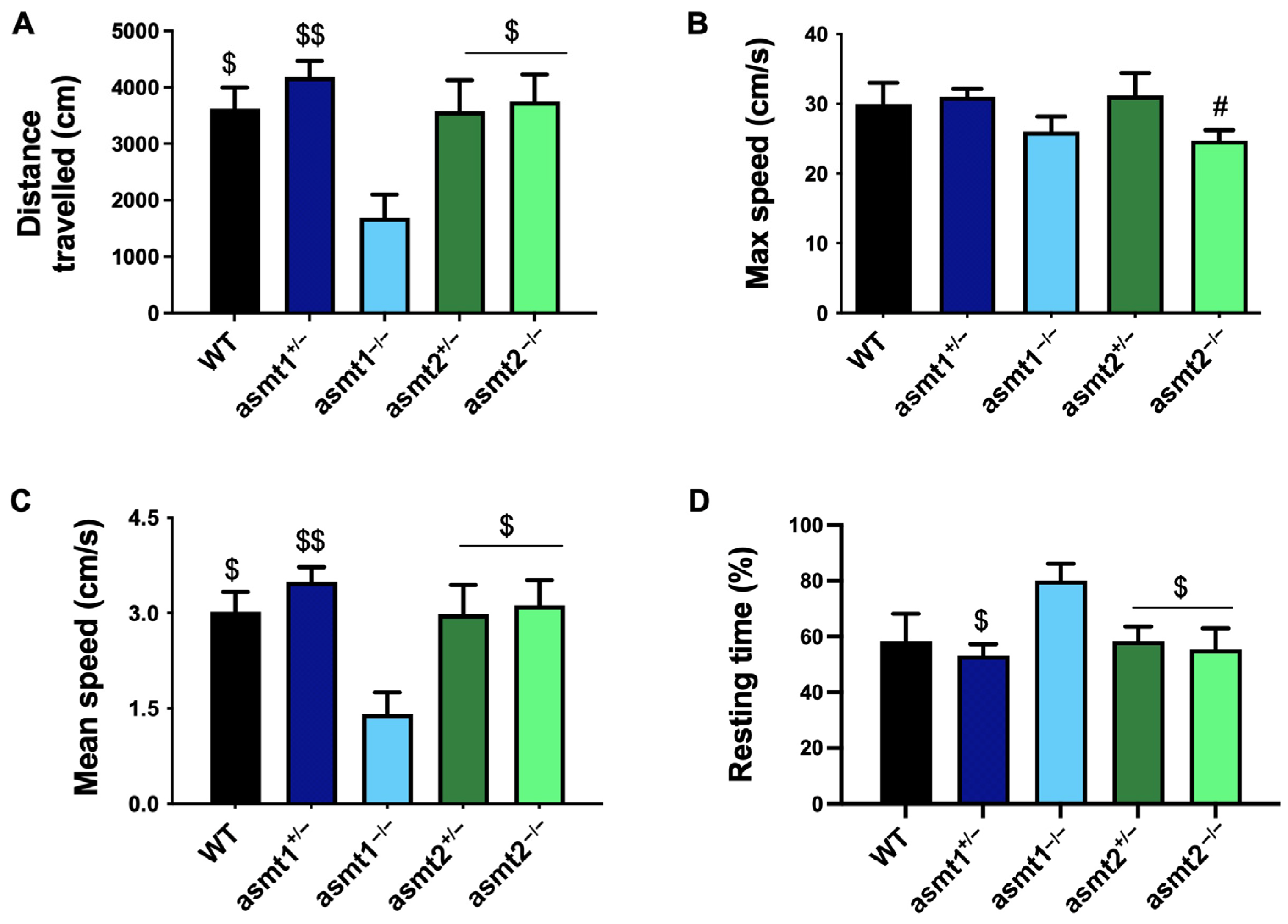
2.3. Physical Activity Was More Compromised in the asmt1 Homozygous Zebrafish than in the asmt2 Group
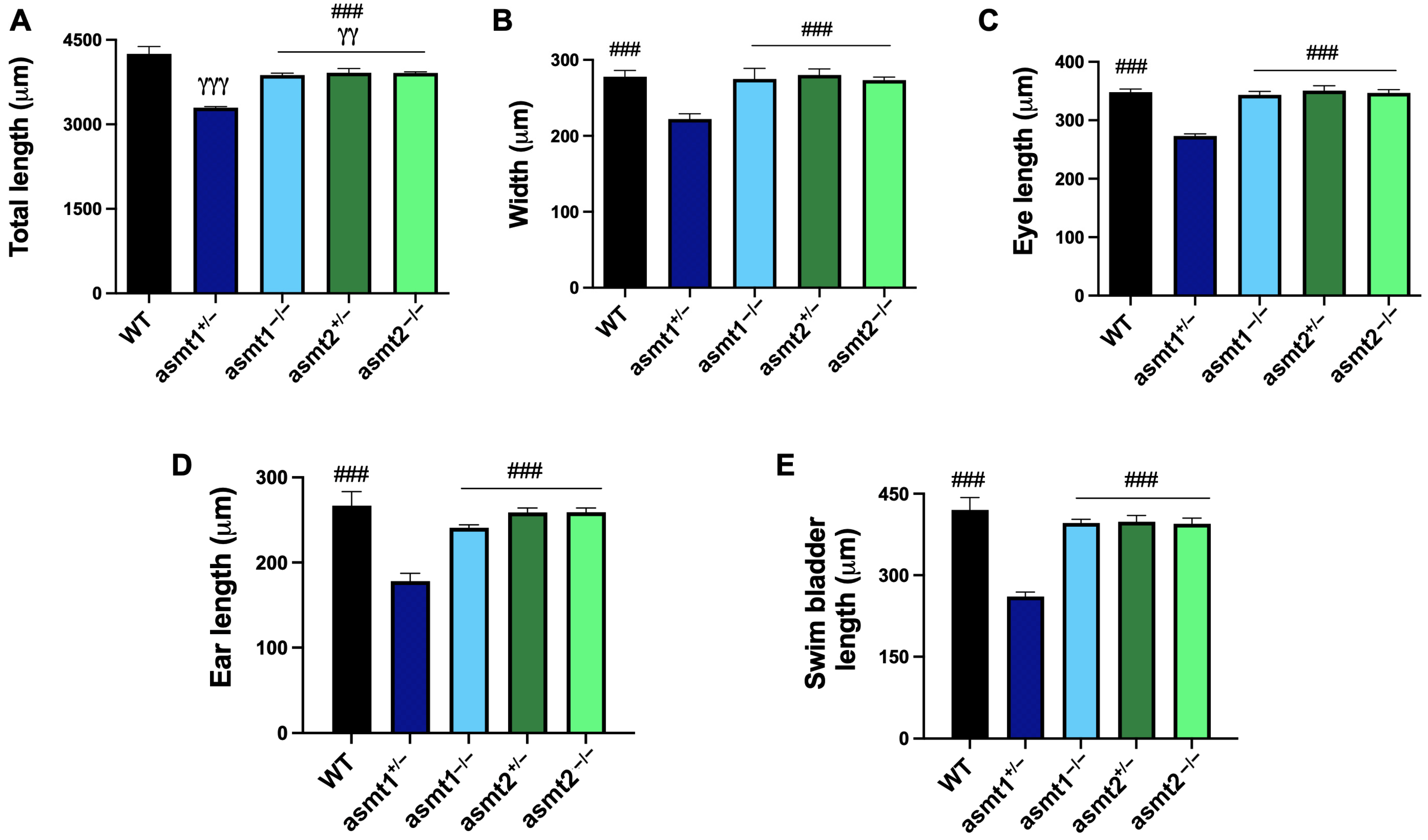
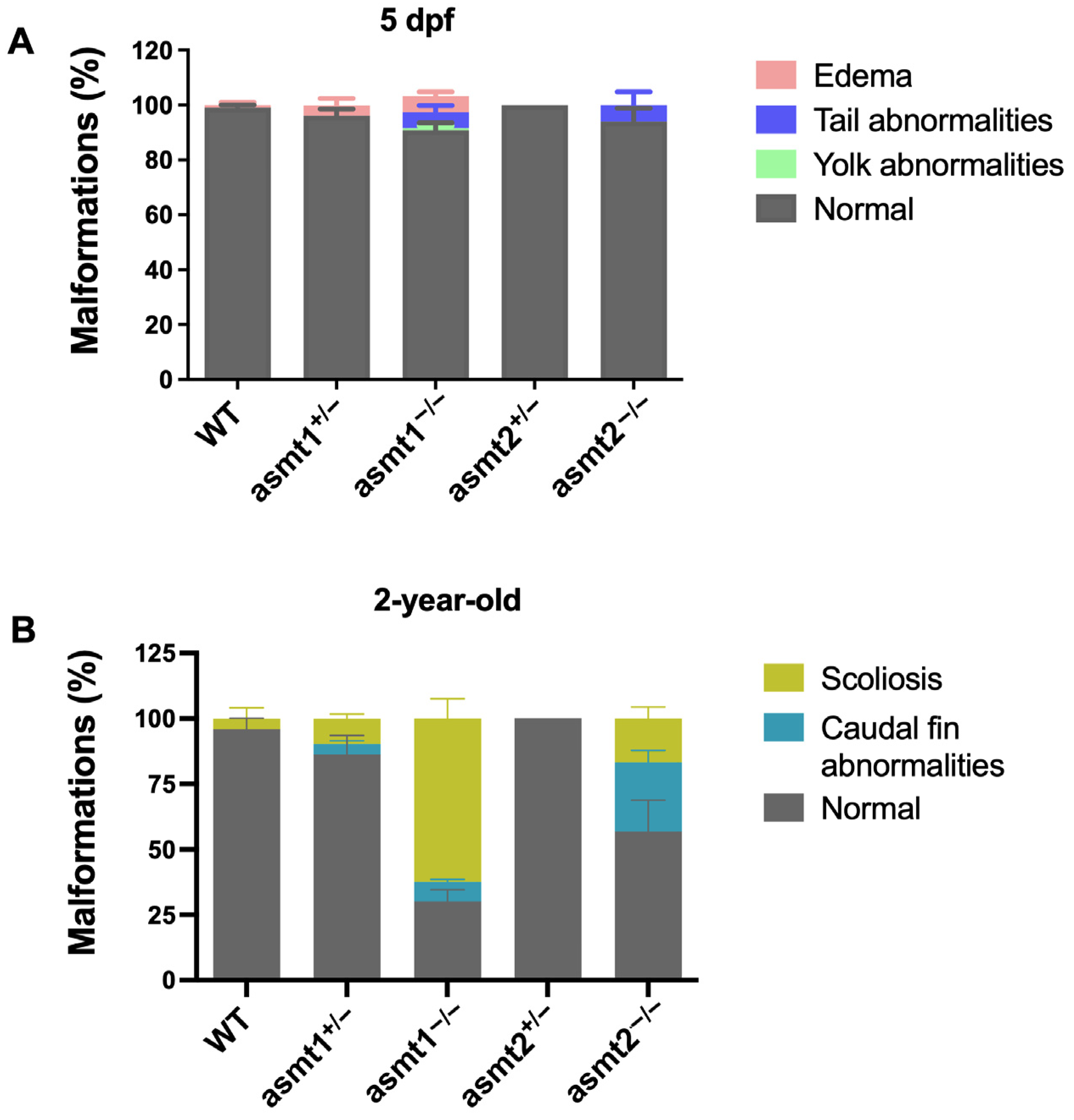
2.4. Mutation in asmt1 but Not in asmt2 Produced Morphological Changes in Zebrafish Larvae
2.5. The Mutant Zebrafish Lines Showed Malformations During the Larval Stage That Increased in Adult Zebrafish
3. Discussion
4. Materials and Methods
4.1. Fish Maintenance
4.2. Generation and Genotyping of Zebrafish asmt Mutant Lines
4.3. Determination of Melatonin Concentration
4.4. Sleep/Wake Rhythm Analysis
4.5. Morphometric Analysis and Malformation Quantification
4.6. Assessment of Locomotor Activity
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Tan, D.X.; Chuffa, L.G.A.; da Silva, D.G.H.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Dual Sources of Melatonin and Evidence for Different Primary Functions. Front. Endocrinol. 2024, 15, 1414463. [Google Scholar] [CrossRef]
- Acuña Castroviejo, D.; Escames, G.; Carazo, A.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondrial homeostasis and mitochondrial-related diseases. Curr. Top. Med. Chem. 2002, 2, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Prokopenko, I.; Langenberg, C.; Florez, J.C.; Saxena, R.; Soranzo, N.; Thorleifsson, G.; Loos, R.J.; Manning, A.K.; Jackson, A.U.; Aulchenko, Y.; et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 2009, 41, 77–81. [Google Scholar] [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Rodríguez-Santana, C.; Rusanova, I.; Escames, G.; Acuña-Castroviejo, D. Chronodisruption and Loss of Melatonin Rhythm, Associated with Alterations in Daily Motor Activity and Mitochondrial Dynamics in Parkinsonian Zebrafish, Are Corrected by Melatonin Treatment. Antioxidants 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, J.; Ramírez-Casas, Y.; Yang, Y.; Aranda-Martínez, P.; Martínez-Ruiz, L.; Escames, G.; Acuña-Castroviejo, D. From Chronodisruption to Sarcopenia: The Therapeutic Potential of Melatonin. Biomolecules 2023, 13, 1779. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Vigo, D.E. Chronobiotic and Cytoprotective Activity of Melatonin in the Cardiovascular System. Doses Matter. npj Biol. Timing Sleep 2024, 1, 7. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Li, J.; Bian, C.; Qin, C.; Shi, Q. A Comparative Genomics Study on the Molecular Evolution of Serotonin/Melatonin Biosynthesizing Enzymes in Vertebrates. Front. Mol. Biosci. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ruan, Z.; Li, J.; Bian, C.; You, X.; Coon, S.L.; Shi, Q. A Comparative Genomic and Transcriptomic Survey Provides Novel Insights into N-Acetylserotonin Methyltransferase (ASMT) in Fish. Molecules 2017, 22, 1653. [Google Scholar] [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Human Diseases. Int. J. Mol. Sci. 2022, 23, 7438. [Google Scholar] [CrossRef]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, J.; Ramírez-Casas, Y.; Aranda-Martínez, P.; López-Rodríguez, A.; Sayed, R.K.A.; Escames, G.; Acuña-Castroviejo, D. iMS-Bmal1−/− mice show evident signs of sarcopenia that are counteracted by exercise and melatonin therapies. J. Pineal Res. 2024, 76, e12912. [Google Scholar] [CrossRef]
- Danilova, N.; Krupnik, V.E.; Sugden, D.; Zhdanova, I.V. Melatonin stimulates cell proliferation in zebrafish larva and accelerates its development. FASEB 2004, 18, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of Zebrafish Models in Understanding Human Diseases—A Review of Models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Zohar, Y. Photoperiodism in Fish. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 400–408. [Google Scholar] [CrossRef]
- Rath, M.F.; Coon, S.L.; Amaral, F.G.; Weller, J.L.; Møller, M.; Klein, D.C. Melatonin Synthesis: Acetylserotonin O-Methyltransferase (ASMT) Is Strongly Expressed in a Subpopulation of Pinealocytes in the Male Rat Pineal Gland. Endocrinology 2016, 157, 2028–2040. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Xia, J.; Cui, Z.; Li, L.; Qi, Z.; Liu, W. Gene Expression Profile Associated with Asmt Knockout-Induced Depression-like Behaviors and Exercise Effects in Mouse Hypothalamus. Biosci. Rep. 2022, 42, BSR20220800. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Boudebesse, C.; Henrion, A.; Jamain, S.; Henry, C.; Leboyer, M.; Bellivier, F.; Etain, B. An ASMT Variant Associated with Bipolar Disorder Influences Sleep and Circadian Rhythms: A Pilot Study. Genes Brain Behav. 2014, 13, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Z.; Zhang, Y.; Zhang, S.; Cui, Z.; Liu, W.; Li, L.; Xia, J.; Zou, Y.; Qi, Z. ASMT Determines Gut Microbiota and Increases Neurobehavioral Adaptability to Exercise in Female Mice. Commun. Biol. 2023, 6, 1126. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Yu, L.; Lopez-Patino, M.; Shang, E.; Kishi, S.; Guelin, E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res. Bull. 2008, 75, 433–441. [Google Scholar] [CrossRef]
- Lananna, B.V.; Musiek, E.S. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832. [Google Scholar] [CrossRef]
- Gerhard, G.S.; Kauffman, E.J.; Wang, X.; Stewart, R.; Moore, J.L.; Kasales, C.J.; Demidenko, E.; Cheng, K.C. Life spans and senescent phenotypes in two strains of Zebrafish (Danio Rerio). Exp. Gerontol. 2002, 37, 1055–1068. [Google Scholar] [CrossRef]
- Zilberman-Peled, B.; Ron, B.; Gross, A.; Finberg, J.P.; Gothilf, Y. A possible new role for fish retinal serotonin-N-acetyltransferase-1 (AANAT1): Dopamine metabolism. Brain Res. 2006, 1073–1074, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Gothilf, Y.; Coon, S.L.; Toyama, R.; Chitnis, A.; Namboodiri, M.A.; Klein, D.C. Zebrafish serotonin N-acetyltransferase-2: Marker for development of pineal photoreceptors and circadian clock function. Endocrinology 1999, 140, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Moreno-Mateos, M.A.; Vejnar, C.E.; Beaudoin, J.D.; Fernandez, J.P.; Mis, E.K.; Khokha, M.K.; Giraldez, A.J. CRISPRscan: Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 2015, 12, 982–988. [Google Scholar] [CrossRef]
- Lozano-Lorca, M.; Olmedo-Requena, R.; Rodríguez-Barranco, M.; Redondo-Sánchez, D.; Jiménez-Pacheco, A.; Vázquez-Alonso, F.; Arana-Asensio, E.; Sánchez, M.J.; Fernández-Martínez, J.; Acuña-Castroviejo, D.; et al. Salivary Melatonin Rhythm and Prostate Cancer: CAPLIFE Study. J. Urol. 2022, 207, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Doldur-Balli, F.; Zimmerman, A.J.; Keenan, B.T.; Shetty, Z.Y.; Grant, S.F.A.; Seiler, C.; Veatch, O.J.; Pack, A.I. Pleiotropic effects of a high confidence Autism Spectrum Disorder gene, arid1b, on zebrafish sleep. Neurobiol. Sleep Circadian Rhythm. 2023, 14, 100096. [Google Scholar] [CrossRef]
- Liu, C.X.; Li, C.Y.; Hu, C.C.; Wang, Y.; Lin, J.; Jiang, Y.H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Martínez, P.; Fernández-Martínez, J.; Díaz-Casado, M.E.; Ramírez-Casas, Y.; Martín-Estebané, M.; López-Rodríguez, A.; Escames, G.; Acuña-Castroviejo, D. Lack of asmt1 or asmt2 Yields Different Phenotypes and Malformations in Larvae to Adult Zebrafish. Int. J. Mol. Sci. 2025, 26, 3912. https://doi.org/10.3390/ijms26083912
Aranda-Martínez P, Fernández-Martínez J, Díaz-Casado ME, Ramírez-Casas Y, Martín-Estebané M, López-Rodríguez A, Escames G, Acuña-Castroviejo D. Lack of asmt1 or asmt2 Yields Different Phenotypes and Malformations in Larvae to Adult Zebrafish. International Journal of Molecular Sciences. 2025; 26(8):3912. https://doi.org/10.3390/ijms26083912
Chicago/Turabian StyleAranda-Martínez, Paula, José Fernández-Martínez, María Elena Díaz-Casado, Yolanda Ramírez-Casas, María Martín-Estebané, Alba López-Rodríguez, Germaine Escames, and Darío Acuña-Castroviejo. 2025. "Lack of asmt1 or asmt2 Yields Different Phenotypes and Malformations in Larvae to Adult Zebrafish" International Journal of Molecular Sciences 26, no. 8: 3912. https://doi.org/10.3390/ijms26083912
APA StyleAranda-Martínez, P., Fernández-Martínez, J., Díaz-Casado, M. E., Ramírez-Casas, Y., Martín-Estebané, M., López-Rodríguez, A., Escames, G., & Acuña-Castroviejo, D. (2025). Lack of asmt1 or asmt2 Yields Different Phenotypes and Malformations in Larvae to Adult Zebrafish. International Journal of Molecular Sciences, 26(8), 3912. https://doi.org/10.3390/ijms26083912







