Abstract
The global rise in antimicrobial resistance, particularly among ESKAPE pathogens, has intensified the demand for alternative therapeutic strategies. Silver nanoparticles (AgNPs) have exhibited broad-spectrum antimicrobial activity and represent a promising approach to combat multidrug-resistant infections. This study aimed to synthesize and functionalize AgNPs using various polymeric agents—ethylene glycol (EG), polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), and their combinations—and to evaluate their antimicrobial and antibiofilm efficacy against clinically relevant bacterial strains. AgNPs were synthesized via chemical reduction and functionalized as Ag@EG, Ag@PEG, Ag@EG/PVP, and Ag@PEG/PVP. A total of 68 clinical isolates—including Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus lugdunensis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa—were tested. Antimicrobial susceptibility was assessed using disc diffusion and broth microdilution assays, while antibiofilm activity was evaluated via the crystal violet method. Among all tested formulations, Ag@EG/PVP exhibited the highest antimicrobial and antibiofilm activity, with notably low minimum inhibitory concentrations (MIC50) and minimum biofilm eradication concentrations (MBEC50) for Ps. aeruginosa and K. pneumoniae. In contrast, AgNPs functionalized with PEG or EG alone showed limited efficacy. Biofilm-forming isolates, particularly Staphylococcus spp., required higher concentrations for inhibition. These results highlight the critical role of functionalization in modulating the antimicrobial properties of AgNPs, with Ag@EG/PVP demonstrating potent activity against both planktonic and biofilm-associated multidrug-resistant bacteria. Overall, this study supports further developing AgNPs-based formulations as adjuncts or alternatives to conventional antibiotics, particularly for managing biofilm-related infections. Future research should focus on formulation optimization, safety assessment, and translational potential.
1. Introduction
Nanomedicine is focused on the manipulation of materials at the nanoscale (1–100 nm) and exploits different physicochemical characteristics of nanoparticles—particularly their high surface-area-to-volume ratio—to enhance biochemical reactivity and catalytic performance compared to their bulk equivalents [1,2,3,4]. Due to the escalating global challenge of antimicrobial resistance, developing novel and effective therapeutic alternatives has become an essential priority [5,6,7]. Among inorganic nanomaterials, silver nanoparticles (AgNPs) have demonstrated a broad spectrum of antimicrobial, antifungal, antiviral, and anti-inflammatory activities [8,9]. These properties have led to their integration into various biomedical applications such as drug delivery systems, wound dressings, catheter coatings to prevent biofilm formation, and topical formulations such as creams and ointments designed to inhibit opportunistic infections [10,11,12]. As a result, AgNPs have gained prominence as potential agents in addressing multidrug-resistant (MDR) infections, particularly those caused by ESKAPE pathogens—Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species [13,14]. These pathogens are known for resisting various antibiotic classes and for their frequent involvement in healthcare-associated infections, posing a substantial global public health threat [13,14].
Beyond their intrinsic antimicrobial capabilities, AgNPs have shown a substantial potential as delivery vehicles for various therapeutic agents, including anti-inflammatory, antioxidant, antimicrobial, and anticancer compounds [15]. Clinically, AgNPs have been incorporated into various products such as wound dressings, catheter and implant coatings, and bone graft materials due to their combined antibacterial and anti-inflammatory effects [15,16,17,18,19,20,21,22,23,24,25,26]. Additionally, their utility has expanded into non-medical domains, including antimicrobial textiles, food packaging, and water purification systems [27,28,29,30,31,32].
Recent advances in nanotechnology have underscored the potential of nanoparticles—particularly silver-based nanomaterials—in combating biofilm-associated infections caused by multidrug-resistant pathogens [8,9]. Several reviews and experimental studies have highlighted the role of AgNPs in targeting biofilm-associated infections caused by multidrug-resistant bacteria. Notably, Kumar et al. emphasized recent nanotechnology-based strategies to inhibit biofilm formation and disrupt microbial communities resistant to antibiotics [33]. Afrasiabi and Partoazar explored how nanoparticle-based delivery systems can modulate gene expression in biofilm-forming bacteria, potentially enhancing treatment precision [34]. Similarly, Sarkar et al. reviewed the antibiofilm potential of nanoparticles against ESKAPE pathogens, underscoring the critical need for novel nanotherapeutics [35]. Proteomics-based insights into the mechanisms of AgNPs’ antibacterial action have also emerged, as shown by Rodrigues et al., who reported key metabolic and structural disruptions induced by silver-based agents [36]. Furthermore, the size of biosynthesized AgNPs has been shown to play a significant role in antibacterial and antibiofilm activity, with smaller particles exhibiting greater efficacy [37].
Other recent advances include innovative combination therapies. Mukherjee et al. described synergistic approaches using nanotechnology to target biofilms of ESKAPE pathogens through enhanced penetration and local delivery [38]. Khairnar et al. demonstrated that conjugating AgNPs with vancomycin improved antimicrobial efficacy against Gram-positive bacteria and enabled better biofilm eradication [39]. Moreover, Szymczak et al. presented a novel strategy combining bacteriophages with silver nanoparticles, showing significantly enhanced antibiofilm performance compared to either therapy alone [40]. Collectively, these studies underscore the therapeutic potential of AgNPs and highlight the need to optimize their functionalization for maximal antimicrobial benefit.
All scientific findings support the continued investigation and optimization of functionalized AgNPs as a versatile platform for managing biofilm-associated and drug-resistant infections. However, limitations such as potential cytotoxicity and environmental impact pose challenges to the therapeutic application of AgNPs [16,17]. These nanoparticles can accumulate in vital organs and may cross biological barriers, including the blood–brain barrier, raising safety concerns [41,42,43,44]. To address these issues, emerging strategies such as conjugation with peptides, antibiotics, or dendrimers aim to enhance efficacy while reducing host toxicity and resistance development [45,46,47,48,49]. Synergistic combinations of AgNPs with antibiotics have shown superior antibacterial activity, enabling lower antibiotic doses and potentially curbing resistance evolution [50,51,52]. Additionally, green synthesis methods using plant extracts or microbial agents offer eco-friendly alternatives with lower cytotoxicity profiles [53,54,55].
Comprehensive biocompatibility assessments and controlled release systems are essential to ensure their safe clinical use. Functionalization with organic or inorganic substances further improves AgNPs biocompatibility and stability. The surface functionalization of AgNPs plays a critical role in determining their stability, dispersion, and interaction with microbial membranes. Polymers such as polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), and ethylene glycol (EG) are widely used to improve nanoparticle biocompatibility and solubility [15,16,17,18,19,20,21]. While prior studies have explored AgNPs functionalized with individual polymers, there remains a significant gap in comparative data evaluating the antimicrobial and antibiofilm performance of AgNPs functionalized with both individual and combined polymers, particularly against biofilm-producing, drug-resistant clinical isolates [15,16,17,18,19,20,21]. Moreover, few studies have systematically assessed these effects using both minimum inhibitory concentrations (MIC) and minimum biofilm eradication concentrations (MBEC) endpoints across a diverse panel of clinically relevant strains.
Our study addresses this gap by synthesizing AgNPs functionalized with PEG, PVP, EG, and their combinations and evaluating their antimicrobial and antibiofilm activity against multidrug-resistant strains—including ESKAPE pathogens—isolated from difficult-to-treat infections. Through side-by-side comparisons using MIC and MBEC assays, we identify the most effective formulation, Ag@EG/PVP, and provide preliminary insights into its potential for clinical translation. This work contributes to the optimization of AgNPs-based antimicrobial strategies and supports their use as viable adjuncts or alternatives to conventional antibiotics in the global effort to combat antimicrobial resistance.
2. Results
This study evaluated the antimicrobial and antibiofilm properties of AgNPs functionalized with four different polymeric formulations. A total of 68 bacterial isolates were included, representing clinically relevant infections such as ventilator-associated pneumonia, catheter-associated urinary tract infections, diabetic foot ulcers, central line-associated bloodstream infections, and prosthetic joint infections. The bacterial panel consisted of 11 strains of E. coli, 11 strains of K. pneumoniae, 23 strains of Ps. aeruginosa, 12 strains of S. aureus, 6 strains of S. epidermidis, and 6 strains of S. lugdunensis.
2.1. Qualitative Antimicrobial Assessment Using the Disc Diffusion Method
Among all AgNPs formulations tested, only Ag@EG/PVP at 1 mg/mL demonstrated clear antimicrobial activity.
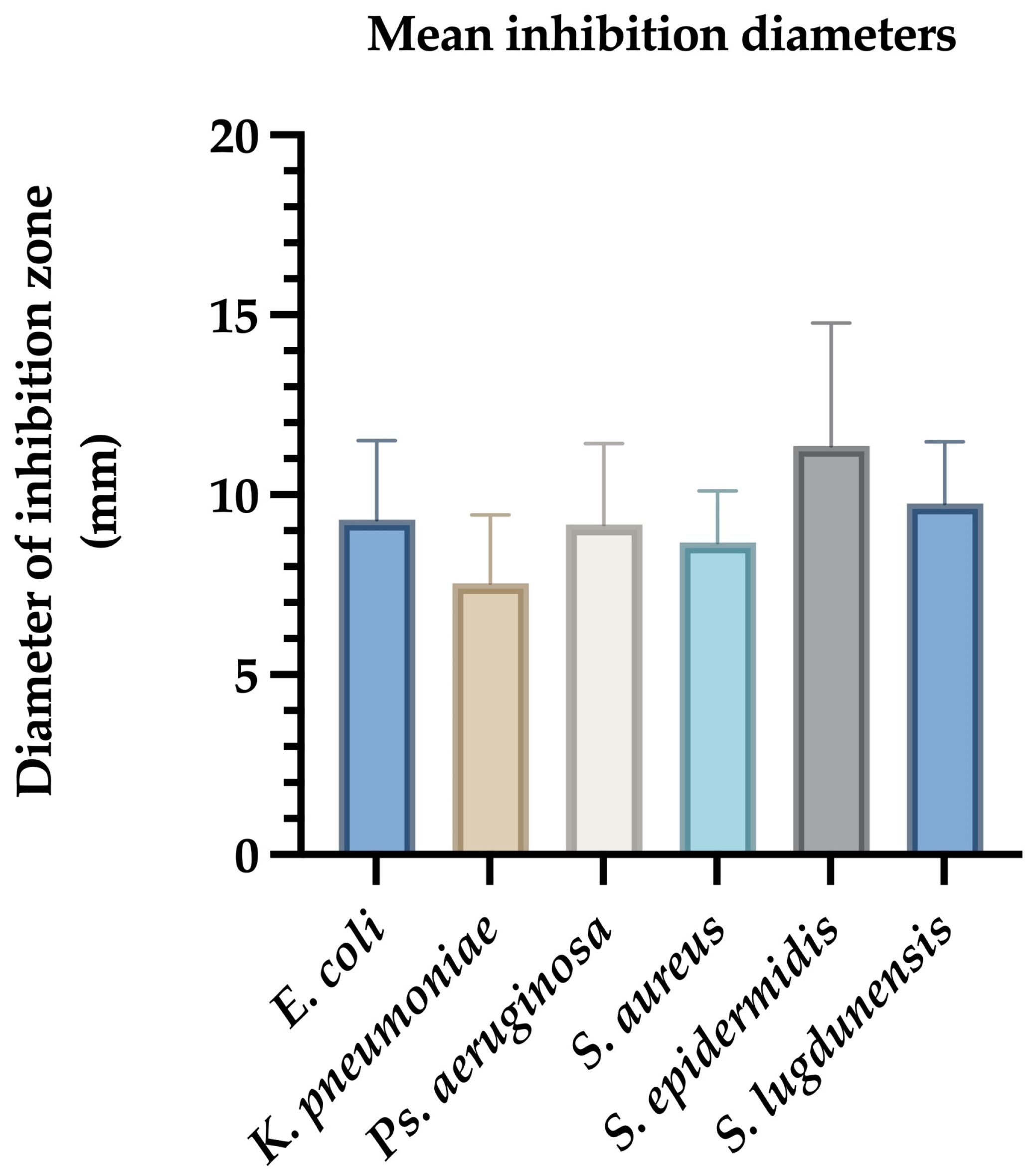
For E. coli, inhibition zones ranged from 7 mm to 12.5 mm, with a mean diameter of 9.09 ± 0.85 mm. Reference strains ATCC 25922 and NCTC 13846 showed inhibition zones of 8 mm and 7 mm, respectively (Figure 1).
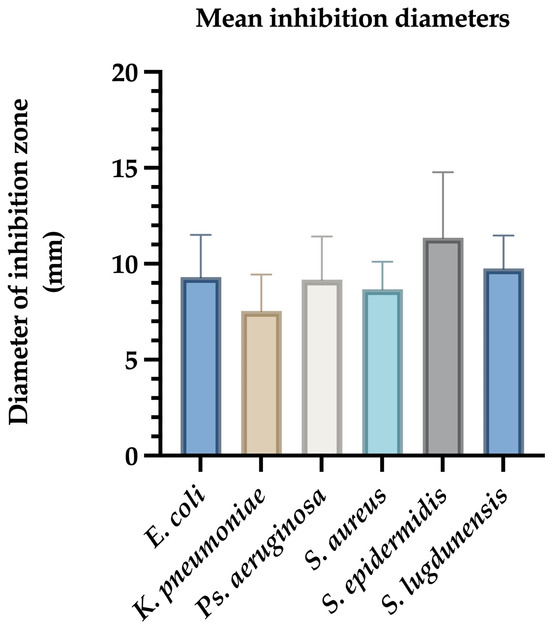
Figure 1.
Mean inhibition zone diameters (mm) of Ag@EG/PVP against clinical bacterial isolates. The bar graph illustrates the average diameters of growth inhibition zones produced by AgNPs functionalized with ethylene glycol and polyvinylpyrrolidone (Ag@EG/PVP) against various clinically relevant bacterial strains. Data represent the mean ± standard deviation of triplicate assays conducted in three independent experiments. S. epidermidis exhibited the largest mean inhibition zone, indicating high susceptibility, while K. pneumoniae showed the smallest zone, consistent with its multidrug-resistant phenotype.
K. pneumoniae isolates exhibited inhibition zones between 4 mm and 11 mm, with a mean diameter of 7.54 ± 1.03 mm (Figure 1).
Ps. aeruginosa ATCC 27853 displayed an 8 mm zone, while clinical isolates showed inhibition zones ranging from 6 mm to 15 mm (mean: 9.2 ± 1.58 mm) (Figure 1).
For Gram-positive bacteria, S. aureus ATCC 12600 had a 7 mm inhibition zone. Clinical S. aureus strains showed inhibition zones ranging from 6 mm to 11 mm (mean: 8.66 ± 0.92 mm). S. epidermidis demonstrated the highest average inhibition (8–17 mm, mean: 12.08 ± 1.75 mm), while S. lugdunensis showed inhibition zones between 7 mm and 12 mm (mean: 9.75 ± 1.12 mm) (Figure 1).
Ag@EG/PVP demonstrated the most consistent and potent antimicrobial activity across all tested strains. The largest inhibition zone (17 mm) was observed against coagulase-negative Staphylococci (CNS), underscoring the superior efficacy of this formulation. Conversely, the least pronounced antimicrobial effect was recorded for K. pneumoniae, which may reflect its known multidrug-resistant phenotype. In contrast, the other AgNPs formulations—Ag@PEG, Ag@EG, and Ag@PEG/PVP—exhibited minimal to no inhibitory activity, producing only marginal effects against K. pneumoniae with inhibition diameters not exceeding 4–5 mm (Table 1).

Table 1.
Comparative analysis of antimicrobial and antibiofilm activity of AgNPs functionalized with different polymeric substances against clinical bacterial isolates. This table summarizes the MIC50, MIC90, MBEC50, and MBEC90 values for five clinically relevant bacterial strains (E. coli, K. pneumoniae, Ps. aeruginosa, S. aureus, and coagulase-negative Staphylococci (CNS)) treated with AgNPs functionalized with PEG, EG, PEG/PVP, and EG/PVP. The results demonstrate that Ag@EG/PVP consistently exhibited the lowest MIC and MBEC values across both Gram-negative and Gram-positive strains, particularly for K. pneumoniae and Ps. aeruginosa, where MIC50 and MBEC50 reached as low as 0.0039 mg/mL. In contrast, all other formulations showed significantly higher values, often approaching or exceeding 0.5 mg/mL. Notably, S. aureus and CNS exhibited the highest resistance to biofilm eradication, with MBEC90 values >0.5 mg/mL across all nanoparticle types.
2.2. Minimal Inhibitory Concentration (MIC)
Ag@EG/PVP yielded the lowest MIC50 values: 0.0156 mg/mL for E. coli and 0.0039 mg/mL for K. pneumoniae, Ps. aeruginosa, and coagulase-negative staphylococci (CNS). S. aureus strains were less susceptible, requiring higher MIC50 values (0.5 mg/mL).
MIC90 values also confirmed the superior activity of Ag@EG/PVP, remaining at 0.0039 mg/mL for Ps. aeruginosa and 0.0156 mg/mL for K. pneumoniae. S. aureus again exhibited the highest MIC90 values, exceeding 0.5 mg/mL. MIC50 and MIC90 values were identical for Ps. aeruginosa, indicating uniform susceptibility. Other formulations often required concentrations beyond the upper detection limit (>0.5 mg/mL) to achieve inhibition (Table 1).
2.3. Minimum Biofilm Eradication Concentration (MBEC)
Biofilm assays showed that K. pneumoniae isolates had the lowest MBEC50 values (0.0039–0.0156 mg/mL), followed by Ps. aeruginosa (0.0039–0.25 mg/mL). Ag@EG/PVP consistently exhibited the most potent biofilm-disruptive effects across species.
Biofilms formed by Staphylococcus spp. were more resistant, requiring MBEC50 values exceeding 0.5 mg/mL. Interestingly, Ag@PEG was relatively more effective against E. coli biofilms (MBEC50: 0.0078 mg/mL), whereas other formulations exceeded 0.5 mg/mL.
MBEC90 results had the following pattern: values >0.5 mg/mL were required for Staphylococcus spp. and E. coli, while Ag@EG/PVP achieved MBEC90 values of 0.0156 mg/mL for K. pneumoniae and 0.0039 mg/mL for Ps. aeruginosa. In most cases, MBEC90 values were higher than MIC90, except in K. pneumoniae, where they were equivalent (Table 1).
These findings collectively support the potential of EG/PVP-functionalized AgNPs as potent agents against multidrug-resistant pathogens, particularly in biofilm-associated infections.
3. Discussions
Although various AgNPs formulations have been explored for antimicrobial applications, few studies have systematically compared AgNPs functionalized with both individual and combined polymeric agents such as EG, PEG, and PVP. Our study provides a unique comparative framework for assessing the efficacy of these formulations, identifying Ag@EG/PVP as a particularly promising candidate due to its superior performance against both planktonic and biofilm-associated bacterial phenotypes.
Infectious diseases remain a leading cause of global morbidity and mortality, accounting for nearly one-fifth of deaths in 2016 alone [56,57,58]. Respiratory, enteric, and systemic infections caused by bacterial, viral, fungal, and parasitic pathogens impose significant clinical and economic burdens [58,59]. Antibiotics are among the most frequently prescribed drug classes, yet up to 50% of these prescriptions—especially in primary care—are considered inappropriate, further exacerbating AMR [60]. The rapid emergence of MDR pathogens and the scarcity of new antibiotics underscore the urgent need for alternative antimicrobial strategies.
Silver nanoparticles have garnered substantial attention as potent antimicrobial agents, offering broad-spectrum activity against Gram-positive and Gram-negative bacteria [18,61]. AgNPs can be synthesized using top-down approaches such as mechanical milling or bottom-up methods involving physical (e.g., thermoplasma, pyrolytic spray, etc.), chemical (e.g., inverse microemulsion), or biological techniques [16,17]. Among these, chemical reduction is the most widely adopted due to its reproducibility and ability to produce stable colloidal dispersions [16,17]. More recently, biological synthesis using plant extracts or microbial agents has emerged as an eco-friendly alternative, leveraging natural reducing agents such as polyphenols and proteins [62,63,64]. Despite these advancements, challenges remain in fully understanding AgNPs synthesis pathways, optimizing environmental safety, and elucidating molecular interactions with bacterial targets [65].
The antimicrobial activity of AgNPs stems from multiple mechanisms. These include disruption of bacterial membranes, interference with enzymatic processes, and the generation of ROS [66,67,68,69,70,71,72,73,74]. AgNPs increase membrane permeability and structural damage by adhering to the bacterial surface, bind to thiol groups on metabolic enzymes to impair respiration, and interfere with DNA replication and protein synthesis. Additionally, they disrupt quorum sensing and destabilize the extracellular polymeric substance (EPS) matrix within biofilms, leading to disassembly and eradication of mature biofilms [69,75,76]. The efficacy of AgNPs is strongly influenced by physicochemical properties such as particle size, morphology, surface charge, and solubility [77,78]. Notably, spherical or quasi-spherical nanoparticles exhibit greater silver ion release due to their increased surface area, and particles smaller than 10 nm demonstrate heightened antimicrobial activity due to enhanced penetration and reactivity [77,79,80,81,82]. Synergistic use with antibiotics can further enhance antimicrobial effects, reduce required dosages, and potentially mitigate resistance development while minimizing environmental impact [83,84].
Biofilm formation poses a substantial challenge in clinical settings due to its role in conferring high resistance to antibiotics and disinfectants, often up to 1000-fold greater than planktonic cells [85,86,87,88]. Biofilms are a major cause of chronic and nosocomial infections, including respiratory illnesses in cystic fibrosis patients, endocarditis, chronic prostatitis, otitis media, and oral diseases such as periodontitis and caries [85,87,88,89,90,91,92]. Medical devices like catheters, prosthetic valves, and sutures are frequent sites of biofilm colonization, leading to recurrent infections [88,90,91,92,93]. Furthermore, biofilms are implicated in foodborne diseases, forming on surfaces of meat and poultry products, with pathogens like E. coli, Listeria monocytogenes, and Salmonella spp. being notable culprits [94,95,96].
The biofilm matrix, primarily composed of polysaccharides, proteins, and nucleic acids, ensures robust adherence to surfaces and protection against hostile environments [85,97,98,99]. Biofilm development initiates with surface attachment, facilitated by flagella, pili, or lipopolysaccharides, and progresses through microcolony formation regulated by quorum sensing [100,101,102,103]. This process culminates in heightened bacterial resistance and immune evasion, complicating eradication efforts [100,101,102,103].
AgNPs effectively disrupt biofilm formation by inhibiting bacterial adhesion, neutralizing extracellular polymeric substances, and inducing ROS-mediated damage [68,75,85,104,105]. Disruption of bacterial cytoskeletal proteins such as MreB and interaction with cell membranes lead to morphological alterations and cell lysis [68,75,104,105]. Intracellularly, AgNPs bind to thiol groups of enzymes, impairing respiration and metabolism, and interact with DNA, affecting replication and transcription processes [70,76]. Additionally, AgNPs interfere with phosphorylation pathways essential for bacterial growth and can induce apoptosis via p53 and caspase3 activation [67,75]. Notably, the smaller the nanoparticles, the greater their antimicrobial efficacy due to enhanced cellular uptake [16].
Numerous studies have confirmed the antibiofilm potential of AgNPs. For instance, significant inhibition of S. aureus and E. coli was observed at MIC values of 100 µg/mL, with marked morphological damage [84]. AgNPs (8.3 nm) inhibited Ps. aeruginosa PAO1 biofilms at concentrations as low as 4–5 µg/mL [106]. In resistant Ps. aeruginosa strains, 20 µg/mL AgNPs reduced biofilm formation by up to 67% [17]. Furthermore, AgNPs suppressed quorum-sensing-regulated virulence factors in Chromobacterium violaceum and Ps. aeruginosa, highlighting their broad-spectrum activity [15].
Advanced analyses, including SEM and TMT-labeled proteomics, revealed that AgNPs disrupt biofilm architecture, impair bacterial motility and adhesion, trigger oxidative stress responses, and downregulate key metabolic pathways [107]. AgNPs synthesized by Cedecea sp. demonstrated strong bactericidal and antibiofilm effects, with notable stability and efficacy against E. coli and Ps. aeruginosa [108].
In addition to demonstrating efficacy, our findings contribute to the early-stage optimization of AgNPs functionalization strategies. By integrating MIC and MBEC assessments across a diverse panel of MDR clinical isolates—many of which belong to the ESKAPE group—we provide data that may inform the rational design of AgNPs-based therapeutics. These insights are particularly relevant for developing adjunct or alternative therapies aimed at combating the growing threat of antimicrobial resistance in hospital-acquired infections.
In our study, we examined the efficacy of AgNPs formulations against several clinically relevant MDR pathogens. S. aureus and coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are major contributors to nosocomial and device-associated infections. These organisms, although typically part of the skin microbiota, can become pathogenic in immunocompromised individuals or when the epithelial barrier is disrupted [109,110,111,112]. Their virulence is mediated by surface adhesins, enzymes, and toxins and is particularly enhanced by their ability to form biofilms, which enable persistence on abiotic surfaces and lead to treatment failure and recurrence [111,113,114,115,116]. Methicillin-resistant S. aureus strains further complicate therapy, often necessitating last-line agents such as linezolid or vancomycin [117,118]. Infections like hidradenitis suppurativa and chronic abscesses are frequently associated with biofilm-producing Staphylococcus species [119,120,121,122]. Our findings confirm previous reports that AgNPs can effectively disrupt staphylococcal biofilms [113,115,116,123,124,125], with S. epidermidis showing the highest susceptibility to Ag@EG/PVP in both diffusion and MBEC assays. This supports its potential as a targeted strategy against biofilm-associated staphylococcal infections.
Acinetobacter baumannii is a Gram-negative opportunist known for its exceptional genomic plasticity and ability to accumulate diverse resistance determinants. The World Health Organization designates carbapenem-resistant A. baumannii as a critical priority pathogen [126,127]. The clinical isolates in our study harbored OXA-type carbapenemases (e.g., OXA-51, OXA-40, etc.) and aminoglycoside resistance genes such as aac(6′)-Iad, reflecting its high resistance burden [128,129,130,131,132]. Although it was not the most susceptible species to AgNPs in our assays, its inclusion remains essential given its notorious biofilm formation and limited treatment options. Continued testing of AgNPs formulations, particularly Ag@EG/PVP, is warranted to assess efficacy against persistent and resistant strains.
Pseudomonas aeruginosa is a major cause of ventilator-associated pneumonia, catheter-related infections, and chronic wound infections. Its resistance mechanisms include the production of carbapenemases (e.g., VIM-2 and IMP-13) and overexpression of multidrug efflux pumps like MexAB-OprM [125,133,134,135,136,137,138]. Moreover, it forms robust, structured biofilms that impede antibiotic penetration and alter bacterial metabolic states, reducing treatment efficacy [125]. Our study demonstrated that Ag@EG/PVP exhibited excellent antibiofilm and antimicrobial activity against Ps. aeruginosa, consistent with previous work on AgNPs-mediated disruption of biofilms and quorum sensing [138,139,140]. These findings highlight its relevance in combating Pseudomonas-related infections, particularly in intensive care settings.
Klebsiella pneumoniae presents a formidable clinical challenge due to its ability to acquire and disseminate resistance genes through horizontal gene transfer. In our isolates, extended-spectrum β-lactamase production and carbapenemase expression (e.g., NDM and OXA-48) were frequently observed, mirroring global resistance patterns [139,140,141,142]. This species is also known for its capacity to form dense biofilms on mucosal surfaces and medical devices. Interestingly, K. pneumoniae demonstrated the lowest MBEC50 values in our assays, indicating that Ag@EG/PVP is particularly effective in eradicating its biofilms. This aligns with the existing literature on AgNPs-mediated biofilm disruption in Enterobacteriaceae [124,139,140,141,142].
The high antimicrobial and antibiofilm efficacy observed with Ag@EG and Ag@PEG/PVP formulations underscores their therapeutic potential. MIC values were consistent with those required for biofilm eradication, reinforcing their activity against planktonic and sessile forms. Ag@EG/PVP in particular emerged as the most effective formulation, especially against K. pneumoniae and S. epidermidis. The enhanced performance is likely due to the synergistic effects of EG and PVP. PVP serves as a steric stabilizer, preventing aggregation and enhancing colloidal stability, while EG, as a polyol and reducing agent, promotes uniform nanoparticle size and improves aqueous dispersion [143,144]. This combination supports a controlled and sustained release of Ag+ ions—the primary antimicrobial component [145]. Furthermore, the increased surface functionalization provided by these polymers enhances nanoparticle interaction with bacterial membranes, promoting oxidative stress, membrane disruption, and inhibition of vital cellular processes [145].
The reproducibility of our findings was ensured through the use of multiple independent replicates and triplicate assays. Antimicrobial and antibiofilm results were consistent across all tested strains, and statistical analysis confirmed the significant superiority of Ag@EG/PVP compared to other formulations (p < 0.05). These results support the continued investigation of AgNPs-based strategies for clinical use, including wound healing, orthopedic implants, and cardiovascular device coatings [15,16,17]. Our study contributes to the growing evidence that polymer-functionalized AgNPs—particularly those utilizing combined stabilizers—represent a viable path forward in the fight against multidrug-resistant, biofilm-forming pathogens.
4. Materials and Methods
4.1. Synthesis and Functionalization of Silver Nanoparticles
AgNPs were synthesized and functionalized using four different formulations containing either individual or combined polymeric agents, according to previous published results: EG, PEG, and PVP. The resulting formulations were designated as Ag@EG, Ag@PEG, Ag@EG/PVP, and Ag@PEG/PVP [146,147].
To synthesize AgNPs, 1 g of AgNO3 was dissolved in 300 mL of ultrapure water. Separately, 20 g of NaOH was dissolved in 400 mL of ultrapure water, followed by the addition of one of the following: 3 g EG, 3 g PEG, 1.5 g EG + 1.5 g PVP, or 1.5 g PEG + 1.5 g PVP. Each mixture was stirred continuously at 80 °C. The silver nitrate solution was gradually added dropwise into each polymer solution under constant stirring to facilitate nanoparticle formation. The suspensions were then vacuum-filtered, washed three times with sterile distilled water, and air-dried. The concentration of AgNPs was adjusted to 1 mg/mL in all biological assays.
All inhibition zone measurements presented in this study refer to diameters and are reported in millimeters (mm). Values are expressed with a single decimal place, consistent with the precision of the measuring instrument used.
4.2. Bacterial Strains and Growth Conditions
A total of 68 clinical bacterial isolates were included in this study. These were obtained from hospitalized patients diagnosed with infections that are challenging to treat, including urinary tract infections, respiratory infections, and wound-associated infections. Strains were collected from Romanian healthcare settings, where the prevalence of MDR pathogens is high and contributes to elevated morbidity and mortality rates.
The bacterial panel comprised E. coli (n = 11), K. pneumoniae (n = 11), Ps. aeruginosa (n = 23), S. aureus (n = 12), S. epidermidis (n = 6), and S. lugdunensis (n = 6). MDR profiles were confirmed based on resistance to at least three distinct antibiotic classes. Isolates were initially characterized for antimicrobial resistance and virulence factors to ensure clinical relevance and strain diversity.
Antibiotic susceptibility testing was performed using disc diffusion or broth microdilution methods, and resistance patterns were interpreted according to CASFM/EUCAST guidelines (Table S1). The antibiotic discs used for each bacterial species were as follows:
- Staphylococcus spp.: cefoxitin (FOX) 30 µg, gentamicin (CN) 10 µg, erythromycin (E) 15 µg, clindamycin (DA) 2 µg, quinupristin-dalfopristin (QD) 15 µg, norfloxacin (NOR) 10 µg, linezolid (LZD) 10 µg, fusidic acid (FD) 10 µg, cotrimoxazole (SXT) 1.25 µg, rifampicin (RD) 5 µg, kanamycin (K) 30 µg, penicillin G (P) 6 µg, and tetracycline (TE) 30 µg;
- E. coli and K. pneumoniae: amoxicillin/clavulanic acid (AMC) 20/10 µg, amoxicillin (AML) 20 µg, ticarcillin (TIC) 75 µg, piperacillin (PRL) 100 µg, imipenem (IPM) 10 µg, cefotaxime (CTX) 5 µg, cefoxitin (FOX) 30 µg, ceftazidime (CAZ) 10 µg, ticarcillin/clavulanic acid (TIM) 75/100 µg, ciprofloxacin (CIP) 5 µg, levofloxacin (LEV) 5 µg, nalidixic acid (NA) 20 µg, aztreonam (ATM) 30 µg, norfloxacin (NOR) 10 µg, and moxifloxacin (MOX) 5 µg;
- Ps. aeruginosa: imipenem (IPM) 10 µg, meropenem (MEM) 10 µg, aztreonam (ATM) 30 µg, cefepime (FEP) 30 µg, ceftazidime (CAZ) 10 µg, ticarcillin/clavulanic acid (TIM) 75/100 µg, ticarcillin (TIC) 75 µg, fosfomycin (FOT) 200 µg, and ceftazidime/avibactam (CZA) 10/4 µg.
Reference strains used for quality control included S. aureus subsp. aureus Rosenbach ATCC® 12600™, E. coli ATCC® 25922™, E. coli NCTC 13846, and Ps. aeruginosa ATCC® 27853™.
All isolates were stored at −80 °C in nutrient broth supplemented with 20% glycerol. Before use, strains were subcultured on nutrient agar and incubated at 35 °C for 24 h. Bacterial suspensions were prepared in 0.9% saline and adjusted to a 0.5 McFarland turbidity standard (approximately 1–3 × 10⁸ CFU/mL).
4.3. Qualitative Antimicrobial Activity: Disc Diffusion Assay
The antimicrobial activity of each AgNPs formulation was evaluated using a modified disc diffusion assay. Mueller–Hinton agar plates were inoculated with the bacterial suspensions using sterile swabs in three directions at 45° angles, following CLSI guidelines. A 5 µL volume of each AgNPs suspension (1 mg/mL) was spotted on the agar surface. Plates were allowed to sit for 15 min to permit diffusion and were then incubated at 37 °C for 20 h. Inhibition zones were measured in millimeters. Each isolate was tested in triplicate across three independent experiments to ensure reproducibility.
4.4. MIC Determination
MICs were assessed via broth microdilution in 96-well plates. An initial AgNPs concentration of 5 mg/mL was serially diluted two-fold to obtain concentrations ranging from 0.5 mg/mL to 0.0039 mg/mL. A volume of 15 µL of bacterial suspension was added to each well containing 135 µL of Mueller–Hinton broth and AgNPs. Plates were incubated for 24 h at 37 °C without shaking. MICs were recorded based on visible turbidity and confirmed by spectrophotometric absorbance readings at 620 nm. Assays were performed in triplicate and repeated independently three times.
4.5. Evaluation of AgNPs on Biofilm Formation
Biofilm inhibition was quantified using a crystal violet assay. AgNPs were serially diluted as described above. A 15 µL aliquot of bacterial suspension was added to 135 µL of the AgNPs dilution in a 96-well polystyrene microplate. Plates were incubated at 37 °C for 24 h. Wells were gently washed with sterile water to remove planktonic cells, fixed with cold methanol for 5 min, stained with 1% crystal violet for 20 min, and rinsed. The bound dye was solubilized using 33% acetic acid, and absorbance was measured at 492 nm. All experiments were performed in triplicate and repeated independently on three occasions.
4.6. Statistical Analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using GraphPad Prism (v9.5) and IBM SPSS Statistics (v27). One-way ANOVA followed by Tukey’s post hoc test was used for multiple group comparisons, while two-group comparisons employed Student’s t-test. A p-value of <0.05 was considered statistically significant. The normality of data distribution was assessed prior to analysis.
The number of clinical isolates was selected to ensure sufficient biological variability across species and infection types, reflecting the microbial diversity encountered in clinical practice. Triplicate assays and independent repetitions were employed to enhance statistical power and experimental reliability.
4.7. Methodological Considerations and Study Limitations
One limitation of this study is the absence of unmodified AgNPs as a comparative control group. Although sterile distilled water was used as a negative control, future work should include bare AgNPs to delineate better the impact of polymeric functionalization on antimicrobial and antibiofilm efficacy.
In addition, dynamic light scattering (DLS) and zeta potential measurements were not performed. These parameters are essential for assessing nanoparticle size distribution and surface charge, which influence colloidal stability, cellular uptake, and bioactivity. Future studies should include comprehensive physicochemical characterization to better understand the relationship between nanoparticle structure and antimicrobial performance.
Furthermore, all experiments were conducted in vitro. While these findings offer valuable insight into AgNPs antimicrobial and antibiofilm potential, in vivo studies are necessary to evaluate biodistribution, toxicity, pharmacokinetics, and therapeutic relevance. Animal models and preclinical trials are recommended for future investigations to support clinical translation.
5. Conclusions
Our study demonstrated that AgNPs functionalized with polymeric agents exhibit significant antimicrobial and antibiofilm efficacy, particularly when coated with EG and PVP. Among 68 multidrug-resistant clinical isolates, Ag@EG/PVP consistently outperformed other formulations across both planktonic and biofilm conditions, notably against Ps. aeruginosa and K. pneumoniae.
Our findings underscore the importance of surface functionalization in enhancing the bioactivity of AgNPs and support the development of polymer-modified nanomaterials as adjuncts or alternatives to conventional antibiotics. The demonstrated dual efficacy against resistant pathogens and biofilms positions Ag@EG/PVP as a promising candidate for biomedical applications, including wound dressings, implant coatings, and anti-infective surfaces. Future studies should focus on in vivo validation, toxicity profiling, and formulation strategies to advance clinical translation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26093930/s1.
Author Contributions
Conceptualization, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., L.S.C.M. and M.M.M.; methodology, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., L.S.C.M. and M.M.M.; software, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., L.S.C.M. and M.M.M.; validation, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., A.A., L.S.C.M. and M.M.M.; formal analysis, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., L.S.C.M. and M.M.M.; investigation, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., A.A., L.S.C.M. and M.M.M.; resources, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., L.S.C.M. and M.M.M.; data curation, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., A.A., L.S.C.M. and M.M.M.; writing—original draft preparation, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., A.A., L.S.C.M. and M.M.M.; writing—review and editing, M.S., A.M.H., B.B.-G., L.M.D., A.M.G., A.A., L.S.C.M. and M.M.M.; visualization, M.S. and M.M.M.; supervision, M.S. and M.M.M.; project administration, M.S. and M.M.M.; funding acquisition, A.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Carol Davila University of Medicine and Pharmacy, Bucharest, Romania, postdoctoral program contract number 28567/02.10.2023.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (17088/28.06.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Acknowledgments
Alina-Maria Holban acknowledges the financial support of the French Institute in Romania through the Programme de Bourses France Excellence Roumanie.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations and Symbols
| AgNPs | Silver nanoparticles |
| IL | Interleukin |
| MRS | Methicillin-resistant Staphylococcus |
| WHO | World Health Organization |
| SCN | Coagulase-negative Staphylococcus |
| TLR | Toll-like receptor |
| Antibiotics | |
| AK | Amikacin |
| AMC | Amoxicillin |
| AMP | Ampicillin |
| ATM | Aztreonam |
| C | Chloramphenicol |
| CAZ | Ceftazidime |
| CIP | Ciprofloxacin |
| CT | Colistin |
| CXM | Cefuroxime |
| DA | Clindamycin |
| E | Erythromycin |
| FEP | Cefepime |
| FOX | Cefoxitin |
| GN | Gentamicin |
| IMP | Imipenem |
| LEV | Levofloxacin |
| LZD | Linezolid |
| MEM | Meropenem |
| NET | Netilmicin |
| P | Penicillin |
| SXT | Trimethoprim sulfamethoxazole |
| TEC | Teicoplanin |
| TIM | Ticarcillin-clavulanate |
| TOB | Tobramycin |
References
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.; Poinern, G. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of Silver Nanoparticles Synthesized Using Urtica dioica Linn. Leaves and Their Synergistic Effects with Antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Popa, L.G.; Giurcaneanu, C.; Mihai, M.M.; Beiu, C.; Orzan, O.A.; Negoita, S.; Burcea, M.; Turlea, R.I.; Enachescu, C.I. The use of cadaveric skin allografts in the management of extensive wounds. Rom. J. Leg. Med. 2021, 29, 37–44. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Chircov, C.; Dolete, G.; Oprea, O.-C.; Vasile, B.-S.; Crainiceanu, S.A.; Nicoara, A.-I.; Marinas, I.C.; Stan, M.S.; et al. Antioxidant, Antitumoral, Antimicrobial, and Prebiotic Activity of Magnetite Nanoparticles Loaded with Bee Pollen/Bee Bread Extracts and 5-Fluorouracil. Antioxidants 2024, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.; Călugăreanu, A.; Orzan, O.A. Recent advances in diagnosis and therapy of skin cancers through nanotechnological approaches. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Cambridge, UK, 2017; pp. 285–306. [Google Scholar] [CrossRef]
- Mihai, M.M.; Bălăceanu-Gurău, B.; Ion, A.; Holban, A.M.; Gurău, C.-D.; Popescu, M.N.; Beiu, C.; Popa, L.G.; Popa, M.I.; Dragomirescu, C.C.; et al. Host-Microbiome Crosstalk in Chronic Wound Healing. Int. J. Mol. Sci. 2024, 25, 4629. [Google Scholar] [CrossRef]
- Mihai, M.M.; Popa, M.I.; Holban, A.M.; Gheorghe-Barbu, I.; Popa, L.G.; Chifiriuc, M.-C.; Giurcăneanu, C.; Bleotu, C.; Cucu, C.I.; Lazăr, V.; et al. Clinical and microbiological features of host-bacterial interplay in chronic venous ulcers versus other types of chronic skin ulcers. Front. Microbiol. 2024, 14, 1326904. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed] [PubMed Central]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia Calcarata: Potential Source for the Fabrication of Bioactive Silver Nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral Silver Nanocrystals with Distinct Scattering Signatures. Angew. Chem. Int. Ed. 2006, 45, 4597–4601. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert. Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Wilkinson, L.J.; White, R.J.; Chipman, J.K. Silver and Nanoparticles of Silver in Wound Dressings: A Review of Efficacy and Safety. J. Wound Care 2011, 20, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnæs, J.; Mijakovic, I. Silver Nanoparticles Produced from Cedecea Sp. Exhibit Antibiofilm Activity and Remarkable Stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Huang, G.; Wei, Z.; Nie, K.; Liu, Z.; Deng, C.; Wang, D. IL-10 Gene-Modified Human Amniotic Mesenchymal Stem Cells Augment Regenerative Wound Healing by Multiple Synergistic Effects. Stem Cells Int. 2019, 2019, 9158016. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Bozaci, E.; Akar, E.; Ozdogan, E.; Demir, A.; Altinisik, A.; Seki, Y. Application of Carboxymethylcellulose Hydrogel Based Silver Nanocomposites on Cotton Fabrics for Antibacterial Property. Carbohydr. Polym. 2015, 134, 128–135. [Google Scholar] [CrossRef]
- Emam, H.E.; Saleh, N.H.; Nagy, K.S.; Zahran, M.K. Functionalization of Medical Cotton by Direct Incorporation of Silver Nanoparticles. Int. J. Biol. Macromol. 2015, 78, 249–256. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Sun, H.; Wang, S.; Zhang, R. PH-Responsive Release Behavior and Anti-Bacterial Activity of Bacterial Cellulose-Silver Nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial Wound Dressing Nanofiber Mats from Multicomponent (Chitosan/Silver-NPs/Polyvinyl Alcohol) Systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.R.; Figueira, D.R.; de Sá, K.D.; Miguel, S.P.; Fradique, R.G.; Mendonça, A.G.; Correia, I.J. 3D Printed Scaffolds with Bactericidal Activity Aimed for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2016, 93, 1432–1445. [Google Scholar] [CrossRef]
- Slane, J.; Vivanco, J.; Rose, W.; Ploeg, H.-L.; Squire, M. Mechanical, Material, and Antimicrobial Properties of Acrylic Bone Cement Impregnated with Silver Nanoparticles. Mater. Sci. Eng. C 2015, 48, 188–196. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-Biocomposite Scaffolds of Chitosan, Carboxymethyl Cellulose and Silver Nanoparticle Modified Cellulose Nanowhiskers for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef]
- Strydom, S.J.; Rose, W.E.; Otto, D.P.; Liebenberg, W.; de Villiers, M.M. Poly(Amidoamine) Dendrimer-Mediated Synthesis and Stabilization of Silver Sulfonamide Nanoparticles with Increased Antibacterial Activity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 85–93. [Google Scholar] [CrossRef]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver Nanoparticles for Water Pollution Monitoring and Treatments: Ecosafety Challenge and Cellulose-Based Hybrids Solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.G.; Torres, J.G.T.; Vidal, H.P.; Rocha, M.A.L.; Pérez, J.C.A.; López, I.C.; Romero, D.D.L.C.; Reyna, A.E.E.D.L.M.; Sosa, J.G.P.; Pavón, A.A.S.; et al. Application of Silver Nanoparticles for Water Treatment. In Silver Nanoparticles—Fabrication, Characterization and Applications; Maaz, K., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Kueh, A.B.H.; Salim, M.R.; Zaini, M.A.A. Silver Nanoparticles in the Water Environment in Malaysia: Inspection, Characterization, Removal, Modeling, and Future Perspective. Sci. Rep. 2018, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations. Microorganisms 2020, 8, 132. [Google Scholar] [CrossRef]
- Mikołajczuk-Szczyrba, A.; Kieliszek, M.; Giurgiulescu, L.; Sokołowska, B. Characteristics and application of silver nanoparticles in the food industry—Review. Carpathian J. Food Sci. Technol. 2019, 11, 153–160. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A Review of Silver Nanoparticles in Food Packaging Technologies: Regulation, Methods, Properties, Migration, and Future Challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Kumar, L.; Bisen, M.; Harjai, K.; Chhibber, S.; Azizov, S.; Lalhlenmawia, H.; Kumar, D. Advances in Nanotechnology for Biofilm Inhibition. ACS Omega 2023, 8, 21391–21409. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Partoazar, A. Targeting bacterial biofilm-related genes with nanoparticle-based strategies. Front. Micrbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, A.; Mitra, R.; Kundu, S.; Banerjee, P.; Chowdhury, A.A.; Ghosh, S. Escaping the ESKAPE pathogens: A review on antibiofilm potential of nanoparticles. Microb. Pathog. 2024, 194, 106842. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Y.; Alani, A.K.; Ahmed, B.O.; Hamid, L.L. Effect of biosynthesized silver nanoparticle size on antibacterial and anti-biofilm activity against pathogenic multidrug resistant bacteria. OpenNano 2024, 20, 100213. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bose, S.; Shaoo, A.; Das, S.K. Nanotechnology-based therapeutic approaches: An advanced strategy to target the biofilm of ESKAPE pathogens. Mater. Adv. 2023, 4, 2544–2572. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Das, A.; Oupický, D.; Sadykov, M.; Romanova, S. Strategies to overcome antibiotic resistance: Silver nanoparticles and vancomycin in pathogen eradication. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Szymczak, M.; Pankowski, J.A.; Kwiatek, A.; Grygorcewicz, B.; Karczewska-Golec, J.; Sadowska, K.; Golec, P. An effective antibiofilm strategy based on bacteriophages armed with silver nanoparticles. Sci. Rep. 2024, 14, 9088. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Water-Soluble Fluorescent Silver Nanoclusters. Adv. Mater. 2010, 22, 1078–1082. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in Health Care: Antimicrobial Effects and Safety in Use. In Current Problems in Dermatology; Hipler, U.-C., Elsner, P., Eds.; KARGER: Basel, Switzerland, 2006; Volume 33, pp. 17–34. [Google Scholar]
- Saha, S.K.; Das, S.; Chowdhury, P.; Saha, S.K. Biocompatibility of a Sonochemically Synthesized Poly(N-Isopropyl Acrylamide)/Silica Nanocomposite. RSC Adv. 2014, 4, 14457. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef]
- Parisien, A.; Allain, B.; Zhang, J.; Mandeville, R.; Lan, C.Q. Novel Alternatives to Antibiotics: Bacteriophages, Bacterial Cell Wall Hydrolases, and Antimicrobial Peptides. J. Appl. Microbiol. 2008, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poblete, H.; Agarwal, A.; Thomas, S.S.; Bohne, C.; Ravichandran, R.; Phopase, J.; Comer, J.; Alarcon, E.I. New Insights into Peptide–Silver Nanoparticle Interaction: Deciphering the Role of Cysteine and Lysine in the Peptide Sequence. Langmuir 2016, 32, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Retout, M.; Gosselin, B.; Mattiuzzi, A.; Ternad, I.; Jabin, I.; Bruylants, G. Peptide-Conjugated Silver Nanoparticles for the Colorimetric Detection of the Oncoprotein Mdm2 in Human Serum. ChemPlusChem 2022, 87, e202100450. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging Trends in Clinical Implications of Bio-Conjugated Silver Nanoparticles in Drug Delivery. Colloid Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Willner, I.; Baron, R.; Willner, B. Growing Metal Nanoparticles by Enzymes. Adv. Mater. 2006, 18, 1109–1120. [Google Scholar] [CrossRef]
- Sweet, M.J.; Chessher, A.; Singleton, I. Review: Metal-Based Nanoparticles; Size, Function, and Areas for Advancement in Applied Microbiology. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 80, pp. 113–142. [Google Scholar]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial Silver: Uses, Toxicity and Potential for Resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Sampath, G.; Chen, Y.-Y.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K.; Shyu, D.J.H. Biologically Synthesized Silver Nanoparticles and Their Diverse Applications. Nanomaterials 2022, 12, 3126. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Shittu, E.O.; Akpor, O.B.; Rotimi, D.; Batiha, G.E.-S. Silver Nanoparticles Restrict Microbial Growth by Promoting Oxidative Stress and DNA Damage. EXCLI J. 2020, 19, 492. [Google Scholar] [CrossRef] [PubMed]
- Beiu, C.; Giurcaneanu, C.; Mihai, M.; Popa, L.; Hage, R. Darier Disease—A Clinical Illustration of Its High Variable Expressivity. Cureus 2019, 11, e6292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitipir, C.; Barbu, M.A.; Popa, L.G.; Mihai, M.M.; Radu, I.; Mirea, D.; Giurcaneanu, C.; Scăunașu, R.V. Management of papulo-pustular rash induced by epidermal growth factor receptor inhibitors. Farmacia 2015, 63, 875–881. [Google Scholar]
- Furuse, Y. Analysis of research intensity on infectious disease by disease burden reveals which infectious diseases are neglected by researchers. Proc. Natl. Acad. Sci. USA 2019, 116, 478–483. [Google Scholar] [CrossRef]
- Savulescu, S.E.; Berteanu, M.; Filipescu, I.; Beiu, C.; Mihai, M.M.; Popa, L.G.; Popescu, S.I.; Balescu, I.; Bacalbasa, N.; Popescu, M.N. Repetitive Peripheral Magnetic Stimulation (rPMS) in Subjects With Lumbar Radiculopathy: An Electromyography-guided Prospective, Randomized Study. Vivo 2021, 35, 623–627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Low, M.; Almog, R.; Balicer, R.D.; Liberman, N.; Raz, R.; Peretz, A.; Nitzan, O. Infectious disease burden and antibiotic prescribing in primary care in Israel. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem. Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef]
- Bala, A.; Rani, G. A Review on Phytosynthesis, Affecting Factors and Characterization Techniques of Silver Nanoparticles Designed by Green Approach. Int. Nano Lett. 2020, 10, 159–176. [Google Scholar] [CrossRef]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Merghni, A.; Lassoued, M.A.; Noumi, E.; Hadj Lajimi, R.; Adnan, M.; Mastouri, M.; Snoussi, M. Cytotoxic Activity and Antibiofilm Efficacy of Biosynthesized Silver Nanoparticles against Methicillin-Resistant Staphylococcus aureus Strains Colonizing Cell Phones. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9410024. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green Synthesized Silver Nanoparticles Destroy Multidrug Resistant Bacteria via Reactive Oxygen Species Mediated Membrane Damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of Plant-Mediated Synthesis of Silver Nanoparticles—A Review on Biomolecules Involved, Characterisation and Antibacterial Activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yu, X.; Xu, C.; Li, X.; Li, Z.; Wei, D.; Liu, Y. New Toxicity Mechanism of Silver Nanoparticles: Promoting Apoptosis and Inhibiting Proliferation. PLoS ONE 2015, 10, e0122535. [Google Scholar] [CrossRef]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of Silver-Coated Fabric to Prevent Bacterial Colonization and Subsequent Device-Based Biofilm Formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Abd El-Mongy, M.; Eid, K.F. Comparative Study between Two Red Algae for Biosynthesis Silver Nanoparticles Capping by SDS: Insights of Characterization and Antibacterial Activity. Microb. Pathog. 2019, 129, 224–232. [Google Scholar] [CrossRef]
- Mukundan, D.; Mohankumar, R.; Vasanthakumari, R. Comparative Study of Synthesized Silver and Gold Nanoparticles Using Leaves Extract of Bauhinia tomentosa Linn and Their Anticancer Efficacy. Bull. Mater. Sci. 2017, 40, 335–344. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria: Activity of Silver Nanoparticles against MDR Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Gaddam, S.A.; Subba Rao, Y.; Prasad, T.N.V.K.V.; Varada Reddy, A.; Sai Gopal, D.V.R. Biofabrication of Silver Nanoparticles Using Andrographis paniculata. Eur. J. Med. Chem. 2014, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An Enhancement of Antimicrobial Efficacy of Biogenic and Ceftriaxone-Conjugated Silver Nanoparticles: Green Approach. Environ. Sci. Pollut. Res. 2018, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver Nanoparticles in Dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-Negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Kalwar, K.; Shan, D. Antimicrobial Effect of Silver Nanoparticles (AgNPs) and Their Mechanism—A Mini Review. Micro Nano Lett. 2018, 13, 277–280. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Liao, S.; Jiang, C.; Wang, L.; Tang, Y.; Wu, G.; Dai, G.; Chen, L. Quantitative Proteomics Reveals the Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2020, 19, 3109–3122. [Google Scholar] [CrossRef]
- Mihai, M.M.; Holban, A.M.; Ion, A.; Bălăceanu, B.; Gurău, C.D.; Lazăr, V. Chapter 4—Nano-targeted drug delivery approaches for biofilm-associated infections. In Micro and Nano Technologies, Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–138. ISBN 9780323907927. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The Biofilm-associated Bacterial Infections Unrelated to Indwelling Devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Rimoldi, S.G.; Cavallo, I.; D’Agosto, G.; Trento, E.; Cagnoni, G.; Palazzin, A.; Pagani, C.; Romeri, F.; De Vecchi, E.; et al. Microbial Biofilm Correlates with an Increased Antibiotic Tolerance and Poor Therapeutic Outcome in Infective Endocarditis. BMC Microbiol. 2019, 19, 228. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; Camargo, E.R.D.; Barbosa, D.B. The Growing Importance of Materials That Prevent Microbial Adhesion: Antimicrobial Effect of Medical Devices Containing Silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S.K. Significance of Microbial Biofilms in Food Industry: A Review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing Implant-Related Infections: Active Release Strategies. Chem. Soc. Rev. 2006, 35, 780. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.B.; Johnson, M.E.; Wong, A.C.L. Biofilm Formation and Contamination of Cheese by Nonstarter Lactic Acid Bacteria in The Dairy Environment. J. Dairy Sci. 2001, 84, 1926–1936. [Google Scholar] [CrossRef]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm Formation by Lactic Acid Bacteria and Resistance to Environmental Stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef]
- Brandl, M.T. Fitness of Human Enteric Pathogens on Plants and Implications for Food Safety. Annu. Rev. Phytopathol. 2006, 44, 367–392. [Google Scholar] [CrossRef]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental Survival Mechanisms of the Foodborne Pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A Foodborne Pathogen That Knows How to Survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Jefferson, K.K. What Drives Bacteria to Produce a Biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Mashwani, Z.-R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in Plants and Plant Extracts of Silver Nanoparticles with Potent Antimicrobial Properties: Current Status and Future Prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic Gold–Silver Nanoparticles Mediate Bacterial Killing by Disrupting the Actin Cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vega-Baudrit, J.; Gamboa, S.M.; Rojas, E.R.; Martinez, V.V. Synthesis and Characterization of Silver Nanoparticles and Their Application as an Antibacterial Agent. Int. J. Biosen. Bioelectron. 2019, 5, 172. [Google Scholar] [CrossRef]
- Abdollahi, H.; Noaparast, M.; Shafaei, S.Z.; Manafi, Z.; Muñoz, J.A.; Tuovinen, O.H. Silver-Catalyzed Bioleaching of Copper, Molybdenum and Rhenium from a Chalcopyrite–Molybdenite Concentrate. Int. Biodeterior. Biodegrad. 2015, 104, 194–200. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A Facile Synthesis Method of Hydroxyethyl Cellulose-Silver Nanoparticle Scaffolds for Skin Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-P.; Li, S.-C.; Wang, R.-Y. Development of Biosynthesized Silver Nanoparticles Based Formulation for Treating Wounds during Nursing Care in Hospitals. J. Photochem. Photobiol. B Biol. 2018, 183, 137–141. [Google Scholar] [CrossRef]
- Parlet, C.P.; Brown, M.M.; Horswill, A.R. Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019, 27, 497–507. [Google Scholar] [CrossRef]
- Williams, M.R.; Costa, S.K.; Zaramela, L.S.; Khalil, S.; Todd, D.A.; Winter, H.L.; Sanford, J.A.; O’neill, A.M.; Liggins, M.C.; Nakatsuji, T.; et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 2019, 11, eaat8329. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, C.W.; Lee, H.K. Interactions between Host Immunity and Skin-Colonizing Staphylococci: No Two Siblings Are Alike. Int. J. Mol. Sci. 2019, 20, 718. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Bhattarai, N.R.; Khanal, B. Comparative evaluation of methods for the detection of biofilm formation in coagulase-negative staphylococci and correlation with antibiogram. Infect. Drug Resist. 2018, 11, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, I.; Popa, M.; Gabriela Măruţescu, L. Molecular Features of Virulence and Resistance Mechanisms in Nosocomial and Community-Acquired Staphylococcus aureus. In Staphylococcus aureus; Hemeg, H., Ozbak, H., Afrin, F., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Blicharz, L.; Rudnicka, L.; Samochocki, Z. Staphylococcus aureus: An underestimated factor in the pathogenesis of atopic dermatitis? Postępy Dermatol. Alergol. 2019, 36, 11–17. [Google Scholar] [CrossRef]
- Theos, K.R.; Johnson, K.M.; Johnson, D.W. Staphylococcus aureus Antibiotic Susceptibilities in Infections in an Outpatient Dermatology Office on O’ahu. Hawaii J. Med. Public Health 2019, 78, 163–168. [Google Scholar] [PubMed] [PubMed Central]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef]
- Ibler, K.S.; Kromann, C.B. Recurrent furunculosis—Challenges and management: A review. Clin. Cosmet. Investig. Dermatol. 2014, 7, 59–64. [Google Scholar] [CrossRef]
- Cheng, A.G.; DeDent, A.C.; Schneewind, O.; Missiakas, D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; Nicholson, C.L.; Parks-Miller, A.; Hamzavi, I.H. Hidradenitis suppurativa: An update on connecting the tracts. F1000Research 2017, 6, 1272. [Google Scholar] [CrossRef]
- Ardon, C.; Prens, E.; Fuursted, K.; Ejaz, R.N.; Shailes, J.; Jenssen, H.; Jemec, G. Biofilm production and antibiotic susceptibility of Staphylococcus epidermidis strains from Hidradenitis Suppurativa lesions. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Holmes, A.E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 5 February 2025). [PubMed] [PubMed Central]
- Boutal, H.; Vogel, A.; Bernabeu, S.; Devilliers, K.; Creton, E.; Cotellon, G.; Plaisance, M.; Oueslati, S.; Dortet, L.; Jousset, A.; et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 909–915. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Kanj, S.S. Acinetobacter Infection: Treatment and Prevention—UpToDate. Available online: https://www.uptodate.com/contents/treatment-and-prevention-of-acinetobacter-infection (accessed on 4 April 2025).
- Butler, D.A.; Biagi, M.; Tan, X.; Qasmieh, S.; Bulman, Z.P.; Wenzler, E. Multidrug Resistant Acinetobacter baumannii: Resistance by Any Other Name Would Still be Hard to Treat. Curr. Infect. Dis. Rep. 2019, 21, 46. [Google Scholar] [CrossRef]
- Hu, S.; Niu, L.; Zhao, F.; Yan, L.; Nong, J.; Wang, C.; Gao, N.; Zhu, X.; Wu, L.; Bo, T.; et al. Identification of Acinetobacter baumannii and its carbapenem-resistant gene blaOXA-23-like by multiple cross displacement amplification combined with lateral flow biosensor. Sci. Rep. 2019, 9, 17888. [Google Scholar] [CrossRef]
- Palmieri, M.; D’andrea, M.M.; Pelegrin, A.C.; Perrot, N.; Mirande, C.; Blanc, B.; Legakis, N.; Goossens, H.; Rossolini, G.M.; van Belkum, A. Abundance of Colistin-Resistant, OXA-23- and ArmA-Producing Acinetobacter baumannii Belonging to International Clone 2 in Greece. Front. Microbiol. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Wachino, J.-I.; Yamane, K.; Shibata, N.; Yagi, T.; Shibayama, K.; Kato, H.; Arakawa, Y. Spread of novel aminoglycoside resistance gene aac(6′)-Iad among Acinetobacter clinical isolates in Japan. Antimicrob. Agents Chemother. 2004, 48, 2075–2080. [Google Scholar] [CrossRef]
- Cerezales, M.; Xanthopoulou, K.; Wille, J.; Krut, O.; Seifert, H.; Gallego, L.; Higgins, P.G. Mobile Genetic Elements Harboring Antibiotic Resistance Determinants in Acinetobacter baumannii Isolates from Bolivia. Front. Microbiol. 2020, 11, 919. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- El Zowalaty, M.E.; Al Thani, A.A.; Webster, T.J.; Schweizer, H.P.; Nasrallah, G.K.; Marei, H.E.; Ashour, H.M. Pseudomonas aeruginosa: Arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol. 2015, 10, 1683–1706. [Google Scholar] [CrossRef]
- Fujii, A.; Seki, M.; Higashiguchi, M.; Tachibana, I.; Kumanogoh, A.; Tomono, K. Community-acquired, hospital-acquired, and healthcare-associated pneumonia caused by Pseudomonas aeruginosa. Respir. Med. Case Rep. 2014, 12, 30–33. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Surveillance of Antimicrobial Resistance in Europe—2018; ECDC: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2018 (accessed on 18 February 2025).
- Potron, A.; Fournier, D.; Emeraud, C.; Triponney, P.; Plésiat, P.; Naas, T.; Dortet, L. Evaluation of the Immunochromatographic NG-Test Carba 5 for Rapid Identification of Carbapenemase in Nonfermenters. Antimicrob. Agents Chemother. 2019, 63, e00968-19. [Google Scholar] [CrossRef]
- Morales, S.; Gallego, M.A.; Vanegas, J.M.; Jiménez, J.N. Detection of carbapenem resistance genes in Pseudomonas aeruginosa isolates with several phenotypic susceptibility profiles. CES Med. 2018, 32, 203–214. [Google Scholar] [CrossRef]
- Shigemura, K.; Osawa, K.; Kato, A.; Tokimatsu, I.; Arakawa, S.; Shirakawa, T.; Fujisawa, M. Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients. J. Antibiot. 2015, 68, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.D.; Taee, S.; Dezfuli, A.A.; Meghdadi, H.; Shafie, F. Investigation of the prevalence of genes conferring resistance to carbapenems in Pseudomonas aeruginosa isolates from burn patients. Infect. Drug Resist. 2019, 12, 1153–1159. [Google Scholar] [CrossRef]
- Szabó, D.; Szentandrássy, J.; Juhász, Z.; Katona, K.; Nagy, K.; Rókusz, L. Imported PER-1 producing Pseudomonas aeruginosa, PER-1 producing Acinetobacter baumanii and VIM-2-producing Pseudomonas aeruginosa strains in Hungary. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 12. [Google Scholar] [CrossRef]
- Empel, J.; Filczak, K.; Mrówka, A.; Hryniewicz, W.; Livermore, D.M.; Gniadkowski, M. Outbreak of Pseudomonas aeruginosa infections with PER-1 extended-spectrum beta-lactamase in Warsaw, Poland: Further evidence for an international clonal complex. J. Clin. Microbiol. 2007, 45, 2829–2834. [Google Scholar] [CrossRef]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology 2008, 19, 075104. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on antimicrobial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Popescu, E.L.; Bălăşoiu, M.; Cristea, O.M.; Stoica, A.E.; Oprea, O.C.; Vasile, B.Ş.; Grumezescu, A.M.; Băncescu, G.; Busuioc, C.J.; Mogoşanu, G.D.; et al. Study of antimicrobial effects of functionalized silver nanoparticles. Rom. J. Morphol. Embryol. 2019, 60, 939–946. [Google Scholar] [PubMed]
- Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Paoliello, M.; Mogosanu, G.D.; Streba, C.T.; Popescu, E.L.; Stoica, A.E.; Bîrcă, A.C.; et al. The Effect of Silver Nanoparticles on Antioxidant/Pro-Oxidant Balance in a Murine Model. Int. J. Mol. Sci. 2020, 21, 1233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).