Long-Term Elite Controllers of HIV-1 Infection Exhibit a Deep Perturbation of Monocyte Homeostasis
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Groups
2.2. Levels of Monocytes and Monocyte Subsets
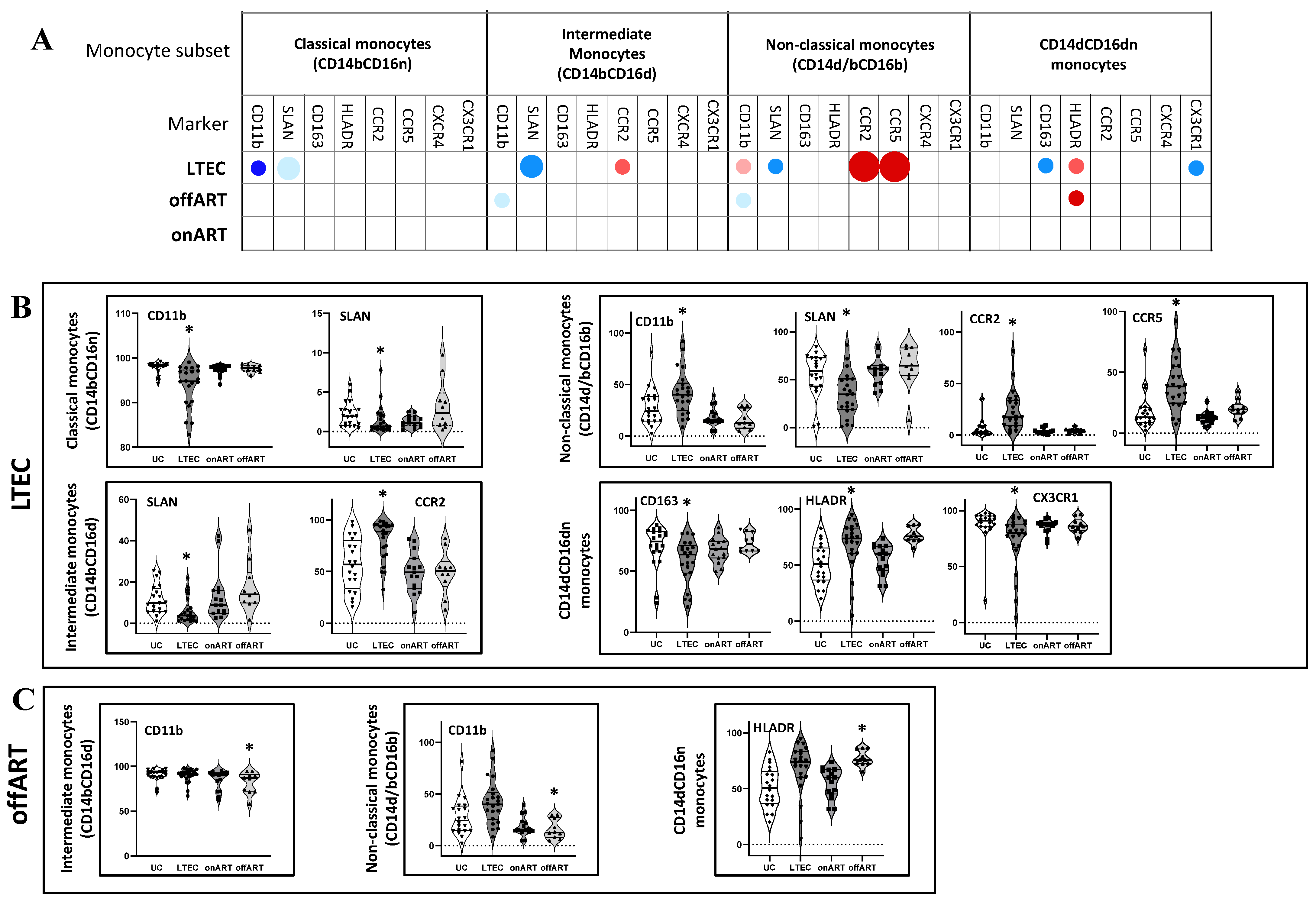
2.3. Profound Alterations in Single Marker Expression Profiles in the LTEC Group
- Next, using manual analysis with FCS Express v.7, the expression levels of eight different markers (CD11b, CD163, HLADR, SLAN, CCR2, CCR5, CXCR4, and CX3CR1) were compared between the PLWH groups and the UC reference group. Supplementary Figure S1 illustrates the single-marker expression profile for different monocyte subsets in the UC group. Interestingly, the LTEC group exhibited the most pronounced alteration of single-marker expression across the monocyte subsets (Figure 2 and Supplementary Figure S2). The description of significantly altered markers in the LTEC group is as follows. Markers: Classical monocytes: Decreased CD11b and SLAN expressions;
- Intermediate monocytes: Decreased SLAN and increased CCR2 expressions;
- Non-classical monocytes: Decreased SLAN and increased CD11b, CCR2, and CCR5 expressions;
- CD14⁺CD16⁻ monocytes: Increased HLA-DR and decreased CD163 and CX3CR1 expressions.
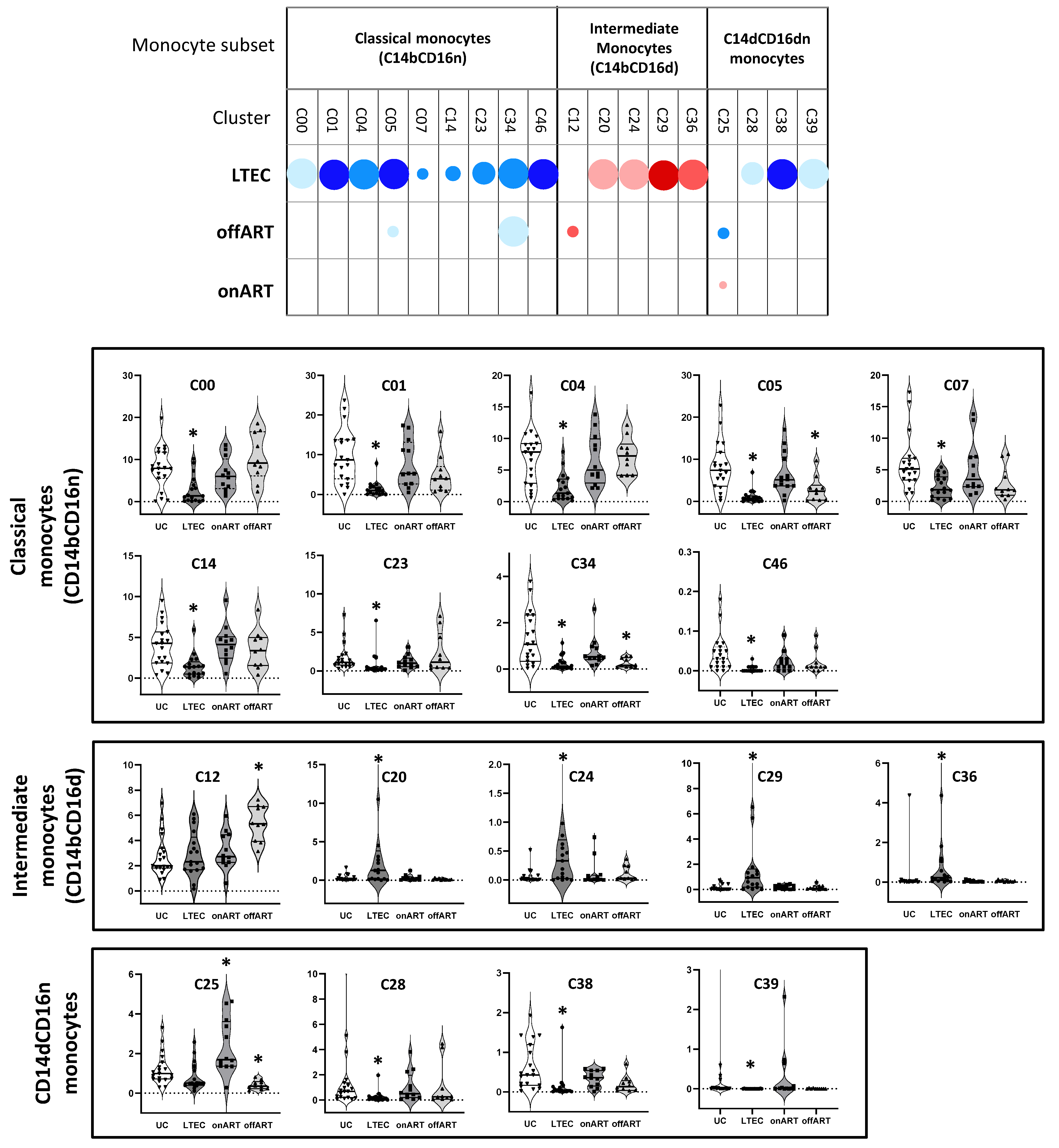
2.4. Unsupervised Multidimensional Analysis of Flow Cytometry Data Identifies Monocyte Clusters That Distinguish Groups
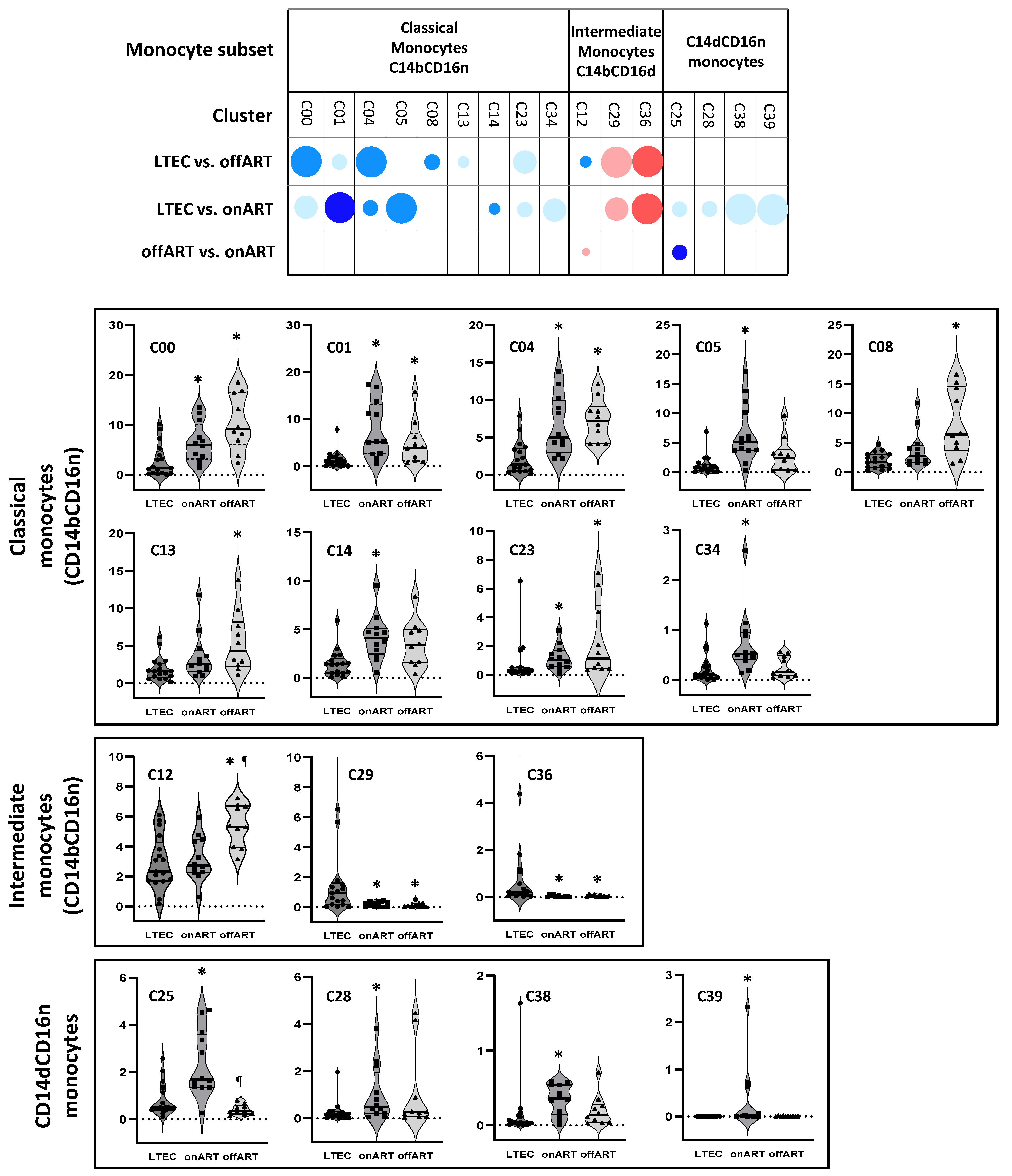
2.5. LTECs Show the Most Profound Alterations in Monocyte Clusters
- -
- LTECs vs. offART: A total of nine clusters were differentially expressed (seven decreased and two increased). All but one of the decreased clusters belonged to the classical monocyte subset, while the increased clusters belonged to the intermediate monocyte subset. Four of these clusters (C10, C20, C16, and C26) were also differentially expressed in LTEC vs. UC comparisons;
- -
- LTECs vs. onART: A total of thirteen clusters were differentially expressed (eleven decreased and two increased). Seven decreased clusters were from the classical monocyte subset, and four were from the CD14dCD16n subset, while the two increased clusters belonged to the intermediate monocyte subset. All but one of these clusters were also significantly altered in LTEC vs. UC comparisons (Figure 4).
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Cell Samples
4.3. Multiparameter Flow Cytometry
4.4. Unsupervised Analysis of Flow Cytometry Data
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Autran, B.; Descours, B.; Avettand-Fenoel, V.; Rouzioux, C. Elite controllers as a model of functional cure. Curr. Opin. HIV AIDS 2011, 6, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Lewin, S.R.; Ross, A.L.; Ananworanich, J.; Benkirane, M.; Cannon, P.; Chomont, N.; Douek, D.; Lifson, J.D.; Lo, Y.R.; et al. International AIDS Society global scientific strategy: Towards an HIV cure 2016. Nat. Med. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Muñoz, M.A.; Restrepo, C.; Benito, J.M.; Rallón, N. Elite controllers: A heterogeneous group of HIV-infected patients. Virulence 2020, 11, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Grabar, S.; Selinger-Leneman, H.; Abgrall, S.; Pialoux, G.; Weiss, L.; Costagliola, D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 2009, 23, 1163–1169. [Google Scholar] [CrossRef]
- Pereyra, F.; Lo, V.; Triant, V.A.; Wei, J.; Buzón, M.J.; Fich, K.V.; Hwang, J.; Campbell, J.H.; Burdo, T.H.; Williams, K.C.; et al. Increased Coronary Atherosclerosis and Immune Activation in HIV-1 Elite Controllers. AIDS 2012, 26, 2409–2412. [Google Scholar] [CrossRef]
- Crowell, T.A.; Gebo, K.A.; Blankson, J.N.; Korthuis, P.T.; Yehia, B.R.; Rytsein, R.M.; Moore, R.D.; Sharp, V.; Nijhawna, A.E.; Mathews, W.C.; et al. Hospitalization Rates and Reasons Among HIV Elite Controllers and Persons with Medically Controlled HIV Infection. J. Infect. Dis. 2015, 211, 1692–1702. [Google Scholar] [CrossRef]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; McCune, J.M.; Roland, M.; Page-Shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Lampiris, H.; et al. Relationship Between T Cell Activation and CD4+ T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. J. Infect. Dis. 2008, 197, 126–133. [Google Scholar] [CrossRef]
- Hunt, P.W.; Landay, A.L.; Sinclair, E.; Martinson, J.A.; Hatano, H.; Emu, B.; Norris, P.J.; Busch, M.; Martin, J.N.; Brooks, C.; et al. A Low T Regulatory Cell Response May Contribute to Both Viral Control and Generalized Immune Activation in HIV Controllers. PLoS ONE 2011, 6, e15924. [Google Scholar] [CrossRef]
- Krishnan, S.; Wilson, E.M.P.; Sheikh, V.; Rupert, A.; Mendoza, D.; Yang, J.; Lempicki, R.; Migueles, S.A.; Sereti, I. Evidence for Innate Immune System Activation in HIV Type 1-Infected Elite Controllers. J. Infect. Dis. 2014, 209, 931–939. [Google Scholar] [CrossRef]
- Platten, M.; Jung, N.; Trapp, S.; Flossdorf, P.; Meyer-Olson, D.; Zur Wiesch, J.S.; Stephan, C.; Mauss, S.; Weiss, V.; von Bergwelt-Baildon, M.; et al. Cytokine and Chemokine Signature in Elite Versus Viremic Controllers Infected with HIV. AIDS Res. Human. Retroviruses 2016, 32, 579–587. [Google Scholar] [CrossRef]
- Nasi, M.; Pinti, M.; Mussini, C.; Cossarizza, A. Persistent inflammation in HIV infection: Established concepts, new perspectives. Immunol. Lett 2014, 161, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, M.S.; Soares, L.S.; Galvão-Lima, L.J.; Zambuzi, F.A.; Cacemiro, M.A.; Brauer, V.S.; Frantz, F.G. HIV infection: Focus on the innate immune cells. Immunol. Res. 2016, 64, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Wacleche, V.S.; Tremblay, C.L.; Routy, J.P.; Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Geissmann, F. Toward a functional characterization of blood monocytes. Immunol. Cell Biol. 2011, 89, 2–4. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- Michailidis, C.; Giannopoulos, G.; Vigklis, V.; Armenis, K.; Tsakris, A.; Gargalianos, P. Impaired phagocytosis among patients infected by the human immunodeficiency virus: Implication for a role of highly active anti-retroviral therapy. Clin. Exp. Immunol. 2012, 167, 499–504. [Google Scholar] [CrossRef]
- Jiang, W.; Lederman, M.M.; Salkowitz, J.R.; Rodriguez, B.; Harding, C.V.; Sieg, S.F. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. JVI 2005, 79, 4109–4119. [Google Scholar] [CrossRef]
- Gama, L.; Shirk, E.N.; Russell, J.N.; Carvalho, K.I.; Li, M.; Queen, S.E.; Kalil, J.; Zink, M.C.; Clements, J.E.; Kallas, E.G. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J. Leucoc. Biol. 2012, 91, 803–816. [Google Scholar] [CrossRef]
- Anzinger, J.J.; Butterfield, T.R.; Angelovich, T.A.; Crowe, S.M.; Palmer, C.S. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J. Immunol. Res. 2014, 2014, 569819. [Google Scholar] [CrossRef]
- Ansari, A.W.; Meyer-Olson, D.; Schmidt, R.E. Selective expansion of pro-inflammatory chemokine CCL2-loaded CD14+CD16+ monocytes subset in HIV-infected therapy naïve individuals. J. Clin. Immunol. 2013, 33, 302–306. [Google Scholar] [CrossRef]
- Dutertre, C.A.; Amraoui, S.; DeRosa, A.; Jourdain, J.P.; Vimeux, L.; Goguet, M.; Degrelle, S.; Feuillet, V.; Liovat, A.S.; Muller-Trutwin, M.; et al. Pivotal role of M-DC8+ monocytes from viremic HIV-infected patients in TNF overproduction in response to microbial products. Blood 2012, 120, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.P.; Singh, A.; Hullsiek, K.H.; Gibson, D.; Henry, W.K.; Lichtenstein, K.; Onen, N.F.; Kojic, E.; Patel, P.; Brooks, J.T.; et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J. Infect. Dis. 2014, 210, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Su, B.; Zhang, T.; Zhu, X.; Xia, W.; Fu, Y.; Zhao, G.; Xia, H.; Dai, L.; Sun, L.; et al. Perturbations of Monocyte subsets and Their association with T helper cell Differentiation in acute and chronic HIV-1-infected Patients. Front. Immunol. 2017, 8, 272. [Google Scholar] [CrossRef]
- Tamene, W.; Marconi, V.C.; Abebe, M.; Wassie, L.; Belay, Y.; Kebede, A.; Sack, U.; Howe, R. Differential expression of chemokine receptors on monocytes in TB and HIV S. Heliyon 2023, 9, e17202. [Google Scholar] [CrossRef]
- Prabhu, V.M.; Singh, A.K.; Padwal, V.; Nagar, V.; Patil, P.; Patel, V. Monocyte Based Correlates of Immune Activation and Viremia in HIV-Infected Long-Term Non-Progressors. Front. Immunol. 2019, 10, 2849. [Google Scholar] [CrossRef]
- McCausland, M.R.; Juchnowski, S.M.; Zidar, D.A.; Kuritzkes, D.R.; Andrade, A.; Sieg, S.F.; Lederman, M.M.; Funderburg, N.T. Altered Monocyte Phenotype in HIV-1 Infection Tends to Normalize with Integrase- Inhibitor-Based Antiretroviral Therapy. PLoS ONE 2015, 10, e0139474. [Google Scholar] [CrossRef]
- Huamana, M.A.; Juchnowskib, S.M.; Zidarb, D.A.; Kityoe, C.M.; Nalukwagoe, S.; Nazzinda, R.; Fichtenbaum, C.J.; Longenecker, C.T. Monocyte activation in persons living with HIV and tuberculosis coinfection. AIDS 2021, 35, 447–452. [Google Scholar] [CrossRef]
- Gascon, R.L.; Narvaez, A.B.; Zhang, R.; Kahn, J.O.; Hecht, F.M.; Herndier, B.G.; McGrath, M.S. Increased HLA-DR expression on peripheral blood monocytes in subsets of subjects with primary HIV infection is associated with elevated CD4 T-cell apoptosis and CD4 T-cell depletion. J. Acquir. Immune Defic. Syndr. 2002, 30, 146–153. [Google Scholar] [CrossRef]
- Mueller, K.A.L.; Hanna, D.B.; Ehinger, E.; Xue, X.; Baas, L.; Gawaz, M.P.; Geisler, T.; Anastos, K.; Cohen, M.H.; Gange, S.J.; et al. Loss of CXCR4 on non-classical monocytes in participants of theWomen’s Interagency HIV Study (WIHS) with subclinical atherosclerosis. Cardiovasc. Res. 2019, 115, 1029–1040. [Google Scholar] [CrossRef]
- Booiman, T.; Wit, F.W.; Maurer, I.; De Francesco, D.; Sabin, C.A.; Harskamp, A.M.; Prins, M.; Garagnani, P.; Pirazzini, C.; Franceschi, C.; et al. High Cellular Monocyte Activation in People Living With Human Immunodeficiency Virus on Combination Antiretroviral Therapy and Lifestyle-Matched Controls Is Associated with Greater Inflammation in Cerebrospinal Fluid. Open Forum Infect. Dis. 2017, 4, ofx108. [Google Scholar] [CrossRef]
- Teer, E.; Joseph, D.E.; Glashoff, R.H.; Essop, M.F. Monocyte/Macrophage-Mediated Innate Immunity in HIV-1 Infection: From Early Response to Late Dysregulation and Links to Cardiovascular Diseases Onset. Virol. Sin. 2021, 36, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Kramski, M.; Schorcht, A.; Johnston, A.P.R.; Lichtfuss, G.F.; Jegaskanda, S.; De Rose, R.; Stratov, I.; Kelleher, A.D.; French, M.A.; Center, R.J.; et al. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. J. Immunol. Methods 2012, 384, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, R.; Morin, S.; Hans Sass, H.J.; Grzesiek, S.; Vekemans, M.; Florence, E.; Tran, H.T.T.; Imiru, R.G.; Heyndrickx, L.; Vanham, G.; et al. Monocytes Contribute to Differential Immune Pressure on R5 Versus X4 HIV Through the Adipocytokine Visfatin/ NAMPT. PLoS ONE 2012, 7, e35074. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.R.; Fliser, D.; Heine, G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef]
- Kwaa, A.K.; Blankson, J.N. Immune Responses in Controllers of HIV Infection. Annu. Rev. Immunol. 2024, 42, 21–33. [Google Scholar] [CrossRef]
- Huang, J.; Burke, P.S.; Cung, T.D.H.; Pereyra, F.; Toth, I.; Walker, B.D.; Borges, L.; Lichterfeld, M.; Yu, X.G. Leukocyte immunoglobulin-like receptors maintain unique antigen-presenting properties of circulating myeloid dendritic cells in HIV-1-infected elite controllers. J. Virol. 2010, 84, 9463–9471. [Google Scholar] [CrossRef]
- Rubin, L.H.; Shirk, E.N.; Pohlenz, L.; Romero, H.; Roti, E.; Dastgheyb, R.M.; Santiuste, I.; Coughlin, J.M.; Brown, T.T.; Clements, J.E. Intact HIV reservoir in monocytes is associated with cognitive function in virally suppressed women with HIV. J. Infect. Dis. 2025, 231, 165–174. [Google Scholar] [CrossRef]
- Chow, D.C.; Saiki, K.M.W.; Siriwardhana, C.; Lozano-Gerona, J.; Vanapruks, S.; Ogle, J.; Premeaux, T.A.; Ndhlovu, L.C.; Boisvert, W.A. Increased transmigration of intermediate monocytes associated with atherosclerotic burden in people with HIV on antiretroviral therapy. AIDS 2023, 37, 1177–1179. [Google Scholar] [CrossRef]
- Veenhuis, R.T.; Williams, D.W.; Shirk, E.N.; Abreu, C.M.; Ferreira, E.A.; Coughlin, J.M.; Brown, T.T.; Maki, P.M.; Anastos, K.; Berman, J.W.; et al. Higher circulating intermediate monocytes are associated with cognitive function in women with HIV. JCI Insight 2021, 6, e146215. [Google Scholar] [CrossRef]
- Barbour, J.D.; Jalbert, E.C.; Chowa, D.C.; Gangcuangco, L.M.A.; Norris, P.J.; Keating, S.M.; Heitman, J.; Nagamine, L.; Seto, T.; Ndhlovu, L.C.; et al. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis 2014, 232, 52–58. [Google Scholar] [CrossRef]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 Integrins as Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, N.; Haeffner-Cavaillon, N.; Ledur, A.; L’Age-Stehr, J.; Ziegler-Heitbrock, H.W.; Kazatchkine, M.D. CR1 (CD35) and CR3 (CD11b/CD18) mediate infection of human monocytes and monocytic cell lines with complement-opsonized HIV independently of CD4. Clin. Exp. Immunol. 1993, 92, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, R.; Imbert, V.; Chicheportiche, R.; Dayer, J.M. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood 2001, 97, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Maisaa, A.; Chenga, W.J.; Angelovich, T.A.; Lichtfuss, G.F.; Palmer, C.S.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 2012, 26, 843–853. [Google Scholar] [CrossRef]
- Hofer, T.P.; van de Loosdrecht, A.A.; Stahl-Hennig, C.; Cassatella, M.A.; Ziegler-Heitbrock, L. 6-Sulfo LacNAc (Slan) as a Marker for Non-Classical Monocytes. Front. Immunol. 2019, 10, 2052. [Google Scholar] [CrossRef]
- Covino, D.A.; Sabbatucci, M.; Fantuzzi, L. The CCL2/CCR2 Axis in the Pathogenesis of HIV-1 Infection: A New Cellular Target for Therapy? Curr. Drug Targets 2016, 17, 76–110. [Google Scholar] [CrossRef]
- Kedzierska, K.; Crowe, S.M.; Turville, S.; Cunningham, A.L. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 2003, 13, 39–56. [Google Scholar] [CrossRef]
- Dentone, C.; Fenoglio, D.; Parodi, A.; Altosole, T.; Di Biagio, A.; Bozzano, F. Intermediate monocytes expansion and homing markers expression in COVID-19 patients associate with kidney dysfunction. Clin. Exp. Med. 2023, 23, 1235–1242. [Google Scholar] [CrossRef]
- França, C.N.; Izar, M.C.O.; Marinella NS Hortêncio, M.N.S.; do Amaral, J.B.; Ferreira, C.E.S.; Tuleta, I.D.; Fonseca, F.A.H. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef]
- Williams, D.W.; Byrd, D.; Rubin, L.H.; Anastos, K.; Morgello, S.; Berman, J.W. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e36. [Google Scholar] [CrossRef]
- Cheadle, W.G. The human leukocyte antigens and their relationship to infection. Am. J. Surg. 1993, 165 (Suppl. S2), 75S–81S. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, E.; Godfrey, S.B.; Stamegna, P.; Ogbechie, T.; Kerrigan, C.; Zhang, M.; Walker, B.; Le Gall, S. Differential HIV epitope processing in monocytes and CD4 T cells affects cytotoxic T lymphocyte recognition. J. Infect. Dis. 2009, 200, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Lentz, M.R.; Autissier, P.; Krishnan, A.; Halpern, E.; Letendre, S.; Rosenberg, E.S.; Ellis, R.J.; Williams, K.C. Soluble CD163 Made by Monocyte/Macrophages Is a Novel Marker of HIV Activity in Early and Chronic Infection Prior to and After Anti-Retroviral Therapy. J. Infect. Dis. 2011, 204, 154–163. [Google Scholar] [CrossRef]
- Burdo, T.H.; Lo, J.; Abbara, S.; Wei, J.; DeLelys, M.E.; Preffer, F.; Rosenberg, E.S.; Williams, K.C.; Grinspoon, S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011, 204, 1227–1236. [Google Scholar] [CrossRef]
- Burdo, T.H.; Weiffenbach, A.; Woods, S.P.; Letendre, S.; Ellis, R.J.; Williams, K.C. Elevated sCD163 in plasma but not cerebrospinal fluid is amarker of neurocognitive impairment in HIV infection. AIDS 2013, 27, 1387–1395. [Google Scholar] [CrossRef]
- Knudsen, T.B.; Ertner, G.; Petersen, J.; Møller, H.J.; Moestrup, S.K.; Eugen-Olsen, J.; Kronborg, G.; Benfield, T. Plasma Soluble CD163 Level Independently Predicts All-Cause Mortality in HIV-1-Infected Individuals. J. Infect. Dis. 2016, 214, 1198–1204. [Google Scholar] [CrossRef]
- Ancuta, P.; Kamat, A.; Kunstman, K.J.; Kim, E.Y.; Autissier, P.; Wurcel, A. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 2008, 3, e2516. [Google Scholar] [CrossRef]
- Moestrup, S.K.; Møller, H.J. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 2004, 36, 347–354. [Google Scholar] [CrossRef]
- Tippett, E.; Cheng, W.J.; Westhorpe, C.; Cameron, P.U.; Brew, B.J.; Lewin, S.R.; Jaworowski, A.; Crowe, S.M. Differential Expression of CD163 on Monocyte Subsets in Healthy and HIV-1 Infected Individuals. PLoS ONE 2006, 6, e19968. [Google Scholar] [CrossRef]
- Leon, A.; Perez, I.; Ruiz-Mateos, E.; Benito, J.M.; Leal, M.; Lopez-Galindez, C.; Rallon, N.; Alcami, J.; Lopez-Aldeguer, J.; Viciana, P.; et al. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016, 30, 1209–1220. [Google Scholar] [CrossRef]

| Characteristic | LTEC (n = 22) | onART (n = 15) | offART (n = 10) | UC (n = 20) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 44 [35–49] | 44 [42–49] | 43 [36–49] | 43 [36–49] | 0.266 |
| Sex (% of males) | 59 | 80 | 100 | 50 | 0.025 |
| Years since HIV diagnosis | 15 [6–20] | 12 [5–15] | 5 [3–9] | NA | 0.027 |
| Years as EC | 13 [7–16] | NA | NA | NA | NA |
| Years on ART | NA | 6 [3–9] | NA | NA | NA |
| Plasma HIV load (copies/mL) | 50 | 50 | 57937 [28,817–80,676] | NA | NA |
| CD4 count (cells/μL) | 837 [603–1210] | 820 [599–1127] | 625 [518–920] | NA | 0.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito, J.M.; Jiménez-Carretero, D.; Valentín-Quiroga, J.; Mahillo, I.; Ligos, J.M.; Restrepo, C.; Cabello, A.; López-Collazo, E.; Sánchez-Cabo, F.; Górgolas, M.; et al. Long-Term Elite Controllers of HIV-1 Infection Exhibit a Deep Perturbation of Monocyte Homeostasis. Int. J. Mol. Sci. 2025, 26, 3926. https://doi.org/10.3390/ijms26093926
Benito JM, Jiménez-Carretero D, Valentín-Quiroga J, Mahillo I, Ligos JM, Restrepo C, Cabello A, López-Collazo E, Sánchez-Cabo F, Górgolas M, et al. Long-Term Elite Controllers of HIV-1 Infection Exhibit a Deep Perturbation of Monocyte Homeostasis. International Journal of Molecular Sciences. 2025; 26(9):3926. https://doi.org/10.3390/ijms26093926
Chicago/Turabian StyleBenito, José M., Daniel Jiménez-Carretero, Jaime Valentín-Quiroga, Ignacio Mahillo, José M. Ligos, Clara Restrepo, Alfonso Cabello, Eduardo López-Collazo, Fátima Sánchez-Cabo, Miguel Górgolas, and et al. 2025. "Long-Term Elite Controllers of HIV-1 Infection Exhibit a Deep Perturbation of Monocyte Homeostasis" International Journal of Molecular Sciences 26, no. 9: 3926. https://doi.org/10.3390/ijms26093926
APA StyleBenito, J. M., Jiménez-Carretero, D., Valentín-Quiroga, J., Mahillo, I., Ligos, J. M., Restrepo, C., Cabello, A., López-Collazo, E., Sánchez-Cabo, F., Górgolas, M., & Rallón, N. (2025). Long-Term Elite Controllers of HIV-1 Infection Exhibit a Deep Perturbation of Monocyte Homeostasis. International Journal of Molecular Sciences, 26(9), 3926. https://doi.org/10.3390/ijms26093926








