Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA
Abstract
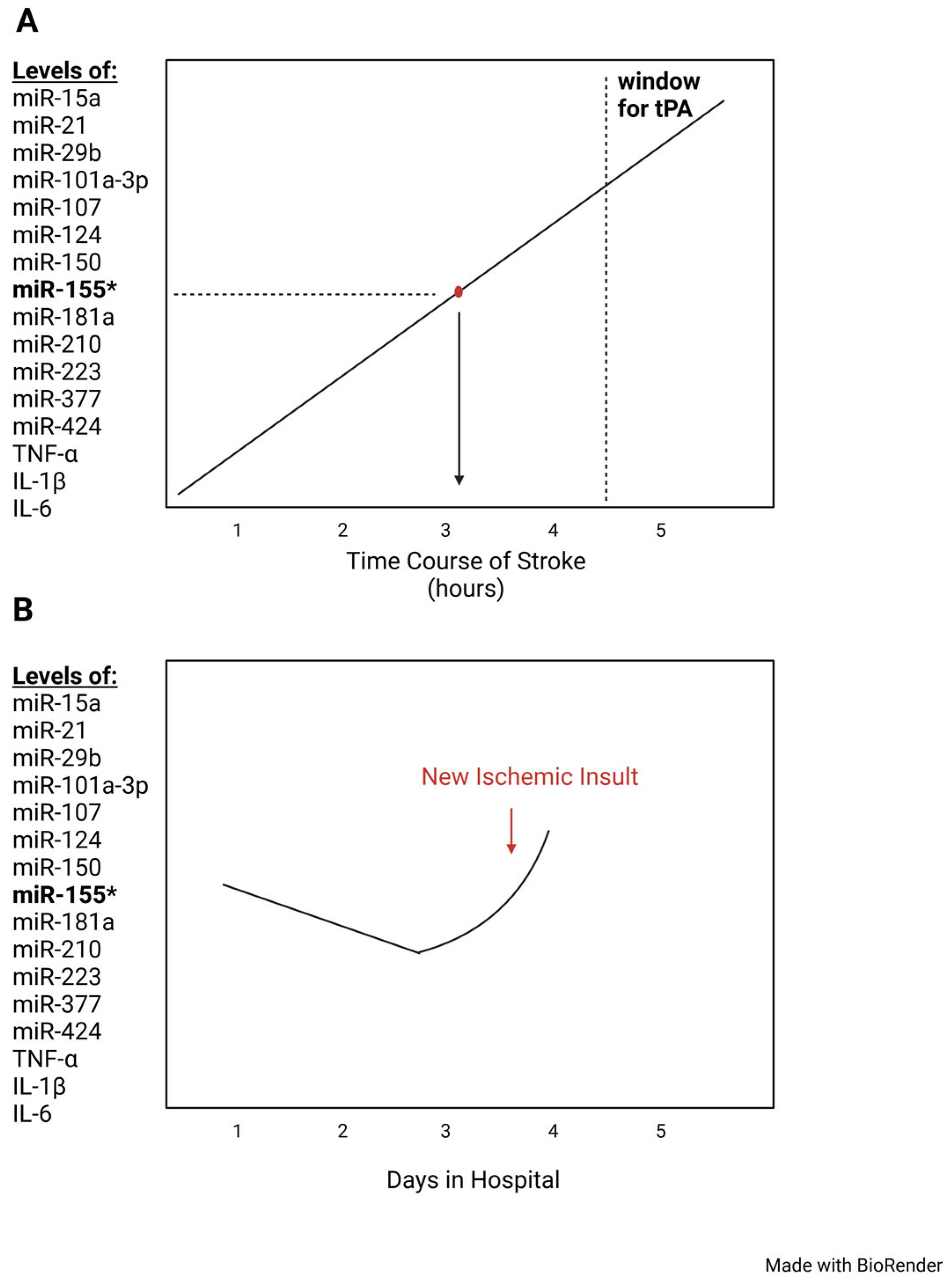
:1. Introduction
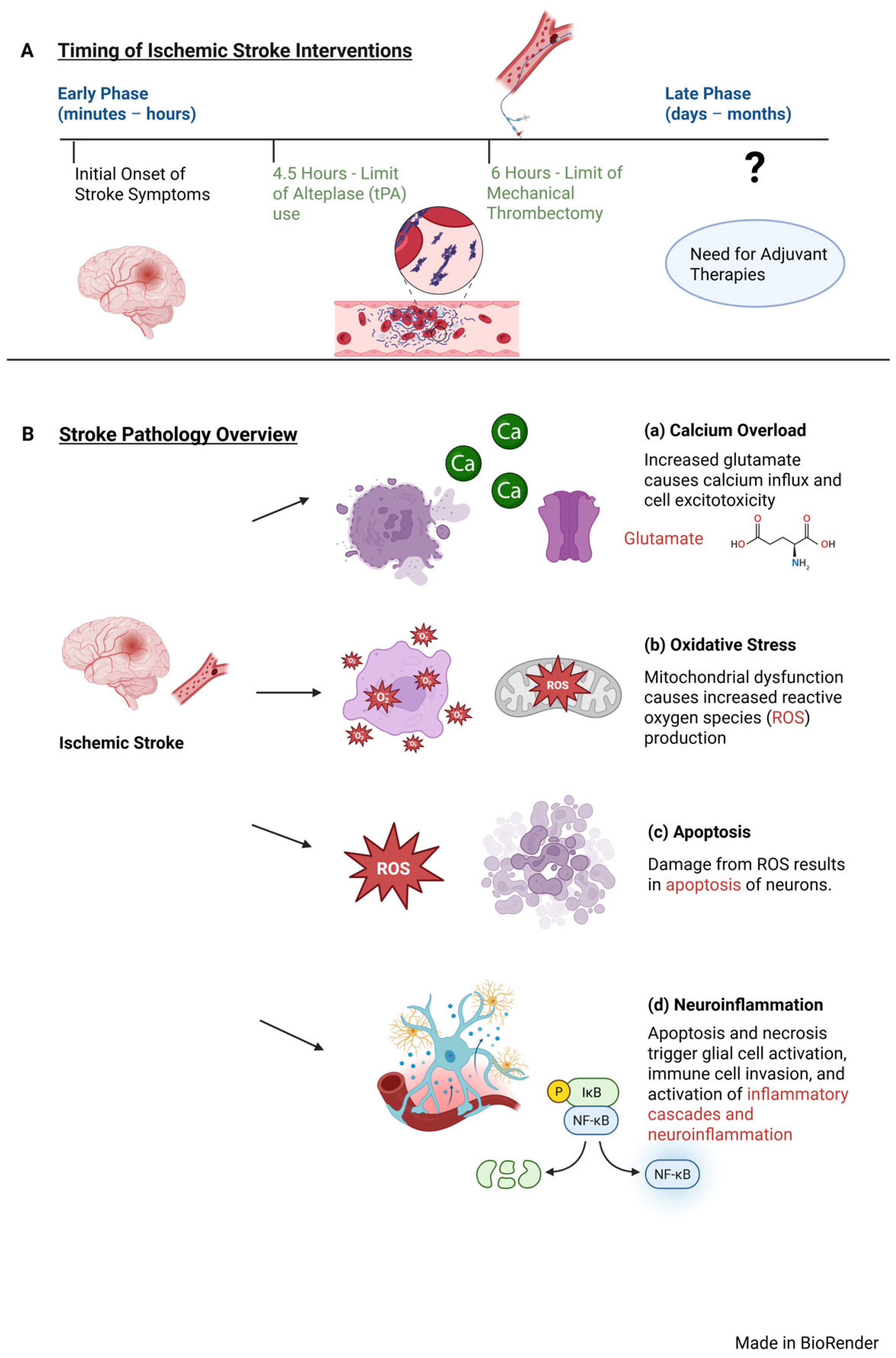
2. Overview of Stroke Pathophysiology
3. Stroke Management Timing Considerations
4. Non-Coding RNAs: Promising Biomarkers for Stroke Progression
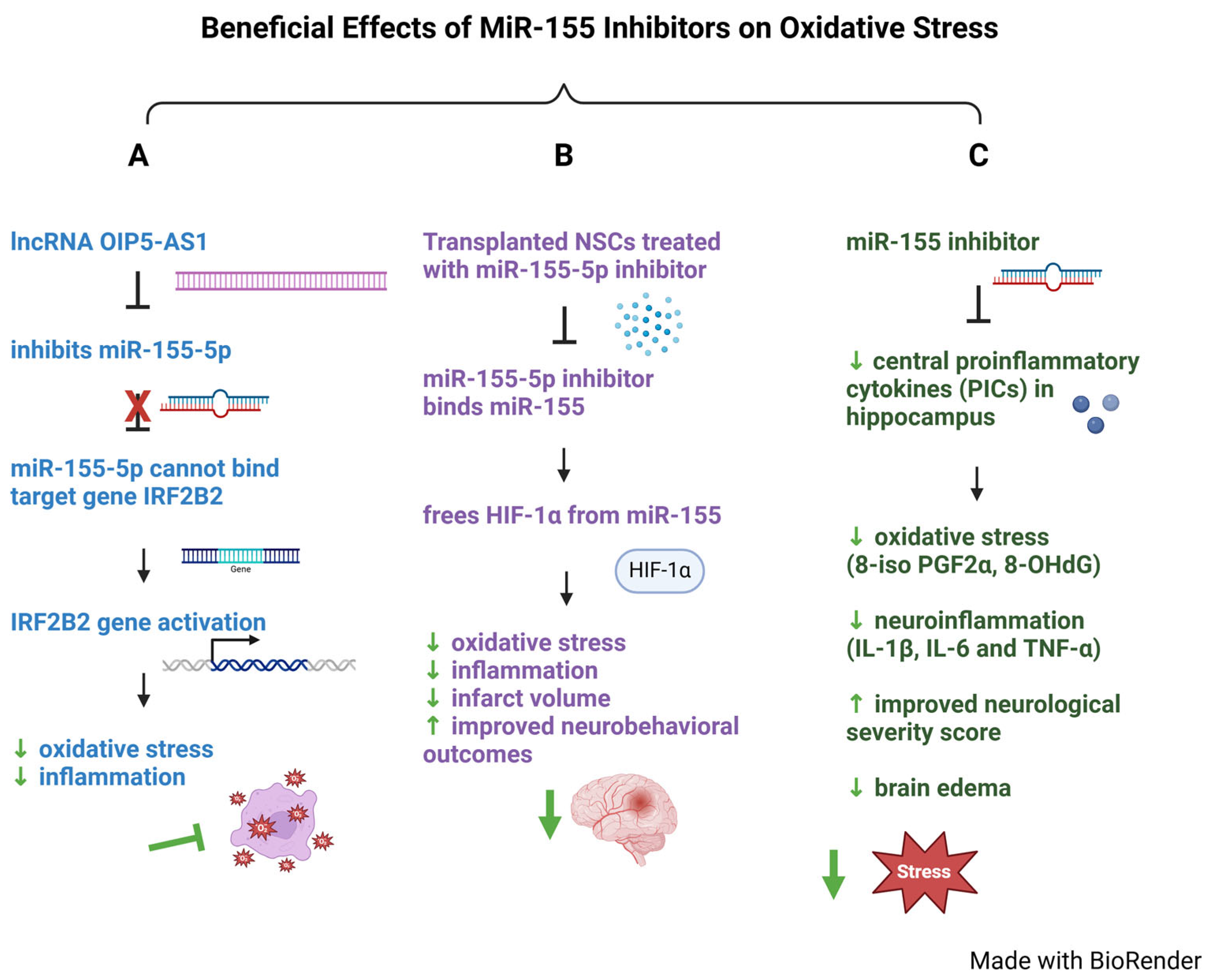
4.1. Role of MiR-155 in Oxidative Stress
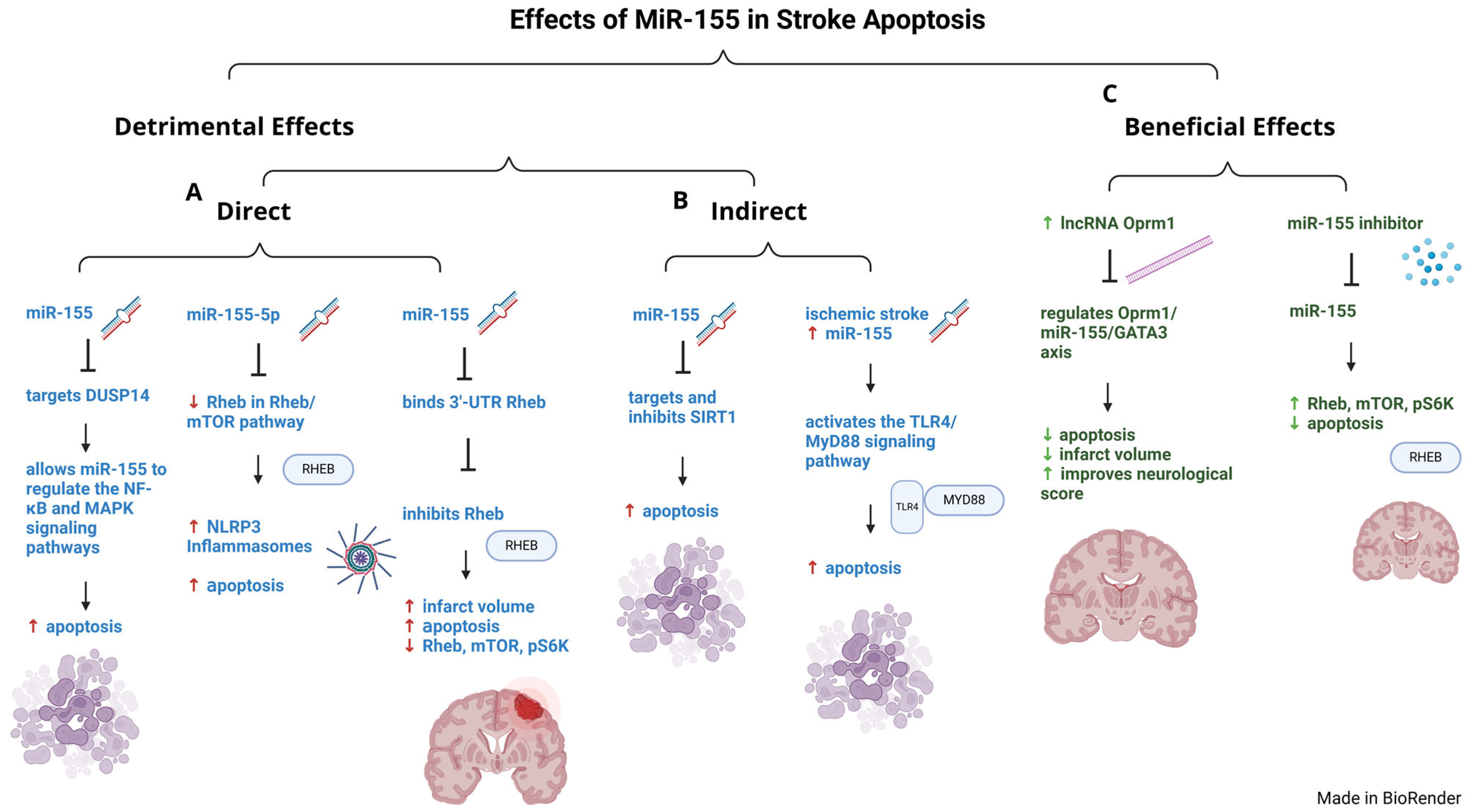
4.2. Role of MiR-155 in Stroke-Related Apoptosis
4.3. Role of MiR-155 in Neuroinflammation
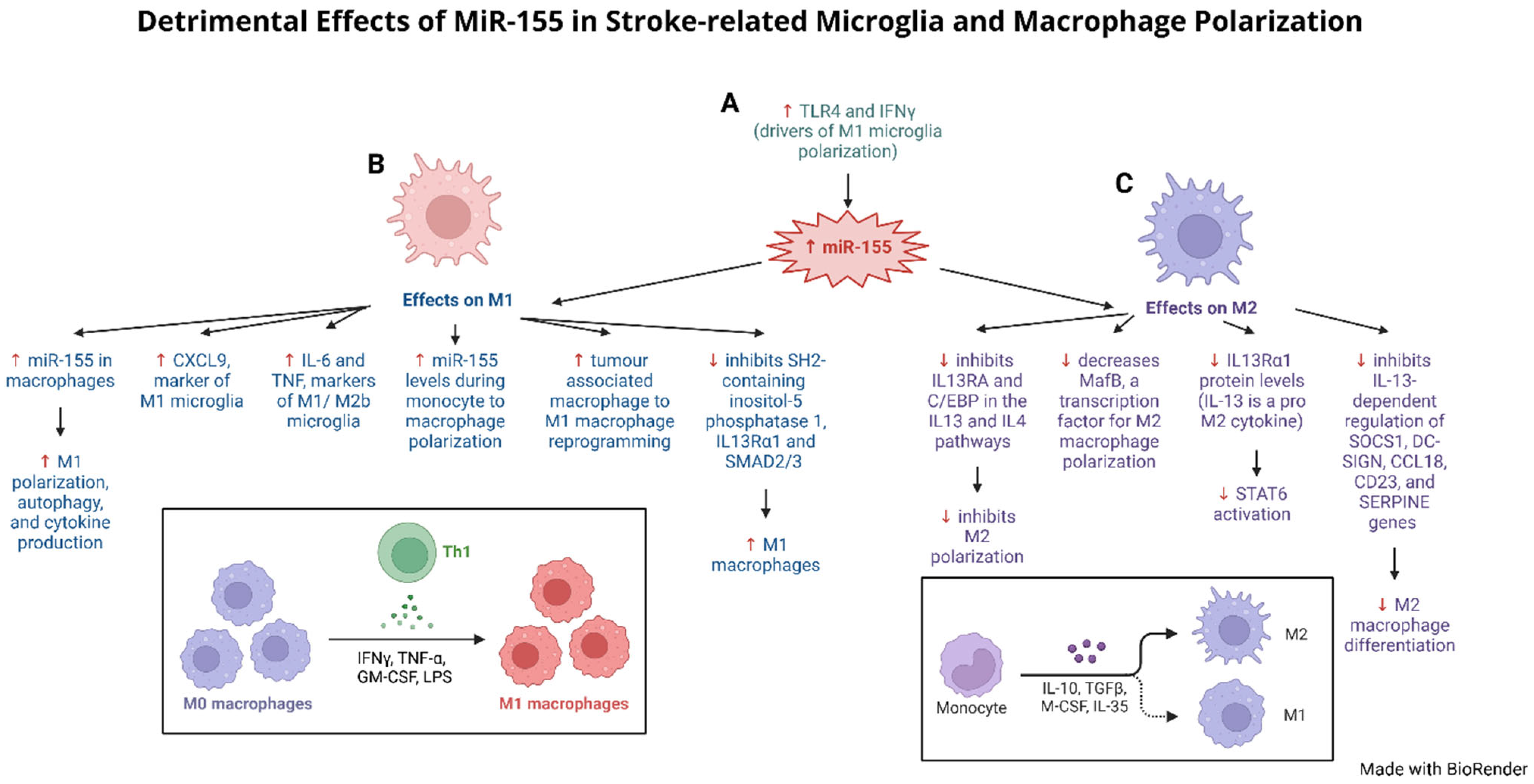
5. Influence of MiR-155 Modulating Microglia and Astrocytes After Stroke
6. Proposal to Use NcRNA MiR-155 as a Biomarker for Ischemic Stroke
6.1. Urgent Clinical Need for Time-Sensitive Biomarkers to Manage Stroke
6.2. Artificial Intelligence and Stroke Biomarkers
7. Discussion
7.1. Central Role of MiR-155: Could the Pathology of Acute Ischemic Stroke Be Reversed by miRNA-155 Specific Inhibitors
7.2. MiR-155 Inhibitors (anti-RNA) as Therapeutic for Ischemic Stroke: Antisense Oligonucleotides
7.3. MiR-155 as Biomarker for Ischemic Stroke
7.4. Outstanding Challenges and Future Stroke Research Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective Strategies for Ischemic Stroke-Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4334. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. 2), S6–S16. [Google Scholar] [CrossRef]
- Maida, C.D.; Norrito, R.L.; Rizzica, S.; Mazzola, M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 6297. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abejie, A.N.; et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Close, S.M. Stroke Facts What to know. In Stroke; CDC: Atlanta, GA, USA, 2024. [Google Scholar]
- World Health Organization. The Atlas of Heart Disease and Stroke/Judith Mackay and George Mensah with Shanthi Mendis and Kurt Greenland; World Health Organization: Geneva, Switzerland, 2004.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk factors for ischemic stroke: Differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Tirschwell, D.L.; Smith, N.L.; Heckbert, S.R.; Lemaitre, R.N.; Longstreth, W.T., Jr.; Psaty, B.M. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 2004, 63, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Bielecki, J.; Kocabas, E.; Singh, S.; Senff, J.R.; Casaubon, L.K.; Rosand, J.; Rac, V.E.; Pikula, A. Lifestyle approaches to hypertension for prevention of stroke and vascular cognitive impairment: A realist review protocol. BMJ Open 2024, 14, e088631. [Google Scholar] [CrossRef] [PubMed]
- Wassertheil-Smoller, S. Stroke in women. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Appelros, P.; Stegmayr, B.; Terént, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef]
- Kelly-Hayes, M. Influence of age and health behaviors on stroke risk: Lessons from longitudinal studies. J. Am. Geriatr. Soc. 2010, 58 (Suppl. 2), S325–S328. [Google Scholar] [CrossRef]
- Stewart, J.A.; Dundas, R.; Howard, R.S.; Rudd, A.G.; Wolfe, C.D. Ethnic differences in incidence of stroke: Prospective study with stroke register. BMJ 1999, 318, 967–971. [Google Scholar] [CrossRef]
- Sohrabji, F.; Okoreeh, A.; Panta, A. Sex hormones and stroke: Beyond estrogens. Horm. Behav. 2019, 111, 87–95. [Google Scholar] [CrossRef]
- Putaala, J. Ischemic Stroke in Young Adults. Continuum 2020, 26, 386–414. [Google Scholar] [CrossRef]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef]
- Zhang, X.; Hamblin, M.H.; Yin, K.J. Noncoding RNAs and Stroke. Neuroscientist 2019, 25, 22–26. [Google Scholar] [CrossRef]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Momeni, F.; Saadatpour, L.; Sahebkar, A.; Goodarzi, M.; Masoudifar, A.; Kouhpayeh, S.; Salehi, H.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J. Cell Physiol. 2018, 233, 856–865. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, C.; Yang, P.; He, S.; Liao, Q.; Kang, S.; Zhao, Y. Clustered microRNAs’ coordination in regulating protein-protein interaction network. BMC Syst. Biol. 2009, 3, 65. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Witten, L.; Slack, F.J. miR-155 as a novel clinical target for hematological malignancies. Carcinogenesis 2020, 41, 2–7. [Google Scholar] [CrossRef]
- Zheng, B.; Jeong, S.; Zhu, Y.; Chen, L.; Xia, Q. miRNA and lncRNA as biomarkers in cholangiocarcinoma(CCA). Oncotarget 2017, 8, 100819–100830. [Google Scholar] [CrossRef]
- Long, F.; Lin, Z.; Li, L.; Ma, M.; Lu, Z.; Jing, L.; Li, X.; Lin, C. Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol. Cancer 2021, 20, 26. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The definition of stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Morotti, A.; Poli, L.; Costa, P. Acute Stroke. Semin. Neurol. 2019, 39, 61–72. [Google Scholar] [CrossRef]
- Ohashi, S.N.; DeLong, J.H.; Kozberg, M.G.; Mazur-Hart, D.J.; van Veluw, S.J.; Alkayed, N.J.; Sansing, L.H. Role of Inflammatory Processes in Hemorrhagic Stroke. Stroke 2023, 54, 605–619. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef]
- Matys, P.; Mirończuk, A.; Starosz, A.; Grubczak, K.; Kochanowicz, J.; Kułakowska, A.; Kapica-Topczewska, K. Expanding Role of Interleukin-1 Family Cytokines in Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 10515. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kang, R.; Yu, Y.; Liu, J.; Zhang, Y.; Shen, C.; Wang, J.; Wu, P.; Shen, C.; Wang, Z. Crosstalk between miRNAs and their regulated genes network in stroke. Sci. Rep. 2016, 6, 20429. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Hénaut, L.; Grissi, M.; Brazier, F.; Assem, M.; Poirot-Leclercq, S.; Lenglet, G.; Boudot, C.; Avondo, C.; Boullier, A.; Choukroun, G.; et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci. Rep. 2019, 9, 6432. [Google Scholar] [CrossRef]
- Nakamura, K.; Shichita, T. Cellular and molecular mechanisms of sterile inflammation in ischaemic stroke. J. Biochem. 2019, 165, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Gasull, T.; Arboix, A. Molecular Mechanisms and Pathophysiology of Acute Stroke: Emphasis on Biomarkers in the Different Stroke Subtypes. Int. J. Mol. Sci. 2022, 23, 9476. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.; Ding, Y.; Li, M.; Yao, J.; Wang, L.; Gao, Z. Phased Treatment Strategies for Cerebral Ischemia Based on Glutamate Receptors. Front. Cell. Neurosci. 2019, 13, 168. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawakita, F.; Asada, R. Neuroelectric Mechanisms of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2022, 23, 3102. [Google Scholar] [CrossRef]
- Wen, B.; Xu, K.; Huang, R.; Jiang, T.; Wang, J.; Chen, J.; Chen, J.; He, B. Preserving mitochondrial function by inhibiting GRP75 ameliorates neuron injury under ischemic stroke. Mol. Med. Rep. 2022, 25, 165. [Google Scholar] [CrossRef]
- Rahi, V.; Kaundal, R.K. Exploring the intricacies of calcium dysregulation in ischemic stroke: Insights into neuronal cell death and therapeutic strategies. Life Sci. 2024, 347, 122651. [Google Scholar] [CrossRef]
- Ferrari, F.; Gorini, A.; Hoyer, S.; Villa, R.F. Glutamate metabolism in cerebral mitochondria after ischemia and post-ischemic recovery during aging: Relationships with brain energy metabolism. J. Neurochem. 2018, 146, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Arumugam, T.V.; Stokes, K.Y.; Granger, D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006, 113, 2105–2112. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Gregersen, R.; Meldgaard, M.; Clausen, B.H.; Heibøl, E.K.; Ladeby, R.; Knudsen, J.; Frandsen, A.; Owens, T.; Finsen, B. A role for interferon-gamma in focal cerebral ischemia in mice. J. Neuropathol. Exp. Neurol. 2004, 63, 942–955. [Google Scholar] [CrossRef]
- Guruswamy, R.; ElAli, A. Complex Roles of Microglial Cells in Ischemic Stroke Pathobiology: New Insights and Future Directions. Int. J. Mol. Sci. 2017, 18, 496. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.; Besselmann, M.; Müller, M.; Strecker, J.K.; Ringelstein, E.B.; Kiefer, R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: An investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2005, 196, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J.; Hayward, J.A.; Huang, C.; Huma, Z.E.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef]
- Liu, T.; Clark, R.K.; McDonnell, P.C.; Young, P.R.; White, R.F.; Barone, F.C.; Feuerstein, G.Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994, 25, 1481–1488. [Google Scholar] [CrossRef]
- Wang, X.; Yue, T.L.; Barone, F.C.; White, R.F.; Gagnon, R.C.; Feuerstein, G.Z. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol. Chem. Neuropathol. 1994, 23, 103–114. [Google Scholar] [CrossRef]
- Murakami, Y.; Saito, K.; Hara, A.; Zhu, Y.; Sudo, K.; Niwa, M.; Fujii, H.; Wada, H.; Ishiguro, H.; Mori, H.; et al. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J. Neurochem. 2005, 93, 1616–1622. [Google Scholar] [CrossRef]
- Del Zoppo, G.J.; Saver, J.L.; Jauch, E.C.; Adams, H.P., Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the American Heart Association/American Stroke Association. Stroke 2009, 40, 2945–2948. [Google Scholar] [CrossRef]
- Hughes, R.E.; Tadi, P.; Bollu, P.C. TPA Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Kohli, V.; Koltz, M.T. Indications for Surgical Intervention in the Treatment of Ischemic Stroke. In Stroke; Dehkharghani, S., Ed.; Exon Publications: Brisbane, AU, USA, 2021. [Google Scholar]
- Nie, J.H.; Li, T.X.; Zhang, X.Q.; Liu, J. Roles of Non-Coding RNAs in Normal Human Brain Development, Brain Tumor, and Neuropsychiatric Disorders. Noncoding RNA 2019, 5, 36. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, X.; Qian, W.; Cai, H.; Sun, X.; Zhang, W.; Tan, S.; Wu, Z.; Qian, P.; Ding, K.; et al. CCAR1 5’ UTR as a natural miRancer of miR-1254 overrides tamoxifen resistance. Cell Res. 2016, 26, 655–673. [Google Scholar] [CrossRef]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-cell communication: microRNAs as hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef]
- Drula, R.; Pardini, B.; Fu, X.; De Los Santos, M.C.; Jurj, A.; Pang, L.; El-Daly, S.M.; Fabris, L.; Knutsen, E.; Dragomir, M.P.; et al. 17β-estradiol promotes extracellular vesicle release and selective miRNA loading in ERα-positive breast cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2122053120. [Google Scholar] [CrossRef]
- Fabbri, M. TLRs as miRNA receptors. Cancer Res. 2012, 72, 6333–6337. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. microRNAs are ligands of Toll-like receptors. RNA 2013, 19, 737–739. [Google Scholar] [CrossRef]
- Ziats, M.N.; Rennert, O.M. Identification of differentially expressed microRNAs across the developing human brain. Mol. Psychiatry 2014, 19, 848–852. [Google Scholar] [CrossRef]
- Oh, Y.M.; Lee, S.W.; Kim, W.K.; Chen, S.; Church, V.A.; Cates, K.; Li, T.; Zhang, B.; Dolle, R.E.; Dahiya, S.; et al. Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy. Nat. Neurosci. 2022, 25, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.R.; Latourelle, J.C.; Bockholt, J.H.; Bregu, J.; Smock, J.; Paulsen, J.S.; Myers, R.H. MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology 2018, 90, e264–e272. [Google Scholar] [CrossRef]
- Maes, O.C.; Chertkow, H.M.; Wang, E.; Schipper, H.M. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr. Genom. 2009, 10, 154–168. [Google Scholar] [CrossRef]
- Scheper, M.F.; Iyer, A.; Anink, J.J.; Mesarosova, L.; Mills, J.D.; Aronica, E. Dysregulation of miR-543 in Parkinson’s disease: Impact on the neuroprotective gene SIRT1. Neuropathol. Appl. Neurobiol. 2023, 49, e12864. [Google Scholar] [CrossRef]
- Hezroni, H.; Perry, R.B.T.; Ulitsky, I. Long Noncoding RNAs in Development and Regeneration of the Neural Lineage. Cold Spring Harb. Symp. Quant. Biol. 2019, 84, 165–177. [Google Scholar] [CrossRef]
- Schneider, M.F.; Müller, V.; Müller, S.A.; Lichtenthaler, S.F.; Becker, P.B.; Scheuermann, J.C. LncRNA RUS shapes the gene expression program towards neurogenesis. Life Sci. Alliance 2022, 5, e202201504. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Chang, K.Y.; Li, Z.; Gates, K.; Rana, Z.A.; Dang, J.; Zhang, D.; Han, T.; Yang, C.S.; Cunningham, T.J.; et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol. Cell 2014, 53, 1005–1019. [Google Scholar] [CrossRef]
- Wu, D.P.; Zhao, Y.D.; Yan, Q.Q.; Liu, L.L.; Wei, Y.S.; Huang, J.L. Circular RNAs: Emerging players in brain aging and neurodegenerative diseases. J. Pathol. 2023, 259, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Fu, B.; Bao, J.; Su, R.; Zhao, H.; Liu, Z. Novel circular RNA 2960 contributes to secondary damage of spinal cord injury by sponging miRNA-124. J. Comp. Neurol. 2021, 529, 1456–1464. [Google Scholar] [CrossRef]
- Doxakis, E. Insights into the multifaceted role of circular RNAs: Implications for Parkinson’s disease pathogenesis and diagnosis. npj Park. Dis. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Dube, U.; Del-Aguila, J.L.; Li, Z.; Budde, J.P.; Jiang, S.; Hsu, S.; Ibanez, L.; Fernandez, M.V.; Farias, F.; Norton, J.; et al. An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat. Neurosci. 2019, 22, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T. MicroRNAs and Regeneration in Animal Models of CNS Disorders. Neurochem. Res. 2020, 45, 188–203. [Google Scholar] [CrossRef]
- Tam, W. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene 2001, 274, 157–167. [Google Scholar] [CrossRef]
- Mashima, R. Physiological roles of miR-155. Immunology 2015, 145, 323–333. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Kelly, P.J.; Lemmens, R.; Tsivgoulis, G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke 2021, 52, 2697–2706. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, K.; Wang, Y.; Shi, F.D. Neurovascular Inflammation and Complications of Thrombolysis Therapy in Stroke. Stroke 2023, 54, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Maranini, B.; Ciancio, G.; Ferracin, M.; Cultrera, R.; Negrini, M.; Sabbioni, S.; Govoni, M. microRNAs and Inflammatory Immune Response in SARS-CoV-2 Infection: A Narrative Review. Life 2022, 12, 288. [Google Scholar] [CrossRef]
- Roganović, J.R. microRNA-146a and -155, upregulated by periodontitis and type 2 diabetes in oral fluids, are predicted to regulate SARS-CoV-2 oral receptor genes. J. Periodontol. 2021, 92, 35–43. [Google Scholar] [CrossRef]
- Tsitsiou, E.; Lindsay, M.A. microRNAs and the immune response. Curr. Opin. Pharmacol. 2009, 9, 514–520. [Google Scholar] [CrossRef]
- Ceppi, M.; Pereira, P.M.; Dunand-Sauthier, I.; Barras, E.; Reith, W.; Santos, M.A.; Pierre, P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2735–2740. [Google Scholar] [CrossRef]
- Alwani, A.; Andreasik, A.; Szatanek, R.; Siedlar, M.; Baj-Krzyworzeka, M. The Role of miRNA in Regulating the Fate of Monocytes in Health and Cancer. Biomolecules 2022, 12, 100. [Google Scholar] [CrossRef]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Simpson, D.A.; Leonard, M.O.; Tambuwala, M.M.; Cummins, E.P.; et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell. Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef]
- Louafi, F.; Martinez-Nunez, R.T.; Sanchez-Elsner, T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta}. J. Biol. Chem. 2010, 285, 41328–41336. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.D.; Feng, S.Y.; Huang, A.F. Role of miR-155 in inflammatory autoimmune diseases: A comprehensive review. Inflamm. Res. 2022, 71, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef]
- An, H.; Xu, H.; Zhang, M.; Zhou, J.; Feng, T.; Qian, C.; Qi, R.; Cao, X. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood 2005, 105, 4685–4692. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, L.; Huang, H.; Lei, D.; Lou, L.; Jin, Y.; Sun, J.; Wang, L. Downregulated FTO Promotes MicroRNA-155-mediated Inflammatory Response in Cerebral Ischemia/Reperfusion Injury. Neuroscience 2023, 526, 305–313. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef]
- Dunne, A.; O’Neill, L.A. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 2005, 579, 3330–3335. [Google Scholar] [CrossRef]
- Ke, F.; Wang, H.; Geng, J.; Jing, X.; Fang, F.; Fang, C.; Zhang, B.H. MiR-155 promotes inflammation and apoptosis via targeting SIRT1 in hypoxic-ischemic brain damage. Exp. Neurol. 2023, 362, 114317. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, K.; Xu, K.; Liu, Q.H. MiR-155-5p accelerates cerebral ischemia-reperfusion injury via targeting DUSP14 by regulating NF-κB and MAPKs signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1408–1419. [Google Scholar]
- Chen, W.; Wang, L.; Liu, Z. MicroRNA-155 influences cell damage in ischemic stroke via TLR4/MYD88 signaling pathway. Bioengineered 2021, 12, 2449–2458. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, X.; Gao, Z.; Chu, L. miR-155-5p in Extracellular Vesicles Derived from Choroid Plexus Epithelial Cells Promotes Autophagy and Inflammation to Aggravate Ischemic Brain Injury in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 8603427. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.W.; Dickson, A.M.; Clay, G.; McCaffrey, A.P.; Wilson, M.E. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012, 287, 21816–21825. [Google Scholar] [CrossRef]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.Y.; Zen, K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef]
- Martinez-Nunez, R.T.; Louafi, F.; Sanchez-Elsner, T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J. Biol. Chem. 2011, 286, 1786–1794. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Puig-Kröger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabéu, C.; Vega, M.A.; Corbí, A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef]
- Kim, H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci. Rep. 2017, 7, 7591. [Google Scholar] [CrossRef]
- Wahl, S.M.; Allen, J.B.; Costa, G.L.; Wong, H.L.; Dasch, J.R. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor beta. J. Exp. Med. 1993, 177, 225–230. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Chen, T.; Peng, L.; Wang, C.; Xue, G.; Yu, S. DJ-1 regulates astrocyte activation through miR-155/SHP-1 signaling in cerebral ischemia/reperfusion injury. J. Neurochem. 2025, 169, e16230. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Chen, M.; Sun, Q.; Chen, H.; Li, Y. Up-regulating lncRNA OIP5-AS1 protects neuron injury against cerebral hypoxia-ischemia induced inflammation and oxidative stress in microglia/macrophage through activating CTRP3 via sponging miR-186-5p. Int. Immunopharmacol. 2021, 92, 107339. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Hari, A.; Qin, Z.; Couture, P.; Huang, H.; Lagace, D.C.; Stewart, A.F.R.; Chen, H.H. Loss of IRF2BP2 in Microglia Increases Inflammation and Functional Deficits after Focal Ischemic Brain Injury. Front. Cell. Neurosci. 2017, 11, 201. [Google Scholar] [CrossRef]
- Zhang, J.K.; Li, Y.; Yu, Z.T.; Jiang, J.W.; Tang, H.; Tu, G.L.; Xia, Y. OIP5-AS1 Inhibits Oxidative Stress and Inflammation in Ischemic Stroke Through miR-155-5p/IRF2BP2 Axis. Neurochem. Res. 2023, 48, 1382–1394. [Google Scholar] [CrossRef]
- Chinenov, Y.; Coppo, M.; Gupte, R.; Sacta, M.A.; Rogatsky, I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genom. 2014, 15, 656. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, S.; Jiang, P.; Liu, R.; Liu, X.; Qian, J.; Zheng, X.; Chu, Y. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: A novel anti-inflammation mechanism. Free Radic. Biol. Med. 2012, 52, 1307–1317. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Bai, L.; Du, Y.; Liu, L.; Chen, X. Effects of Inhibition of miR-155-5p in Neural Stem Cell Subarachnoid Transplant on Rats with Cerebral Infarction. Hum. Gene Ther. Methods 2019, 30, 184–193. [Google Scholar] [CrossRef]
- Sun, L.; Ji, S.; Xing, J. Inhibition of microRNA-155 Alleviates Neurological Dysfunction Following Transient Global Ischemia and Contribution of Neuroinflammation and Oxidative Stress in the Hippocampus. Curr. Pharm. Des. 2019, 25, 4310–4317. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Liu, L.; Jia, Y.; Yao, H.; Ma, F. Overexpression of the long non-coding RNA Oprm1 alleviates apoptosis from cerebral ischemia-reperfusion injury through the Oprm1/miR-155/GATA3 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Luo, Z.; Zhong, C.; Pan, X.; Xu, X. Influence of miR-155 on Cell Apoptosis in Rats with Ischemic Stroke: Role of the Ras Homolog Enriched in Brain (Rheb)/mTOR Pathway. Med. Sci. Monit. 2016, 22, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, F.; Lu, H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020, 260, 118305. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef]
- Sun, H.; Wang, C.; Zhou, Y.; Cheng, X. Long Noncoding RNA OIP5-AS1 Overexpression Promotes Viability and Inhibits High Glucose-Induced Oxidative Stress of Cardiomyocytes by Targeting MicroRNA-34a/SIRT1 Axis in Diabetic Cardiomyopathy. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2017–2027. [Google Scholar] [CrossRef]
- Yoon, D.; Pastore, Y.D.; Divoky, V.; Liu, E.; Mlodnicka, A.E.; Rainey, K.; Ponka, P.; Semenza, G.L.; Schumacher, A.; Prchal, J.T. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J. Biol. Chem. 2006, 281, 25703–25711. [Google Scholar] [CrossRef]
- Shahjouei, S.; Cai, P.Y.; Ansari, S.; Sharififar, S.; Azari, H.; Ganji, S.; Zand, R. Middle Cerebral Artery Occlusion Model of Stroke in Rodents: A Step-by-Step Approach. J. Vasc. Interv. Neurol. 2016, 8, 1–8. [Google Scholar]
- Li, Y.; Sun, J.; Wu, R.; Bai, J.; Hou, Y.; Zeng, Y.; Zhang, Y.; Wang, X.; Wang, Z.; Meng, X. Mitochondrial MPTP: A Novel Target of Ethnomedicine for Stroke Treatment by Apoptosis Inhibition. Front. Pharmacol. 2020, 11, 352. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Yu, T.; Nie, E.; Hu, Q.; Wu, W.; Zhi, T.; Jiang, K.; Wang, X.; Lu, X.; et al. Blocking MIR155HG/miR-155 axis inhibits mesenchymal transition in glioma. Neuro Oncol. 2017, 19, 1195–1205. [Google Scholar] [CrossRef]
- Liang, S.; Hu, J.; Zhang, A.; Li, F.; Li, X. miR-155 induces endothelial cell apoptosis and inflammatory response in atherosclerosis by regulating Bmal1. Exp. Ther. Med. 2020, 20, 128. [Google Scholar] [CrossRef]
- Silva, J.P.D.; Lizarte Neto, F.S.; Cirino, M.L.A.; Carvalho, C.A.M.; Carlotti, C.G., Jr.; Colli, B.O.; Tirapelli, D.; Tirapelli, L.F. Analysis of Caspase-9 protein and microRNAs miR-21, miR-126 and miR-155 related to the apoptosis mechanism in the cerebellum of rats submitted to focal cerebral ischemia associated with an alcoholism model. Arq. Neuropsiquiatr. 2019, 77, 689–695. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Y.; Xie, F.; Zhong, Y.; Wang, Y.; Xu, M.; Feng, J.; Charish, J.; Monnier, P.P.; Qin, X. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018, 25, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Pena-Philippides, J.C.; Caballero-Garrido, E.; Lordkipanidze, T.; Roitbak, T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J. Neuroinflammation 2016, 13, 287. [Google Scholar] [CrossRef]
- Hou, J.; Wang, P.; Lin, L.; Liu, X.; Ma, F.; An, H.; Wang, Z.; Cao, X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009, 183, 2150–2158. [Google Scholar] [CrossRef]
- Kundu, M.; Basu, J. The Role of microRNAs and Long Non-Coding RNAs in the Regulation of the Immune Response to Mycobacterium tuberculosis Infection. Front. Immunol. 2021, 12, 687962. [Google Scholar] [CrossRef]
- Nejad, C.; Stunden, H.J.; Gantier, M.P. A guide to miRNAs in inflammation and innate immune responses. FEBS J. 2018, 285, 3695–3716. [Google Scholar] [CrossRef]
- Sheedy, F.J.; Palsson-McDermott, E.; Hennessy, E.J.; Martin, C.; O’Leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’Neill, L.A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, X.; Hu, H.; Lin, X. The inhibitory effect of Gualou Guizhi Decoction on post-ischemic neuroinflammation via miR-155 in MCAO rats. Ann. Palliat. Med. 2021, 10, 1370–1379. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Forrest, A.R.; Kanamori-Katayama, M.; Tomaru, Y.; Lassmann, T.; Ninomiya, N.; Takahashi, Y.; de Hoon, M.J.; Kubosaki, A.; Kaiho, A.; Suzuki, M.; et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia 2010, 24, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.L.; Etzrodt, M.; De Palma, M.; Pittet, M.J. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013, 34, 350–359. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, L.; Zu, J.; Wang, Z.; Han, B.; Chen, B.; Cheng, M.; Ju, M.; Li, M.; Shu, G.; et al. Circulating Circular RNAs as Biomarkers for the Diagnosis and Prediction of Outcomes in Acute Ischemic Stroke. Stroke 2020, 51, 319–323. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, K.; Hassan, S.H.; Zhang, X.; Tang, X.; Pu, H.; Stetler, R.A.; Chen, J.; Yin, K.J. Endothelium-Targeted Deletion of microRNA-15a/16-1 Promotes Poststroke Angiogenesis and Improves Long-Term Neurological Recovery. Circ. Res. 2020, 126, 1040–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.Y.; Li, Y.J.; Hong, Z.; Wei, W.S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J. Neuroinflammation 2012, 9, 211. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Dong, L.; Zhao, J.; Zhang, C.; Zhu, C. Acetylbritannilactone Modulates MicroRNA-155-Mediated Inflammatory Response in Ischemic Cerebral Tissues. Mol. Med. 2015, 21, 197–209. [Google Scholar] [CrossRef]
- Tan, K.S.; Armugam, A.; Sepramaniam, S.; Lim, K.Y.; Setyowati, K.D.; Wang, C.W.; Jeyaseelan, K. Expression profile of MicroRNAs in young stroke patients. PLoS ONE 2009, 4, e7689. [Google Scholar] [CrossRef]
- Moon, J.M.; Xu, L.; Giffard, R.G. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J. Cereb. Blood Flow Metab. 2013, 33, 1976–1982. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Gao, Y.; Baral, S.; Zhou, Y.; Hu, B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci. Rep. 2015, 5, 13316. [Google Scholar] [CrossRef]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary Release of Exosomes From Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery After Stroke in Rats Treated With Exosomes Harvested From MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017, 26, 243–257. [Google Scholar]
- Xu, X.; Wen, Z.; Zhao, N.; Xu, X.; Wang, F.; Gao, J.; Jiang, Y.; Liu, X. MicroRNA-1906, a Novel Regulator of Toll-Like Receptor 4, Ameliorates Ischemic Injury after Experimental Stroke in Mice. J. Neurosci. 2017, 37, 10498–10515. [Google Scholar] [CrossRef]
- Tao, Z.; Zhao, H.; Wang, R.; Liu, P.; Yan, F.; Zhang, C.; Ji, X.; Luo, Y. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J. Neurol. Sci. 2015, 355, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.J.; Deng, Z.; Huang, H.; Hamblin, M.; Xie, C.; Zhang, J.; Chen, Y.E. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol. Dis. 2010, 38, 17–26. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gao, L.; Wang, R.; Liu, X.; Gao, Z.; Tao, Z.; Xu, C.; Song, J.; Ji, X.; et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 2013, 44, 1706–1713. [Google Scholar] [CrossRef]
- Rahmati, M.; Ferns, G.A.; Mobarra, N. The lower expression of circulating miR-210 and elevated serum levels of HIF-1α in ischemic stroke; Possible markers for diagnosis and disease prediction. J. Clin. Lab. Anal. 2021, 35, e24073. [Google Scholar] [CrossRef]
- Tiedt, S.; Dichgans, M. Role of Non-Coding RNAs in Stroke. Stroke 2018, 49, 3098–3106. [Google Scholar] [CrossRef]
- Srinivasan, S.; Yeri, A.; Cheah, P.S.; Chung, A.; Danielson, K.; De Hoff, P.; Filant, J.; Laurent, C.D.; Laurent, L.D.; Magee, R.; et al. Small RNA Sequencing across Diverse Biofluids Identifies Optimal Methods for exRNA Isolation. Cell 2019, 177, 446–462.e416. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, Y.; Wang, H.; Khadka, V.S.; Hu, L.; Ai, J.; Dou, Y.; Li, Y.; Dai, S.; Mason, C.E.; et al. Ratio-Based Method To Identify True Biomarkers by Normalizing Circulating ncRNA Sequencing and Quantitative PCR Data. Anal. Chem. 2019, 91, 6746–6753. [Google Scholar] [CrossRef]
- Chen, G.; Qian, H.M.; Chen, J.; Wang, J.; Guan, J.T.; Chi, Z.L. Whole transcriptome sequencing identifies key circRNAs, lncRNAs, and miRNAs regulating neurogenesis in developing mouse retina. BMC Genom. 2021, 22, 779. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Yoneoka, Y.; Takahashi, K.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.I.; Sonoda, T.; et al. Integrated extracellular microRNA profiling for ovarian cancer screening. Nat. Commun. 2018, 9, 4319. [Google Scholar] [CrossRef] [PubMed]
- Todo, K.; Iwata, T.; Doijiri, R.; Yamagami, H.; Morimoto, M.; Hashimoto, T.; Sonoda, K.; Yamazaki, H.; Koge, J.; Okazaki, S.; et al. Frequent Premature Atrial Contractions in Cryptogenic Stroke Predict Atrial Fibrillation Detection with Insertable Cardiac Monitoring. Cerebrovasc. Dis. 2020, 49, 144–150. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Denk, J.; Oberhauser, F.; Kornhuber, J.; Wiltfang, J.; Fassbender, K.; Schroeter, M.L.; Volk, A.E.; Diehl-Schmid, J.; Prudlo, J.; Danek, A.; et al. Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS ONE 2018, 13, e0197329. [Google Scholar] [CrossRef]
- Piscopo, P.; Grasso, M.; Puopolo, M.; D’Acunto, E.; Talarico, G.; Crestini, A.; Gasparini, M.; Campopiano, R.; Gambardella, S.; Castellano, A.E.; et al. Circulating miR-127-3p as a Potential Biomarker for Differential Diagnosis in Frontotemporal Dementia. J. Alzheimers Dis. 2018, 65, 455–464. [Google Scholar] [CrossRef]
- Ahlbrecht, J.; Martino, F.; Pul, R.; Skripuletz, T.; Sühs, K.W.; Schauerte, C.; Yildiz, Ö.; Trebst, C.; Tasto, L.; Thum, S.; et al. Deregulation of microRNA-181c in cerebrospinal fluid of patients with clinically isolated syndrome is associated with early conversion to relapsing-remitting multiple sclerosis. Mult. Scler. 2016, 22, 1202–1214. [Google Scholar] [CrossRef]
- Vistbakka, J.; Elovaara, I.; Lehtimäki, T.; Hagman, S. Circulating microRNAs as biomarkers in progressive multiple sclerosis. Mult. Scler. 2017, 23, 403–412. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef]
- Takousis, P.; Sadlon, A.; Schulz, J.; Wohlers, I.; Dobricic, V.; Middleton, L.; Lill, C.M.; Perneczky, R.; Bertram, L. Differential expression of microRNAs in Alzheimer’s disease brain, blood, and cerebrospinal fluid. Alzheimers Dement. 2019, 15, 1468–1477. [Google Scholar] [CrossRef]
- Zailaie, S.A.; Siddiqui, J.J.; Al Saadi, R.M.; Anbari, D.M.; Alomari, A.S.; Cupler, E.J. Serum Based miRNA as a Diagnostic Biomarker for Multiple Sclerosis: A Systematic Review and Meta-Analysis. Immunol. Investig. 2022, 51, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, T.; Matsuzaki, J.; Yamamoto, Y.; Sakurai, T.; Aoki, Y.; Takizawa, S.; Niida, S.; Ochiya, T. Serum MicroRNA-Based Risk Prediction for Stroke. Stroke 2019, 50, 1510–1518. [Google Scholar] [CrossRef]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef]
- Larrabeiti-Etxebarria, A.; Lopez-Santillan, M.; Santos-Zorrozua, B.; Lopez-Lopez, E.; Garcia-Orad, A. Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma. Cancers 2019, 11, 144. [Google Scholar] [CrossRef]
- Ghafoor, M.; Kamal, M.; Nadeem, U.; Husain, A.N. Educational Case: Myocardial Infarction: Histopathology and Timing of Changes. Acad. Pathol. 2020, 7, 2374289520976639. [Google Scholar] [CrossRef]
- Bejleri, J.; Jirström, E.; Donovan, P.; Williams, D.J.; Pfeiffer, S. Diagnostic and Prognostic Circulating MicroRNA in Acute Stroke: A Systematic and Bioinformatic Analysis of Current Evidence. J. Stroke 2021, 23, 162–182. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Long Non-coding RNA in CNS Injuries: A New Target for Therapeutic Intervention. Mol. Ther. Nucleic Acids 2019, 17, 754–766. [Google Scholar] [CrossRef]
- Marto, J.P.; Carvalho, A.S.; Mollet, I.G.; Mendonça, M.; Salavisa, M.; Meira, B.; Fernandes, M.; Serrazina, F.; Cabral, G.; Ventura, R.; et al. Proteomics to Identify New Blood Biomarkers for Diagnosing Patients With Acute Stroke. J. Am. Heart Assoc. 2023, 12, e030021. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, L.; Chan, C.L.; Zhang, Y.; Tong, S.W.; Zhang, X.; Ho, J.W.K.; Jiao, Y.; Rainer, T.H. Multi-Level Biomarkers for Early Diagnosis of Ischaemic Stroke: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 13821. [Google Scholar] [CrossRef]
- di Biase, L.; Bonura, A.; Pecoraro, P.M.; Carbone, S.P.; Di Lazzaro, V. Unlocking the Potential of Stroke Blood Biomarkers: Early Diagnosis, Ischemic vs. Haemorrhagic Differentiation and Haemorrhagic Transformation Risk: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 11545. [Google Scholar] [CrossRef]
- Soun, J.E.; Chow, D.S.; Nagamine, M.; Takhtawala, R.S.; Filippi, C.G.; Yu, W.; Chang, P.D. Artificial Intelligence and Acute Stroke Imaging. AJNR Am. J. Neuroradiol. 2021, 42, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Grefkes, C. Precision medicine in stroke: Towards personalized outcome predictions using artificial intelligence. Brain 2022, 145, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, N.A.; Baig, A.A.; Waqas, M.; Patel, T.R.; Dossani, R.H.; Wilson, M.; Cappuzzo, J.M.; Siddiqui, A.H.; Tutino, V.M.; Levy, E.I. Artificial Intelligence for Large-Vessel Occlusion Stroke: A Systematic Review. World Neurosurg. 2022, 159, 207–220.e201. [Google Scholar] [CrossRef]
- Caballero-Garrido, E.; Pena-Philippides, J.C.; Lordkipanidze, T.; Bragin, D.; Yang, Y.; Erhardt, E.B.; Roitbak, T. In Vivo Inhibition of miR-155 Promotes Recovery after Experimental Mouse Stroke. J. Neurosci. 2015, 35, 12446–12464. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Anthiya, S.; Griveau, A.; Loussouarn, C.; Baril, P.; Garnett, M.; Issartel, J.P.; Garcion, E. MicroRNA-Based Drugs for Brain Tumors. Trends Cancer 2018, 4, 222–238. [Google Scholar] [CrossRef]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef]
- Tang, L.; Chen, H.Y.; Hao, N.B.; Tang, B.; Guo, H.; Yong, X.; Dong, H.; Yang, S.M. microRNA inhibitors: Natural and artificial sequestration of microRNA. Cancer Lett. 2017, 407, 139–147. [Google Scholar] [CrossRef]
- Jiang, F.; Du, L.; Chen, Z.J.; Wang, X.; Ge, D.; Liu, N. LNP-miR-155 cy5 Inhibitor Regulates the Copper Transporter via the β-Catenin/TCF4/SLC31A1 Signal for Colorectal Cancer Therapy. Mol. Pharm. 2023, 20, 4138–4152. [Google Scholar] [CrossRef]
- Sayyed, A.A.; Gondaliya, P.; Mali, M.; Pawar, A.; Bhat, P.; Khairnar, A.; Arya, N.; Kalia, K. MiR-155 Inhibitor-Laden Exosomes Reverse Resistance to Cisplatin in a 3D Tumor Spheroid and Xenograft Model of Oral Cancer. Mol. Pharm. 2021, 18, 3010–3025. [Google Scholar] [CrossRef]
- Lv, H.; Guo, J.; Li, S.; Jiang, D. miR-155 inhibitor reduces the proliferation and migration in osteosarcoma MG-63 cells. Exp. Ther. Med. 2014, 8, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- van der Ree, M.H.; van der Meer, A.J.; van Nuenen, A.C.; de Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment. Pharmacol. Ther. 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Paoletti, A.; Rohmer, J.; Ly, B.; Pascaud, J.; Rivière, E.; Seror, R.; Le Goff, B.; Nocturne, G.; Mariette, X. Monocyte/Macrophage Abnormalities Specific to Rheumatoid Arthritis Are Linked to miR-155 and Are Differentially Modulated by Different TNF Inhibitors. J. Immunol. 2019, 203, 1766–1775. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Gomes da Silva, A.M.; Silbiger, V.N. miRNAs as biomarkers of atrial fibrillation. Biomarkers 2014, 19, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Butz, H.; Kinga, N.; Racz, K.; Patocs, A. Circulating miRNAs as biomarkers for endocrine disorders. J. Endocrinol. Investig. 2016, 39, 1–10. [Google Scholar] [CrossRef]
- Yan, H.; Ma, F.; Zhang, Y.; Wang, C.; Qiu, D.; Zhou, K.; Hua, Y.; Li, Y. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and meta-analysis. Medicine 2017, 96, e6825. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Adcock, I.M.; Garssen, J.; Mortaz, E.; Varahram, M.; Mirsaeidi, M.; Velayati, A. The roles of miRNAs as potential biomarkers in lung diseases. Eur. J. Pharmacol. 2016, 791, 395–404. [Google Scholar] [CrossRef]
- Herrold, A.A.; Kletzel, S.L.; Foecking, E.M.; Saban, K.L.; Przybycien-Szymanska, M.M.; Zilliox, M.; Bhaumik, D.; Lange, D.; Radke, J.R.; Salinas, I.; et al. miRNAs as Potential Biomarkers for Traumatic Brain Injury: Pathway From Diagnosis to Neurorehabilitation. J. Head Trauma Rehabil. 2021, 36, E155–E169. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, K.; Osei, J.; Kelly, W.; Poulsen, A.; Donahue, K.; Wang, J.; Hunter, M.; Bard, J.; Wang, J.; Poulsen, D. Circulating microRNAs as biomarkers in traumatic brain injury. Neuropharmacology 2019, 145, 199–208. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Anwar, F.; Zamzami, M.A.; Choudhry, H.; Shaik, M.M.; Tamargo, I.A.; Kamal, M.A. miRNAs as Circulating Biomarkers for Alzheimer’s Disease and Parkinson’s Disease. Med. Chem. 2016, 12, 217–225. [Google Scholar] [CrossRef]
- El-Koussy, M.; Schroth, G.; Brekenfeld, C.; Arnold, M. Imaging of acute ischemic stroke. Eur. Neurol. 2014, 72, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Huţanu, A.; Iancu, M.; Bălaşa, R.; Maier, S.; Dobreanu, M. Predicting functional outcome of ischemic stroke patients in Romania based on plasma CRP, sTNFR-1, D-Dimers, NGAL and NSE measured using a biochip array. Acta Pharmacol. Sin. 2018, 39, 1228–1236. [Google Scholar] [CrossRef]
- Sotgiu, S.; Zanda, B.; Marchetti, B.; Fois, M.L.; Arru, G.; Pes, G.M.; Salaris, F.S.; Arru, A.; Pirisi, A.; Rosati, G. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur. J. Neurol. 2006, 13, 505–513. [Google Scholar] [CrossRef]
- Satoh, T.; Otsuka, A.; Contassot, E.; French, L.E. The inflammasome and IL-1β: Implications for the treatment of inflammatory diseases. Immunotherapy 2015, 7, 243–254. [Google Scholar] [CrossRef]
- Loddick, S.A.; Turnbull, A.V.; Rothwell, N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 176–179. [Google Scholar] [CrossRef]
- Nappi, F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 3630. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Yu, T.; Deng, J.; Li, X.; Jin, J. Non-Coding RNA Regulatory Network in Ischemic Stroke. Front. Neurol. 2022, 13, 820858. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, Z.; Shi, Z.S. Non-Coding RNA in Acute Ischemic Stroke: Mechanisms, Biomarkers and Therapeutic Targets. Cell Transplant. 2018, 27, 1763–1777. [Google Scholar] [CrossRef] [PubMed]

| Signaling Pathway | miR-155 Levels | Key Target Gene | Function | Reference |
|---|---|---|---|---|
| Neuroinflammation | ||||
| Ras/NFkB | Upregulated | KRAS | Upregulation of miR-155 target the KRAS gene inhibiting Ras/NFkB signaling and reducing proinflammatory cytokines (IL-17, IL-22, IFN-γ, TNF-α, IL-6 and 2). | [100,101] |
| NFkB | Upregulated | CD33 | miR-155 upregulation targets the CD33 gene leading to increased levels of proinflammatory cytokines (IL-1β, IL-8, TNF-α). | [100,101] |
| NFkB | Upregulated | N/A | miR-155 upregulation leads to increased inflammation through NFkB pathway activation, causing high levels of TLR-signaling inflammatory cytokines and type I interferons (IFNs). | [102,103] |
| NFkB and TGFβ | N/A | MIRHA155 Gene | Regulatory elements in the MIRHA155 gene are stimulated by IL10 and IFNγ; and modulated by the NFkB pathway and the TGFβ pathway through SMAD4. | [95,104,105,106] |
| TGFβ | Upregulated | SMAD2 | miR-155 targets SMAD2, a TGFβ signaling mediator, to decrease SMAD2 protein levels, TGF-β-induced SMAD-2 phosphorylation, and SMAD-2-dependent activation. | [107] |
| TLR/MyD88, NF-κB, JNK/STAT, PI3K/Akt, and MAPK | Upregulated | SOCS1 and SHIP1 | miR-155 targeting of SOCS1 and SHIP1 activates the TLR/MyD88, NF-κB, JNK/STAT, PI3K/Akt, and MAPK signaling pathways to promote secretion of inflammatory cytokines and chemokines. | [72,108] |
| Wnt/β-catenin | Upregulated | HBP1 | miR-155 regulates the Wnt/β-catenin signaling pathway by inhibiting HBP1. | [72,108] |
| Ischemic stroke | Upregulated | hypoxia-inducible factor (HIF) | Increased miR-155 levels decrease HIF-1α mRNA, protein, and transcriptional activity in hypoxic conditions. | [106] |
| Monocyte/macrophage | Upregulated | N/A | miR-155 enhanced monocyte and macrophage production of proinflammatory cytokine CCL2. | [104,109] |
| NFkB | Upregulated | N/A | miR-155 inhibits BCL6, an inhibitor of NFkB. | [104,105,109,110,111] |
| FTO/m6A RNA methylation, IL-1b/TNF-a | Upregulated | N/A | Downregulation of FTO expression increases N6—methyladenosine (m6 A) RNA modification of miR-155, which worsens infarct volume, neurological deficit, and inflammatory IL-1b and TNF-a levels. | [112] |
| TLR | Upregulated | N/A | TLR2, TLR3, TLR4, and TLR9 induce miR-155 elevation. | [113] |
| TLR | Upregulated | N/A | MyD88 and TRIF signaling pathways induce miR-155 expression. MyD88 is necessary for TLR2 and TLR9 signaling, and TRIF for TLR3 signaling. | [114] |
| TNF-alpha | Upregulated | N/A | TNF-alpha is an miR-155 inducer. IFNs require TNF-alpha in macrophages to increase miR-155 levels. | [113] |
| JNK Pathway | Upregulated | N/A | TNF-alpha or poly(I:C) stimulation of the JNK pathway increases miR-155 upregulation. | [113] |
| BIC | Upregulated | BIC gene | Upregulation of poly(I:C) or IFN- increases BIC mRNA involved in miR-155 upregulation. miR-155 is a downstream target of IFN- and an early gene target for poly(I:C). | [113] |
| Apoptosis | ||||
| Apoptosis | Upregulated | SIRT1 | miR-155 targets and inhibits SIRT1, promoting apoptosis. | [115] |
| Apoptosis | Upregulated | miR-155-5p directly targets and inhibits DUSP14 by binding the 3′UTR, thereby preventing DUSP14 from inhibiting the NF-κB and MAPKs signaling pathways, resulting in increased apoptosis. | [116] | |
| Apoptosis (TLR4/MyD88) | Upregulated | Increased miR-155 levels activate the TLR4/MyD88 signaling pathway, worsening the inflammatory response following stroke. | [117] | |
| Apoptosis (Rheb/mTOR) | Upregulated | miR-155-5p promotes apoptosis by suppressing Rheb expression and by promoting NLRP3-mediated inflammasomes. | [118] | |
| Microglia | ||||
| M1 microglia polarization | Upregulated | High miR-155 levels increase cytokine production, autophagy, and M1 pro-inflammatory polarization. | [108] | |
| M1 and M2 microglia polarization | Upregulated | High levels of passenger strand miR-155 were detected in M1 and M2 microglia polarization. | [119] | |
| M1 and M2b polarization | Upregulated | Transfection of macrophages with miR-155 increased CXCL9 (M1 marker), and IL-6 and TNF (M1 and M2b markers). | [120] | |
| M1 macrophage polarization | Upregulated | IFNγ and TLR4 induce M1 macrophage polarization in vitro, and miR-155 level are upregulated by IFNγ and TLR4. | [105] | |
| M1 macrophage polarization | Upregulated | miR-155 overexpression reprogrammed tumor-associated macrophages into M1 macrophages. | [121] | |
| M1 macrophage polarization | Upregulated | miR-155 targets and suppresses SH2-containing inositol-5-phosphatase 1, IL13Rα1 and SMAD2/3, promoting M1 macrophages. | [122,123] | |
| M2 macrophage polarization | Upregulated | miR-155 inhibits the anti-inflammatory M2 polarization by inhibiting IL13RA and C/EBP in the IL13 and IL4 pathways. | [107,122,124,125] | |
| M2 macrophage polarization | Upregulated | miR-155 downregulated MafB, a transcription factor important for M2 macrophage polarization. | [124] | |
| M2 macrophage polarization | Upregulated | IL-13, a pro-M2 cytokine, functions by binding to IL-13 receptor α1 (IL13Rα1), a part of the Type II IL-4 receptor, to activate signal transducer and activator of transcription 6 (STAT6). miR-155 directly targets and reduces IL13Rα1 protein levels, causing reduced STAT6 activation. | [122] | |
| M2 macrophage polarization | Upregulated | miR-155 impacts IL-13-dependent regulation of SOCS1, DC-SIGN, CCL18, CD23, and SERPINE genes involved in differentiating M2 macrophages. | [122] | |
| Astrocyte polarization | Upregulated | DJ-1 suppresses miR-155, regulating astrocyte activation via miR-155/SHP-1 signaling pathway. DJ-1 inhibits transition of astrocytes to harmful A1 subtypes, promoting beneficial A2 polarization. | [126] |
| miR-155 Levels | Principal Target Gene | Function | Reference | |
|---|---|---|---|---|
| NFkB | Downregulated | N/A | miR-155 overexpression reversed the anti-inflammatory effects of glucocorticoids, while miR-155 inhibition restored them. | [130,131] |
| Oxidative Stress | Downregulated | N/A | lncRNA OIP5-AS1 interacts and represses miR-155-5p, preventing miR-155-5p from binding its target IRF2BP2, which then suppresses oxidative stress. | [129] |
| Oxidative Stress | Downregulated | N/A | miR-155-5p directly targets HIF-1α and negatively regulates its expression, promoting oxidative stress. miR-155-5p inhibitor in NSCs reversed miR-155-5p’s inhibition of HIF-1α, resulting in reduced oxidative stress and inflammation, decreased infarct volume and improved neurobehavioral outcomes. | [132] |
| Oxidative Stress | Downregulated | N/A | miR-155 inhibitor significantly reduced upregulation of hippocampus pro-inflammatory cytokines (PICs), decreased oxidative stress, and improved neurological severity score and reduced brain edema. | [133] |
| Apoptosis | Downregulated | Overexpression of lncRNA Oprm1 decreases apoptosis, through a lncRNA Oprm1/miR-155/GATA3 axis, by significantly decreasing infarct size and improving neurological score. | [134] | |
| Apoptosis | Downregulated | High cerebral infarct volumes and apoptosis were associated with increased miR-155 and decreased Rheb, mTOR, and pS6K, while treatment with miR-155 inhibitors were protected with reduced apoptosis and increased Rheb, mTOR, and p-S6K expression | [135] |
| NcRNA | Function in Ischemic Stroke | Reference |
|---|---|---|
| miR-155 miR-146a/b miR-21 | miR-155, miR-146a/b and miR-21 create a trinity that regulate TLR pathways to control inflammation in stroke | [119,147,148,149,150,151] |
| miR-155 miR-29b miR-146a miR-193b miR-222 | miR-155, in addition to miR-29b, miR-146a, miR-193b, and miR-222, are elevated in monocyte to macrophage differentiation. | [120,153] |
| miR-9 miR-21 miR-24 miR-26a miR-125a, b miR-143 miR-145 miR-146a miR-148 miR-187 miR-223 miR-378-3p miR-511-3p | miR-9, miR-21, miR-24, miR-26a, miR-125a, b, miR-143, miR-145, miR-146a, miR-148, miR-187, miR-223, miR-378-3p, miR-511-3p have been reported to play a role in macrophage polarization. | [104,154] |
| circFUNDC1 circPDS5B circCDC14A | High levels of circFUNDC1, circPDS5B and circCDC14A were found to be positively correlated with infarct volume in acute ischemic stroke. | [155] |
| miR-15a/16-1 cluster | Endothelium-targeted deletion of the miR-15a/16-1 cluster ameliorates blood–brain barrier dysfunction in ischemic stroke and poststroke angiogenesis | [156] |
| miR-181c | miR-181 suppresses TNF-α expression in post-ischemic neuronal damage. | [157] |
| miR-155 | miR-155 exerts both pro- and anti-inflammatory effects by targeting mediators of inflammatory signaling— SHIP1, SOCS1, SMAD2 and TAB2. | [158] |
| miR-126 | Increased in endothelial cell or CV functions in ischemic stroke. | [159] |
| miR-130 | Increased in angiogenesis in ischemic stroke. | [159] |
| miR-181 | Increased in infarct core and decreased in penumbra after focal ischemia. miR-181 was shown to sensitize cells to apoptosis by reducing Bcl-2. | [160] |
| miR-107 | Increased miR-107 levels may regulate post-ischemic stroke angiogenesis. | [161] |
| miR-15a/16–1 | miR-15a/16–1 repress pro-angiogenic factors VEGFA and FGF2 and their receptors VEGFR2 and FGFR1.. | [156] |
| miR-133 | Overexpressing MSCs further stimulates and increases exosomes’ release from astrocytes, possibly by downregulating the RABEPK expression.. | [162] |
| miR-1906 | Is increased in glial cells and decreased in neurons. miR-1906 is involved in abolishment of TLR4 protein expression and could ameliorate brain injury in stroke. | [163] |
| miR-99a | miR-99a prevented apoptosis and blocked cell cycle progression in neuro-2a cells | [164] |
| miR-497 | miR-497 promotes ischemic neuronal death by negatively regulating anti-apoptotic proteins bcl-2 and bcl-w. | [165] |
| miR-424 | miR-424 prevents ischemic brain injury by suppressing microglia activation. | [166] |
| miRNA-210 + HIF-1α | HIF-1α induces miR-210 which could prevent apoptosis and induce angiogenesis. | [167] |
| miR-124, miR-223, miR-107, miR-181a | Involved in regulating excitotoxicity in stroke. | [168] |
| miR-21, miR-210, miR-424, miR-29b, miR-124, miR-15a, miR-181a | Involved in regulating apoptosis and programmed cell death in stroke. | [168] |
| miR-29b, miR-124, miR-150, miR-155 | Involved in promoting blood–brain barrier breakdown after stroke. | [168] |
| miR-21, miR-124, miR-223, miR-424, miR-15a, miR-155, miR-181a, miR-210, miR-377 | Involved in promoting inflammation in stroke. | [168] |
| miR-124 | Involved in protective effects against stroke including angiogenesis and neurogenesis, and damaging effects of stroke including excitotoxicity, programmed cell death, blood–brain barrier breakdown, and inflammation. | [168] |
| miR-155 Research Future Directions | Recommendations |
|---|---|
| Profiling miR-155 stability in patients | Test the stability and abundance of miR-155 in CSF and blood serum IS patient samples as compared to healthy controls at different stages of the disease. |
| Measuring expression miR-155 levels variability in different patient populations with severity of stroke types | Establishing miR-155 differential expression levels in different patient populations. |
| Need to evaluate efficacy in large scale cohort studies | Analyze beneficial/adverse effects of miR-155 administration in large IS patient cohorts. |
| Refine integration of miR-155 with IS standard of care protocols | Analysis of existing stroke diagnostic protocols and potential incorporation of miR-155 into diagnosis algorithm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hering, C.; Conover, G.M. Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA. Int. J. Mol. Sci. 2025, 26, 3947. https://doi.org/10.3390/ijms26093947
Hering C, Conover GM. Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA. International Journal of Molecular Sciences. 2025; 26(9):3947. https://doi.org/10.3390/ijms26093947
Chicago/Turabian StyleHering, Catherine, and Gloria M. Conover. 2025. "Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA" International Journal of Molecular Sciences 26, no. 9: 3947. https://doi.org/10.3390/ijms26093947
APA StyleHering, C., & Conover, G. M. (2025). Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA. International Journal of Molecular Sciences, 26(9), 3947. https://doi.org/10.3390/ijms26093947






