Adsorption and Sensing Performance of Pt(1-3)-Modified TiSe2 for Dissolved Gas (CH4, C2H2, and CO) in Transformer Oil: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
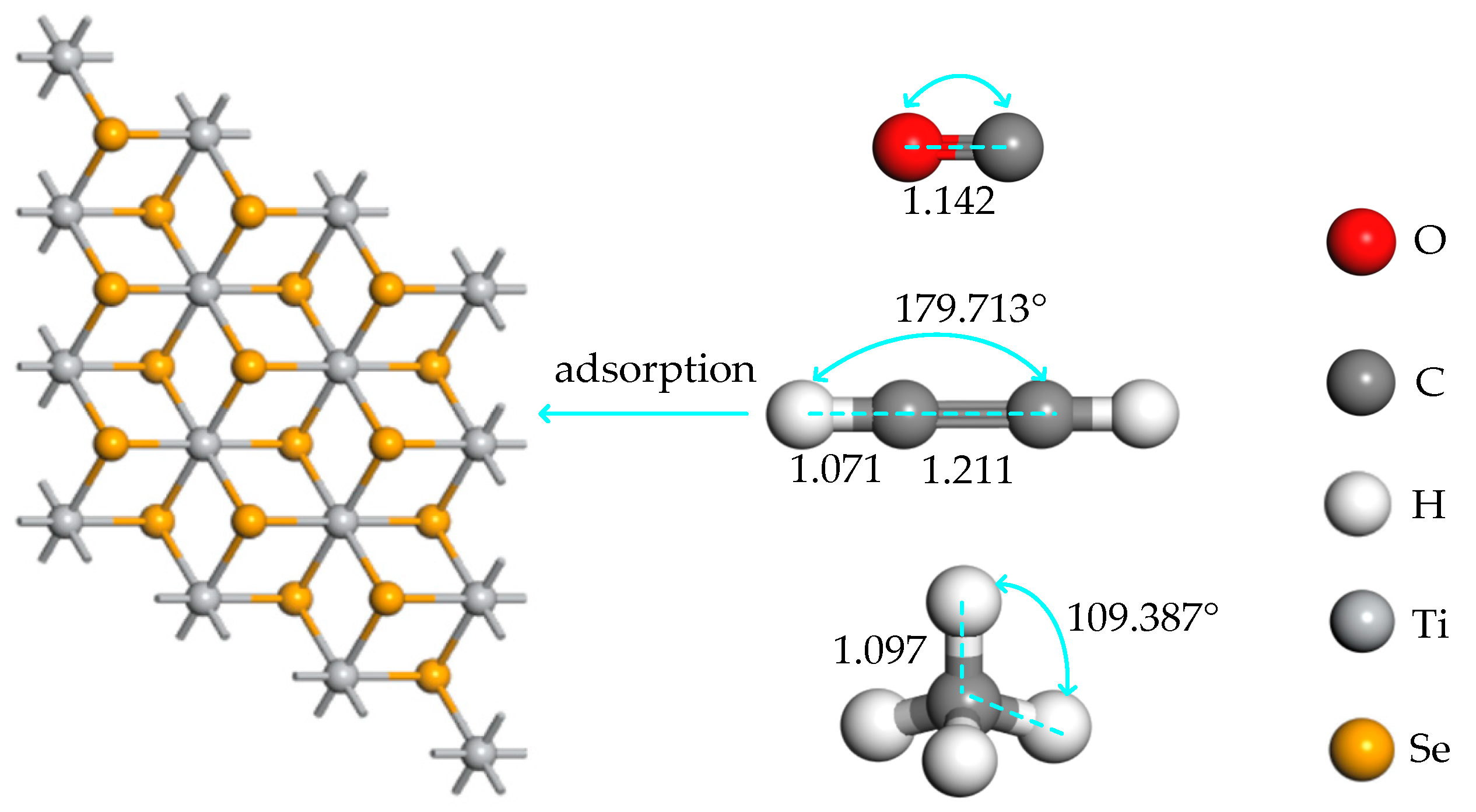
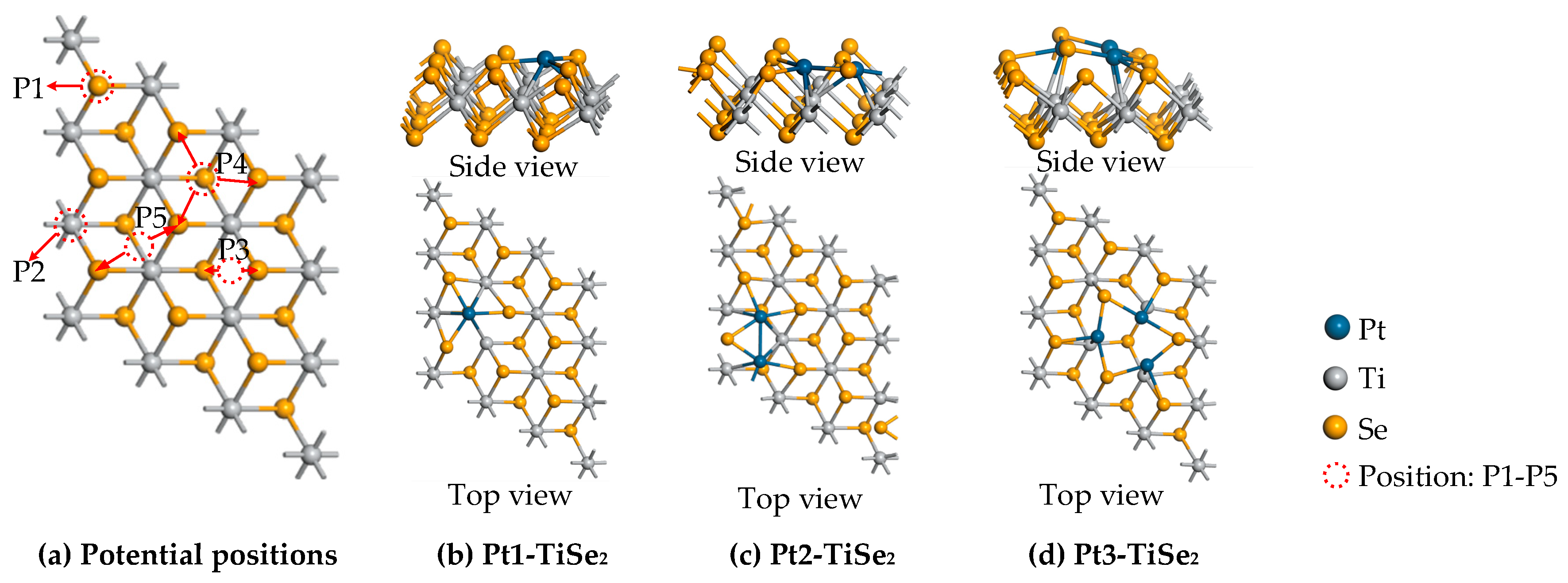
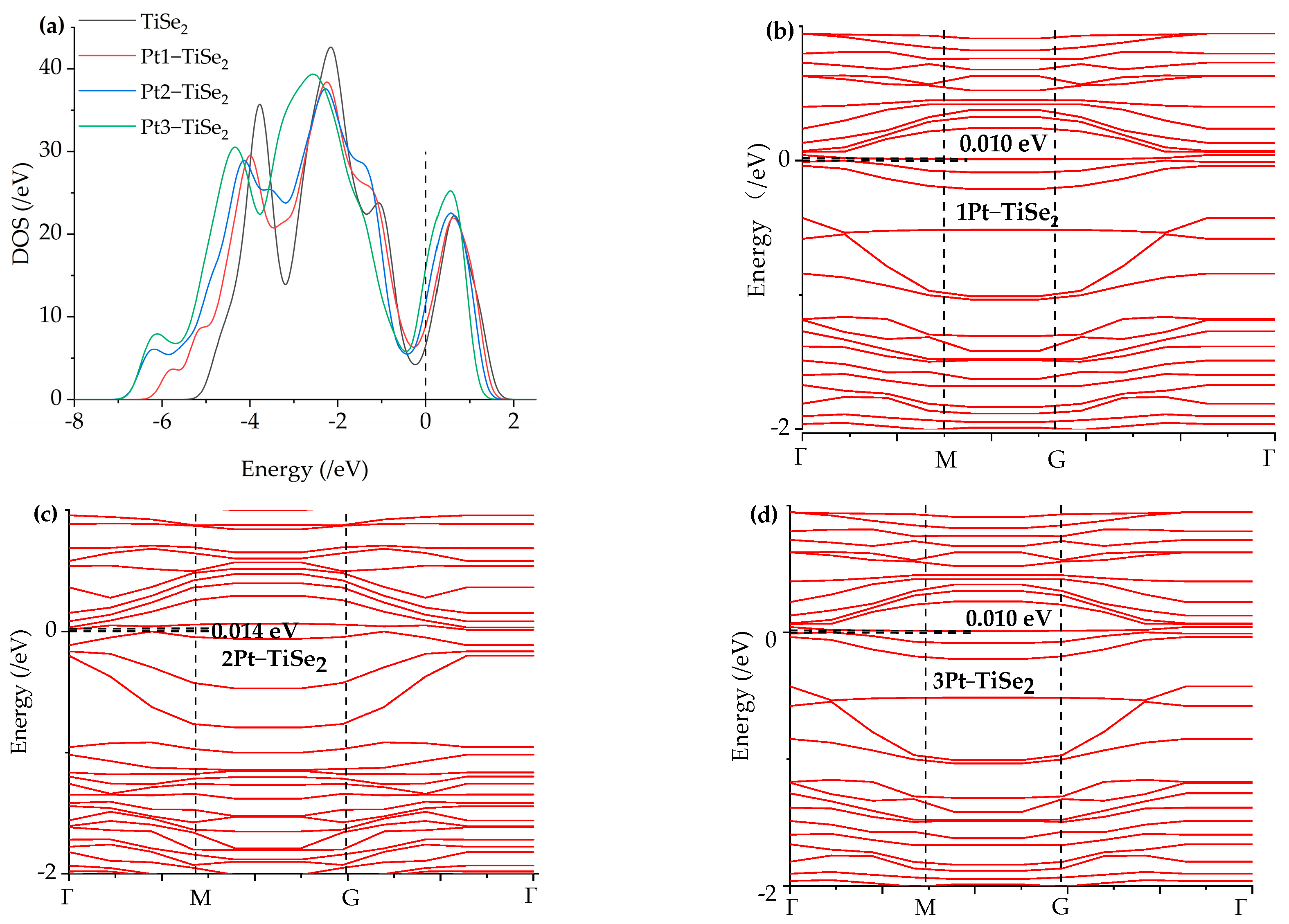
2.1. The Structure of the System and the Optimal Metal Modification Sites
2.2. Gas Adsorption Property Analysis
2.2.1. CH4 Gas Adsorption
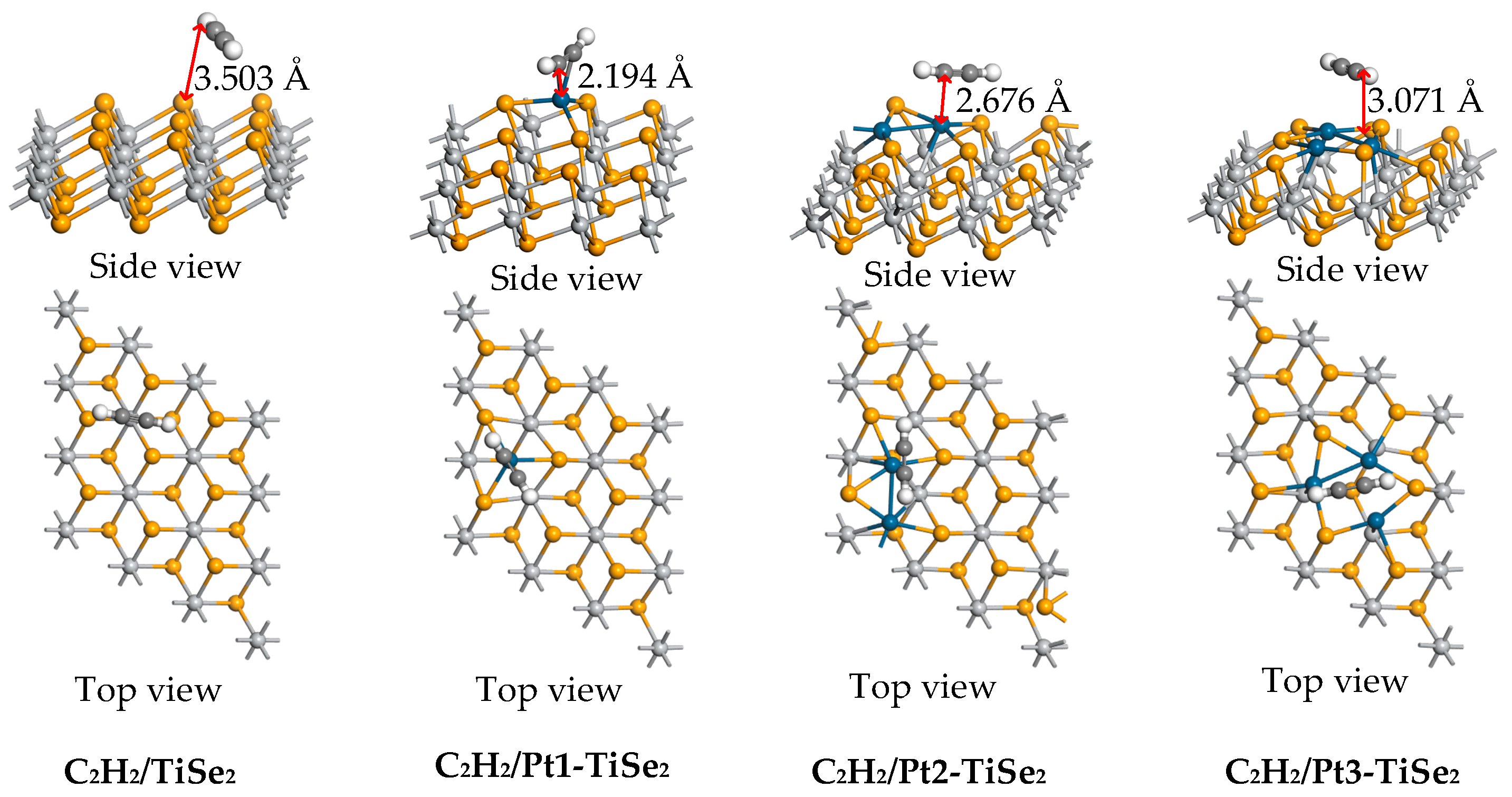
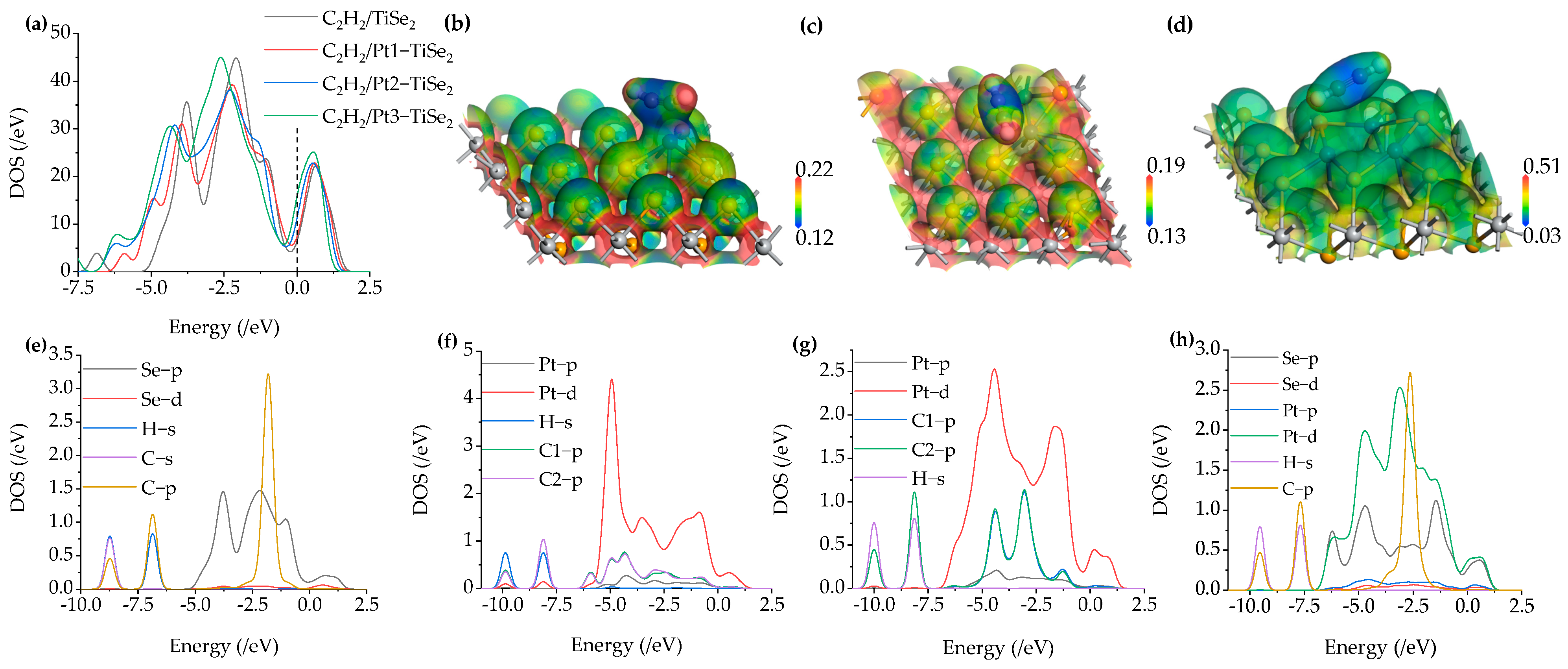
2.2.2. C2H2 Gas Adsorption
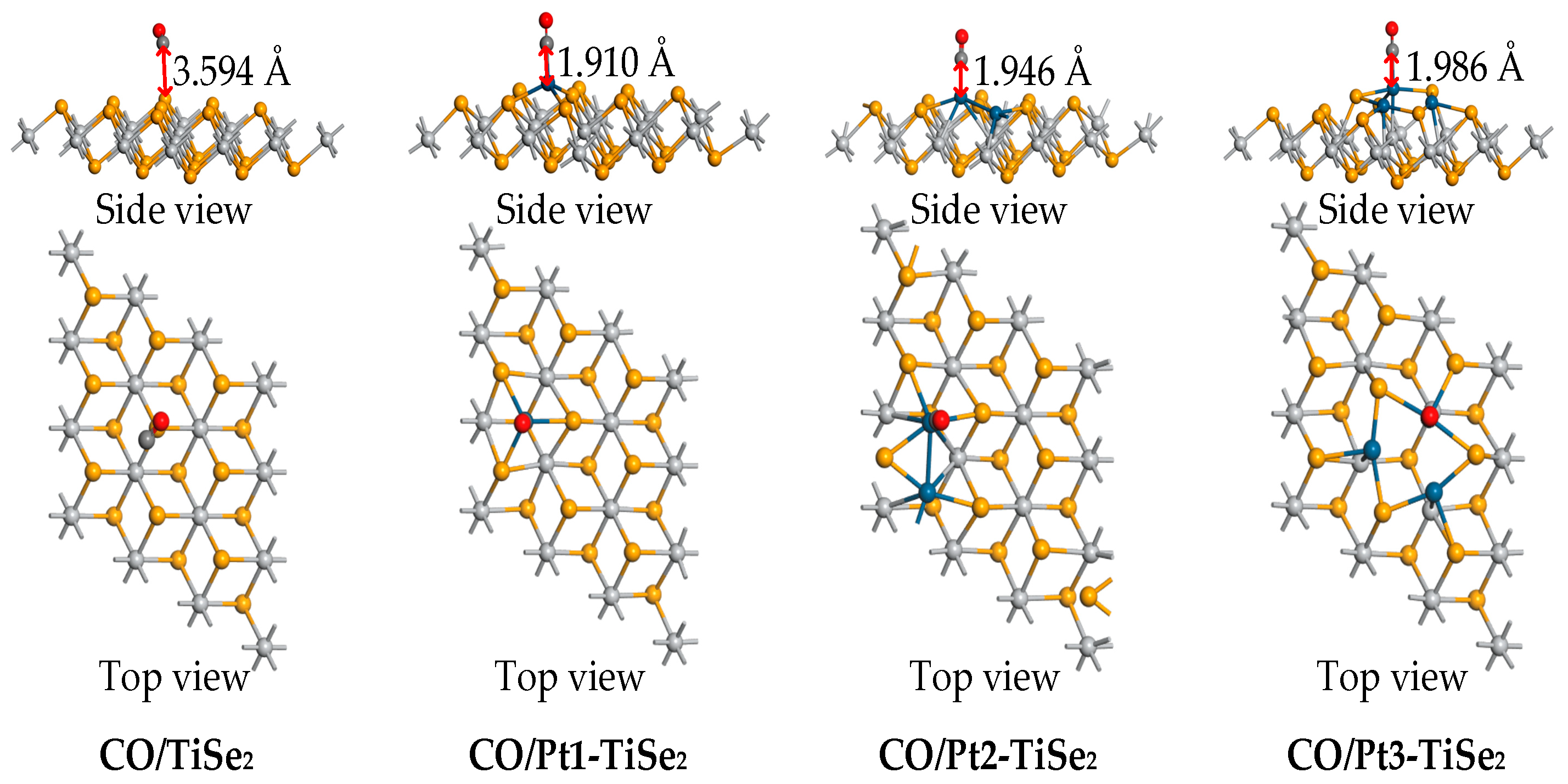
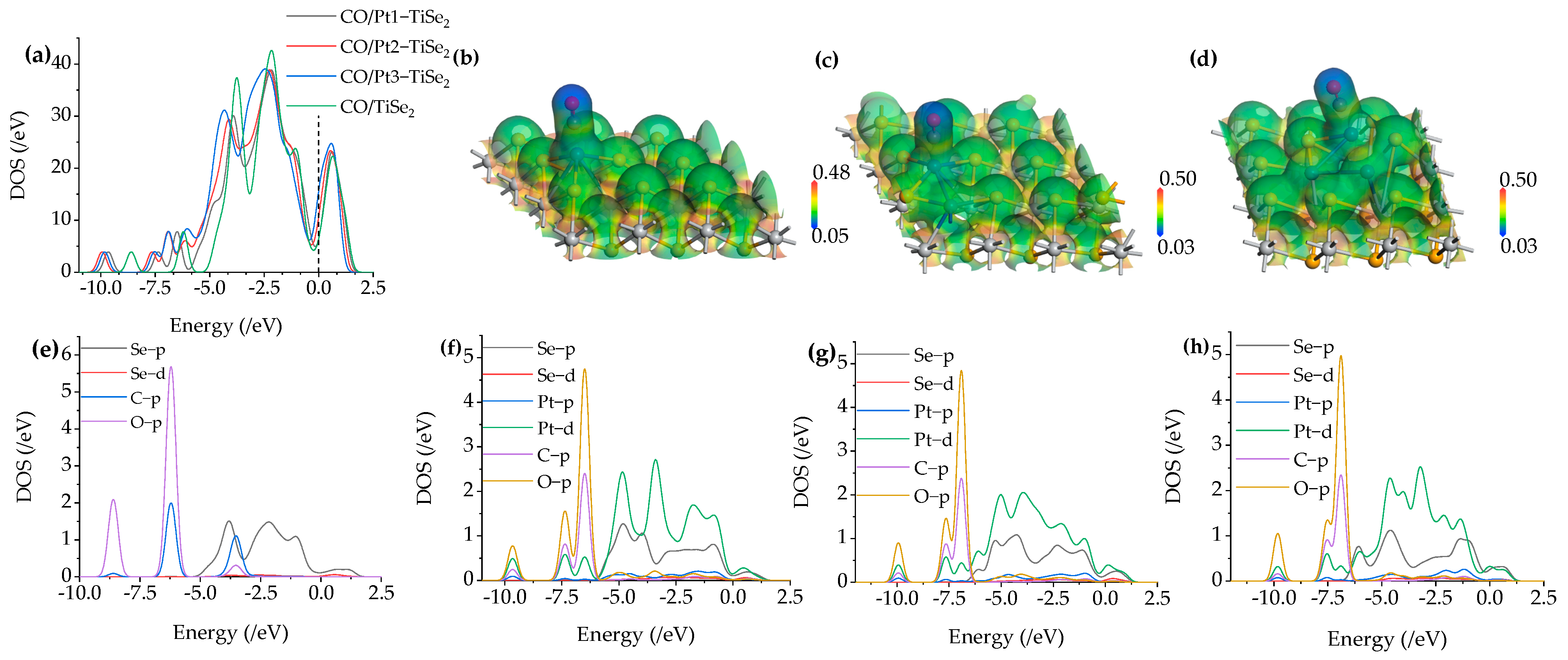
2.2.3. CO Gas Adsorption
2.3. Gas Desorption Property Analysis
3. Calculation Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehmood, M.A.; Nazir, M.T.; Li, J.; Wang, F.; Azam, M.M. Comprehensive investigation on service aged power transformer insulating oil after decades of effective performance in field. Arab. J. Sci. Eng. 2020, 45, 6517–6528. [Google Scholar] [CrossRef]
- Phadungthin, R.; Ekkachai, C.; Haema, J.; Suwanasri, T. Analysis of insulating oil to evaluate the condition of power transformer. In Proceedings of the ECTI-CON2010: The 2010 ECTI International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chiang Mai, Thailand, 19–21 May 2010; pp. 108–111. [Google Scholar]
- Thiviyanathan, V.A.; Ker, P.J.; Leong, Y.S.; Abdullah, F.; Ismail, A.; Jamaludin, M.Z. Power transformer insulation system: A review on the reactions, fault detection, challenges and future prospects. Alex. Eng. J. 2022, 61, 7697–7713. [Google Scholar] [CrossRef]
- Fernández, I.; Delgado, F.; Ortiz, F.; Ortiz, A.; Fernández, C.; Renedo, C.J.; Santisteban, A. Thermal degradation assessment of kraft paper in power transformers insulated with natural esters. Appl. Therm. Eng. 2016, 104, 129–138. [Google Scholar] [CrossRef]
- Golarz, J. Understanding dissolved gas analysis (DGA) techniques and interpretations. In Proceedings of the 2016 IEEE/PES Transmission and Distribution Conference and Exposition (T&D), Dallas, TX, USA, 3–5 May 2016; pp. 1–5. [Google Scholar]
- Jalbert, J.; Rodriguez-Celis, E.M.; Arroyo-Fernández, O.H.; Duchesne, S.; Morin, B. Methanol marker for the detection of insulating paper degradation in transformer insulating oil. Energies 2019, 12, 3969. [Google Scholar] [CrossRef]
- Mehta, A.; Sharma, R.N.; Chauhan, S. Partial discharge study by monitoring key gases of power transformers. In Proceedings of the 2011 3rd International Conference on Electronics Computer Technology, Kanyakumari, India, 8–10 April 2011; pp. 183–186. [Google Scholar]
- Zhou, S.; Iannuzzi, D. Immersion photoacoustic spectrometer (iPAS) for arcing fault detection in power transformers. Opt. Lett. 2019, 44, 3741–3744. [Google Scholar] [CrossRef]
- Bakar, N.A.; Abu-Siada, A.; Islam, S. A review of dissolved gas analysis measurement and interpretation techniques. IEEE Electr. Insul. Mag. 2014, 30, 39–49. [Google Scholar] [CrossRef]
- Bustamante, S.; Manana, M.; Arroyo, A.; Castro, P.; Laso, A.; Martinez, R. Dissolved gas analysis equipment for online monitoring of transformer oil: A review. Sensors 2019, 19, 4057. [Google Scholar] [CrossRef]
- Duval, M. Dissolved gas analysis: It can save your transformer. IEEE Electr. Insul. Mag. 1989, 5, 22–27. [Google Scholar] [CrossRef]
- Ashkezari, A.D.; Saha, T.K.; Ekanayake, C.; Ma, H. Evaluating the accuracy of different DGA techniques for improving the transformer oil quality interpretation. In Proceedings of the AUPEC 2011, Brisbane, QLD, Australia, 25–28 September 2011; pp. 1–6. [Google Scholar]
- Majid, A.; Khadim, B.; Alkhedher, M.; Haider, S.; Akhtar, M.S. Modeling of inert gas sensors using first principles methods. IEEE Sens. J. 2023, 23, 18118–18124. [Google Scholar] [CrossRef]
- Ouyang, B. First-Principles algorithm for air quality electrochemical gas sensors. ACS Sens. 2020, 5, 2742–2746. [Google Scholar] [CrossRef]
- Vargas-Bernal, R. Electrical properties of two-dimensional materials used in gas sensors. Sensors 2019, 19, 1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Varghese, S.S.; Varghese, S.H.; Swaminathan, S.; Singh, K.K.; Mittal, V. Two-dimensional materials for sensing. Graphene Beyond 2015, 4, 651–687. [Google Scholar]
- Wang, B.; Gu, Y.; Chen, L.; Ji, L.; Zhu, H.; Sun, Q. Gas sensing devices based on two-dimensional materials: A review. Nanotechnology 2022, 33, 252001. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, Y.; Zhan, H.; Zhou, Y.; Kang, J. DFT investigation of transition metal-doped graphene for the adsorption of HCl gas. Diam. Relat. Mater. 2023, 136, 109995. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Hu, K.; Li, T.; Yan, Y.; Li, J. The adsorption and sensing performances of Ir-modified MoS2 monolayer toward SF6 decomposition products: A DFT study. Nanomaterials 2021, 11, 100. [Google Scholar] [CrossRef]
- Tan, S.; Bi, M.; Lei, S.; He, X.; Hu, X.; He, J.; Jiang, T. Adsorption of SF6 decomposition gases (H2S, SO2 and SOF2) on TM (Pd and Pt) modified monolayer ZrS2: A DFT study. Comput. Theor. Chem. 2024, 1236, 114586. [Google Scholar] [CrossRef]
- Wang, X.; Gui, Y.; Ding, Z.; Xu, H.; Zeng, H.; Chen, X. Density functional theory study of Pd, Pt, and Au modified GeSe for adsorption and sensing of dissolved gases in transformer oil. Surf. Interfaces 2022, 31, 101994. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Yang, J.; Jin, G.; Yang, P.; Gao, M.; Huang, H. DFT study of metal (Ag, Au, Pt)-modified SnS2 for adsorption of SF6 decomposition gases in gas-insulated switchgear. Langmuir 2024, 40, 7049–7059. [Google Scholar] [CrossRef]
- Shen, J.; Hill, J.M.; Watwe, R.M.; Spiewak, B.E.; Dumesic, J.A. Microcalorimetric, infrared spectroscopic, and DFT studies of ethylene adsorption on Pt/SiO2 and Pt−Sn/SiO2 catalysts. J. Phys. Chem. B 1999, 103, 3923–3934. [Google Scholar] [CrossRef]
- Kolekar, S.; Bonilla, M.; Diaz, H.C.; Hashimoto, M.; Lu, D.; Batzill, M. Controlling the charge density wave transition in monolayer TiSe2: Substrate anddoping effects. Adv. Quantum Technol. 2018, 1, 1800070. [Google Scholar] [CrossRef]
- Xiao, L.; Guo, G.; Zhang, M.; You, M.; Luo, S.; Guo, G.; He, C.; Tang, C.; Zhong, J. Cu- and Al-decorated monolayer TiSe2 for enhanced gas detection sensitivity: A DFT Study. Langmuir 2023, 39, 18631–18643. [Google Scholar] [CrossRef] [PubMed]
- Benesh, G.A.; Woolley, A.M.; Umrigar, C. The pressure dependences of TiS2 and TiSe2 band structures. J. Phys. C Solid State Phys. 1985, 18, 1595. [Google Scholar] [CrossRef]
- Markov, M.; Rezaei, S.E.; Sadeghi, S.N.; Esfarjani, K.; Zebarjadi, M. Thermoelectric properties of semimetals. Phys. Rev. Mater. 2019, 3, 095401. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, Z.; Wang, X. Large-area fabrication of 2D layered topological semimetal films and emerging applications. Adv. Phys. X 2022, 7, 2034529. [Google Scholar] [CrossRef]
- Li, P.; Zheng, X.; Yu, H.; Zhao, G.; Shu, J.; Xu, X.; Sun, W.; Dou, S.X. Electrochemical potassium/lithium-ion intercalation into TiSe2: Kinetics and mechanism. Energy Storage Mater. 2019, 16, 512–518. [Google Scholar] [CrossRef]
- Antonio, J.E.; Cervantes, J.M.; Rosas-Huerta, J.L.; Pilo, J.; Carvajal, E.; Escamilla, R. A first-principles investigation on the electronic and mechanical properties of 1T TiSe2 multilayers for energy storage. J. Electrochem. Soc. 2021, 168, 030531. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Hao, X.; Zhu, T.; Cao, Y.; Wang, W. Insight into the anchoring effect of two-dimensional TiX2 (X=S, Se, Te) materials for Lithium-Sulfur batteries: A DFT study. J. Electrochem. Soc. 2021, 168, 120516. [Google Scholar] [CrossRef]
- Das, S. Quantum Oscillations in Two Dimensional Dirac and Weyl Semimetals. Ph.D. Thesis, The Florida State University, Tallahassee, FL, USA, 2016; 100p. [Google Scholar]
- Rossnagel, K.; Kipp, L.; Skibowski, M. Charge-density-wave phase transition in 1T-TiSe2: Excitonic insulator versus band-type Jahn-Teller mechanism. Phys. Rev. B 2002, 65, 235101. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Haas, P.; Tran, F.; Blaha, P.; Schwarz, K. Construction of an optimal GGA functional for molecules and solids. Phys. Rev. B 2011, 83, 205117. [Google Scholar] [CrossRef]
- Choudhary, K.; Tavazza, F. Convergence and machine learning predictions of Monkhorst-Pack k-points and plane-wave cut-off in high-throughput DFT calculations. Comput. Mater. Sci. 2019, 161, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, I.; Ray, I.; Majumdar, S.; Sen, A.; Maiti, H.S. Improvement of recovery time of nanostructured tin dioxide-based thick film gas sensors through surface modification. Sens. Actuators B Chem. 2007, 127, 554–558. [Google Scholar] [CrossRef]
- D’Arsié, L.; Alijani, V.; Brunelli, S.T.S.; Rigoni, F.; Di Santo, G.; Caputo, M.; Panighel, M.; Freddi, S.; Sangaletti, L.; Goldoni, A. Improved recovery time and sensitivity to H2 and NH3 at room temperature with SnOx vertical nanopillars on ITO. Sci. Rep. 2018, 8, 10028. [Google Scholar] [CrossRef]
- Gui, Y.; Zeng, X.; Hao, J. Adsorption properties of nCu2O-graphene (n = 1, 2, 3) for SOF2 and SO2F2 gas molecules. Diam. Relat. Mater. 2023, 139, 110378. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Wang, G.; Hou, P.; Liu, J.; Chen, X. Physisorption behaviors of deoxyribonucleic acid nucleobases and base pairs on bismuthene from theoretical insights. Appl. Surf. Sci. 2023, 627, 157242. [Google Scholar] [CrossRef]
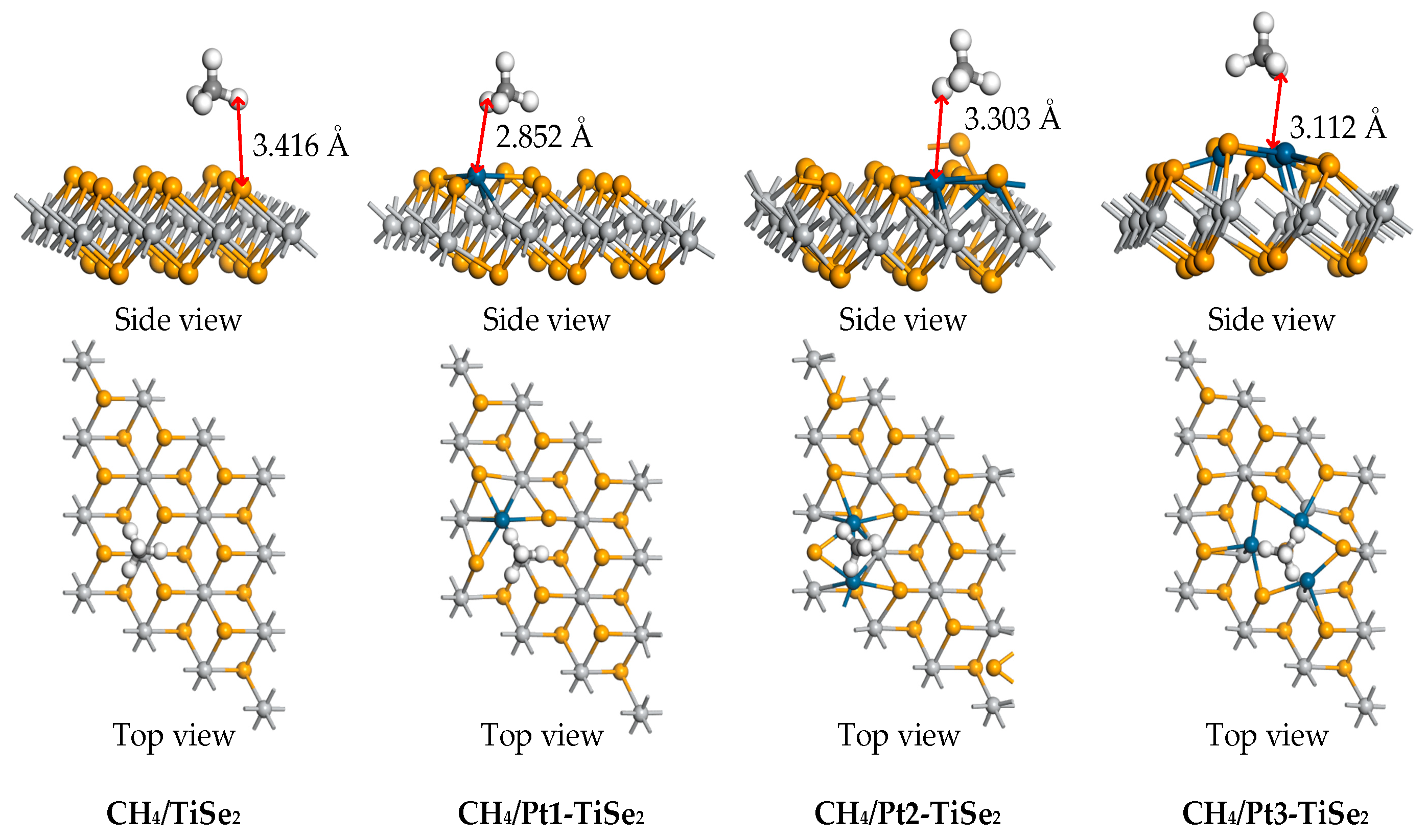


| System | Eads (eV) | Qt (e) | Distance (Å) |
|---|---|---|---|
| CH4/TiSe2 | −0.297 | −0.054 | 3.416 |
| CH4/Pt1-TiSe2 | −0.250 | −0.062 | 2.852 |
| CH4/Pt2-TiSe2 | −0.248 | −0.060 | 3.303 |
| CH4/Pt3-TiSe2 | −0.270 | −0.061 | 3.112 |
| System | Eads (eV) | Qt (e) | Distance (Å) |
|---|---|---|---|
| C2H2/TiSe2 | −0.405 | −0.019 | 3.503 |
| C2H2/Pt1-TiSe2 | −0.940 | 0.031 | 2.194 |
| C2H2/Pt2-TiSe2 | −0.489 | 0.053 | 2.676 |
| C2H2/Pt3-TiSe2 | −0.422 | −0.004 | 3.071 |
| System | Eads (eV) | Qt (e) | Distance (Å) |
|---|---|---|---|
| CO/TiSe2 | −0.219 | −0.002 | 3.594 |
| CO/Pt1-TiSe2 | −1.338 | 0.001 | 1.910 |
| CO/Pt2-TiSe2 | −0.851 | 0.001 | 1.946 |
| CO/Pt3-TiSe2 | −0.703 | 0.004 | 1.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Gui, Y.; Huang, H. Adsorption and Sensing Performance of Pt(1-3)-Modified TiSe2 for Dissolved Gas (CH4, C2H2, and CO) in Transformer Oil: A DFT Study. Int. J. Mol. Sci. 2025, 26, 3985. https://doi.org/10.3390/ijms26093985
Ding J, Gui Y, Huang H. Adsorption and Sensing Performance of Pt(1-3)-Modified TiSe2 for Dissolved Gas (CH4, C2H2, and CO) in Transformer Oil: A DFT Study. International Journal of Molecular Sciences. 2025; 26(9):3985. https://doi.org/10.3390/ijms26093985
Chicago/Turabian StyleDing, Junsheng, Yingang Gui, and Hua Huang. 2025. "Adsorption and Sensing Performance of Pt(1-3)-Modified TiSe2 for Dissolved Gas (CH4, C2H2, and CO) in Transformer Oil: A DFT Study" International Journal of Molecular Sciences 26, no. 9: 3985. https://doi.org/10.3390/ijms26093985
APA StyleDing, J., Gui, Y., & Huang, H. (2025). Adsorption and Sensing Performance of Pt(1-3)-Modified TiSe2 for Dissolved Gas (CH4, C2H2, and CO) in Transformer Oil: A DFT Study. International Journal of Molecular Sciences, 26(9), 3985. https://doi.org/10.3390/ijms26093985







