Diagnostic and Prognostic Potential of SH3YL1 and NOX4 in Muscle-Invasive Bladder Cancer
Abstract
:1. Introduction
2. Results
2.1. ELISA Analysis of SH3YL1 in MIBC Patients
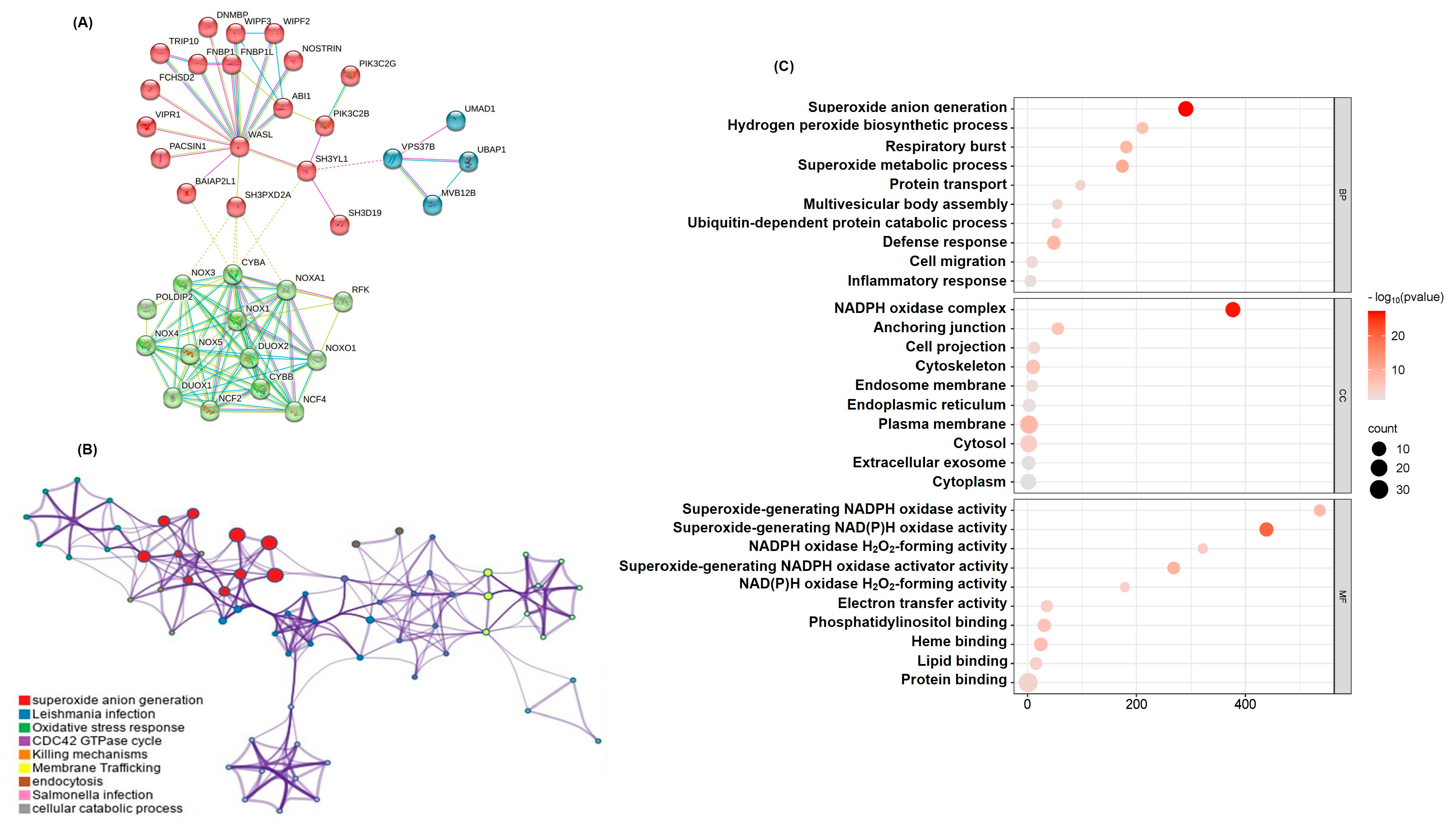
2.2. Functional Network Analysis of SH3YL1-Related Genes Highlights NADPH Pathways
2.3. SH3YL1 and NOX Family Gene Correlation Analysis in NMIBC and MIBC
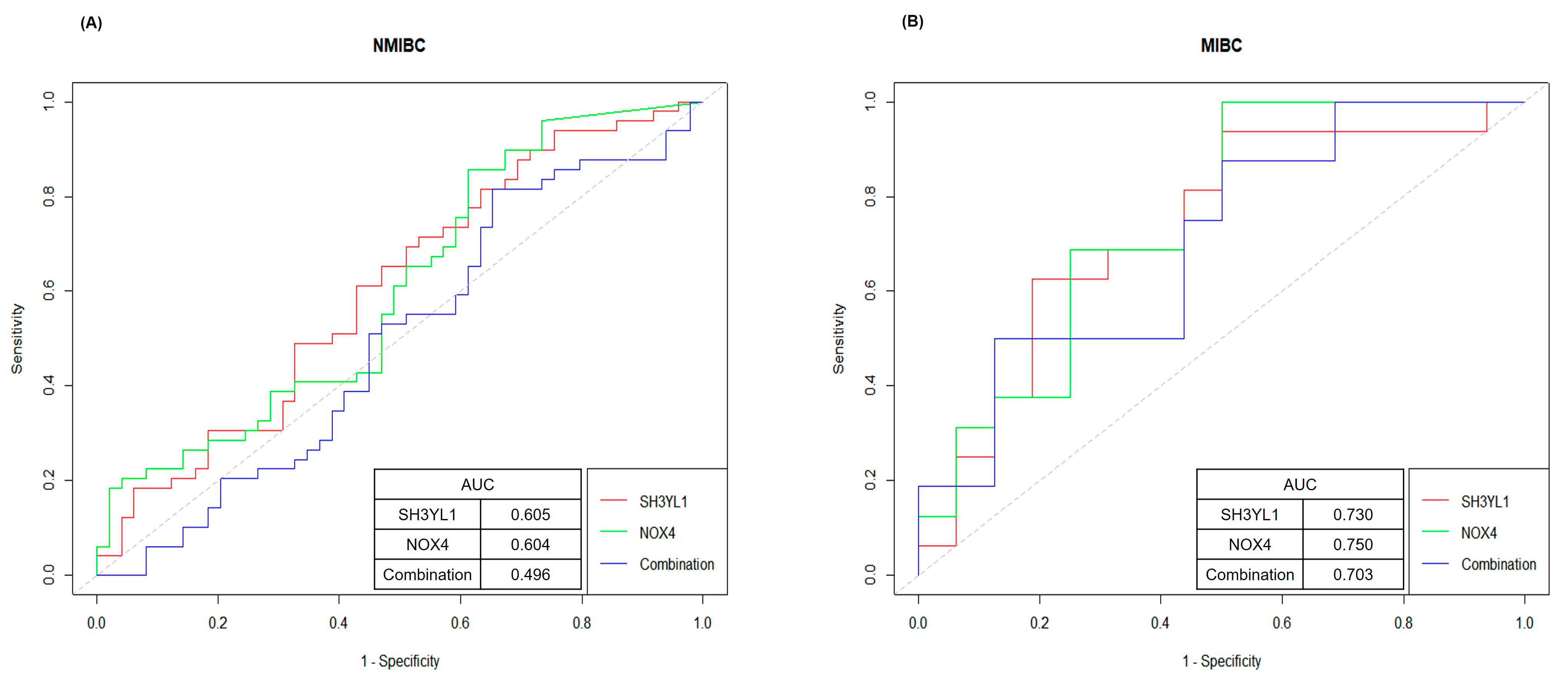
2.4. ROC Curve Analysis for Evaluating SH3YL1 and NOX4 as Biomarkers in NMIBC and MIBC
2.5. Survival Analysis of SH3YL1 and NOX4 Expression in MIBC and NMIBC
3. Discussion
4. Material and Methods
4.1. Medical Samples
4.2. Sample Collection and Processing
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Protein–Protein Interaction (PPI) Network Construction
4.5. GO Analysis
4.6. RNA Transcriptome Sequencing
4.7. Data Acquisition
4.8. Survival Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, screening, and prevention of bladder cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Goutas, D.; Tzortzis, A.; Gakiopoulou, H.; Vlachodimitropoulos, D.; Giannopoulou, I.; Lazaris, A.C. Contemporary molecular classification of urinary bladder cancer. In Vivo 2021, 35, 75–80. [Google Scholar] [CrossRef]
- Schwarzova, L.; Varchulova Novakova, Z.; Danisovic, L.; Ziaran, S. Molecular classification of urothelial bladder carcinoma. Mol. Biol. Rep. 2023, 50, 7867–7877. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, W.; Yang, X.; Wu, C.; Cheng, F. Traditional classification and novel subtyping systems for bladder cancer. Front. Oncol. 2020, 10, 102. [Google Scholar] [CrossRef]
- Giudici, N.; Seiler-Blarer, R. Histologic subtypes of non-muscle invasive bladder cancer. World J. Clin. Oncol. 2024, 15, 835–839. [Google Scholar] [CrossRef]
- Teoh, J.Y.-C.; Kamat, A.M.; Black, P.C.; Grivas, P.; Shariat, S.F.; Babjuk, M. Recurrence mechanisms of non-muscle-invasive bladder cancer—A clinical perspective. Nat. Rev. Urol. 2022, 19, 280–294. [Google Scholar] [CrossRef]
- Taylor, J.; Becher, E.; Steinberg, G.D. Update on the guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2020, 125, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Miron, B.; Ross, E.A.; Anari, F.; O’Neill, J.; Hoffman-Censits, J.H.; Zibelman, M.R.; Kutikov, A.; Viterbo, R.; Greenberg, R.E.; Chen, D. Defects in DNA Repair Genes and Long-Term Survival in Cisplatin-Based Neoadjuvant Chemotherapy for Muscle Invasive Bladder Cancer (MIBC); American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Hamid, A.R.A.; Ridwan, F.R.; Parikesit, D.; Widia, F.; Mochtar, C.A.; Umbas, R. Meta-analysis of neoadjuvant chemotherapy compared to radical cystectomy alone in improving overall survival of muscle-invasive bladder cancer patients. BMC Urol. 2020, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Jiang, D.M.; Gupta, S.; Kitchlu, A.; Meraz-Munoz, A.; North, S.A.; Alimohamed, N.S.; Blais, N.; Sridhar, S.S. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat. Rev. Urol. 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Z.; Chen, W.; Li, D.; Zhang, H.; Zhu, Y.; Mo, Q.; Zhao, X.; Fan, Q.; Deng, F. Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist. Updates 2023, 68, 100938. [Google Scholar] [CrossRef]
- Raj, G.V.; Karavadia, S.; Schlomer, B.; Arriaga, Y.; Lotan, Y.; Sagalowsky, A.; Frenkel, E. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 2011, 117, 276–282. [Google Scholar] [CrossRef]
- Schardt, J.; Roth, B.; Seiler, R. Forty years of cisplatin-based chemotherapy in muscle-invasive bladder cancer: Are we understanding how, who and when? World J. Urol. 2019, 37, 1759–1765. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of cisplatin-induced acute kidney injury: Pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Hamroun, A.; Lenain, R.; Bigna, J.J.; Speyer, E.; Bui, L.; Chamley, P.; Pottier, N.; Cauffiez, C.; Dewaeles, E.; Dhalluin, X. Prevention of cisplatin-induced acute kidney injury: A systematic review and meta-analysis. Drugs 2019, 79, 1567–1582. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Kim, J.-S.; Jeong, K.-H.; Kim, S.-K. Acute kidney injury: Biomarker-guided diagnosis and management. Medicina 2022, 58, 340. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, C.L. Biomarkers in acute kidney injury. Biomark. Kidney Dis. 2017, 48, 241–315. [Google Scholar]
- Teo, S.H.; Endre, Z.H. Biomarkers in acute kidney injury (AKI). Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 331–344. [Google Scholar] [CrossRef]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef]
- Oh, D.-J. A long journey for acute kidney injury biomarkers. Ren. Fail. 2020, 42, 154–165. [Google Scholar] [CrossRef]
- Yoo, J.-Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. LPS-induced acute kidney injury is mediated by Nox4-SH3YL1. Cell Rep. 2020, 33, 108245. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Wang, B.; Zhang, Z.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. NOX4 is a potential therapeutic target in septic acute kidney injury by inhibiting mitochondrial dysfunction and inflammation. Theranostics 2023, 13, 2863. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, H.E.; Yoo, J.-Y.; An, E.J.; Song, S.-J.; Han, K.-H.; Cha, D.R.; Bae, Y.S. Nox4-SH3YL1 complex is involved in diabetic nephropathy. Iscience 2024, 27, 108868. [Google Scholar] [CrossRef]
- Han, S.Y.; Han, S.H.; Ghee, J.Y.; Cha, J.J.; Kang, Y.S.; Cha, D.R. SH3YL1 Protein Predicts Renal Outcomes in Patients with Type 2 Diabetes. Life 2023, 13, 963. [Google Scholar] [CrossRef]
- Gomaa, S.N.S.; Sheir, R.E.-S.; Farhan, H.M.; Ahmed, T.M.; Abdelsattar, A.S. Assessment of SH3YL1 protein as a marker for diabetic nephropathy in type 2 diabetes mellitus. Egypt. J. Med. Res. 2022, 3, 222–237. [Google Scholar] [CrossRef]
- Nagao, K.; Hara, T.; Nishijima, J.; Shimizu, K.; Fujii, N.; Kobayashi, K.; Kawai, Y.; Inoue, R.; Yamamoto, Y.; Matsumoto, H. The efficacy of trimodal chemoradiotherapy with cisplatin as a bladder-preserving strategy for the treatment of muscle-invasive bladder cancer. Urol. Int. 2017, 99, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.; Soloway, M. Cisplatin, neoadjuvant chemotherapy and bladder cancer. Urology 2022, 159, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Alassaf, N.; Attia, H. Autophagy and necroptosis in cisplatin-induced acute kidney injury: Recent advances regarding their role and therapeutic potential. Front. Pharmacol. 2023, 14, 1103062. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Volovat, S.; Apetrii, M.; Stefan, A.; Vlad, C.; Voroneanu, L.; Hogas, M.; Haisan, A.; Volovat, C.; Hogas, S. Cisplatin and AKI: An ongoing battle with new perspectives—A narrative review. Int. Urol. Nephrol. 2023, 55, 1205–1209. [Google Scholar] [CrossRef]
- Meng, X.-M.; Ren, G.-L.; Gao, L.; Yang, Q.; Li, H.-D.; Wu, W.-F.; Huang, C.; Zhang, L.; Lv, X.-w.; Li, J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Investig. 2018, 98, 63–78. [Google Scholar] [CrossRef]
- Matuszczak, M.; Salagierski, M. Diagnostic and prognostic potential of biomarkers CYFRA 21.1, ERCC1, p53, FGFR3 and TATI in bladder cancers. Int. J. Mol. Sci. 2020, 21, 3360. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Bonnot, T.; Gillard, M.B.; Nagel, D.H. A simple protocol for informative visualization of enriched gene ontology terms. Bio-Protocol 2019, 9, e3429. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jung, E.; Song, G.; Joung, J.; Chung, J.; Seo, H.; Lee, H. Diagnostic and Prognostic Potential of SH3YL1 and NOX4 in Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2025, 26, 3959. https://doi.org/10.3390/ijms26093959
Kim M, Jung E, Song G, Joung J, Chung J, Seo H, Lee H. Diagnostic and Prognostic Potential of SH3YL1 and NOX4 in Muscle-Invasive Bladder Cancer. International Journal of Molecular Sciences. 2025; 26(9):3959. https://doi.org/10.3390/ijms26093959
Chicago/Turabian StyleKim, Mingyu, Euihyun Jung, Geehyun Song, Jaeyoung Joung, Jinsoo Chung, Hokyung Seo, and Hyungho Lee. 2025. "Diagnostic and Prognostic Potential of SH3YL1 and NOX4 in Muscle-Invasive Bladder Cancer" International Journal of Molecular Sciences 26, no. 9: 3959. https://doi.org/10.3390/ijms26093959
APA StyleKim, M., Jung, E., Song, G., Joung, J., Chung, J., Seo, H., & Lee, H. (2025). Diagnostic and Prognostic Potential of SH3YL1 and NOX4 in Muscle-Invasive Bladder Cancer. International Journal of Molecular Sciences, 26(9), 3959. https://doi.org/10.3390/ijms26093959




