Understanding the Mechanisms of Chemotherapy-Related Cardiotoxicity Employing hiPSC-Derived Cardiomyocyte Models for Drug Screening and the Identification of Genetic and Epigenetic Variants
Abstract
1. Introduction
2. CTRTOX Pathophysiology and Mechanisms
2.1. Anthracycline-Induced Cardiotoxicity
2.1.1. Doxorubicin
2.1.2. Daunorubicin
2.1.3. Epirubicin
2.1.4. Idarubicin
2.1.5. Mitoxantrone
2.2. Targeted-Therapy-Induced Cardiotoxicity
2.2.1. Trastuzumab
2.2.2. Lapatinib
2.2.3. Sunitinib
2.2.4. Gefitinib
2.2.5. Afatinib
2.2.6. Sorafenib
2.2.7. Erlotinib
2.2.8. Pazopanib
2.3. Macrolide-Induced Cardiotoxicity
2.3.1. Mitomycin C
2.3.2. Erythromycin and Clarithromycin
3. Recent Advancements in Therapeutic Strategies in the Treatment of CTRTOX: Success and Limitations
3.1. Pharmacological Drug Selection
3.2. Delivery Strategies and Targeted Therapies
4. Application of Human iPSC-Derived Cardiomyocytes to Study CTRTOX
4.1. Patient-Specific iPSC-Based 2D and 3D CM Models: Platform to Investigate CTRTOX and Identify Genetic and Epigenetic Variants
4.2. Application of hiPSC-CMs for Identification of the Genetic and Epigenetic Markers of CTRTOX
5. Challenges and Future Outlook of Utilizing hiPSC-CMs in Studies on CTRTOX
5.1. High-Throughput Manufacturing of 2D and 3D CMs from hiPSCs
5.2. Improvement of hiPSC-CM Models
5.3. Exploration of 3D hiPSC-CMs in Studying the Mechanisms of CTRTOX
5.4. High-Throughput Screening of Genetic, Epigenetic, and Proteome Markers Using Patient-Specific hiPSC-CMs
5.5. Utilizing hiPSC-CMs to Assess Cardioprotective Strategies
5.6. Therapeutic Strategies for Restoring Mitochondrial Health in CTRTOX Using hiPSC-CMs
5.7. Isogenic Cardiac Organoid Models
5.8. Exploring 3D and 4D Bioprinting Applications
5.9. Delivery Approaches
5.10. Mathematical Modeling and Multimodal Machine Learning
5.11. Designing Robust Clinical Trials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| AIC | Anthracycline-induced cardiotoxicity |
| Akt | AKT serine/threonine kinase |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2-associated X protein |
| CTRTOX | Chemotherapy-related cardiotoxicity |
| CVDs | Cardiovascular diseases |
| CMs | Cardiomyocytes |
| DOX | Doxorubicin |
| DIC | Doxorubicin-induced cardiotoxicity |
| DNR | Daunorubicin |
| ETC | Electron transport chain |
| EPI | Epirubicin |
| eNOS | Endothelial nitric oxide synthase |
| Klf4 | Kruppel-like factor-4 |
| LVEF | Left ventricular ejection fraction |
| LVD | Left ventricular dysfunction |
| MTX | Mitoxantrone |
| mtDNA | Mitochondrial DNA |
| MAPK | Mitogen-activated protein kinase |
| NOS | Nitric oxide synthase |
| NO | Nitric oxide |
| NOX2 | NAPDH oxidase 2 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| PI3K | Phosphatidylinositol 3 kinase |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SOD | Superoxide dismutase |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| Topo IIβ | Topoisomerase IIβ |
| TNFα | Tumor necrotic factor α |
| TZM | Trastuzumab |
References
- Kremer, L.C.; van der Pal, H.J.; Offringa, M.; van Dalen, E.C.; Voute, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann. Oncol. 2002, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Granger, C.B. Prediction and prevention of chemotherapy-induced cardiomyopathy: Can it be done? Circulation 2006, 114, 2432–2433. [Google Scholar] [CrossRef]
- Yu, K.D.; Ye, F.G.; He, M.; Fan, L.; Ma, D.; Mo, M.; Wu, J.; Liu, G.Y.; Di, G.H.; Zeng, X.H.; et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women with Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1390–1396. [Google Scholar] [CrossRef]
- Frye, R.L.; Simari, R.D.; Gersh, B.J.; Burnett, J.C.; Brumm, S.; Myerle, K.; Jaffe, A.S.; Holmes, D.R.; Lerman, A.; Terzic, A. Ethical issues in cardiovascular research involving humans. Circulation 2009, 120, 2113–2121. [Google Scholar] [CrossRef]
- Asnani, A.; Moslehi, J.J.; Adhikari, B.B.; Baik, A.H.; Beyer, A.M.; de Boer, R.A.; Ghigo, A.; Grumbach, I.M.; Jain, S.; Zhu, H.; et al. Preclinical Models of Cancer Therapy-Associated Cardiovascular Toxicity: A Scientific Statement from the American Heart Association. Circ. Res. 2021, 129, e21–e34. [Google Scholar] [CrossRef]
- Shelburne, N.; Adhikari, B.; Brell, J.; Davis, M.; Desvigne-Nickens, P.; Freedman, A.; Minasian, L.; Force, T.; Remick, S.C. Cancer treatment-related cardiotoxicity: Current state of knowledge and future research priorities. J. Natl. Cancer Inst. 2014, 106, dju232. [Google Scholar] [CrossRef]
- Begley, C.G.; Ellis, L.M. Drug development: Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef]
- Francia, G.; Kerbel, R.S. Raising the bar for cancer therapy models. Nat. Biotechnol. 2010, 28, 561–562. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef]
- Rossant, J. Stem cells and early lineage development. Cell 2008, 132, 527–531. [Google Scholar] [CrossRef]
- Young, R.A. Control of the embryonic stem cell state. Cell 2011, 144, 940–954. [Google Scholar] [CrossRef]
- Xu, C.; Police, S.; Rao, N.; Carpenter, M.K. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 2002, 91, 501–508. [Google Scholar] [CrossRef]
- Ling-Ling, E.; Zhao, Y.S.; Guo, X.M.; Wang, C.Y.; Jiang, H.; Li, J.; Duan, C.M.; Song, Y. Enrichment of cardiomyocytes derived from mouse embryonic stem cells. J. Heart Lung Transplant. 2006, 25, 664–674. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; Campora, A.; Lisi, M.; Mandoli, G.E. Donor shortage in heart transplantation: How can we overcome this challenge? Front. Cardiovasc. Med. 2022, 9, 1001002. [Google Scholar] [CrossRef]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Swijnenburg, R.J.; Tanaka, M.; Vogel, H.; Baker, J.; Kofidis, T.; Gunawan, F.; Lebl, D.R.; Caffarelli, A.D.; de Bruin, J.L.; Fedoseyeva, E.V.; et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 2005, 112 (Suppl. S9), I166–I172. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Clavellina, D.; Balkan, W.; Hare, J.M. Stem cell therapy for acute myocardial infarction: Mesenchymal Stem Cells and induced Pluripotent Stem Cells. Expert. Opin. Biol. Ther. 2023, 23, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Pant, T.; Juric, M.; Bosnjak, Z.J.; Dhanasekaran, A. Recent Insight on the Non-coding RNAs in Mesenchymal Stem Cell-Derived Exosomes: Regulatory and Therapeutic Role in Regenerative Medicine and Tissue Engineering. Front. Cardiovasc. Med. 2021, 8, 737512. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. Induced Pluripotent Stem Cell Generation from Blood Cells Using Sendai Virus and Centrifugation. J. Vis. Exp. 2016, 118, 54650. [Google Scholar] [CrossRef]
- Cheng, L.; Lei, Q.; Yin, C.; Wang, H.Y.; Jin, K.; Xiang, M. Generation of Urine Cell-Derived Non-Integrative Human iPSCs and iNSCs: A Step-by-Step Optimized Protocol. Front. Mol. Neurosci. 2017, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Cui, Y.; Luan, J.; Zhou, X.; Han, J. Urine-derived induced pluripotent stem cells as a modeling tool to study rare human diseases. Intractable Rare Dis. Res. 2016, 5, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Koonce, C.; Aoyama, N.; Einhorn, S.; Fiene, S.; Thompson, A.; Swanson, B.; Anson, B.; Kattman, S. Phenotypic screening with human iPS cell-derived cardiomyocytes: HTS-compatible assays for interrogating cardiac hypertrophy. J. Biomol. Screen. 2013, 18, 1203–1211. [Google Scholar] [CrossRef]
- Drowley, L.; Koonce, C.; Peel, S.; Jonebring, A.; Plowright, A.T.; Kattman, S.J.; Andersson, H.; Anson, B.; Swanson, B.J.; Wang, Q.D.; et al. Human Induced Pluripotent Stem Cell-Derived Cardiac Progenitor Cells in Phenotypic Screening: A Transforming Growth Factor-Beta Type 1 Receptor Kinase Inhibitor Induces Efficient Cardiac Differentiation. Stem Cells Transl. Med. 2016, 5, 164–174. [Google Scholar] [CrossRef]
- Pant, T.; Mishra, M.K.; Bai, X.; Ge, Z.D.; Bosnjak, Z.J.; Dhanasekaran, A. Microarray analysis of long non-coding RNA and mRNA expression profiles in diabetic cardiomyopathy using human induced pluripotent stem cell-derived cardiomyocytes. Diab Vasc. Dis. Res. 2019, 16, 57–68. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gerard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014, 9, 810–821. [Google Scholar] [CrossRef]
- Doherty, K.R.; Talbert, D.R.; Trusk, P.B.; Moran, D.M.; Shell, S.A.; Bacus, S. Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. Toxicol. Appl. Pharmacol. 2015, 285, 51–60. [Google Scholar] [CrossRef]
- Talbert, D.R.; Doherty, K.R.; Trusk, P.B.; Moran, D.M.; Shell, S.A.; Bacus, S. A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicol. Sci. 2015, 143, 147–155. [Google Scholar] [CrossRef]
- Kikuchi, C.; Bienengraeber, M.; Canfield, S.; Koopmeiner, A.; Schafer, R.; Bosnjak, Z.J.; Bai, X. Comparison of Cardiomyocyte Differentiation Potential Between Type 1 Diabetic Donor- and Nondiabetic Donor-Derived Induced Pluripotent Stem Cells. Cell Transplant. 2015, 24, 2491–2504. [Google Scholar] [CrossRef]
- Zwelling, L.A.; Kerrigan, D.; Michaels, S. Cytotoxicity and DNA strand breaks by 5-iminodaunorubicin in mouse leukemia L1210 cells: Comparison with adriamycin and 4′-(9-acridinylamino)methanesulfon-m-anisidide. Cancer Res. 1982, 42, 2687–2691. [Google Scholar] [PubMed]
- Sarvazyan, N. Visualization of doxorubicin-induced oxidative stress in isolated cardiac myocytes. Am. J. Physiol. 1996, 271, H2079–H2085. [Google Scholar] [CrossRef] [PubMed]
- Marcillat, O.; Zhang, Y.; Davies, K.J. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem. J. 1989, 259, 181–189. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; Zuppinger, C.; Wallimann, T.; Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006, 41, 389–405. [Google Scholar] [CrossRef]
- Kalivendi, S.V.; Kotamraju, S.; Zhao, H.; Joseph, J.; Kalyanaraman, B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. Effect of antiapoptotic antioxidants and calcium. J. Biol. Chem. 2001, 276, 47266–47276. [Google Scholar] [CrossRef]
- Luo, D.; Vincent, S.R. Inhibition of nitric oxide synthase by antineoplastic anthracyclines. Biochem. Pharmacol. 1994, 47, 2111–2112. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Martasek, P.; Hogg, N.; Masters, B.S.; Pritchard, K.A., Jr.; Kalyanaraman, B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 1997, 36, 11293–11297. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Hogg, N.; Martasek, P.; Karoui, H.; Tordo, P.; Pritchard, K.A., Jr.; Kalyanaraman, B. Effect of redox-active drugs on superoxide generation from nitric oxide synthases: Biological and toxicological implications. Free Radic Res. 1999, 31, 607–617. [Google Scholar] [CrossRef]
- Duquaine, D.; Hirsch, G.A.; Chakrabarti, A.; Han, Z.; Kehrer, C.; Brook, R.; Joseph, J.; Schott, A.; Kalyanaraman, B.; Vasquez-Vivar, J.; et al. Rapid-onset endothelial dysfunction with adriamycin: Evidence for a dysfunctional nitric oxide synthase. Vasc. Med. 2003, 8, 101–107. [Google Scholar] [CrossRef]
- McLaughlin, D.; Zhao, Y.; O’Neill, K.M.; Edgar, K.S.; Dunne, P.D.; Kearney, A.M.; Grieve, D.J.; McDermott, B.J. Signalling mechanisms underlying doxorubicin and Nox2 NADPH oxidase-induced cardiomyopathy: Involvement of mitofusin-2. Br. J. Pharmacol. 2017, 174, 3677–3695. [Google Scholar] [CrossRef]
- Lin, J.; Fang, L.; Li, H.; Li, Z.; Lyu, L.; Wang, H.; Xiao, J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharmacol. 2019, 859, 172490. [Google Scholar] [CrossRef] [PubMed]
- Gratia, S.; Kay, L.; Potenza, L.; Seffouh, A.; Novel-Chate, V.; Schnebelen, C.; Sestili, P.; Schlattner, U.; Tokarska-Schlattner, M. Inhibition of AMPK signalling by doxorubicin: At the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress. Cardiovasc. Res. 2012, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Lubieniecka, J.M.; Graham, J.; Heffner, D.; Mottus, R.; Reid, R.; Hogge, D.; Grigliatti, T.A.; Riggs, W.K. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front. Genet. 2013, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Arozal, W.; Watanabe, K.; Veeraveedu, P.T.; Ma, M.; Thandavarayan, R.A.; Sukumaran, V.; Suzuki, K.; Kodama, M.; Aizawa, Y. Protective effect of carvedilol on daunorubicin-induced cardiotoxicity and nephrotoxicity in rats. Toxicology 2010, 274, 18–26. [Google Scholar] [CrossRef]
- Srankova, J.; Doka, G.; Pivackova, L.; Mesarosova, L.; Kyselovic, J.; Klimas, J.; Krenek, P. Daunorubicin Down-Regulates the Expression of Stem Cell Markers and Factors Involved in Stem Cell Migration and Homing in Rat Heart in Subchronic but not Acute Cardiomyopathy. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 443–452. [Google Scholar] [CrossRef]
- Kucerova, D.; Doka, G.; Kruzliak, P.; Turcekova, K.; Kmecova, J.; Brnoliakova, Z.; Kyselovic, J.; Kirchhefer, U.; Muller, F.U.; Krenek, P.; et al. Unbalanced upregulation of ryanodine receptor 2 plays a particular role in early development of daunorubicin cardiomyopathy. Am. J. Transl. Res. 2015, 7, 1280–1294. [Google Scholar]
- Li, W.; Lu, M.; Zhang, Y.; Xia, D.; Chen, Z.; Wang, L.; Yin, N.; Wang, Z. Puerarin attenuates the daunorubicin-induced apoptosis of H9c2 cells by activating the PI3K/Akt signaling pathway via the inhibition of Ca2+ influx. Int. J. Mol. Med. 2017, 40, 1889–1894. [Google Scholar] [CrossRef][Green Version]
- Guven, A.; Yavuz, O.; Cam, M.; Ercan, F.; Bukan, N.; Comunoglu, C. Melatonin protects against epirubicin-induced cardiotoxicity. Acta Histochem. 2007, 109, 52–60. [Google Scholar] [CrossRef]
- Li, H.; Mao, Y.; Zhang, Q.; Han, Q.; Man, Z.; Zhang, J.; Wang, X.; Hu, R.; Zhang, X.; Irwin, D.M.; et al. Xinmailong mitigated epirubicin-induced cardiotoxicity via inhibiting autophagy. J. Ethnopharmacol. 2016, 192, 459–470. [Google Scholar] [CrossRef]
- Galetta, F.; Franzoni, F.; Cervetti, G.; Cecconi, N.; Carpi, A.; Petrini, M.; Santoro, G. Effect of epirubicin-based chemotherapy and dexrazoxane supplementation on QT dispersion in non-Hodgkin lymphoma patients. Biomed. Pharmacother. 2005, 59, 541–544. [Google Scholar] [CrossRef]
- Mantovani, G.; Madeddu, C.; Cadeddu, C.; Dessi, M.; Piras, A.; Massa, E.; Serpe, R.; Antoni, G.; Mercuro, G. Persistence, up to 18 months of follow-up, of epirubicin-induced myocardial dysfunction detected early by serial tissue Doppler echocardiography: Correlation with inflammatory and oxidative stress markers. Oncologist 2008, 13, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, Z.; Li, Y.; Lv, D.; Zhao, X.; Gao, J.; Teng, H. Identification of differential gene expression related to epirubicin-induced cardiomyopathy in breast cancer patients. Hum. Exp. Toxicol. 2020, 39, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chuang, M.H.; Li, D.K. The development of congestive heart failure and ventricular tachycardia after first exposure to idarubicin in a patient with acute myeloid leukaemia. Br. J. Clin. Pharmacol. 2010, 69, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.; Serra, J.; Ribera, J.M. Cardiac arrest in a patient diagnosed with acute myeloid leukemia after the first dose of idarubicin. Med. Clin. 2018, 151, 506–507. [Google Scholar] [CrossRef]
- Ahmed, Z.; Davaro, E.; Batanian, J.; Verma, N. First-dose idarubicin cardiomyopathy: A case of new heart failure after induction chemotherapy for acute myeloid leukaemia. BMJ Case Rep. 2019, 12, e228149. [Google Scholar] [CrossRef]
- Bezwoda, W.R.; Hesdorffer, C.S. Mitoxantrone, methotrexate, and 5-fluorouracil combination chemotherapy as first-line treatment in stage IV breast cancer. Cancer 1986, 57, 218–221. [Google Scholar] [CrossRef]
- Rossato, L.G.; Costa, V.M.; de Pinho, P.G.; Arbo, M.D.; de Freitas, V.; Vilain, L.; de Lourdes Bastos, M.; Palmeira, C.; Remiao, F. The metabolic profile of mitoxantrone and its relation with mitoxantrone-induced cardiotoxicity. Arch. Toxicol. 2013, 87, 1809–1820. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Gomes, A.S.; Carvalho, R.A.; Carvalho, F.; Remiao, F.; Pinto, M.; Bastos, M.L.; Sousa, E.; Costa, V.M. Naphthoquinoxaline metabolite of mitoxantrone is less cardiotoxic than the parent compound and it can be a more cardiosafe drug in anticancer therapy. Arch. Toxicol. 2017, 91, 1871–1890. [Google Scholar] [CrossRef]
- Costa, V.M.; Capela, J.P.; Sousa, J.R.; Eleuterio, R.P.; Rodrigues, P.R.S.; Dores-Sousa, J.L.; Carvalho, R.A.; Lourdes Bastos, M.; Duarte, J.A.; Remiao, F.; et al. Mitoxantrone impairs proteasome activity and prompts early energetic and proteomic changes in HL-1 cardiomyocytes at clinically relevant concentrations. Arch. Toxicol. 2020, 94, 4067–4084. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Dores-Sousa, J.L.; Padrao, A.I.; Duarte-Araujo, M.; Duarte, J.A.; Seabra, V.; Goncalves-Monteiro, S.; Remiao, F.; Carvalho, F.; Sousa, E.; et al. Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals 2021, 14, 510. [Google Scholar] [CrossRef]
- Brandao, S.R.; Reis-Mendes, A.; Duarte-Araujo, M.; Neuparth, M.J.; Rocha, H.; Carvalho, F.; Ferreira, R.; Costa, V.M. Cardiac Molecular Remodeling by Anticancer Drugs: Doxorubicin Affects More Metabolism While Mitoxantrone Impacts More Autophagy in Adult CD-1 Male Mice. Biomolecules 2023, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Sennhauser, S.; May, L.; Simone, N.; Beuningen, A.; Bhatt, K. A Case of Acute Mitoxantrone Mediated Myocarditis in Refractory Acute Myeloid Leukemia (AML). J. Heart Lung Transpl. 2023, 42, S199–S200. [Google Scholar] [CrossRef]
- Alderton, P.M.; Gross, J.; Green, M.D. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res. 1992, 52, 194–201. [Google Scholar] [PubMed]
- Alexander, J.; Dainiak, N.; Berger, H.J.; Goldman, L.; Johnstone, D.; Reduto, L.; Duffy, T.; Schwartz, P.; Gottschalk, A.; Zaret, B.L. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N. Engl. J. Med. 1979, 300, 278–283. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Marcus, C.; Smith, T.L.; Blumenschein, G.R. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer 1985, 55, 2761–2765. [Google Scholar] [CrossRef]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Middleman, E.; Luce, J.; Frei, E., III. Clinical trials with adriamycin. Cancer 1971, 28, 844–850. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View from the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef]
- Bachur, N.R.; Gordon, S.L.; Gee, M.V. Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation. Mol. Pharmacol. 1977, 13, 901–910. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Fukazawa, R.; Arstall, M.A.; Kelly, R.A. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ. Res. 1999, 84, 257–265. [Google Scholar] [CrossRef]
- Chandran, K.; Aggarwal, D.; Migrino, R.Q.; Joseph, J.; McAllister, D.; Konorev, E.A.; Antholine, W.E.; Zielonka, J.; Srinivasan, S.; Avadhani, N.G.; et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: Cardioprotection by Mito-Q. Biophys. J. 2009, 96, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [PubMed]
- Goormaghtigh, E.; Huart, P.; Praet, M.; Brasseur, R.; Ruysschaert, J.M. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys. Chem. 1990, 35, 247–257. [Google Scholar] [CrossRef]
- Chang, J.S.; Ha, K. A truncated PPAR gamma 2 localizes to mitochondria and regulates mitochondrial respiration in brown adipocytes. PLoS ONE 2018, 13, e0195007. [Google Scholar] [CrossRef]
- Gervasi, P.G.; Agrillo, M.R.; Lippi, A.; Bernardini, N.; Danesi, R.; Del Tacca, M. Superoxide anion production by doxorubicin analogs in heart sarcosomes and by mitochondrial NADH dehydrogenase. Res. Commun. Chem. Pathol. Pharmacol. 1990, 67, 101–115. [Google Scholar]
- Kowalik-Jankowska, T.; Ruta, M.; Wisniewska, K.; Lankiewicz, L.; Dyba, M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1–10, 1–16 fragments of human and mouse beta-amyloid peptide. J. Inorg. Biochem. 2004, 98, 940–950. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.L.; Chen, H.L.; Wu, D.; Chen, J.X.; Wang, X.X.; Li, R.L.; He, J.H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef]
- Herman, E.H.; Ferrans, V.J. Influence of vitamin E and ICRF-187 on chronic doxorubicin cardiotoxicity in miniature swine. Lab. Investig. 1983, 49, 69–77. [Google Scholar]
- Tesoriere, L.; Ciaccio, M.; Valenza, M.; Bongiorno, A.; Maresi, E.; Albiero, R.; Livrea, M.A. Effect of vitamin A administration on resistance of rat heart against doxorubicin-induced cardiotoxicity and lethality. J. Pharmacol. Exp. Ther. 1994, 269, 430–436. [Google Scholar] [CrossRef]
- Shimpo, K.; Nagatsu, T.; Yamada, K.; Sato, T.; Niimi, H.; Shamoto, M.; Takeuchi, T.; Umezawa, H.; Fujita, K. Ascorbic acid and adriamycin toxicity. Am. J. Clin. Nutr. 1991, 54 (Suppl. S6), 1298S–1301S. [Google Scholar] [CrossRef]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, K.F.; Materon, E.M.; Oliveira, O.N., Jr. Influence of cytochrome P450 3A4 and membrane lipid composition on doxorubicin activity. Colloids Surf. B Biointerfaces 2022, 220, 112886. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, A.M.; Zordoky, B.N.; El-Sherbeni, A.A.; El-Kadi, A.O. Chronic doxorubicin cardiotoxicity modulates cardiac cytochrome P450-mediated arachidonic acid metabolism in rats. Drug Metab. Dispos. 2012, 40, 2126–2135. [Google Scholar] [CrossRef]
- Di Marco, A.; Cassinelli, G.; Arcamone, F. The discovery of daunorubicin. Cancer Treat. Rep. 1981, 65 (Suppl. S4), 3–8. [Google Scholar]
- Goldin, A.; Venditti, J.M.; Geran, R. The effectiveness of the anthracycline analog 4′-epidoxorubicin in the treatment of experimental tumors: A review. Investig. New Drugs 1985, 3, 3–21. [Google Scholar] [CrossRef]
- Carella, A.M.; Berman, E.; Maraone, M.P.; Ganzina, F. Idarubicin in the treatment of acute leukemias. An overview of preclinical and clinical studies. Haematologica 1990, 75, 159–169. [Google Scholar]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-mediated cardiotoxicity: Current understanding, challenges, and frontiers. Antib. Ther. 2018, 1, 13–17. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Northfelt, D.W.; Gentile, F.; Zamorano, J.L.; Khandheria, B.K. Trastuzumab-induced cardiotoxicity: Heart failure at the crossroads. Mayo Clin. Proc. 2008, 83, 197–203. [Google Scholar] [CrossRef]
- Farolfi, A.; Melegari, E.; Aquilina, M.; Scarpi, E.; Ibrahim, T.; Maltoni, R.; Sarti, S.; Cecconetto, L.; Pietri, E.; Ferrario, C.; et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: A retrospective study of possible risk and protective factors. Heart 2013, 99, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Hamirani, Y.; Fanous, I.; Kramer, C.M.; Wong, A.; Salerno, M.; Dillon, P. Anthracycline- and trastuzumab-induced cardiotoxicity: A retrospective study. Med. Oncol. 2016, 33, 82. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.F.; Yadav, N.U.; Lung, B.Y.; Eaton, A.A.; Thaler, H.T.; Hudis, C.A.; Dang, C.T.; Steingart, R.M. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res. Treat. 2015, 149, 489–495. [Google Scholar] [CrossRef]
- Koulaouzidis, G.; Yung, A.E.; Yung, D.E.; Skonieczna-Zydecka, K.; Marlicz, W.; Koulaouzidis, A.; Charisopoulou, D. Conventional cardiac risk factors associated with trastuzumab-induced cardiotoxicity in breast cancer: Systematic review and meta-analysis. Curr. Probl. Cancer 2021, 45, 100723. [Google Scholar] [CrossRef]
- ElZarrad, M.K.; Mukhopadhyay, P.; Mohan, N.; Hao, E.; Dokmanovic, M.; Hirsch, D.S.; Shen, Y.; Pacher, P.; Wu, W.J. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS ONE 2013, 8, e79543. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Olorundare, O.E.; Adeneye, A.A.; Akinsola, A.O.; Ajayi, A.M.; Agede, O.A.; Soyemi, S.S.; Mgbehoma, A.I.; Okoye, I.I.; Albrecht, R.M.; Ntambi, J.M.; et al. Therapeutic Potentials of Selected Antihypertensive Agents and Their Fixed-Dose Combinations Against Trastuzumab-Mediated Cardiotoxicity. Front. Pharmacol. 2020, 11, 610331. [Google Scholar] [CrossRef]
- Dirican, A.; Levent, F.; Alacacioglu, A.; Kucukzeybek, Y.; Varol, U.; Kocabas, U.; Senoz, O.; Emren, S.V.; Demir, L.; Coban, E.; et al. Acute cardiotoxic effects of adjuvant trastuzumab treatment and its relation to oxidative stress. Angiology 2014, 65, 944–949. [Google Scholar] [CrossRef]
- Anjos, M.; Fontes-Oliveira, M.; Costa, V.M.; Santos, M.; Ferreira, R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021, 280, 119760. [Google Scholar] [CrossRef]
- Negro, A.; Brar, B.K.; Lee, K.F. Essential roles of Her2/erbB2 in cardiac development and function. Recent. Prog. Horm. Res. 2004, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dokmanovic, M.; King, K.E.; Mohan, N.; Endo, Y.; Wu, W.J. Cardiotoxicity of ErbB2-targeted therapies and its impact on drug development, a spotlight on trastuzumab. Expert Opin. Drug Metab. Toxicol. 2017, 13, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Shen, Y.; Endo, Y.; ElZarrad, M.K.; Wu, W.J. Trastuzumab, but Not Pertuzumab, Dysregulates HER2 Signaling to Mediate Inhibition of Autophagy and Increase in Reactive Oxygen Species Production in Human Cardiomyocytes. Mol. Cancer Ther. 2016, 15, 1321–1331. [Google Scholar] [CrossRef]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Wu, W.J. Implications of Autophagy and Oxidative Stress in Trastuzumab-Mediated Cardiac Toxicities. Austin Pharmacol. Pharm. 2017, 2, 1005. [Google Scholar]
- Schneider, J.W.; Chang, A.Y.; Garratt, A. Trastuzumab cardiotoxicity: Speculations regarding pathophysiology and targets for further study. Semin. Oncol. 2002, 29 (Suppl. S3–S11), 22–28. [Google Scholar] [CrossRef]
- Pecoraro, M.; Pinto, A.; Popolo, A. Trastuzumab-induced cardiotoxicity and role of mitochondrial connexin43 in the adaptive response. Toxicol. Vitr. 2020, 67, 104926. [Google Scholar] [CrossRef]
- Jiang, J.; Mohan, N.; Endo, Y.; Shen, Y.; Wu, W.J. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget 2018, 9, 6095–6108. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Zeglinski, M.; Ludke, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A ‘dual-hit’. Exp. Clin. Cardiol. 2011, 16, 70–74. [Google Scholar]
- Fukazawa, R.; Miller, T.A.; Kuramochi, Y.; Frantz, S.; Kim, Y.D.; Marchionni, M.A.; Kelly, R.A.; Sawyer, D.B. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell. Cardiol. 2003, 35, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Yoon, Y.K.; Kim, J.W.; Han, S.W.; Hur, H.S.; Park, J.; Lee, J.H.; Oh, D.Y.; Im, S.A.; Bang, Y.J.; et al. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS ONE 2009, 4, e5933. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Yorgun, H.; Petekkaya, I.; Ozer, N.; Altundag, K.; Ozisik, Y. Evaluation of cardiac safety of lapatinib therapy for ErbB2-positive metastatic breast cancer: A single center experience. Med. Oncol. 2012, 29, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Koehler, M.; Byrne, J.; Preston, A.J.; Rappold, E.; Ewer, M.S. Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin. Proc. 2008, 83, 679–686. [Google Scholar] [CrossRef]
- Fedele, C.; Riccio, G.; Coppola, C.; Barbieri, A.; Monti, M.G.; Arra, C.; Tocchetti, C.G.; D’Alessio, G.; Maurea, N.; De Lorenzo, C. Comparison of preclinical cardiotoxic effects of different ErbB2 inhibitors. Breast Cancer Res. Treat. 2012, 133, 511–521. [Google Scholar] [CrossRef]
- Gordon, L.I.; Burke, M.A.; Singh, A.T.; Prachand, S.; Lieberman, E.D.; Sun, L.; Naik, T.J.; Prasad, S.V.; Ardehali, H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009, 284, 2080–2087. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Xu, D.; Yu, S.; Zhang, L.; Li, X. Lapatinib induces mitochondrial dysfunction to enhance oxidative stress and ferroptosis in doxorubicin-induced cardiomyocytes via inhibition of PI3K/AKT signaling pathway. Bioengineered 2022, 13, 48–60. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Patel, D.; Wu, X. The dual-targeted HER1/HER2 tyrosine kinase inhibitor lapatinib strongly potentiates the cardiac myocyte-damaging effects of doxorubicin. Cardiovasc. Toxicol. 2013, 13, 33–47. [Google Scholar] [CrossRef]
- Hsu, W.T.; Huang, C.Y.; Yen, C.Y.T.; Cheng, A.L.; Hsieh, P.C.H. The HER2 inhibitor lapatinib potentiates doxorubicin-induced cardiotoxicity through iNOS signaling. Theranostics 2018, 8, 3176–3188. [Google Scholar] [CrossRef]
- Fedele, C.; Riccio, G.; Malara, A.E.; D’Alessio, G.; De Lorenzo, C. Mechanisms of cardiotoxicity associated with ErbB2 inhibitors. Breast Cancer Res. Treat. 2012, 134, 595–602. [Google Scholar] [CrossRef]
- Schmid, T.A.; Gore, M.E. Sunitinib in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 348–371. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Keefe, S.; Haas, N.; Wang, L.; Puzanov, I.; Putt, M.; Catino, A.; Fang, J.; Agarwal, N.; Hyman, D.; et al. Prospective Evaluation of Sunitinib-Induced Cardiotoxicity in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 3601–3609. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Bojic, M.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Telli, M.L.; Witteles, R.M.; Fisher, G.A.; Srinivas, S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol. 2008, 19, 1613–1618. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Autorino, R.; Bruni, G.; Carteni, G.; Ricevuto, E.; Tudini, M.; Ficorella, C.; Romano, C.; Aieta, M.; Giordano, A.; et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann. Oncol. 2009, 20, 1535–1542. [Google Scholar] [CrossRef]
- Ky, B.; French, B.; Ruparel, K.; Sweitzer, N.K.; Fang, J.C.; Levy, W.C.; Sawyer, D.B.; Cappola, T.P. The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J. Am. Coll. Cardiol. 2011, 58, 386–394. [Google Scholar] [CrossRef]
- Hamnvik, O.P.; Choueiri, T.K.; Turchin, A.; McKay, R.R.; Goyal, L.; Davis, M.; Kaymakcalan, M.D.; Williams, J.S. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015, 121, 311–319. [Google Scholar] [CrossRef]
- Izzedine, H.; Ederhy, S.; Goldwasser, F.; Soria, J.C.; Milano, G.; Cohen, A.; Khayat, D.; Spano, J.P. Management of hypertension in angiogenesis inhibitor-treated patients. Ann. Oncol. 2009, 20, 807–815. [Google Scholar] [CrossRef]
- Kappers, M.H.; van Esch, J.H.; Sluiter, W.; Sleijfer, S.; Danser, A.H.; van den Meiracker, A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010, 56, 675–681. [Google Scholar] [CrossRef]
- Cui, G.; Chen, H.; Cui, W.; Guo, X.; Fang, J.; Liu, A.; Chen, Y.; Lee, S.M. FGF2 Prevents Sunitinib-Induced Cardiotoxicity in Zebrafish and Cardiomyoblast H9c2 Cells. Cardiovasc. Toxicol. 2016, 16, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chintalgattu, V.; Rees, M.L.; Culver, J.C.; Goel, A.; Jiffar, T.; Zhang, J.; Dunner, K., Jr.; Pati, S.; Bankson, J.A.; Pasqualini, R.; et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci. Transl. Med. 2013, 5, 187ra69. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cavalcanti, E.; Taibi, R.; Montopoli, M.; Botti, G.; Maurea, N. Polydatin Reduces Cardiotoxicity and Enhances the Anticancer Effects of Sunitinib by Decreasing Pro-Oxidative Stress, Pro-Inflammatory Cytokines, and NLRP3 Inflammasome Expression. Front. Oncol. 2021, 11, 680758. [Google Scholar] [CrossRef]
- Cooper, S.L.; Sandhu, H.; Hussain, A.; Mee, C.; Maddock, H. Involvement of mitogen activated kinase kinase 7 intracellular signalling pathway in Sunitinib-induced cardiotoxicity. Toxicology 2018, 394, 72–83. [Google Scholar] [CrossRef]
- Blanca, A.J.; Ruiz-Armenta, M.V.; Zambrano, S.; Miguel-Carrasco, J.L.; Arias, J.L.; Arevalo, M.; Mate, A.; Aramburu, O.; Vazquez, C.M. Inflammatory and fibrotic processes are involved in the cardiotoxic effect of sunitinib: Protective role of L-carnitine. Toxicol. Lett. 2016, 241, 9–18. [Google Scholar] [CrossRef]
- McMullen, C.J.; Chalmers, S.; Wood, R.; Cunningham, M.R.; Currie, S. Sunitinib and Imatinib Display Differential Cardiotoxicity in Adult Rat Cardiac Fibroblasts That Involves a Role for Calcium/Calmodulin Dependent Protein Kinase II. Front. Cardiovasc. Med. 2020, 7, 630480. [Google Scholar] [CrossRef]
- Korashy, H.M.; Al-Suwayeh, H.A.; Maayah, Z.H.; Ansari, M.A.; Ahmad, S.F.; Bakheet, S.A. Mitogen-activated protein kinases pathways mediate the sunitinib-induced hypertrophy in rat cardiomyocyte H9c2 cells. Cardiovasc. Toxicol. 2015, 15, 41–51. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Ansari, M.A.; El Gendy, M.A.; Al-Arifi, M.N.; Korashy, H.M. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Arch. Toxicol. 2014, 88, 725–738. [Google Scholar] [CrossRef]
- Bouitbir, J.; Alshaikhali, A.; Panajatovic, M.V.; Abegg, V.F.; Paech, F.; Krahenbuhl, S. Mitochondrial oxidative stress plays a critical role in the cardiotoxicity of sunitinib: Sunitinib and oxidative stress in hearts. Toxicology 2019, 426, 152281. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Song, C.; Tian, X.; Zhang, H.; Zhang, J.; Zhao, X. Sunitinib induces cardiotoxicity through modulating oxidative stress and Nrf2-dependent ferroptosis in vitro and in vivo. Chem. Biol. Interact. 2024, 388, 110829. [Google Scholar] [CrossRef]
- Kerkela, R.; Woulfe, K.C.; Durand, J.B.; Vagnozzi, R.; Kramer, D.; Chu, T.F.; Beahm, C.; Chen, M.H.; Force, T. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2009, 2, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.; Cooper, S.; Hussain, A.; Mee, C.; Maddock, H. Attenuation of Sunitinib-induced cardiotoxicity through the A3 adenosine receptor activation. Eur. J. Pharmacol. 2017, 814, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Patel, D.; O’Hara, K.A. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol. Pharmacol. 2008, 74, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Liu, L.; Qi, Y.; Bu, P. Trimetazidine ameliorates sunitinib-induced cardiotoxicity in mice via the AMPK/mTOR/autophagy pathway. Pharm. Biol. 2019, 57, 625–631. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, T.; Yang, X.; Zhu, H.; Ding, X.; Lou, L.; Lu, W.; Yang, B.; He, Q. Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicol. Appl. Pharmacol. 2010, 248, 20–27. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, Y.; Gao, Z.; Zeng, Y.; Du, J.; Yan, H.; Chen, X.; Ping, L.; Lin, N.; Yang, B.; et al. Autophagic degradation of CCN2 (cellular communication network factor 2) causes cardiotoxicity of sunitinib. Autophagy 2022, 18, 1152–1173. [Google Scholar] [CrossRef]
- Mooney, L.; Skinner, M.; Coker, S.J.; Currie, S. Effects of acute and chronic sunitinib treatment on cardiac function and calcium/calmodulin-dependent protein kinase II. Br. J. Pharmacol. 2015, 172, 4342–4354. [Google Scholar] [CrossRef]
- Chen, M.H.; Kerkela, R.; Force, T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 2008, 118, 84–95. [Google Scholar] [CrossRef]
- Hervent, A.S.; De Keulenaer, G.W. Molecular mechanisms of cardiotoxicity induced by ErbB receptor inhibitor cancer therapeutics. Int. J. Mol. Sci. 2012, 13, 12268–12286. [Google Scholar] [CrossRef]
- Alhoshani, A.; Alanazi, F.E.; Alotaibi, M.R.; Attwa, M.W.; Kadi, A.A.; Aldhfyan, A.; Akhtar, S.; Hourani, S.; Agouni, A.; Zeidan, A.; et al. EGFR Inhibitor Gefitinib Induces Cardiotoxicity through the Modulation of Cardiac PTEN/Akt/FoxO3a Pathway and Reactive Metabolites Formation: In Vivo and In Vitro Rat Studies. Chem. Res. Toxicol. 2020, 33, 1719–1728. [Google Scholar] [CrossRef]
- Alanazi, W.A.; Alhamami, H.N.; Alharbi, M.; Alhazzani, K.; Alanazi, A.S.; Alsanea, S.; Ali, N.; Alasmari, A.F.; Alanazi, A.Z.; Alotaibi, M.R.; et al. Angiotensin II type 1 receptor blockade attenuates gefitinib-induced cardiac hypertrophy via adjusting angiotensin II-mediated oxidative stress and JNK/P38 MAPK pathway in a rat model. Saudi Pharm. J. 2022, 30, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.J.; Li, Y.D.; Zhang, H.Q.; Mao, L.; Xie, H.B.; Zhou, F.G.; Zhou, T.L.; Xie, D.; Lin, J.L.; Li, G.Y.; et al. Mechanisms of gefitinib-induced QT prolongation. Eur. J. Pharmacol. 2021, 910, 174441. [Google Scholar] [CrossRef] [PubMed]
- Maimaitituersun, G.; Abulimiti, B.; Jin, M.; Dong, X.; Fu, Z. Gefitinib Increases the Incidence of QT Prolongation in Patients with Non-Small Cell Lung Cancer. Int. Heart J. 2023, 64, 365–373. [Google Scholar] [CrossRef]
- Yang, J.C.; Shih, J.Y.; Su, W.C.; Hsia, T.C.; Tsai, C.M.; Ou, S.H.; Yu, C.J.; Chang, G.C.; Ho, C.L.; Sequist, L.V.; et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Patel, K.; O’Brien, D.; Lorence, R.M. Cardiac safety of afatinib: A review of data from clinical trials. Cardiooncology 2015, 1, 3. [Google Scholar] [CrossRef]
- Ramos, G.E.; Caglevic, C.; Bulnes, J.F.; Panay, S.E.; Zapata, M.I.; Daniele, A.J.; Rodriguez, M.E. Takotsubo cardiomyopathy Afatinib-related in a non-small cell lung cancer patient: Case report. Front. Cardiovasc. Med. 2022, 9, 1060813. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, J.; He, Z.; Boswell, S.A.; Chung, M.; You, F.; Han, S. Three tyrosine kinase inhibitors cause cardiotoxicity by inducing endoplasmic reticulum stress and inflammation in cardiomyocytes. BMC Med. 2023, 21, 147. [Google Scholar] [CrossRef]
- Duran, J.M.; Makarewich, C.A.; Trappanese, D.; Gross, P.; Husain, S.; Dunn, J.; Lal, H.; Sharp, T.E.; Starosta, T.; Vagnozzi, R.J.; et al. Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circ. Res. 2014, 114, 1700–1712. [Google Scholar] [CrossRef]
- Chen, Y.T.; Masbuchin, A.N.; Fang, Y.H.; Hsu, L.W.; Wu, S.N.; Yen, C.J.; Liu, Y.W.; Hsiao, Y.W.; Wang, J.M.; Rohman, M.S.; et al. Pentraxin 3 regulates tyrosine kinase inhibitor-associated cardiomyocyte contraction and mitochondrial dysfunction via ERK/JNK signalling pathways. Biomed. Pharmacother. 2023, 157, 113962. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Ge, T.; Liu, J.; Wang, C.; Yu, Q. Understanding Sorafenib-Induced Cardiovascular Toxicity: Mechanisms and Treatment Implications. Drug Des. Devel. Ther. 2024, 18, 829–843. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Zhao, Q.; Zhang, Y.; Zhang, Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front. Pharmacol. 2022, 13, 904314. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, M.; Umemoto, N.; Shimada, Y.; Nishimura, Y.; Zhang, B.; Kuroyanagi, J.; Miyabe, M.; Tanaka, T. Downregulation of stanniocalcin 1 is responsible for sorafenib-induced cardiotoxicity. Toxicol. Sci. 2015, 143, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yue, S.; Guo, Y.; Han, J.Y.; Wang, H. Sorafenib induces cardiotoxicity through RBM20-mediated alternative splicing of sarcomeric and mitochondrial genes. Pharmacol. Res. 2023, 198, 107017. [Google Scholar] [CrossRef]
- Ma, W.; Liu, M.; Liang, F.; Zhao, L.; Gao, C.; Jiang, X.; Zhang, X.; Zhan, H.; Hu, H.; Zhao, Z. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin. Pharmacol. Toxicol. 2020, 126, 166–180. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, K.; Ma, W.; Zhan, H.; Sun, Q.; Xie, L.; Zhao, Z. Impaired autophagy and mitochondrial dynamics are involved in Sorafenib-induced cardiomyocyte apoptosis. Toxicology 2022, 481, 153348. [Google Scholar] [CrossRef]
- Bouitbir, J.; Panajatovic, M.V.; Krahenbuhl, S. Mitochondrial Toxicity Associated with Imatinib and Sorafenib in Isolated Rat Heart Fibers and the Cardiomyoblast H9c2 Cell Line. Int. J. Mol. Sci. 2022, 23, 2282. [Google Scholar] [CrossRef]
- Schneider, C.; Wallner, M.; Kolesnik, E.; Herbst, V.; Machler, H.; Pichler, M.; von Lewinski, D.; Sedej, S.; Rainer, P.P. The Anti-Cancer Multikinase Inhibitor Sorafenib Impairs Cardiac Contractility by Reducing Phospholamban Phosphorylation and Sarcoplasmic Calcium Transients. Sci. Rep. 2018, 8, 5295. [Google Scholar] [CrossRef]
- Will, Y.; Dykens, J.A.; Nadanaciva, S.; Hirakawa, B.; Jamieson, J.; Marroquin, L.D.; Hynes, J.; Patyna, S.; Jessen, B.A. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol. Sci. 2008, 106, 153–161. [Google Scholar] [CrossRef]
- Zaafar, D.; Khalil, H.M.A.; Rasheed, R.A.; Eltelbany, R.F.A.; Zaitone, S.A. Hesperetin mitigates sorafenib-induced cardiotoxicity in mice through inhibition of the TLR4/NLRP3 signaling pathway. PLoS ONE 2022, 17, e0271631. [Google Scholar] [CrossRef]
- Nagashio, K.; Tajiri, K.; Sato, K.; Ieda, M. Erlotinib-Induced Cardiomyopathy in a Patient with Metastatic Non-Small Cell Lung Cancer. Int. Heart J. 2021, 62, 1171–1175. [Google Scholar] [CrossRef]
- Kus, T.; Aktas, G.; Sevinc, A.; Kalender, M.E.; Camci, C. Could erlotinib treatment lead to acute cardiovascular events in patients with lung adenocarcinoma after chemotherapy failure? Onco Targets Ther. 2015, 8, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.R.; Wappel, R.L.; Talbert, D.R.; Trusk, P.B.; Moran, D.M.; Kramer, J.W.; Brown, A.M.; Shell, S.A.; Bacus, S. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 2013, 272, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Chmielinska, J.J.; Kramer, J.H.; Mak, I.T.; Spurney, C.F.; Weglicki, W.B. Substance P receptor blocker, aprepitant, inhibited cutaneous and other neurogenic inflammation side effects of the EGFR1-TKI, erlotinib. Mol. Cell. Biochem. 2020, 465, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Stuhlmiller, T.J.; Zawistowski, J.S.; Chen, X.; Sciaky, N.; Angus, S.P.; Hicks, S.T.; Parry, T.L.; Huang, W.; Beak, J.Y.; Willis, M.S.; et al. Kinome and Transcriptome Profiling Reveal Broad and Distinct Activities of Erlotinib, Sunitinib, and Sorafenib in the Mouse Heart and Suggest Cardiotoxicity from Combined Signal Transducer and Activator of Transcription and Epidermal Growth Factor Receptor Inhibition. J. Am. Heart Assoc. 2017, 6, e006635. [Google Scholar] [CrossRef]
- Grabowska, M.E.; Chun, B.; Moya, R.; Saucerman, J.J. Computational model of cardiomyocyte apoptosis identifies mechanisms of tyrosine kinase inhibitor-induced cardiotoxicity. J. Mol. Cell. Cardiol. 2021, 155, 66–77. [Google Scholar] [CrossRef]
- Li, W.; Croce, K.; Steensma, D.P.; McDermott, D.F.; Ben-Yehuda, O.; Moslehi, J. Vascular and Metabolic Implications of Novel Targeted Cancer Therapies: Focus on Kinase Inhibitors. J. Am. Coll. Cardiol. 2015, 66, 1160–1178. [Google Scholar] [CrossRef]
- Mazzei, T.; Mini, E.; Novelli, A.; Periti, P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31 (Suppl. SC), 1–9. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Pillozzi, S.; Masselli, M.; Gasparoli, L.; D’Amico, M.; Polletta, L.; Veltroni, M.; Favre, C.; Basso, G.; Becchetti, A.; Arcangeli, A. Macrolide antibiotics exert antileukemic effects by modulating the autophagic flux through inhibition of hERG1 potassium channels. Blood Cancer J. 2016, 6, e423. [Google Scholar] [CrossRef]
- Govi, S.; Dognini, G.P.; Licata, G.; Crocchiolo, R.; Resti, A.G.; Ponzoni, M.; Ferreri, A.J. Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: Final results of a single-centre phase II trial. Br. J. Haematol. 2010, 150, 226–229. [Google Scholar] [CrossRef]
- Wu, Y.; Bi, W.T.; Qu, L.P.; Fan, J.; Kong, X.J.; Ji, C.C.; Chen, X.M.; Yao, F.J.; Liu, L.J.; Cheng, Y.J.; et al. Administration of macrolide antibiotics increases cardiovascular risk. Front. Cardiovasc. Med. 2023, 10, 1117254. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Schuller, J.L.; Network, C.C.R. Macrolide antibiotics and the risk of cardiac arrhythmias. Am. J. Respir. Crit. Care Med. 2014, 189, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Nie, X.Y.; Chen, X.M.; Lin, X.X.; Tang, K.; Zeng, W.T.; Mei, W.Y.; Liu, L.J.; Long, M.; Yao, F.J.; et al. The Role of Macrolide Antibiotics in Increasing Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 66, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, E.; Kolesar, J.; Young, D.; Allen, J.; Villalona-Calero, M.; Shapiro, C.L. Phase II study of sequentially administered low-dose mitomycin-C (MMC) and irinotecan (CPT-11) in women with metastatic breast cancer (MBC). Ann. Oncol. 2008, 19, 1417–1422. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeon, Y.; Lee, H.W.; Kang, J.; Baik, S.H.; Park, E.J. Impact of Mitomycin-C-Induced Neutropenia After Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery in Colorectal Cancer Patients with Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2022, 29, 2077–2086. [Google Scholar] [CrossRef]
- Luo, M.; Wang, F.; Zhang, H.; To, K.K.W.; Wu, S.; Chen, Z.; Liang, S.; Fu, L. Mitomycin C enhanced the efficacy of PD-L1 blockade in non-small cell lung cancer. Signal Transduct. Target. Ther. 2020, 5, 141. [Google Scholar] [CrossRef]
- Bregman, C.L.; Buroker, R.A.; Bradner, W.T.; Hirth, R.S.; Madissoo, H. Cardiac, renal, and pulmonary toxicity of several mitomycin derivatives in rats. Fundam. Appl. Toxicol. 1989, 13, 46–64. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Legha, S.S.; Tashima, C.K.; Hortobagyi, G.N.; Yap, H.Y.; Krutchik, A.N.; Luna, M.A.; Blumenschein, G.R. Adriamycin and mitomycin C: Possible synergistic cardiotoxicity. Cancer Treat. Rep. 1978, 62, 1005–1008. [Google Scholar]
- Tashakori Beheshti, A.; Mostafavi Toroghi, H.; Hosseini, G.; Zarifian, A.; Homaei Shandiz, F.; Fazlinezhad, A. Carvedilol Administration Can Prevent Doxorubicin-Induced Cardiotoxicity: A Double-Blind Randomized Trial. Cardiology 2016, 134, 47–53. [Google Scholar] [CrossRef]
- Nabati, M.; Janbabai, G.; Baghyari, S.; Esmaili, K.; Yazdani, J. Cardioprotective Effects of Carvedilol in Inhibiting Doxorubicin-induced Cardiotoxicity. J. Cardiovasc. Pharmacol. 2017, 69, 279–285. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; das Dores Cruz, F.; Goncalves Brandao, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Ramirez, M.C.; Nes, K.; Schuster, A.; Aguayo, R.; Morales, M.; Ramos, C.; Hasson, D.; Sotomayor, C.G.; Henriquez, P.; et al. Prevention of doxorubicin-induced Cardiotoxicity by pharmacological non-hypoxic myocardial preconditioning based on Docosahexaenoic Acid (DHA) and carvedilol direct antioxidant effects: Study protocol for a pilot, randomized, double-blind, controlled trial (CarDHA trial). Trials 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Imbaby, S.; Ewais, M.; Essawy, S.; Farag, N. Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Hum. Exp. Toxicol. 2014, 33, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.G.; Ozkan, M.; Gunebakmaz, O.; Akkaya, H.; Kaya, E.G.; Akpek, M.; Kalay, N.; Dikilitas, M.; Yarlioglues, M.; Karaca, H.; et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. Int. J. Cardiol. 2013, 167, 2306–2310. [Google Scholar] [CrossRef]
- Hasinoff, B.B. The use of dexrazoxane for the prevention of anthracycline extravasation injury. Expert Opin. Investig. Drugs 2008, 17, 217–223. [Google Scholar] [CrossRef]
- Sotiropoulou, I.M.; Manetas-Stavrakakis, N.; Kourek, C.; Xanthopoulos, A.; Magouliotis, D.; Giamouzis, G.; Skoularigis, J.; Briasoulis, A. Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2419. [Google Scholar] [CrossRef]
- Dickey, J.S.; Gonzalez, Y.; Aryal, B.; Mog, S.; Nakamura, A.J.; Redon, C.E.; Baxa, U.; Rosen, E.; Cheng, G.; Zielonka, J.; et al. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS ONE 2013, 8, e70575. [Google Scholar] [CrossRef]
- Kim, D.J.; Jeena, M.T.; Kim, O.H.; Hong, H.E.; Seo, H.; Ryu, J.H.; Kim, S.J. Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6126. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997, 54 (Suppl. S4), 15–21. [Google Scholar] [CrossRef]
- Gabizon, A.; Shiota, R.; Papahadjopoulos, D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J. Natl. Cancer Inst. 1989, 81, 1484–1488. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, J.H.; Zheng, Y.B.; Cao, W.M.; Shao, X.Y.; Chen, J.Q.; Huang, Y.; Li, G.L.; Sharma, K.; Zhou, H.H.; et al. Cardiac Safety in Breast Cancer Patients Receiving Pegylated Liposome Doxorubicin Sequential Anti-HER2 Monoclonal Antibody Therapy. Front. Pharmacol. 2022, 13, 883600. [Google Scholar] [CrossRef]
- Creutzig, U.; Zimmermann, M.; Bourquin, J.P.; Dworzak, M.N.; Fleischhack, G.; Graf, N.; Klingebiel, T.; Kremens, B.; Lehrnbecher, T.; von Neuhoff, C.; et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: Results from Study AML-BFM 2004. Blood 2013, 122, 37–43. [Google Scholar] [CrossRef]
- Santucci, L.; Mencarelli, A.; Renga, B.; Ceccobelli, D.; Pasut, G.; Veronese, F.M.; Distrutti, E.; Fiorucci, S. Cardiac safety and antitumoral activity of a new nitric oxide derivative of pegylated epirubicin in mice. Anti-Cancer Drugs 2007, 18, 1081–1091. [Google Scholar] [CrossRef]
- Chang, R.S.; Kim, J.; Lee, H.Y.; Han, S.E.; Na, J.; Kim, K.; Kwon, I.C.; Kim, Y.B.; Oh, Y.K. Reduced dose-limiting toxicity of intraperitoneal mitoxantrone chemotherapy using cardiolipin-based anionic liposomes. Nanomedicine 2010, 6, 769–776. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Fadda, L.; Alhusaini, A.; Ahmad, R.; Hasan, I.H.; Mahmoud, A.M. Liposomal Resveratrol and/or Carvedilol Attenuate Doxorubicin-Induced Cardiotoxicity by Modulating Inflammation, Oxidative Stress and S100A1 in Rats. Antioxidants 2020, 9, 159. [Google Scholar] [CrossRef]
- Zara, G.P.; Bargoni, A.; Cavalli, R.; Fundaro, A.; Vighetto, D.; Gasco, M.R. Pharmacokinetics and tissue distribution of idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats. J. Pharm. Sci. 2002, 91, 1324–1333. [Google Scholar] [CrossRef]
- Polgar, L.; Lajko, E.; Soos, P.; Lang, O.; Manea, M.; Merkely, B.; Mezo, G.; Kohidai, L. Drug targeting to decrease cardiotoxicity—Determination of the cytotoxic effect of GnRH-based conjugates containing doxorubicin, daunorubicin and methotrexate on human cardiomyocytes and endothelial cells. Beilstein J. Org. Chem. 2018, 14, 1583–1594. [Google Scholar] [CrossRef]
- Chang, H.-M.; Hsu, J.-Y.; Chen, J.; Yeh, E.T. Abstract 12348: Targeted Degradation of Topoisomerase 2b by Dexrazoxane for Prevention of Doxorubicin-Induced Cardiotoxicity: Dose and Time Course Study in Human. Circulation 2023, 148 (Suppl. S1), A12348. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, M.K.; Song, I.S. Recent Advances in Doxorubicin Formulation to Enhance Pharmacokinetics and Tumor Targeting. Pharmaceuticals 2023, 16, 802. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, M.; Hudis, C. Cardiac profiles of liposomal anthracyclines—Greater cardiac safety versus conventional doxorubicin? Cancer 2004, 100, 2052–2063. [Google Scholar] [CrossRef]
- Franco, Y.L.; Vaidya, T.R.; Ait-Oudhia, S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Fukuda, A.; Tahara, K.; Hane, Y.; Matsui, T.; Sasaoka, S.; Hatahira, H.; Motooka, Y.; Hasegawa, S.; Naganuma, M.; Abe, J.; et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS ONE 2017, 12, e0185654. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Gugerell, A.; Pavo, N.; Traxler, D.; Pils, D.; Maurer, G.; Jakab, A.; et al. Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc. Res. 2020, 116, 970–982. [Google Scholar] [CrossRef]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef]
- Burridge, P.W.; Li, Y.F.; Matsa, E.; Wu, H.; Ong, S.G.; Sharma, A.; Holmstrom, A.; Chang, A.C.; Coronado, M.J.; Ebert, A.D.; et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 2016, 22, 547–556. [Google Scholar] [CrossRef]
- Louisse, J.; Wust, R.C.I.; Pistollato, F.; Palosaari, T.; Barilari, M.; Macko, P.; Bremer, S.; Prieto, P. Assessment of acute and chronic toxicity of doxorubicin in human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Vitr. 2017, 42, 182–190. [Google Scholar] [CrossRef]
- Chaudhari, U.; Ellis, J.K.; Wagh, V.; Nemade, H.; Hescheler, J.; Keun, H.C.; Sachinidis, A. Metabolite signatures of doxorubicin induced toxicity in human induced pluripotent stem cell-derived cardiomyocytes. Amino Acids 2017, 49, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Karhu, S.T.; Kinnunen, S.M.; Tolli, M.; Valimaki, M.J.; Szabo, Z.; Talman, V.; Ruskoaho, H. GATA4-targeted compound exhibits cardioprotective actions against doxorubicin-induced toxicity in vitro and in vivo: Establishment of a chronic cardiotoxicity model using human iPSC-derived cardiomyocytes. Arch. Toxicol. 2020, 94, 2113–2130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Burridge, P.W.; McKeithan, W.L.; Serrano, R.; Shukla, P.; Sayed, N.; Churko, J.M.; Kitani, T.; Wu, H.; Holmstrom, A.; et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 2017, 9, eaaf2584. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.K.; Shang, M.R.; Yin, R.T.; George, S.C. Modeling trastuzumab-related cardiotoxicity in vitro using human stem cell-derived cardiomyocytes. Toxicol. Lett. 2018, 285, 74–80. [Google Scholar] [CrossRef]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients with Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef]
- Chaudhari, U.; Nemade, H.; Wagh, V.; Gaspar, J.A.; Ellis, J.K.; Srinivasan, S.P.; Spitkovski, D.; Nguemo, F.; Louisse, J.; Bremer, S.; et al. Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: An in vitro repeated exposure toxicity approach for safety assessment. Arch. Toxicol. 2016, 90, 2763–2777. [Google Scholar] [CrossRef]
- Chaudhari, U.; Nemade, H.; Gaspar, J.A.; Hescheler, J.; Hengstler, J.G.; Sachinidis, A. MicroRNAs as early toxicity signatures of doxorubicin in human-induced pluripotent stem cell-derived cardiomyocytes. Arch. Toxicol. 2016, 90, 3087–3098. [Google Scholar] [CrossRef]
- Singh, P.; Wang, X.; Hageman, L.; Chen, Y.; Magdy, T.; Landier, W.; Ginsberg, J.P.; Neglia, J.P.; Sklar, C.A.; Castellino, S.M.; et al. Association of GSTM1 null variant with anthracycline-related cardiomyopathy after childhood cancer-A Children’s Oncology Group ALTE03N1 report. Cancer 2020, 126, 4051–4058. [Google Scholar] [CrossRef]
- Magdy, T.; Jiang, Z.; Jouni, M.; Fonoudi, H.; Lyra-Leite, D.; Jung, G.; Romero-Tejeda, M.; Kuo, H.H.; Fetterman, K.A.; Gharib, M.; et al. RARG variant predictive of doxorubicin-induced cardiotoxicity identifies a cardioprotective therapy. Cell Stem Cell 2021, 28, 2076–2089.e7. [Google Scholar] [CrossRef]
- Magdy, T.; Jouni, M.; Kuo, H.H.; Weddle, C.J.; Lyra-Leite, D.; Fonoudi, H.; Romero-Tejeda, M.; Gharib, M.; Javed, H.; Fajardo, G.; et al. Identification of Drug Transporter Genomic Variants and Inhibitors That Protect Against Doxorubicin-Induced Cardiotoxicity. Circulation 2022, 145, 279–294. [Google Scholar] [CrossRef]
- Huang, H.; Christidi, E.; Shafaattalab, S.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. RARG S427L attenuates the DNA repair response to doxorubicin in induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, F.; Jiang, G.; Xue, G.; Philips, S.; Gardner, L.; Cunningham, G.; Bales, C.; Cantor, E.; Schneider, B.P. A non-coding GWAS variant impacts anthracycline-induced cardiotoxic phenotypes in human iPSC-derived cardiomyocytes. Nat. Commun. 2022, 13, 7171. [Google Scholar] [CrossRef]
- Singh, P.; Zhou, L.; Shah, D.A.; Cejas, R.B.; Crossman, D.K.; Jouni, M.; Magdy, T.; Wang, X.; Sharafeldin, N.; Hageman, L.; et al. Identification of novel hypermethylated or hypomethylated CpG sites and genes associated with anthracycline-induced cardiomyopathy. Sci. Rep. 2023, 13, 12683. [Google Scholar] [CrossRef]
- Boden, W.E.; More, G.; Sharma, S.; Bough, E.W.; Korr, K.S.; Young, P.M.; Shulman, R.S. No increase in serum digoxin concentration with high-dose diltiazem. Am. J. Med. 1986, 81, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Shah, D.A.; Jouni, M.; Cejas, R.B.; Crossman, D.K.; Magdy, T.; Qiu, S.; Wang, X.; Zhou, L.; Sharafeldin, N.; et al. Altered Peripheral Blood Gene Expression in Childhood Cancer Survivors with Anthracycline-Induced Cardiomyopathy—A COG-ALTE03N1 Report. J. Am. Heart Assoc. 2023, 12, e029954. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Juric, M.; Zielonka, J.; Benjamin, I.J.; Bosnjak, Z.J. Abstract 14000: Identification of Circulating Surrogate Biomarkers for Risk Prediction of Breast Cancer Treatment-Related Cardiotoxicity. Circulation 2021, 144 (Suppl. S1), A14000. [Google Scholar] [CrossRef]
- Manstein, F.; Ullmann, K.; Kropp, C.; Halloin, C.; Triebert, W.; Franke, A.; Farr, C.M.; Sahabian, A.; Haase, A.; Breitkreuz, Y.; et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med. 2021, 10, 1063–1080. [Google Scholar] [CrossRef]
- Ullmann, K.; Manstein, F.; Triebert, W.; Kriedemann, N.; Franke, A.; Teske, J.; Mertens, M.; Lupanow, V.; Gohring, G.; Haase, A.; et al. Matrix-free human pluripotent stem cell manufacturing by seed train approach and intermediate cryopreservation. Stem Cell Res. Ther. 2024, 15, 89. [Google Scholar] [CrossRef]
- Kriedemann, N.; Manstein, F.; Hernandez-Bautista, C.A.; Ullmann, K.; Triebert, W.; Franke, A.; Mertens, M.; Stein, I.; Leffler, A.; Witte, M.; et al. Protein-free media for cardiac differentiation of hPSCs in 2000 mL suspension culture. Stem Cell Res. Ther. 2024, 15, 213. [Google Scholar] [CrossRef]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C.; American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; et al. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2018, 11, e000043. [Google Scholar] [CrossRef]
- Hyams, N.A.; Kerr, C.M.; Arhontoulis, D.C.; Ruddy, J.M.; Mei, Y. Improving human cardiac organoid design using transcriptomics. Sci. Rep. 2024, 14, 20147. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, C.; Vitiello, L.; de Windt, L.J.; Rampazzo, A.; Calore, M. Modeling Cardiovascular Diseases with hiPSC-Derived Cardiomyocytes in 2D and 3D Cultures. Int. J. Mol. Sci. 2020, 21, 3404. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.A.; Cotovio, J.P.; Rodrigues, C.A.V.; Vaz, S.H.; Fernandes, T.G.; Moreira, L.M.; Cabral, J.M.S.; Diogo, M.M. Transcriptomic Analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells Reveals Faster Cardiomyocyte Maturation Compared to 2D Culture. Sci. Rep. 2019, 9, 9229. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Salerno, L.; Amato, N.; Quercia, C.; Siracusa, C.; Filardo, A.; Chiefalo, A.; Pagano, L.; Misdea, G.; et al. Adult Multipotent Cardiac Progenitor-Derived Spheroids: A Reproducible Model of In Vitro Cardiomyocyte Commitment and Specification. Cells 2023, 12, 1793. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879.e11. [Google Scholar] [CrossRef]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Pereira-Sousa, D.; Vrbsky, J.; Vinarsky, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and maturation of human iPSC-derived 3D organotypic cardiac microtissues in long-term culture. Sci. Rep. 2022, 12, 17409. [Google Scholar] [CrossRef]
- Liu, S.; Fang, C.; Zhong, C.; Li, J.; Xiao, Q. Recent advances in pluripotent stem cell-derived cardiac organoids and heart-on-chip applications for studying anti-cancer drug-induced cardiotoxicity. Cell Biol. Toxicol. 2023, 39, 2527–2549. [Google Scholar] [CrossRef]
- Gintant, G.; Burridge, P.; Gepstein, L.; Harding, S.; Herron, T.; Hong, C.; Jalife, J.; Wu, J.C. Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Preclinical Cancer Drug Cardiotoxicity Testing: A Scientific Statement from the American Heart Association. Circ. Res. 2019, 125, e75–e92. [Google Scholar] [CrossRef]
- Kieda, J.; Shakeri, A.; Landau, S.; Wang, E.Y.; Zhao, Y.; Lai, B.F.; Okhovatian, S.; Wang, Y.; Jiang, R.; Radisic, M. Advances in cardiac tissue engineering and heart-on-a-chip. J. Biomed. Mater. Res. A 2024, 112, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Archer, C.R.; Sargeant, R.; Basak, J.; Pilling, J.; Barnes, J.R.; Pointon, A. Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology. Sci. Rep. 2018, 8, 10160. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.J.; Hall, A.R.; Lloyd, G.R.; Weber, R.J.M.; Wilson, A.; Pointon, A.; Viant, M.R. An Extensive Metabolomics Workflow to Discover Cardiotoxin-Induced Molecular Perturbations in Microtissues. Metabolites 2021, 11, 644. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Pan, G.; Lin, L.; Liu, D.; Liu, Z.; Mei, S.; Zhang, L.; Hu, Z.; Chen, J.; et al. Modulatory effect of metformin on cardiotoxicity induced by doxorubicin via the MAPK and AMPK pathways. Life Sci. 2020, 249, 117498. [Google Scholar] [CrossRef]
- Sheta, A.; Elsakkar, M.; Hamza, M.; Solaiman, A. Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in adult male albino rats. Hum. Exp. Toxicol. 2016, 35, 1227–1239. [Google Scholar] [CrossRef]
- Aguilar, D.; Chan, W.Y.; Bozkurt, B.; Ramasubbu, K.; Deswal, A. Metformin Use and Mortality in Ambulatory Patients with Diabetes and Heart Failure. J. Card. Fail. 2010, 16, S83–S84. [Google Scholar] [CrossRef]
- Norwood, D.K.; Chilipko, A.A.; Amin, S.M.; Macharia, D.; Still, K.L. Evaluating the potential benefits of metformin in patients with cardiovascular disease and heart failure. Consult. Pharm. 2013, 28, 579–583. [Google Scholar] [CrossRef]
- Bhamra, G.S.; Hausenloy, D.J.; Davidson, S.M.; Carr, R.D.; Paiva, M.; Wynne, A.M.; Mocanu, M.M.; Yellon, D.M. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic. Res. Cardiol. 2008, 103, 274–284. [Google Scholar] [CrossRef]
- Gundewar, S.; Calvert, J.W.; Jha, S.; Toedt-Pingel, I.; Ji, S.Y.; Nunez, D.; Ramachandran, A.; Anaya-Cisneros, M.; Tian, R.; Lefer, D.J. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 2009, 104, 403–411. [Google Scholar] [CrossRef]
- Mohsin, A.A.; Chen, Q.; Quan, N.; Rousselle, T.; Maceyka, M.W.; Samidurai, A.; Thompson, J.; Hu, Y.; Li, J.; Lesnefsky, E.J. Mitochondrial Complex I Inhibition by Metformin Limits Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 369, 282–290. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care 2020, 43, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors-Does It All Come Down to Na(+)? Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef] [PubMed]
- Medina-Hernandez, D.; Cadiz, L.; Mastrangelo, A.; Moreno-Arciniegas, A.; Fernandez Tocino, M.; Cueto Becerra, A.A.; Diaz-Guerra Priego, A.; Skoza, W.A.; Higuero-Verdejo, M.I.; Lopez-Martin, G.J.; et al. SGLT2i Therapy Prevents Anthracycline-Induced Cardiotoxicity in a Large Animal Model by Preserving Myocardial Energetics. JACC CardioOncol 2025, 7, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1alpha Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxid. 2023, 12, 1075. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Luo, Y.; Li, M.; Su, X.; Yu, B.; Teng, J.; Wang, H.; Lv, X. Berberine Alleviates Doxorubicin-Induced Myocardial Injury and Fibrosis by Eliminating Oxidative Stress and Mitochondrial Damage via Promoting Nrf-2 Pathway Activation. Int. J. Mol. Sci. 2023, 24, 3257. [Google Scholar] [CrossRef]
- Uche, N.; Dai, Q.; Lai, S.; Kolander, K.; Thao, M.; Schibly, E.; Sendaydiego, X.; Zielonka, J.; Benjamin, I.J. Carvedilol Phenocopies PGC-1alpha Overexpression to Alleviate Oxidative Stress, Mitochondrial Dysfunction and Prevent Doxorubicin-Induced Toxicity in Human iPSC-Derived Cardiomyocytes. Antioxidants 2023, 12, 1585. [Google Scholar] [CrossRef]
- Wright, A.V.; Nunez, J.K.; Doudna, J.A. Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell 2016, 164, 29–44. [Google Scholar] [CrossRef]
- Wallet, M.A.; Santostefano, K.E.; Terada, N.; Brusko, T.M. Isogenic Cellular Systems Model the Impact of Genetic Risk Variants in the Pathogenesis of Type 1 Diabetes. Front Endocrinol 2017, 8, 276. [Google Scholar] [CrossRef]
- Patino-Guerrero, A.; Ponce Wong, R.D.; Kodibagkar, V.D.; Zhu, W.; Migrino, R.Q.; Graudejus, O.; Nikkhah, M. Development and Characterization of Isogenic Cardiac Organoids from Human-Induced Pluripotent Stem Cells Under Supplement Starvation Regimen. ACS Biomater. Sci. Eng. 2023, 9, 944–958. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, J.; Leung, E.; Flandes-Iparraguirre, M.; Vernon, M.; Silberstein, J.; De-Juan-Pardo, E.M.; Jansen, S. Three-Dimensional Bioprinting in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2023, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Jang, J. Advances in 3D Bioprinting Technologies for Cardiovascular Intervention and Regeneration Therapeutics. J. Cardiovasc. Interv. 2025, 4, 1–28. [Google Scholar] [CrossRef]
- Mittal, R.; Woo, F.W.; Castro, C.S.; Cohen, M.A.; Karanxha, J.; Mittal, J.; Chhibber, T.; Jhaveri, V.M. Organ-on-chip models: Implications in drug discovery and clinical applications. J. Cell. Physiol. 2019, 234, 8352–8380. [Google Scholar] [CrossRef] [PubMed]
- Jang, J. 3D Bioprinting and In Vitro Cardiovascular Tissue Modeling. Bioengineering 2017, 4, 71. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.J.; Jang, W.B.; Kwon, S.M. Development of Biocompatible 3D-Printed Artificial Blood Vessels Through Multidimensional Approaches. J. Funct. Biomater. 2023, 14, 497. [Google Scholar] [CrossRef]
- Kinstlinger, I.S.; Bastian, A.; Paulsen, S.J.; Hwang, D.H.; Ta, A.H.; Yalacki, D.R.; Schmidt, T.; Miller, J.S. Open-Source Selective Laser Sintering (OpenSLS) of Nylon and Biocompatible Polycaprolactone. PLoS ONE 2016, 11, e0147399. [Google Scholar] [CrossRef]
- Elkhoury, K.; Zuazola, J.; Vijayavenkataraman, S. Bioprinting the future using light: A review on photocrosslinking reactions, photoreactive groups, and photoinitiators. SLAS Technol. 2023, 28, 142–151. [Google Scholar] [CrossRef]
- Lam, E.H.Y.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Guldager, E.; Vallgarda, S. Health visiting. The “itinerant women’s network” and professional development. Sygeplejersken 1987, 87, 18–23. [Google Scholar]
- He, Y.; Liu, X.; Xu, Z.; Gao, J.; Luo, Q.; He, Y.; Zhang, X.; Gao, D.; Wang, D. Nanomedicine alleviates doxorubicin-induced cardiotoxicity and enhances chemotherapy synergistic chemodynamic therapy. J. Colloid Interface Sci. 2024, 663, 1064–1073. [Google Scholar] [CrossRef]
- Drinkovic, N.; Beus, M.; Barbir, R.; Debeljak, Z.; Tariba Lovakovic, B.; Kalcec, N.; Curlin, M.; Bekavac, A.; Gorup, D.; Mamic, I.; et al. Novel PLGA-based nanoformulation decreases doxorubicin-induced cardiotoxicity. Nanoscale 2024, 16, 9412–9425. [Google Scholar] [CrossRef]
- de Oliveira, B.L.; Niederer, S. A Biophysical Systems Approach to Identifying the Pathways of Acute and Chronic Doxorubicin Mitochondrial Cardiotoxicity. PLoS Comput. Biol. 2016, 12, e1005214. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Chas, M.; Curtis, M.J.; Niederer, S.A. Mechanism of doxorubicin cardiotoxicity evaluated by integrating multiple molecular effects into a biophysical model. Br. J. Pharmacol. 2018, 175, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Rucker-Martin, C.; Gressette, M.; Lefebvre, F.; Gresikova, M.; Solgadi, A.; Veksler, V.; et al. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: Potential role of energy metabolism remodeling. Circ. Heart Fail. 2015, 8, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Norton, N.; Bruno, K.A.; Di Florio, D.N.; Whelan, E.R.; Hill, A.R.; Morales-Lara, A.C.; Mease, A.A.; Sousou, J.M.; Malavet, J.A.; Dorn, L.E.; et al. Trpc6 Promotes Doxorubicin-Induced Cardiomyopathy in Male Mice with Pleiotropic Differences Between Males and Females. Front. Cardiovasc. Med. 2021, 8, 757784. [Google Scholar] [CrossRef]
- De Oliveira Fischer Bacca, C.; Huntermann, R.; Gomes, R.; Alexandrino, F.; Yoshie Sato, M.; de Sant Anna Melo, E. Abstract 4146264: A Meta-analysis of the Right Ventricle Changes in Cancer Therapy-Induced Cardiotoxicity—The Forgotten Ventricle in Cardio-Oncology. Circulation 2024, 150 (Suppl. S1), A4146264. [Google Scholar] [CrossRef]
- Simek, S.; Lue, B.; Rao, A.; Ravipati, G.; Vallabhaneni, S.; Zhang, K.; Zaha, V.G.; Chandra, A. Gender Differences in Diagnosis, Prevention, and Treatment of Cardiotoxicity in Cardio-Oncology. J. Clin. Med. 2022, 11, 5167. [Google Scholar] [CrossRef]
- Farooq, F.; Cason, D.; Ohri, N.; Kumar, S.; Grann, A.; Litvak, A.; Ganesan, S.; Haffty, B.G.; Toppmeyer, D.; Omene, C.; et al. Evaluating the risk of cardiotoxicity associated with concurrent trastuzumab emtansine (TDM1) and radiation therapy in patients with early-stage HER2 positive breast cancer. Cancer Res. 2023, 83, P5-07-08. [Google Scholar] [CrossRef]
- Wang, A.; Raikhelkar, J.; Raghunathan, R.; McGuinness, J.; Vasan, N.; Trivedi, M.; Kalinsky, K.; Crew, K.; Beauchemin, M.; Wright, J.; et al. Racial and Ethnic Disparities in Anthracycline and HER2-targeted Cardiotoxicity in Patients with Breast Cancer Using Global Longitudinal Strain Imaging Techniques. Cancer Res. 2024, 84, Po1-10-04. [Google Scholar] [CrossRef]
- Baxter, M.A.; Spender, L.C.; Walsh, S.; Bray, S.; Skinner, G.; King, S.; Hall, P.S.; Seymour, M.J.; Petty, R.D.; on behalf of the GO2 Investigators. Female Sex but Not Oestrogen Receptor Expression Predicts Survival in Advanced Gastroesophageal Adenocarcinoma-A Post-hoc Analysis of the GO2 Trial. Cancers 2023, 15, 2591. [Google Scholar] [CrossRef]
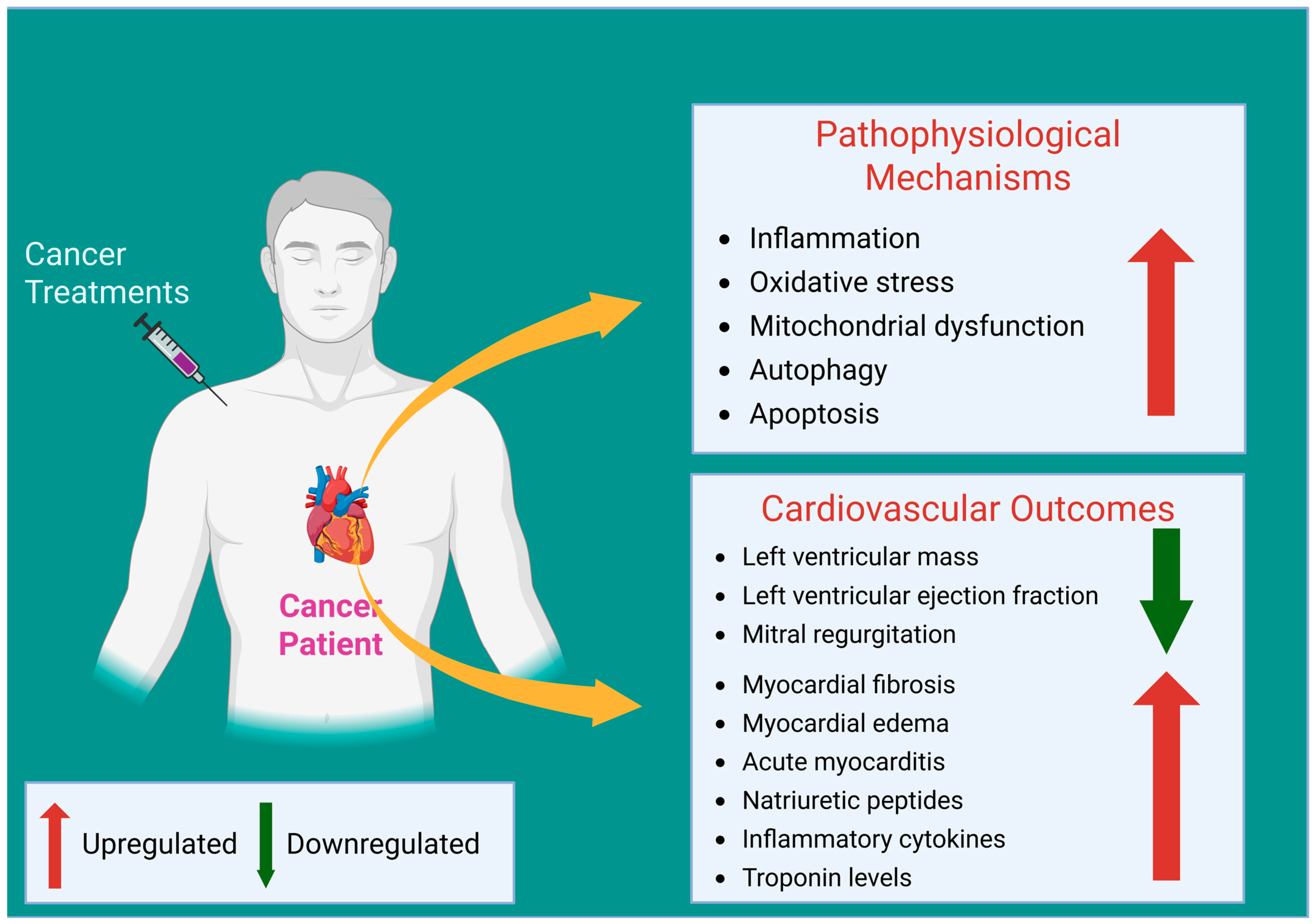
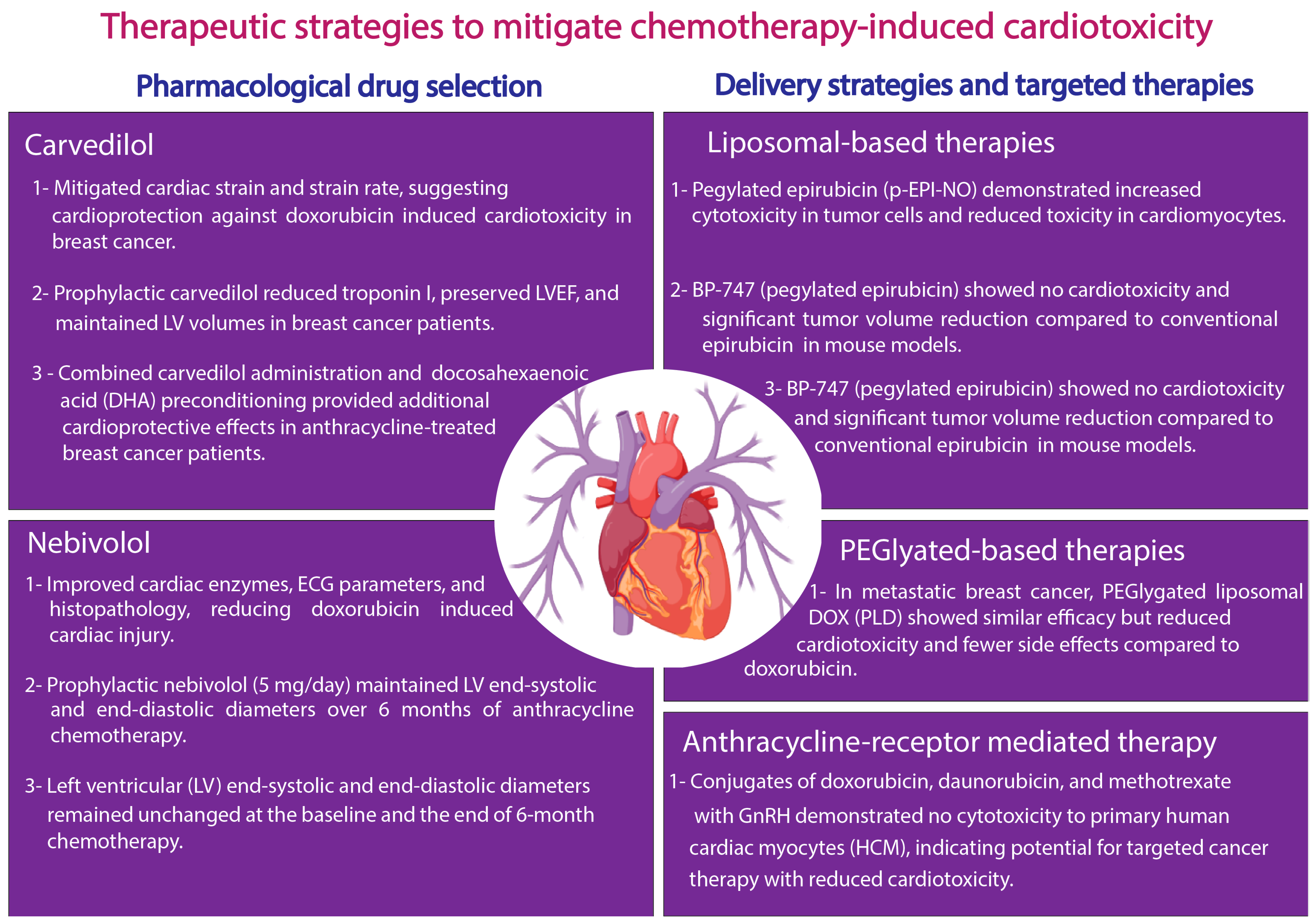
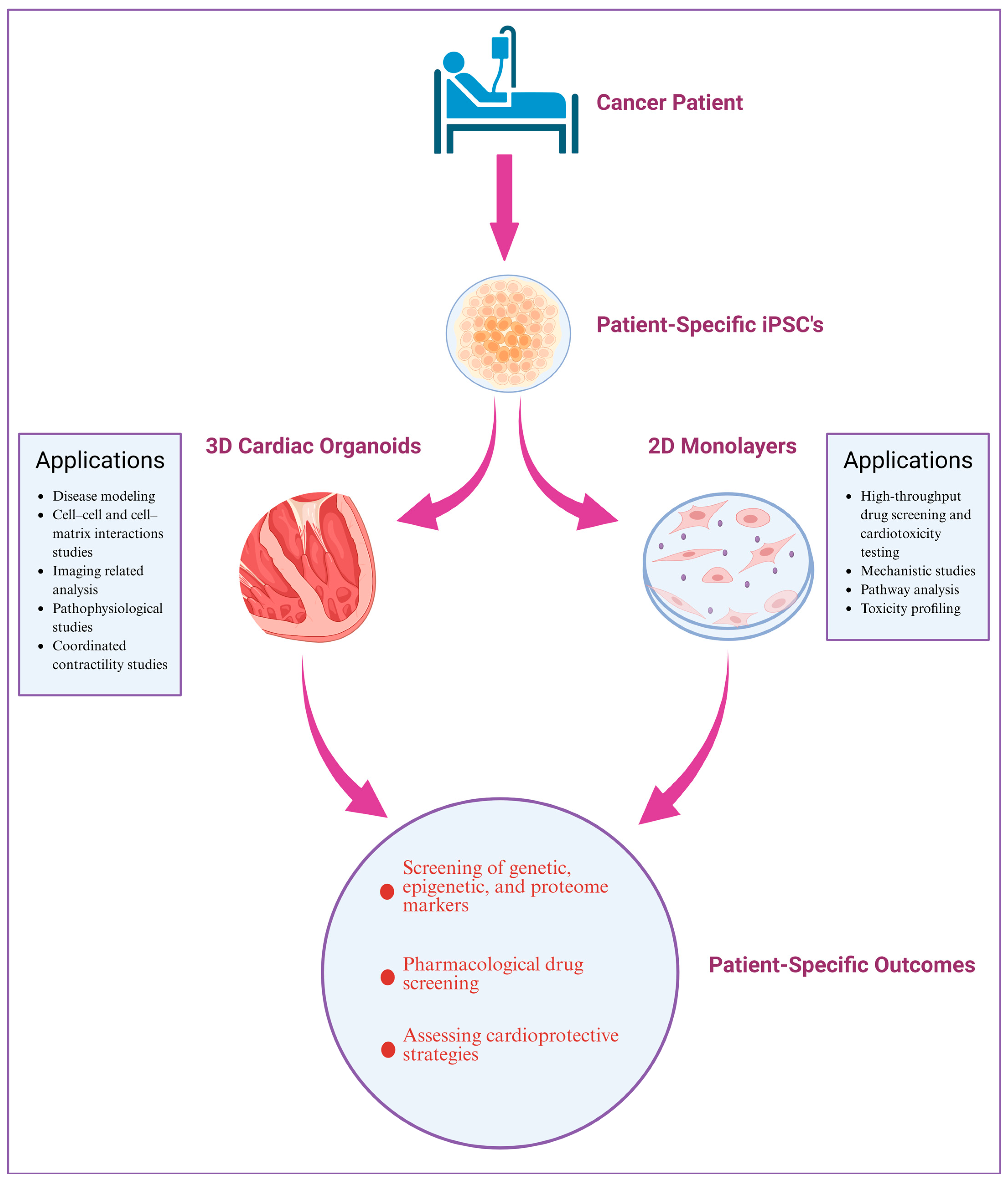
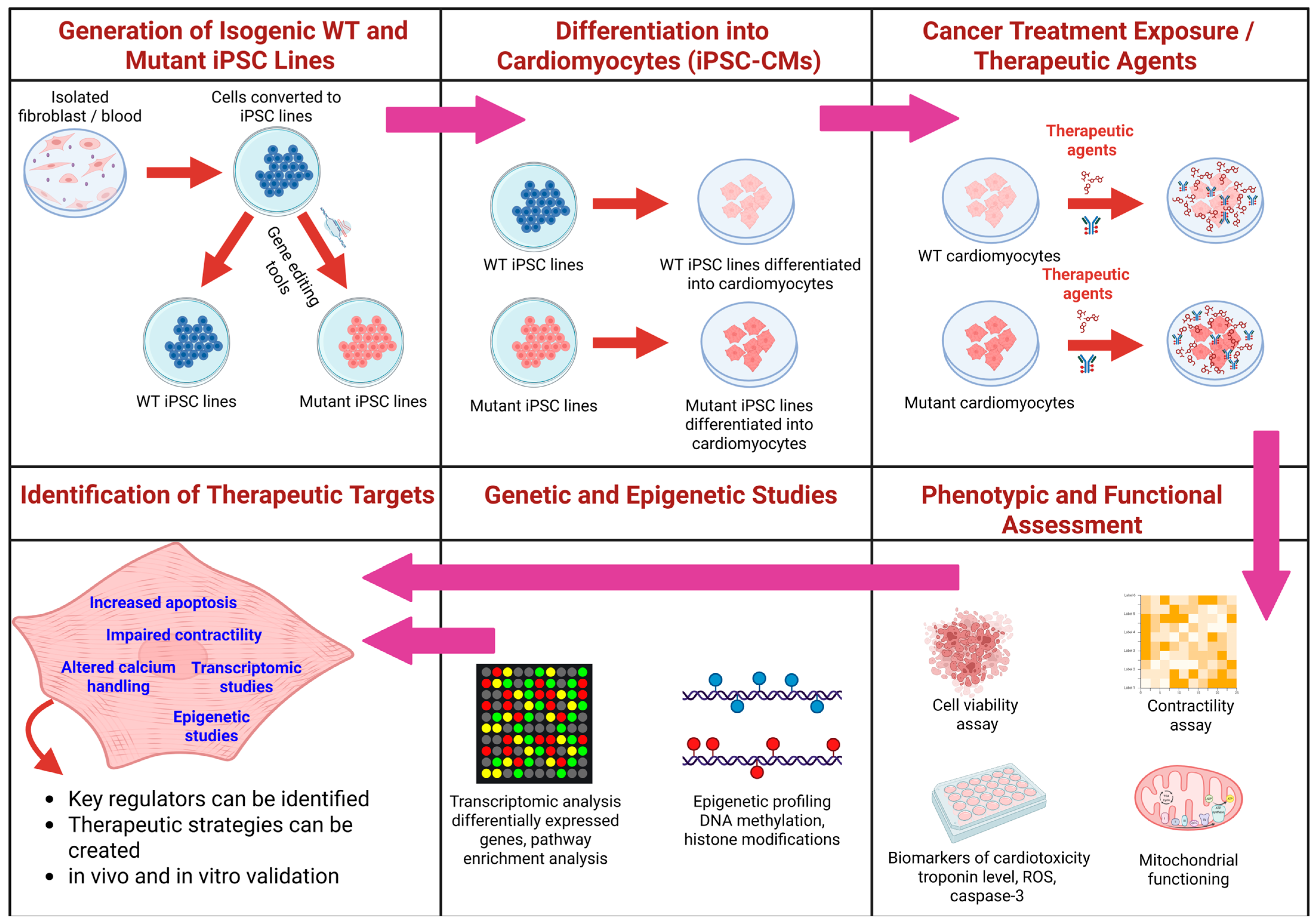
| Chemotherapy Drugs | Experimental Model | Dosage | Effect/Mechanisms | References |
|---|---|---|---|---|
| Doxorubicin (DOX) | Mouse leukemia L1210 cells | 0–5 µM (1 h incubation at 37 °C) | Iminodaunorubicin produces transient DNA breaks; Adriamycin induces persistent DNA breaks | [41] |
| Rat cardiomyocytes | 40–160 µM (20 min incubation at 37 °C) | Increased oxidative stress generation near mitochondria to induce cardiotoxicity | [42] | |
| Bovine heart submitochondrial preparations | 25–50 µM (15 min incubation at 37 °C) and 0.5–1.0 mM (2 h incubation at 37 °C) | Low concentrations induce oxidative damage via redox cycling, inactivating NADH oxidase | [43] | |
| Higher concentrations cause non-oxidative inactivation of ETC complexes by cardiolipin binding | ||||
| Rat cardiomyocytes | 0.1–2 µM (24–48 h incubation at 37 °C) | Impairment of cellular energetics and reduction in ATP levels, affecting mitochondrial respiration and cell viability | [44] | |
| Bovine aortic endothelial cells | 0.5–2 µM (12–24 h incubation at 37 °C) | Increased eNOS transcription and oxidative-stress-mediated apoptosis | [45] | |
| Rat cerebellum homogenates | Doxorubicin (Ki = 24 µM), Aclarubicin (Ki = 50 µM) | Non-competitively inhibits nitric oxide synthase (NOS), causing cardiovascular toxicity | [46] | |
| Purified endothelial nitric oxide synthase | 5–100 µM | Induces eNOS-dependent superoxide generation via doxorubicin redox cycling, causing cardiotoxicity | [47] | |
| Rabbit aortic ring segments and human brachial artery | 10 mg/kg; intravenous single dose | Rapid attenuation of endothelial-dependent dilation, increased superoxide generation via eNOS dysfunction, and rapid depletion of systemic NO levels lead to endothelial dysfunction | [48] | |
| Purified nitric oxide synthase and rat cerebellar homogenates | 50 µM Adriamycin | Generation of superoxide radicals via (NOS), causing dysfunction in NOS activity | [49] | |
| (Nox2−/−) and wild-type (WT) littermate mice | 12 mg/kg DOX or saline control by three weekly intraperitoneal injections (4 mg/kg at 0, 7, and 14 days) | Induced cardiomyopathy characterized by oxidative stress, cardiomyocyte apoptosis, myocardial dysfunction, interstitial fibrosis, and cardiac remodeling via increased Nox2-derived ROS and upregulated mitofusin-2 expression | [50] | |
| C57BL/6 mice | 8 mg/kg weekly; intraperitoneal injection, once a week, for 4 weeks; (total 32 mg/kg cumulative dose) | Cardiac fibrosis, apoptosis, cardiomyocyte injury, oxidative stress induction through upregulation of NOX2 and NOX4. Protective effects observed by Astragaloside IV treatment reducing oxidative stress | [51] | |
| Langendorff-perfused Wistar rat hearts and hearts from doxorubicin-treated Wistar rats | 5–25 µM (during 80 min perfusion, ex vivo) and intraperitoneal administration of 2 mg/kg doxorubicin or an equivalent volume of saline was administered to animals via the implanted catheter three times a week for 2 weeks for a total dose of 12 mg/kg | Inhibition of AMPK despite energetic stress and activation of Akt and MAPK via DNA damage signaling resulted in increased mTOR activation, contributing to chronic cardiac dysfunction and remodeling | [52] | |
| Daunorubicin (DNR) | Acute myeloid leukemia (AML) patients | 50–630 mg/m2 cumulative dose | Identification of POR gene variants as potential markers of DNR-induced cardiotoxicity; the genetic factors account for a significant proportion of LVEF drop via dose–genotype interaction | [53] |
| Eight-week-old male Sprague-Dawley rats | Cumulative dose of 9 mg/kg administered at 3 mg/kg intravenously, given in three equal injections at 48-h intervals | Induces cardiotoxicity and nephrotoxicity manifested as interstitial edema, subendocardial fibrosis, perinuclear vacuolation, and myocardial degeneration, and carvedilol co-administration provides protection | [54] | |
| Ten–twelve-week-old male Wistar rats (comparative acute vs. subchronic cardiomyopathy models) | Acute—Six intraperitoneal injections of 3 mg/kg every 48 h; Subchronic—Single intravenous injection of 15 mg/kg | Both models show reduced left ventricular weight and function with upregulation of natriuretic peptides. The subchronic model shows decreased Myh6 expression and altered stem cell marker expression indicative of impaired regenerative potential | [55] | |
| Ten–twelve-week-old male Wistar rats | Six intraperitoneal doses of 3 mg/kg every 48 h | Early cardiomyopathy characterized by depressed left ventricular pressure and contractility, a twofold upregulation of ryanodine receptor 2 (RyR2), increased NPPA/NPPB expression, and decreased alpha-tubulin gene expression suggesting the disruption of Ca2+ handling in cardiomyocytes | [56] | |
| Rat H9c2 cardiomyocytes (in vitro study) | Cells pre-treated with Puerarin (1–100 µg/mL) for 24 h, then exposed to 1 µM DNR for an additional 24 h | DNR induces apoptosis by increasing Ca2+⁺ influx and activating caspase-3 via the PI3K/Akt signaling pathway. Puerarin pre-treatment attenuates these effects by inhibiting Ca2+⁺ influx and enhancing p-Akt activation, thereby protecting cells from apoptosis | [57] | |
| Epirubicin (EPI) | Male Wistar rats | Single dose of 10 mg/kg; intraperitoneal administration | Induces mitochondrial degeneration, swelling, intracytoplasmic vacuolization, and focal myofilament disarray in cardiomyocytes, leading to cardiotoxicity | [58] |
| Male Sprague-Dawley rats | 8 mg/kg; intraperitoneal administration | Induces cardiotoxicity via the upregulation of genes promoting autophagy and apoptosis | [59] | |
| Non-Hodgkin lymphoma patients | Bolus dose of 40 mg/m2, intravenous infusion (administered for 3 weeks) | Increases QT dispersion and QTc dispersion, reflecting early electrophysiological alterations linked to cardiotoxicity | [60] | |
| Cancer patients in a phase II trial | Cumulative dose of 200 mg/m2 | Induces impairment in systolic left ventricular function, characterized by a reduction in strain rate peak at 3-, 6-, 12-, and 18-months follow-up and correlates with increased IL-6 and oxidative stress markers | [61] | |
| Breast cancer patients | Combination of epirubicin: 100 mg/m2 and cyclophosphamide: 600 mg/m2 at about 3-week intervals for eight cycles | Upregulates apoptosis and downregulates 5′-aminolevulinate synthase 2 (ALAS2), implicating glycine, serine, and threonine metabolism disruptions in cardiomyopathy development | [62] | |
| Idarubicin | Acute myeloid leukemia (AML) patient without cardiac risk factors | 12 mg/m2/day for 3 days (cumulative dose: 36 mg/m2) administered via 30 min intravenous infusion | First exposure resulted in severe subacute congestive heart failure (CHF) and ventricular tachycardia (VT), a prolonged QTc interval with frequent premature ventricular contractions (QTc ~400 ms) | [63] |
| Acute myeloid leukemia (AML) patient | 12 mg/m2/day for 3 days (cumulative dose: 36 mg/m2) administered via 30-min intravenous infusion | Induced cardiac arrest shortly after the first dose of idarubicin in an AML patient | [64] | |
| AML patient without cardiac risk factors | Cumulative dose of 36 mg/m2 administered within 2 weeks | Induced cardiomyopathy with a 25% reduction in left ventricular ejection fraction (LVEF), impaired right ventricular (RV) function, and severe mitral regurgitation | [65] | |
| Mitoxantrone (MTX) | Stage IV breast cancer patients | Mitoxantrone 10 mg/m2, methotrexate 40 mg/m2, 5-fluorouracil 600 mg/m2, given every 3 weeks | Demonstrated broad antitumor activity with a favorable toxicity profile (notably lower cardiotoxicity) compared to other anthracyclines | [66] |
| In vivo in MTX-treated rats and in vitro in H9c2 cells | Not Specified | MTX and its naphthoquinoxaline metabolites accumulate in the heart and liver. CYP450- and CYP2E1-mediated metabolism contributes to cytotoxicity in H9c2 cells | [67] | |
| 7-day differentiated H9c2 cells (in vitro) | 0.01–5 µM | MTX alters energetic pathways (e.g., increased ATP and decreased lactate production), while its NAPHT metabolite is less cardiotoxic, indicating differences in metabolic disruption | [68] | |
| HL-1 cardiomyocytes (in vitro) | 0.1, 1, and 10 µM MTX | Impairs proteasome activity and triggers early energetic and proteomic changes that disturb oxidative stress homeostasis | [69] | |
| Adult and infant mice (in vivo) | Adult mice—7.0 mg/kg cumulative dose; Infant mice—7.0 mg/kg cumulative dose (protocols adjusted for age) | In adult mice, MTX induced myocardial injury, fibrosis, and the increased expression of NF-κB p52 and TNF-α, and decreased IL-6 expression, implicating inflammation in cardiotoxicity; infants showed higher resilience | [70] | |
| Adult CD-1 male mice (in vivo) | 6 mg/kg cumulative dose | Decreases in AMPK and GAPDH content; decreased free carnitine (C0) and increased acetyl carnitine (C2) suggest a shift toward fatty acid oxidation | [71] | |
| AML patient (case report) | Not Specified | Induced acute myocarditis characterized by a 25% reduction in LVEF, diffuse myocardial edema, and delayed gadolinium enhancement, demonstrating acute cardiotoxicity | [72] |
| Therapeutic Strategies | Experimental Model | Dosage | Cardiovascular Outcome | References |
|---|---|---|---|---|
| Carvedilol Prophylaxis | Breast cancer patients receiving anthracycline-based chemotherapy | 6.25 mg carvedilol daily (during chemotherapy) | Mitigation of cardiac strain and strain-rate changes and prevention of DOX-induced cardiotoxicity | [199] |
| Recently diagnosed breast cancer patients | Prophylactic carvedilol (specific dosage not mentioned) | Reduced troponin I level, preserved LVEF, and favorable changes in LVES and LA diameter and the inhibition of AIC | [200] | |
| HER2-negative breast cancer patients with normal LVEF undergoing anthracycline treatment | 6.25 mg carvedilol daily | Significant reduction in troponin levels and diastolic dysfunction | [201] | |
| Breast cancer patients receiving anthracycline treatment | Carvedilol + DHA (administered starting 1 week before and continued for 90 days) | Attenuation of subclinical cardiotoxicity, evidenced by smaller declines in LVEF (measured by CMR), preservation of global longitudinal strain (via ECHO), lower elevations in hs-cTnT and NT-proBNP, and reduced QTc prolongation compared to the placebo | [202] | |
| Nebivolol Prophylaxis | Preclinical study in rats | 1–2 mg/kg orally in rats | Improvement in heart index, cardiac enzymes, histopathology, and ECG parameters, reduction in doxorubicin-induced cardiotoxicity | [203] |
| Breast cancer patients | 5 mg nebivolol daily | Protection of myocardium with stable LV end-systolic and end-diastolic diameters over 6-month chemotherapy | [204] | |
| Dexrazoxane Prophylaxis | Preclinical study in rats | 62.5 mg/kg intraperitoneally at 0, 3, and 6 h | preservation of left ventricular function, lower rates of cardiomyopathy, and low oxidative stress markers compared to untreated groups | [205] |
| Systematic review containing 23 clinical trials, which comprised a total of 14,652 subjects | Not Specified | Reduced cardiovascular risk, 50% risk reduction, threefold lower chance of cardiac dysfunction, 74% decrease in cardiotoxicity | [206] | |
| Mitochondrial-Targeted Antioxidants (MTAs) | Spontaneously hypertensive rat (SHR) implanted with the SST-2 breast tumor cell line | Doxorubicin (DOX), 4 mg/kg, administered intraperitoneally (IP) once weekly for 3 weeks; Mito-Tempol (Mito-T), 1 mg/kg/day, administered intraperitoneally (IP) daily Dexrazoxane (DXZ), 100 mg/kg, administered intraperitoneally (IP) 30 min before DOX injection | Mito-Tempol and dexrazoxane protected the heart from DOX-induced toxicity, preserving LVEF and reducing oxidative stress and apoptosis | [207] |
| AGS gastric cancer cell line (in vitro) and male BALB/c nude mice xenografted with AGS gastric cancer cells (in vivo) | Mito-FF peptide, 10 mg/kg, administered intravenously (IV) every 3 days; 5-Fluorouracil (5-FU), 30 mg/kg, administered intraperitoneally (IP) every 3 days | Mito-FF peptide protected against 5-FU-induced cardiotoxicity, reducing ROS generation and apoptosis in cardiac tissues | [208] | |
| PEGylated Liposomal Doxorubicin (CAELYX) | Preclinical pharmacokinetic studies in animals (rats and beagle dogs) | 0.5 mg/kg | Reduced myocardial accumulation, suggesting a reduced risk of cardiotoxicity, apoptosis, and oxidative stress in the heart tissue | [209] |
| In vivo mouse model (BALB/c mice with implanted tumors) | Single IV bolus of 10 mg/kg for free and liposomal DOX formulations | Approximately fourfold lower doxorubicin concentrations in heart tissue compared to tumor tissue; liposomal formulation effectively minimizes cardiac drug deposition and, consequently, DOX-induced cardiac injury | [210] | |
| Pilot clinical study in cancer patients (n = 16, various tumor types) | Free DOX and liposomal DOX (Doxil) at dose levels of 25 mg/m2 and 50 mg/m2 | Significantly reduced cardiac accumulation of doxorubicin, preserved left ventricular ejection fraction (LVEF); minimizes myocardial exposure, thereby protecting against DOX-induced cardiotoxicity | [211] | |
| PEGylated Liposomal Doxorubicin (PLD) | Phase III clinical trial in women with metastatic breast cancer | PLD—50 mg/m2 every 4 weeks; Conventional DOX—60 mg/m2 every 3 weeks | Preserved left ventricular ejection fraction (LVEF) with a threefold lower incidence of cardiotoxic events compared to conventional DOX | [212] |
| Retrospective study in HER2-positive early breast cancer patients. | 50 mg/m2 every 4 weeks | Clinical cardiotoxicity occurred in 8.6% of patients, subclinical events in 24.3% (10–16% decline), with no treatment interruptions | [213] | |
| Liposomal Daunorubicin Delivery | Pediatric acute myeloid leukemia induction trial | L-DNR, 80 mg/m2 per day for 3 days; Idarubicin, 12 mg/m2 per day for 3 days | Only one patient in the L-DNR arm and three in the idarubicin arm developed subclinical or mild cardiomyopathy | [214] |
| PEGylated Epirubicin with NO-Releasing Moiety (p-EPI-NO) | In vivo mouse models bearing Caco-2 and SKOV-2 tumors | Not Specified | 95% reduction in tumor volume while virtually eliminating clinical, anatomical, and biochemical signs of cardiotoxicity compared to free epirubicin and conventional PEGylated epirubicin | [215] |
| Mitoxantrone (MTO) formulated in Cardiolipin-based Anionic Liposomes | Preclinical intraperitoneal chemotherapy model | Not Specified | Prolonged retention of MTO at tumor sites, resulting in negligible cardiotoxicity versus free MTO | [216] |
| Carvedilol (CAR), Resveratrol (RES), and Liposomal Resveratrol (LIPO-RES) | Rat model of DOX-induced cardiomyopathy | DOX—2 mg/kg twice per week (weeks 2–6); | The combination, particularly CAR/LIPO-RES, significantly reduced serum CK-MB, troponin-I, and LDH levels, preserved cardiac histology, increased S100A1 and SERCA2a expression, and mitigated oxidative stress and inflammation | [217] |
| CAR—30 mg/kg; RES and LIPO-RES: 20 mg/kg for 6 weeks | ||||
| Liposomal Resveratrol (LIPO-RES) | In vivo study in rats | 20 mg/kg for 6 weeks | Protection against DOX-induced oxidative stress, inflammation, and calcium dysregulation | [217] |
| Idarubicin-Loaded Solid Lipid Nanoparticles | In vivo study in rats | 2 mg/kg | Reduced drug uptake in the heart, lowering cardiotoxic potential | [218] |
| GnRH-based Conjugates Containing Doxorubicin, Daunorubicin, and Methotrexate | Human cardiac myocytes (HCMs) and human umbilical vein endothelial cells (HUVECs) | Various concentrations tested | No cytotoxic effect on cardiomyocytes | [219] |
| Chemotherapy Drugs | Experimental Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|
| Doxorubicin (DOX) | hiPSC-CMs derived from breast cancer patients (with/without DIC) were exposed in vitro to doxorubicin (0.1–10 µM) for 24–72 h. The assessments included cell viability, mitochondrial/metabolic function, calcium handling, and transcriptomic profiling | hiPSC-CMs from patients who developed doxorubicin-induced cardiotoxicity (DIC) showed markedly lower viability, impaired mitochondrial function, disrupted calcium handling, decreased antioxidant activity, and increased ROS production compared to cells from non-DIC patients | Limited number of patient-specific lines and potential variability in differentiation efficiency may affect reproducibility | [229] |
| hiPSC-CMs were evaluated using multielectrode array (MEA) analyses for electrical activity (beating rate, field potential duration) and high-content imaging for mitochondrial parameters under single (3 h, 2 days) and repetitive dosing (3 cycles of 2 days each, with washout up to day 14) | Acute exposure up to 6 µM did not alter electrical activity, whereas chronic exposure at nanomolar concentrations significantly reduced cell viability and induced subtle changes in mitochondrial membrane potential and calcium levels | In vitro endpoints might not capture all aspects of long-term cardiotoxicity | [230] | |
| Repeated exposure of hiPSC-CMs to DOX combined with NMR-based metabolic profiling to assess changes in cellular metabolism | Repeated doxorubicin exposure resulted in decreased utilization of pyruvate and acetate, with a concomitant accumulation of formate | NMR sensitivity limits the detection of low-abundance metabolites; further validation | [231] | |
| Low dose of doxorubicin (100 nM for 14 days) in vitro, while in parallel, a rat model was used for in vivo studies | 26% reduction in hiPSC-CM viability | The chronic in vitro exposure model may not fully recapitulate the long-term, multifactorial nature of cardiotoxicity in patients; translation to clinical outcomes remains uncertain | [232] | |
| Tyrosine Kinase Inhibitors (TKIs) | hiPSC-CMs, hiPSC-derived endothelial cells, and cardiac fibroblasts. The assessment includes cell viability, contractility, electrophysiology, and the generation of a “cardiac safety index” | VEGFR2/PDGFR-inhibiting TKIs (sorafenib, regorafenib, ponatinib) exhibited the highest cardiotoxicity (LD50 around 3 µM), and co-treatment with exogenous insulin or IGF1 improved cardiomyocyte viability | In vitro screening may not capture complex in vivo pharmacodynamics and inter-patient variability | [233] |
| Trastuzumab | hiPSC-CMs were treated with 10 μM doxorubicin, 1 μM trastuzumab, 1 ng/mL NRG-1, and 100 ng/mL HB-EGF in a monoculture and in co-culture with endothelial cells | TZM disturbed the calcium balance without causing any cell death; adding neuregulin-1 or HB-EGF restored the normal functioning of cells | In vitro findings require validation in clinical settings; the complexity of energy metabolism in vivo may not be fully replicated in hiPSC-CMs | [234] |
| hiPSC-CMs derived from both healthy individuals and patients with breast cancer who experienced trastuzumab-induced cardiac dysfunction were chronically exposed to trastuzumab. Functional assays include contractility, calcium handling, RNA-seq transcriptomics, and metabolic modulation experiments | Clinically relevant doses of trastuzumab impaired contractility and calcium handling without causing cell death. The transcriptomic analysis revealed mitochondrial dysfunction and altered energy metabolism. hiPSC-CMs from patients with severe cardiotoxicity were more vulnerable, and metabolic modulators improved the phenotype—suggesting that targeting altered energy metabolism may be therapeutic | Small sample size of patient-specific lines and inherent variability in hiPSC-CM maturation | [235] |
| Chemotherapy Drugs | Experimental Procedure and Dosage | Methodology | Genetic/Epigenetic Markers | Major Findings | References |
|---|---|---|---|---|---|
| Doxorubicin (DOX) | 156 nM for a 2-day exposure period (DOX-Day2) or three consecutive exposure periods of DOX for 6 days (DOX-Day6) | miRNA microarray | miR-187-3p, miR-182-5p, miR-486-3p, miR-486-5p, miR-34a-3p, miR-4423-3p, miR-34c-3p, miR-34c-5p, miR-1303, miR-182-5p, miR-4423-3p, and miR-34c-5p | (1) Upregulation of miR-34c-3p and miR-34c-5p may be early indicators of cardiac dysfunction, the development of cardiac pathologies, and future heart failure; (2) miR-187-3p overexpression in cardiomyocytes might be the result of DOX-induced DNA damage; (3) DOX-induced early upregulation of miR-486-5p suggests that the elevation of miR-486-5p may be considered as an early event in the development of cardiovascular diseases | [237] |
| Day 30 hiPSC-CMs were treated for 24 h or 72 h with doxorubicin (0.01–100 μM) | RNA-seq gene expression analysis; Quantitative real-time PCR | SNP (rs2229774) in retinoic acid receptor-γ (RARG) | (1) A rs2229774 patient-specific model recapitulates the patients’ cardiotoxicity phenotype; (2) rs2229774 increased doxorubicin-induced cardiotoxicity susceptibility; (3) RARG agonists attenuate cardiotoxicity without affecting chemotherapy efficacy; (4) Pharmacogenetic screening for rs2229774 and RARG agonists is a clinical option | [239] | |
| Patient-specific DIC was characterized | SLC28A3 locus genetic fine mapping using a MinION nanopore sequencer and RNA sequencing | SNP rs11140490 in the SLC28A3 locus | rs11140490 in the SLC28A3 locus was linked to reduced DIC | [240] | |
| Nine hiPSC-CM lines intrinsically polymorphic (3 lines/genotype) at rs28714259 were treated with the vehicle or 100 nM dexamethasone for 24 h followed by the vehicle or 1 µM doxorubicin treatment for 24 h | RNA sequencing gene expression | SNP rs28714259 in the GR:rs28714259 locus | rs28714259 was linked to anthracycline-induced cardiotoxicity through disruption of GR:rs28714259 locus binding and its subsequent protective signaling | [242] | |
| hiPSC line 19c3 was generated from peripheral blood mononuclear cells from a healthy individual. Effect of doxorubicin (72 h) on hiPSC-CM viability in the control (isotype) and knockouts for EXOC6B, FCHSD2, NIPAL2, SYNPO2, PDXK, SLMAP, and RORA was assessed. Day 30 hiPSC-CMs were treated for 72 h with doxorubicin (0.01–100 μM) | Epigenome-wide association study (EWAS); DNA-methylation EPIC array | EXOC6B, FCHSD2, NIPAL2, SYNPO2, PDXK, and SLMAP | Knockout of genes EXO6CB, FCHSD2, NIPAL2, and SYNPO2 in hiPSC-CMs increased sensitivity to doxorubicin | [243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, A.D.; Dabral, S.; Brajesh, R.G.; Day, B.W.; Juric, M.; Zielonka, J.; Bosnjak, Z.J.; Pant, T. Understanding the Mechanisms of Chemotherapy-Related Cardiotoxicity Employing hiPSC-Derived Cardiomyocyte Models for Drug Screening and the Identification of Genetic and Epigenetic Variants. Int. J. Mol. Sci. 2025, 26, 3966. https://doi.org/10.3390/ijms26093966
Solomon AD, Dabral S, Brajesh RG, Day BW, Juric M, Zielonka J, Bosnjak ZJ, Pant T. Understanding the Mechanisms of Chemotherapy-Related Cardiotoxicity Employing hiPSC-Derived Cardiomyocyte Models for Drug Screening and the Identification of Genetic and Epigenetic Variants. International Journal of Molecular Sciences. 2025; 26(9):3966. https://doi.org/10.3390/ijms26093966
Chicago/Turabian StyleSolomon, Abhishikt David, Swarna Dabral, Raman Gulab Brajesh, Billy W. Day, Matea Juric, Jacek Zielonka, Zeljko J. Bosnjak, and Tarun Pant. 2025. "Understanding the Mechanisms of Chemotherapy-Related Cardiotoxicity Employing hiPSC-Derived Cardiomyocyte Models for Drug Screening and the Identification of Genetic and Epigenetic Variants" International Journal of Molecular Sciences 26, no. 9: 3966. https://doi.org/10.3390/ijms26093966
APA StyleSolomon, A. D., Dabral, S., Brajesh, R. G., Day, B. W., Juric, M., Zielonka, J., Bosnjak, Z. J., & Pant, T. (2025). Understanding the Mechanisms of Chemotherapy-Related Cardiotoxicity Employing hiPSC-Derived Cardiomyocyte Models for Drug Screening and the Identification of Genetic and Epigenetic Variants. International Journal of Molecular Sciences, 26(9), 3966. https://doi.org/10.3390/ijms26093966







