Abstract
Toll-like receptors (TLRs) are key components of the innate immune system in fish, responsible for recognizing pathogen-associated molecular patterns derived from bacteria, viruses, and fungi. The sterlet (Acipenser ruthenus), an endangered sturgeon species valued for its meat and caviar, is a promising model for studying the effects of polyploidy on immune gene regulation. This study examined the expression of Toll-like receptor type 2 (TLR2) and type 13 (TLR13) in the heart, liver, gills, spleen, and kidney of diploid and triploid healthy sterlets using real-time PCR. TLR2 and TLR13 were expressed in all tissues of both diploids and triploids. In diploids, TLR2 expression was the highest in the kidney and the lowest in the liver (p < 0.05). Similarly, TLR13 expression in diploids was highest in the kidney and gills, and lowest in the liver (p < 0.05). In triploids, no significant tissue-specific variation in TLR expression was observed (p > 0.05). Comparisons between diploid and triploid sterlets revealed higher TLR2 expression in the kidney and higher TLR13 expression in the heart and kidney of diploids (p < 0.05). These molecular findings were supported by leukocyte analysis, which showed a significantly lower percentage of lymphocytes and a higher proportion of neutrophils in triploids compared to diploids. Additionally, the proportion of thrombocytes was significantly elevated in triploids (p < 0.05). This study provides the first report of TLR expression in polyploid fish, offering new insights into immune modulation associated with polyploidy in sturgeons.
1. Introduction
The immune system of fish, like that of other vertebrates, comprises both innate and adaptive components that function cooperatively to detect and eliminate pathogens. The innate immune system represents the first line of defense and includes physical barriers, phagocytic cells (e.g., macrophages, neutrophils), antimicrobial peptides, complement proteins, and cytokines such as interleukins and tumor necrosis factor-alpha (TNF-α) [1,2]. Adaptive immunity in fish, although less complex than in mammals, involves T and B lymphocytes, immunoglobulin production, and antigen presentation by specialized cells [2,3]. While adaptive responses are antigen-specific but slower to activate, the innate immune system—particularly through pattern recognition receptors (PRRs)—plays a critical role in rapid pathogen detection and response.
Among PRRs, Toll-like receptors (TLRs) are essential for recognizing pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides (LPS), flagellin, and viral RNA. Upon detection of PAMPs, TLRs activate signaling cascades that lead to the production of pro-inflammatory cytokines, chemokines, and type I interferons, facilitating both innate and adaptive immune responses [4]. In mammals, 10 to 12 TLRs have been identified [5], whereas fish possess over 20 distinct TLRs, classified into six main superfamilies: TLR1, TLR3, TLR4, TLR5, TLR7, and TLR11 [6,7]. This structural and functional diversity arises from genome/gene duplication events and adaptations to specific environmental pressures. The functional variety of TLRs in fish reflects their evolutionary adaptation to aquatic environments, where they encounter pathogen profiles markedly different from those affecting terrestrial animals [8].
TLRs in fish allow teleosts to recognize a wide range of microbial signals. Among them, Toll-like receptor type 2 (TLR2), part of the TLR1 superfamily, is particularly important due to its ability to form functional heterodimers at the cell surface with other TLRs, such as TLR1, TLR4, TLR6, and TLR 10 [9,10,11]. This ability to heterodimerize not only broadens the range of PAMPs that TLR2 can recognize but also diversifies the downstream signaling pathways [12,13], ultimately enhancing innate immune responses, fostering adaptive immunity, and helping to protect against immune complications following pathogen exposure [14]. TLR2 is known to detect a wide range of PAMPs from viruses, bacteria, fungi, and parasites as well as danger-associated molecular patterns (DAMPs) from damaged host cells [14]. Its downstream signaling, primarily through the MyD88-dependent pathway, results in the induction of pro-inflammatory cytokines and subsequent activation of adaptive immunity. While TLR2 has been studied in various teleost species [15,16,17,18,19,20,21,22,23,24,25], research on its role in sturgeons remains limited, with only Acipenser dabryanus investigated to date [26].
Toll-like receptor type 13 (TLR13) is a member of the TLR11 superfamily and plays a critical role in recognizing both bacterial and viral pathogens. It has been shown to detect a highly conserved sequence of bacterial 23S ribosomal RNA [27] and has been implicated in immune responses to vesicular stomatitis virus infections [28]. Like TLR2, TLR13 also signals via the MyD88-dependent pathway, contributing to pro-inflammatory cytokine production (IL-1β, TNF-α, and IL-10), which is critical for alerting and recruiting other immune cells [29]. While increasing research has focused on TLR13 expression in fish [30,31,32,33,34,35,36,37,38], only a single study examined its expression in sturgeons (Acipenser dabryanus; [26]).
Cytokines, secreted after TLR activation, regulate the immune system by influencing the differentiation, activation, and inflammatory responses of immune cells. Fish possess a wide range of cytokine families, and their gene expression is tightly regulated in response to immune stimuli [39,40]. As summarized by Sakai et al. [40], multiple studies have demonstrated increased expression of cytokine genes in fish treated with bacterial components (e.g., lipopolysaccharide) or immunostimulants such as β-glucans, suggesting their pivotal role in early-stage immune responses and inflammation control. Together, TLRs and cytokines form an integrated molecular network that detects pathogens, initiates inflammation, and orchestrates both innate and adaptive immune responses, ensuring efficient pathogen clearance in teleost fish [41]. This functional responsiveness further highlights the importance of monitoring the expression of these immune mediators to understand the molecular basis of fish immune responses under various physiological conditions, including altered ploidy.
Sturgeons (Acipenseridae) are the most valuable fish species, primarily due to their meat and caviar production [42]. Overexploitation of these products has caused a dramatic decline in natural sturgeon populations, with most species now classified as endangered or near extinction [43]. In addition, bacterial and viral diseases pose serious threats to sturgeon species, particularly in aquaculture settings. Bacterial infections from Aeromonas and Flavobacterium can cause skin lesions, respiratory dysfunctions, and internal inflammation, while herpesviruses have been associated with neurological disorders [44].
The sterlet (Acipenser ruthenus), a small sturgeon species native to European and Asian waters, is an ideal model organism for studying polyploidy, the condition of having more than two sets of chromosomes. Polyploidization, especially triploidization, is of particular interest in aquaculture, as triploid fish (especially females) often exhibit faster growth rates and enhanced meat quality due to their sterility [43]. However, polyploidy increases nuclear DNA content, which may affect gene expression, RNA/DNA ratios, cell size, and immune function [45]. The enlargement of cells and nuclei resulting from additional chromosome sets is offset by a decrease in the total number of cells, including immunological cells [46].
A key aspect of fish immunity involves white blood cells (WBC), including granulocytes (neutrophils, eosinophils, basophils), monocytes, and lymphocytes, which participate in both innate and adaptive immune responses. In addition to these leukocytes, fish thrombocytes—historically regarded primarily as mediators of hemostasis—are now recognized as active participants in innate immunity [47]. Unlike anucleate mammalian platelets, fish thrombocytes are nucleated and have been shown to exhibit phagocytic activity, produce cytokines, and express TLRs, such as TLR5 and TLR8 [47]. Leukocyte parameters in sturgeons with different ploidy levels have been examined in previous studies [48,49,50]; however, these investigations did not explore their relationship with TLR expression. Furthermore, to date, no published studies have reported thrombocyte data comparing diploid and triploid sturgeons.
Despite growing interest in fish immunogenetics, TLR expression in sturgeons remains insufficiently characterized, particularly in relation to different ploidy levels. This study aimed to compare the expression of TLR2 and TLR13 across immune-relevant tissues (heart, liver, gills, spleen, and kidney) in healthy diploid and triploid sterlets using real-time PCR. Additionally, leukocyte and thrombocyte profiles were examined to evaluate potential changes in circulating immune cells. This is the first report describing TLR expression and thrombocyte composition in a polyploid sturgeon species, providing new insights into the immune regulation associated with altered ploidy in fish.
2. Results
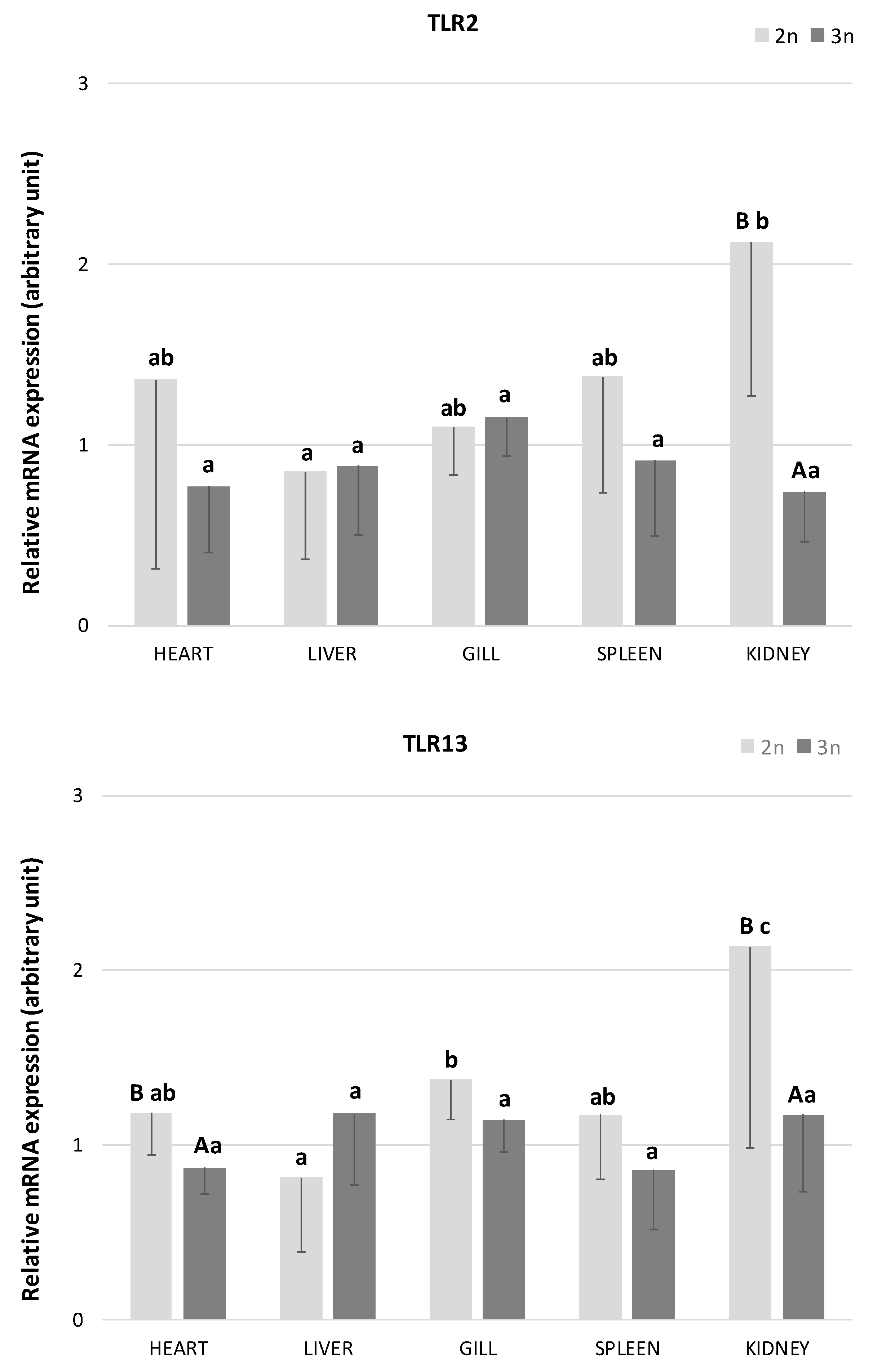
In the present study, the presence of TLR2 and TLR13 transcripts was detected in all examined tissues (heart, liver, gills, spleen, and kidney) of both diploid and triploid individuals of the sterlet A. ruthenus (Figure 1). In diploid sterlets, the expression of both TLRs varied significantly across tissues. The highest TLR2 expression was observed in the kidney, while the liver exhibited the lowest expression levels (p < 0.05). Similarly, the highest TLR13 expression in diploids was detected in the kidney and gills, with significantly the lowest expression in the liver (p < 0.05). In contrast, no significant differences in the expression of either TLR were found across tissues in triploid sterlets (p > 0.05).
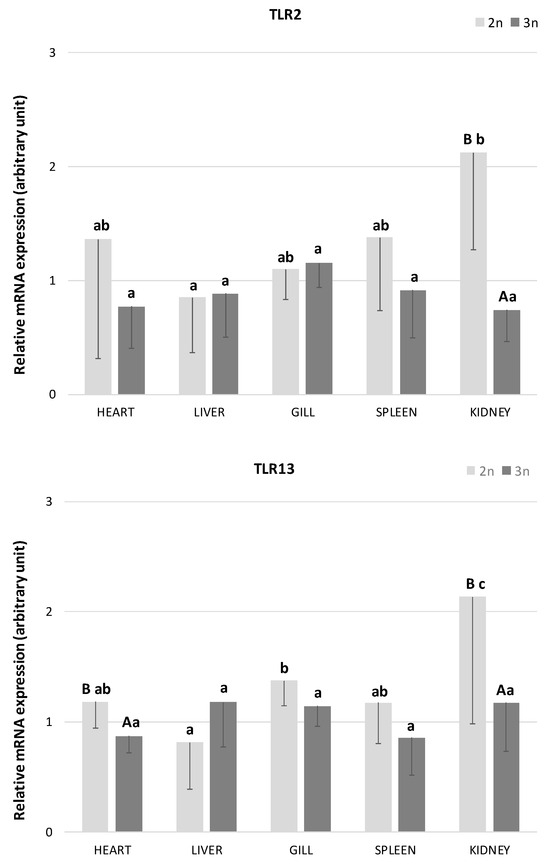
Figure 1.
The relative mRNA expression of Toll-like receptor 2 (TLR2) and 13 (TLR13) in heart, liver, gill, spleen, and kidney in diploid (n = 6 adult fish) and triploid (n = 5 adult fish) sterlet Acipenser ruthenus. Bars indicate average gene expression of fishes at each ploidy level, and error bars indicate standard deviations. Different small letters indicate statistically significant differences between tested tissues within diploid or triploid group (p < 0.05, ANOVA); different capital letters indicate statistically significant differences between diploid and triploid groups within the examined tissue (p < 0.05, independent-samples t-test).
When comparing TLR2 expression between diploid and triploid sterlets, significant differences were observed in the kidney, with diploid sterlets showing higher expression levels (Figure 1; p < 0.05). For TLR13, significant differences in expression were found in the heart and kidney, with diploid sterlets again exhibiting higher mRNA abundance (p < 0.05). Additionally, a trend toward higher TLR13 expression was noted in the gills (p = 0.09) and spleen (p = 0.08) of diploid fish compared to triploid individuals.
Hematological parameters assessed through blood smear analysis are presented in Table 1. The overall percentage of WBC was significantly lower in triploid sterlets compared to diploids (p < 0.001). A marked reduction in lymphocyte proportion was also observed in triploids relative to diploids (p < 0.001). Neutrophil granulocyte percentages were significantly higher in triploids than in diploids (p < 0.0001), as were thrombocyte counts (p < 0.05). No significant differences were found in monocyte or eosinophil percentages between the groups (p > 0.05).

Table 1.
Comparison of hematological parameters between diploid and triploid sterlets (Acipenser ruthenus).
3. Discussion
This study demonstrates the presence of TLR2 and TLR13 transcripts across all examined tissues (heart, liver, gills, spleen, and kidney) in both diploid and triploid sterlets (A. ruthenus). These findings are consistent with prior research, showing that TLRs are broadly expressed in various fish species and tissues. The presence of TLR2 was previously detected in common carp (Cyprinus carpio [22]), grass carp (Ctenopharyngodon idella [23]), rohu (Labeo rohita [19]), channel catfish (Ictalurus punctatus [17]), orange-spotted grouper (Epinephelus coioides, [18]), Japanese flounder (Paralichthys olivaceus [15,25], mandarin fish (Siniperca chuatsi [34]), and Dabry’s sturgeon (Acipenser dabryanus [26]). Similarly, TLR13 has also been reported in several fish, including miiuy croaker (Miichthys miiuy [30]), mandarin fish (Siniperca chuatsi [34]), golden pompano (Trachinotus ovatus [37]), large yellow croaker (Larimichthys crocea [33]), orange-spotted grouper (Epinephelus coioides [31]), Nibea albiflora [38], and soiny mullet (Liza haematocheila [32]). The widespread presence of these receptors across species and tissue types underscores their conserved role in pathogen detection and immune signaling in fish.
In diploid sterlets, TLR2 expression was the highest in the kidney, a result that aligns with previous studies emphasizing the importance of the kidney as a key immune organ in fish, involved in hematopoiesis and immune surveillance [51]. Similar high mRNA expression levels of this receptor have been reported in the kidney of Dabry’s sturgeon [26], turbot [21], common carp [22], grass carp [23], Japanese flounder [15], and mandarin fish [34]. The results from the present as well as the previous studies suggest that the kidney functions as a critical site for immune signaling due to its role in filtering pathogens from the bloodstream [51]. By contrast, the lowest TLR2 expression observed in the liver could be attributed to the liver’s primary metabolic function, which may limit its involvement in frontline immune defense compared to other organs like the kidney or gills. A similar pattern of low TLR2 expression in the liver has been reported in grass carp [23], while Baoprasertkul et al. [17] observed relatively high TLR2 expression in the liver of healthy catfish compared to other tissues. These differences may be due to methodological variations; Baoprasertkul et al. [17] used conventional PCR, a method with lower resolution than real-time PCR.
The expression pattern of TLR13 was highest in the kidney and gills in diploid sterlets. Similar findings were reported by Tang et al. [26], who observed high expression of this receptor in the kidney, gills, and heart of Dabry’s sturgeon; however, there is no information on whether these differences were statistically significant. High levels of TLR13 mRNA have also been found in the kidneys of several other fish species, including miiuy croaker [30], golden pompano [37], large yellow croaker [33], orange-spotted grouper [31], and soiny mullet [32] as well as in the gills of the orange-spotted grouper [31]. In contrast, low levels of TLR13 expression were observed in the gills of miiny croaker [30] and soiny mullet [32].
The high expression of TLR13 in the gills aligns with the role of gills as a primary site of pathogen entry in aquatic organisms, where they serve as the first line of defense against pathogens. TLRs in the gills likely play a crucial role in detecting waterborne pathogens, making the gills an essential component of the fish’s immune system [6]. The high expression of TLR13 in the kidney further supports its involvement in systemic immune responses, especially in detecting bacterial RNA, as TLR13 is known to recognize conserved sequences in bacterial 23S rRNA [27,35]. Lower expression of TLR13 in the liver parallels the patterns observed in this study for TLR2 and is consistent with previous findings in the orange-spotted-grouper [31] and soiny mullet [32]. On the other hand, higher levels of TLR13 expression have been reported in the liver of large yellow croaker [33], Nibea albiflora [38], and miiuy croaker [30]. The variability in TLR13 expression patterns observed across studies suggests that TLR13 expression may be species-specific.
Interestingly, no significant differences in TLR2 or TLR13 expression were observed across tissues in triploid sterlets. To our knowledge, this is the first report about TLR expression in polyploid fishes. This lack of tissue-specific variation in triploids might be due to physiological and genomic changes associated with polyploidy. It has been demonstrated that triploid induction in A. altiparanae specimens resulted in immune impairments and potentially lower resistances to disease and low-quality environments [52]. Moreover, Cadonic et al. [53] provide evidence for epigenetic dysregulation in triploid fishes, which may contribute to their poor performance in response to stress. Thus, the flattened TLR expression profile observed here may reflect diminished immunological plasticity in triploid sterlets.
When comparing gene expressions between diploid and triploid sterlets, TLR2 levels were similar in the heart, liver, gill, and spleen, but significantly higher in the kidney of diploids. Higher TLR2 expression in diploids suggests that polyploidy may influence the immune response capacity of specific organs, with diploids possibly having a more robust immune response in this certain tissue. Christensen et al. [45] found that many genes in the liver exhibit similar expression levels between diploid and triploid coho salmon, likely due to a balance in mRNA transcript production per gene copy (positive gene dosage effects), even in the larger cells of triploids. On the other hand, several genes were differentially expressed between diploid and triploid salmon, indicating that some loci are sensitive to cell size and/or DNA content per cell [45].
For TLR13, diploid sterlets exhibited significantly higher expression in the heart and kidney, with a trend toward higher expression in the gills and spleen in comparison to triploids. The higher expression of TLR13 in diploids may reflect a greater readiness of their immune system to recognize and respond to RNA-based pathogens, which could confer a selective advantage in environments with high pathogen loads [28]. However, more studies are needed to confirm this hypothesis.
Our results showed a significantly lower percentage of WBC and a decreased proportion of lymphocytes as well as a higher percentage of neutrophils and thrombocytes in triploids compared to diploid sterlets. These findings are consistent with previous studies on sturgeon species. Wlasow and Fopp-Bayat [48] as well as Rożyński et al. [49] reported differences in leukocyte parameters between diploid and triploid Siberian sturgeons (A. baerii). Additionally, Salkova et al. [50] observed altered immune cell profiles across species with different ploidy levels, including diploid A. ruthenus, tetraploid A. gueldenstaedtii, and hexaploidy A. brevirostrum. Moreover, these scientists suggest a significant effect of ploidy level on the total number of leukocytes and morphological nuclear changes in the granulocytes and lymphocytes in sturgeon species [50]. To our knowledge, this is the first study to report thrombocyte profiles in triploid sturgeons. Similarly, Gao et al. [54] observed an increased percentage of thrombocytes in triploid and tetraploid loaches (Misgurnus anguillicaudatus); however, the statistical significance of these findings was not provided. This higher proportion of thrombocytes in triploid sterlets may indicate possible differences in hematopoietic regulation. Fish thrombocytes are fascinating because they blur the line between blood clotting and immune function, much more so than in mammals [47].
The altered leukocyte profiles observed in the present study in triploid sterlets might support the hypothesis that polyploidy influences immune system function. Blood smear analysis revealed a significantly lower percentage of WBC in triploid individuals compared to diploids, along with notable shifts in leukocyte and thrombocyte composition. Most strikingly, triploids exhibited a marked reduction in lymphocyte percentage, which may indicate compromised adaptive immune potential, as lymphocytes are essential for antigen-specific responses [39]. At the same time, triploids showed elevated proportions of neutrophils and thrombocytes—cells primarily associated with early-phase, non-specific immune responses [39,47]. These cellular changes, combined with the reduced and less tissue-specific expression of TLR2 and TLR13 observed in triploid sterlets, suggest that polyploidy may result in a less efficient or less tightly regulated immune response. While the increased abundance of thrombocytes and neutrophils in triploids may reflect a compensatory mechanism for lower lymphocyte levels, it remains unclear whether such a response is sufficient to maintain effective immune function.
One important limitation of this study is the lack of histological or morphometric data on cell size and number. Such data could further clarify whether the observed gene expression patterns are associated with structural changes in immune organs. Although we included blood smear analysis, more detailed histological analyses will be needed to evaluate tissue-level adaptations to polyploidy. Furthermore, the study was limited to the assessment of two TLRs. While TLR2 and TLR13 are important components of innate immunity, additional immune markers, such as cytokines, would provide a more comprehensive understanding of the immune landscape in polyploid fish. Further studies using transcriptomics approaches, cytokine assays, and infection models are needed to clarify the functional significance of the observed gene expression patterns.
Despite these limitations, this study presents the first analysis of TLR gene expression alongside immune cell composition in diploid and triploid sterlets. These findings establish important groundwork for future research on the functional immunology of polyploid sturgeons and highlight the need to evaluate their immune competence, particularly in aquaculture settings where disease resistance is critical.
4. Materials and Methods
This study was conducted in strict accordance with the Polish ACT of 21 January 2005 on Animal Experiments (Dz. U. of. 2005, No 33, item 289) and was approved by the Local Ethical Committee for the Experiments on Animals at the University of Warmia and Mazury in Olsztyn, Poland (Permit Number: 75/2012).
4.1. Fish and Tissue Collection
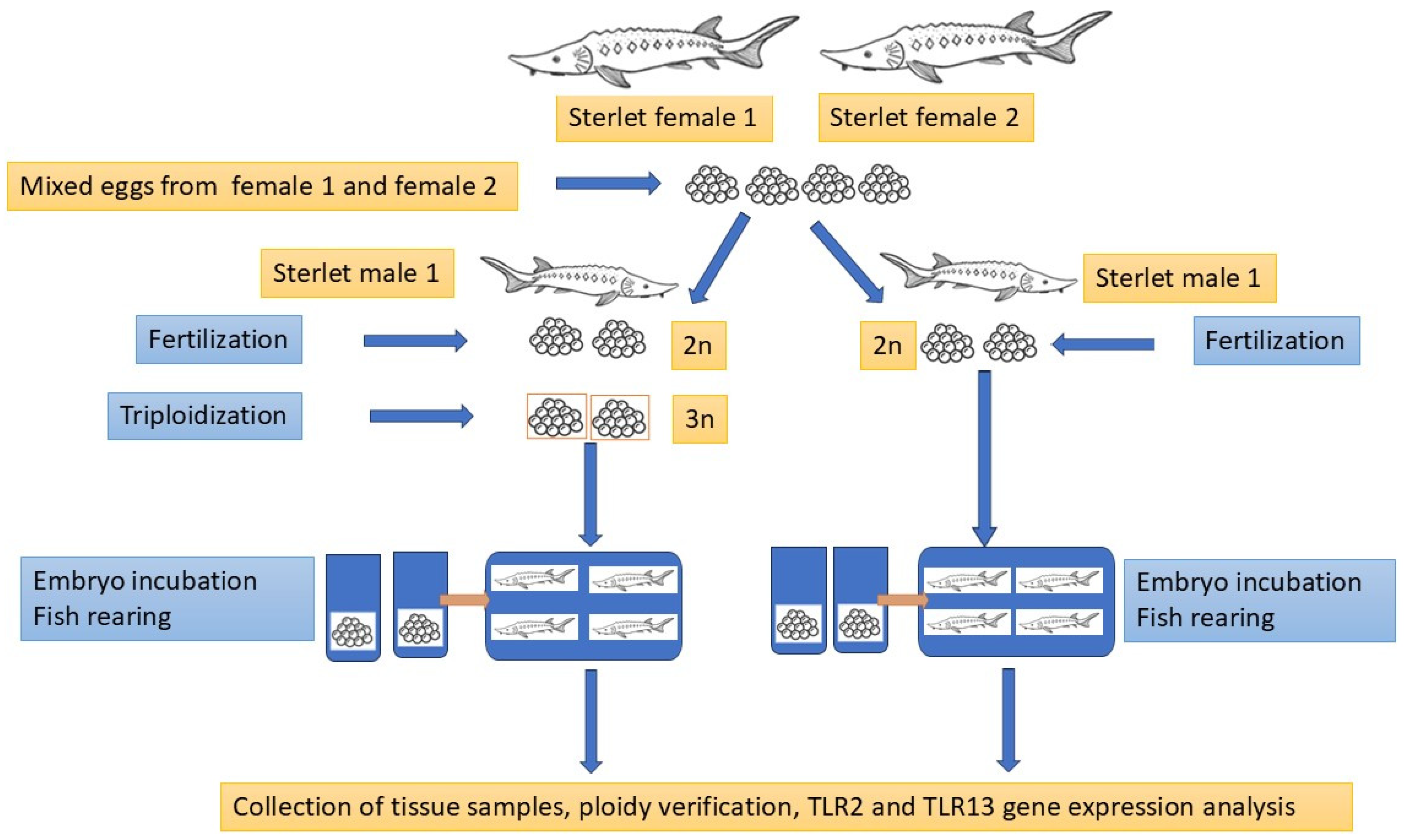
The diploid (2n) and triploid (3n) sterlets were produced according to the procedure published by Fopp-Bayat et al. [55], using sterlet eggs collected from two sterlet females (Female I and Female II) aged 6+ years and fresh sperm collected from sterlet male aged 6+ years. Fish reproduction was conducted under controlled conditions at the Wasosze Fish Farm near Konin, Poland. The portion of 4000 eggs collected from two females were mixed before fertilization and divided into two experimental groups: diploid (2n) and triploid (3n). Sperm collected from two males were examined under a light microscope, and sperm with more than 90% spermatozoa motility were selected to fertilize sterlet eggs. One portion of fertilized eggs (approx. 2000 eggs) was induced to triploidization [55], while the second portion (2n) was the control group. Fertilized eggs from the 2n and 3n groups were incubated in cage incubators [56] at a constant temperature of 17 °C until hatching. The hatched diploid (2n) and triploid (3n) larvae were transferred to two 40 L tanks in a recirculating aquaculture system (RAS) at the Centre for Aquaculture and Environmental Engineering of the University of Warmia and Mazury in Olsztyn. The experimental fish were reared for twelve months post-hatching (mph) according to the procedure described by Fopp-Bayat et al. [57] until sampling.
A total of 11 healthy sterlets Acipenser ruthenus (6 diploids and 5 triploids; F1 generation) with an average body weight of 401 g and 408 g, respectively, were used in the experiment conducted from 2020 to 2021. The ploidy level of each fish was confirmed using fin-clips and flow cytometry. Based on the ploidy analysis, the fish were divided into two experimental groups. Blood for hematological analysis was collected via caudal vein puncture using a heparinized syringe after using MS222 (100 mg/L). After dissection, tissue samples (heart, liver, gill, spleen, and kidney) were immediately collected, frozen in liquid nitrogen, and stored at −80 °C until RNA isolation. An overview of the experimental design is presented in Figure 2.
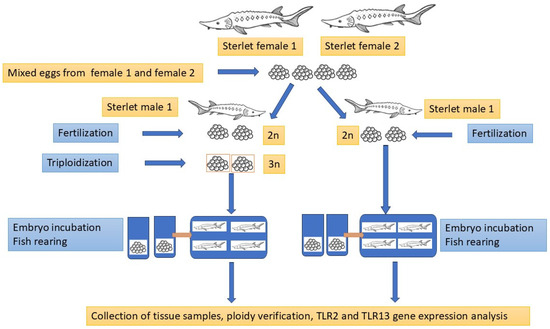
Figure 2.
The graphical illustration of the experimental design.
4.2. Ploidy Verification
The ploidy status of the fish was determined using a CyFlow Ploidy Analyzer (Sysmex) and a ready-to-use kit for nuclei extraction and nuclear DNA staining (CyStain UV Precise T, Sysmex Partec GmbH, Görlitz, Germany). Some modifications were made to the standard protocol. A small piece of dorsal fin (~2 mm) was collected from each fish and placed in a small Petri dish containing 0.3 mL of nuclear extraction buffer. The tissue was mechanically minced using two scalpels. The resulting suspension was transferred to 1.5 mL Eppendorf tubes and incubated at room temperature for 5 min with occasional mixing (by finger vortex). The suspension was then filtered through a 30 µm nylon filter (CellTrics, Sysmex Partec GmbH, Görlitz, Germany) into cytometric tubes. After filtration, 700 µL of staining buffer containing 4′,6-diamidino-2-phenylindole (DAPI) was added to the isolated nuclei, which were incubated for 3 min in reduced light conditions. The samples were then analyzed. Ploidy status was determined by comparing relative DNA content with a standard haploid DNA content (1C) obtained from the sperm of A. ruthenus.
4.3. Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from each tissue using the Total RNA Mini Kit (A&A Biotechnology, Gdynia, Poland) following the manufacturer’s protocol, with slight modifications. Tissue samples weighing 10–20 mg were homogenized in 2 mL tubes containing 800 µL of Phenozol and ceramic beads (Blirt S.A., Gdansk, Poland). Homogenization was performed using a MagNALyser tissue homogenizer (Roche Diagnostics GmbH, Mannheim, Germany). RNA was eluted with 50 µL of RNase- and DNase-free water at 50 °C. RNA integrity was confirmed by agarose gel electrophoresis, and RNA quality and concentrations were assessed spectrophotometrically (Nanodrop, Thermo Fisher Scientific Inc., Waltham, MA, USA) by measuring absorbance at 260 nm and 280 nm. RNA samples were stored at −80 °C until further use. The cDNA template was synthesized from approximately 1 µg of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany) in accordance with the manufacturer’s instructions. The protocol included a genomic DNA removal step using genomic DNA wipe-out buffer provided in the kit. As a control for genomic DNA contamination, reactions without Quantiscript Reverse Transcriptase were performed for each sample. Reverse transcription was carried out for 30 min at 42 °C, followed by a termination step at 95 °C for 3 min (Veriti, Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). The resulting cDNA was diluted 1:10 before being used in real-time PCR analysis.
4.4. Real-Time PCR (qPCR)
Real-time PCR was performed in 96-well plates using a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) with Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA, USA). Two reference genes, EF1α and RPL13, were used for normalization [58]. To evaluate reference gene expression stability, the geNORM software tool (version 3.2, Biogazelle, Zwijnaarde, Belgium) was used. Specific primers for TLR2 and TLR13 were designed using PRIMER3 based on the available mRNA sequences of both sterlet receptors from the NCBI database (accession numbers: XM_034014252.2, XM_034908419.1, respectively). Primer details are provided in Table 2. Primer specificity was confirmed via melting curve analysis and verification of amplicon size by gel electrophoresis. For each assay, primer efficiency was determined by a standard curve of cDNA samples according to the MIQE guidelines for qPCR [59]. The linear correlation coefficient (R2) ranged from 0.985 to 0.998, and the PCR efficiency varied from 99.3% to 116.8%. Non-template control (NTC) samples were included in each run to monitor for contamination. To ensure the absence of genomic DNA amplification, control reactions without reverse transcriptase were performed. Relative mRNA expression levels were calculated using the comparative cycle threshold (CT) method [60].

Table 2.
Primers used for real-time PCR.
4.5. Blood Smears
Blood smears were prepared immediately after collection, air-dried at room temperature, fixed in methanol, and stained using a procedure published by Svobodová et al. [61]. Slides were observed under a light microscope (Eclipse 80i, Nikon, Tokyo, Japan) equipped with NIS-Elements software (version 4.6, Nikon, Tokyo, Japan) using a digital camera (DS-Fi1c, Nikon, Tokyo, Japan). For each specimen, 200 nucleated immune cells were counted and identified based on cell size, nuclear morphology, and cytoplasmic staining. Leukocyte and thrombocyte differentiation was expressed as a percentage of each cell type.
4.6. Statistical Analysis
Statistical analysis was performed using the Statistica software (version 13.3, StatSoft Inc., Tulsa, OK, USA). A one-way ANOVA was used to compare the relative expression of TLR2 and TLR13 between tissues within diploid and triploid sterlets, followed by a least significant difference (LSD) test. To compare the relative expression of TLR2 and TLR13 between diploid and triploid individuals within each tissue, an independent samples t-test was employed. Leukocyte and thrombocyte count data were also analyzed using Student’s t-test. Data were log-transformed when the variances of the compared means differed by at least one order of magnitude. Differences were considered statistically significant at p < 0.05.
Author Contributions
Conceptualization, O.J.; methodology, O.J., S.D., S.G. and A.N.; software, O.J., S.D., S.G. and A.N.; validation, O.J., S.D. and A.N.; formal analysis, O.J.; investigation, O.J., S.D., S.G. and A.N.; resources, O.J. and D.F.-B.; data curation, O.J.; writing—original draft preparation, O.J.; writing—review and editing, O.J. and D.F.-B.; visualization, O.J. and D.F.-B.; supervision, D.F.-B.; project administration, D.F.-B.; funding acquisition, D.F.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UWM in Olsztyn, Poland Statutory Grant No: 11.610.015-110 and by the Minister of Science under “the Regional Initiative of Excellence Program” No: RID/SP/0025/2024/01.
Institutional Review Board Statement
The study was approved by the Local Ethics Committee for Animal Experimentation of the University of Warmia and Mazury in Olsztyn (decision No. 75/2012), and it was conducted based on the guidelines of the Animal Research Act of 21 January 2005 (Journal of Laws, 2005, item 289).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We would like to thank Elzbieta and Andrzej Fopp from Wasosze Fish Farm for the possibility of carrying out experiments using sterlet spawners.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish. Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.M. Innate immunity of fish (overview). Fish Shellfish. Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O. Fishing for mammalian paradigms in the teleost immune system. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish. Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ganguly, B.; Pani, S.; Saha, A.; Samanta, M. A comprehensive review on the dynamic role of toll-like receptors (TLRs) in frontier aquaculture research and as a promising avenue for fish disease management. Int. J. Biol. Macromol. 2023, 253, 126541. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like receptors in bony fish: From genomics to function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The Repertoire for Pattern Recognition of Pathogens by the Innate Immune System Is Defined by Cooperation between Toll-like Receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zhou, Y.; Fang, H.; Lin, S.; Wang, P.-F.; Xiong, R.-P.; Chen, J.; Xiong, X.-Y.; Lv, F.-L.; Liang, Q.-L.; et al. Toll-like Receptor 2/4 Heterodimer Mediates Inflammatory Injury in Intracerebral Hemorrhage. Ann. Neurol. 2014, 75, 876–889. [Google Scholar] [CrossRef]
- Su, S.-B.; Tao, L.; Deng, Z.-P.; Chen, W.; Qin, S.-Y.; Jiang, H.-X. TLR10: Insights, Controversies and Potential Utility as a Therapeutic Target. Scand. J. Immunol. 2021, 93, e12988. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Fumia, A.; D’Angelo, R.; Mangano, A.; Lombardo, G.P.; Giliberti, A.; Messina, E.; Alesci, A.; Lauriano, E.R. Expression and function of toll-like receptor 2 in vertebrate. Acta Histochem. 2023, 125, 152028. [Google Scholar] [CrossRef] [PubMed]
- Colleselli, K.; Stierschneider, A.; Wiesner, C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 12464. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Hirono, I.; Takami, M.; Miyata, M.; Miyazaki, T.; Han, H.-J.; Takano, T.; Endo, M.; Aoki, T. Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics 2004, 56, 38–46. [Google Scholar] [CrossRef]
- Jault, C.; Pichon, L.; Chluba, J. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 2004, 40, 759–771. [Google Scholar] [CrossRef]
- Baoprasertkul, P.; Peatman, E.; Abernathy, J.; Liu, Z. Structural characterization and expression analysis of Toll-like receptor 2 gene from catfish. Fish Shellfish. Immunol. 2007, 22, 418–426. [Google Scholar] [CrossRef]
- Wei, Y.C.; Pan, T.S.; Chang, M.X.; Huang, B.; Xu, Z.; Luo, T.R.; Nie, P. Cloning and expression of Toll-like receptors 1 and 2 from a teleost fish, the orange-spotted grouper Epinephelus coioides. Vet. Immunol. Immunopathol. 2011, 141, 173–182. [Google Scholar] [CrossRef]
- Samanta, M.; Swain, B.; Basu, M.; Panda, P.; Mohapatra, G.B.; Sahoo, B.R.; Maiti, N.K. Molecular characterization of toll-like receptor 2 (TLR2), analysis of its inductive expression and associated down-stream signaling molecules following ligands exposure and bacterial infection in the Indian major carp, rohu (Labeo rohita). Fish Shellfish. Immunol. 2012, 32, 411–425. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Jia, Q.-J.; Yao, C.-L. Characterization and expression analysis of Toll-like receptor 2 gene in large yellow croaker, Larimichthys crocea. Fish Shellfish. Immunol. 2015, 44, 129–137. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, G.; Liu, Q.; Zhang, S. Cloning and expression study of a Toll-like receptor 2 (tlr2) gene from turbot, Scophthalmus maximus. Fish Shellfish. Immunol. 2016, 59, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Fink, I.R.; Pietretti, D.; Voogdt, C.G.P.; Westphal, A.H.; Savelkoul, H.F.J.; Forlenza, M.; Wiegertjes, G.F. Molecular and functional characterization of Toll-like receptor (Tlr)1 and Tlr2 in common carp (Cyprinus carpio). Fish Shellfish. Immunol. 2016, 56, 70–83. [Google Scholar] [CrossRef] [PubMed]
- He, L.B.; Wang, H.; Luo, L.F.; Jiang, S.H.; Liu, L.Y.; Li, Y.M.; Huang, R.; Liao, L.J.; Zhu, Z.Y.; Wang, Y.P. Characterization, expression analysis and localization pattern of toll-like receptor 1 (tlr1) and toll-like receptor 2 (tlr2) genes in grass carp Ctenopharyngodon Idella. J. Fish. Biol. 2016, 89, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, L.; Zhu, K.C.; Guo, H.Y.; Liu, B.; Jiang, S.G.; Zhang, D.C. Genomic structure and molecular characterization of Toll-like receptors 1 and 2 from golden pompano Trachinotus ovatus (Linnaeus, 1758) and their expression response to three types of pathogen-associated molecular patterns. Dev. Comp. Immunol. 2018, 86, 34–40. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Su, B.; Xue, T.; Cao, M.; Li, C. Genome-wide identification, characterization, and expression of the Toll-like receptors in Japanese flounder (Paralichthys olivaceus). Aquaculture 2021, 545, 737127. [Google Scholar] [CrossRef]
- Tang, R.; Wang, S.; Han, P.; Zhang, Q.; Zhang, S.; Xing, X.; Shao, R.; Xu, W.; Xu, Q.; Wei, Q.; et al. Toll-like receptor (TLR) 2 and TLR13 from the endangered primitive-ray finned fish Dabry’s sturgeon (Acipenser dabryanus) and their expression profiling upon immune stimulation. Aquac. Rep. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Oldenburg, M.; Krüger, A.; Ferstl, R.; Kaufmann, A.; Nees, G.; Sigmund, A.; Bathke, B.; Lauterbach, H.; Suter, M.; Dreher, S.; et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 2012, 337, 1111–1115. [Google Scholar] [CrossRef]
- Shi, Z.; Cai, Z.; Sanchez, A.; Zhang, T.; Wen, S.; Wang, J.; Yang, J.; Fu, S.; Zhang, D. A Novel Toll-like Receptor That Recognizes Vesicular Stomatitis Virus. J. Biol. Chem. 2011, 286, 4517–4524. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, X.; Chu, Q.; Xu, T. Discovery of toll-like receptor 13 exists in the teleost fish: Miiuy croaker (Perciformes, Sciaenidae). Dev. Comp. Immunol. 2016, 61, 25–33. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, X.; Yu, X.; Wang, Y.; Zhou, Y.; He, J.; Shi, Y.; Zhang, Y.; Lin, H.; Lu, D. Identification and functional characterization of Toll-like receptor 13 from orange-spotted grouper (Epinephelus coioides). Fish Shellfish. Immunol. 2018, 74, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xu, Y.; Wang, X.; Wang, S.; Zhang, Q.; Wang, Z.; Gao, Q. TLR13, TLR22, TRAF6, and TAK1 in the soiny mullet (Liza haematocheila): Molecular characterization and expression profiling analysis. Dev. Comp. Immunol. 2020, 112, 103774. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pei, L.; Wang, P.; Liu, L.; Li, G.; Liu, B.; Lǚ, Z.; Hiromasa, T.; Pan, H.; Ogura, A. Molecular Characterization and Evolution Analysis of Two Forms of TLR5 and TLR13 Genes Base on Larimichthys crocea Genome Data. Int. J. Genom. 2020, 2020, 4895037. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Chen, S.N.; Huo, H.J.; Nie, P. Identification and expression analysis of sixteen Toll-like receptor genes, TLR1, TLR2a, TLR2b, TLR3, TLR5M, TLR5S, TLR7−9, TLR13a−c, TLR14, TLR21−23 in mandarin fish Siniperca chuatsi. Dev. Comp. Immunol. 2021, 121, 104100. [Google Scholar] [CrossRef]
- Feng-ying, G.; Ji-cai, P.; Miao, W.; Mai-xin, L.; Zhi-gang, L.; Jian-meng, C.; Xiao-li, K.; Meng-meng, Y. Structurally diverse genes encode TLR13 in Nile tilapia: The two receptors can recognize Streptococcus 23S RNA and conduct signal transduction through MyD88. Mol. Immunol. 2021, 132, 60–78. [Google Scholar] [CrossRef]
- Yu, X.; Liang, Y.; Zhou, Y.; He, L.; Liu, Y.; Fu, L.; Lin, H.; Zhang, Y.; Lu, D. 23S rRNA from Vibrio parahaemolyticus regulates the innate immune response via recognition by TLR13 in orange-spotted grouper (Epinephelus coioides). Dev. Comp. Immunol. 2021, 114, 103837. [Google Scholar] [CrossRef]
- Da, F.; Tan, H.; Wan, X.; Lin, G.; Jian, J.; Wen, Z.; Cai, S. Molecular characterization, expression and response to immune challenges of 3 members of the toll-like receptor superfamily 11 in the golden pompano (Trachinotus ovatus). Aquac. Rep. 2022, 25, 101268. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Tang, X.; Xu, D.; Chi, C.; Lv, Z.; Liu, H. Characterization of a novel Toll-like receptor 13 homologue from a marine fish Nibea albiflora, revealing its immunologic function as PRRs. Dev. Comp. Immunol. 2023, 139, 104563. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.; Kono, T. Fish cytokines: Current research and applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Graaf, C.; Van der Meer, J.W.; Kullberg, B.J. Toll-like receptors and the host defense against microbial pathogens: Bringing specificity to the innate-immune system. J. Leukoc. Biol. 2004, 75, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Birstein, V.J.; Doukakis, P.; Sorkin, B.; DeSalle, R. Population Aggregation Analysis of Three Caviar-Producing Species of Sturgeons and Implications for the Species Identification of Black Caviar. Conserv. Biol. 1998, 12, 766–775. [Google Scholar] [CrossRef]
- Chandra, G.; Fopp-Bayat, D. Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 2021, 13, 119–137. [Google Scholar] [CrossRef]
- Radosavljević, V.; Milićević, V.; Maksimović-Zorić, J.; Veljović, L.; Nešić, K.; Pavlović, M.; Ljubojević-Pelić, D.; Marković, Z. Sturgeon diseases in aquaculture. Arh. Vet. Med. 2019, 12, 5–20. [Google Scholar] [CrossRef]
- Christensen, K.A.; Sakhrani, D.; Rondeau, E.B.; Richards, J.; Koop, B.F.; Devlin, R.H. Effect of triploidy on liver gene expression in coho salmon (Oncorhynchus kisutch) under different metabolic states. BMC Genom. 2019, 20, 336. [Google Scholar] [CrossRef]
- Maxime, V. The physiology of triploid fish: Current knowledge and comparison with diploid fish. Fish Fish. 2008, 9, 67–78. [Google Scholar] [CrossRef]
- Ortiz, M.; Esteban, M.A. Biology and functions of fish thrombocytes: A review. Fish Shellfish. Immunol. 2024, 148, 109509. [Google Scholar] [CrossRef]
- Wlasow, T.; Fopp-Bayat, D. The effect of thermal shock on morphological characteristics of blood cells in Siberian sturgeon (Acipenser baerii) triploids. Acta Vet. Brno 2011, 80, 215–218. [Google Scholar] [CrossRef]
- Rożyński, M.; Demska-Zakęś, K.; Fopp-Bayat, D. Hematological and blood gas profiles of triploid Siberian sturgeon (Acipenser baerii Brandt). Arch. Pol. Fish. 2015, 23, 197–203. [Google Scholar] [CrossRef]
- Salkova, E.; Gela, D.; Pecherkova, P.; Flajshans, M. Examination of white blood cell indicators for three different ploidy level sturgeon species reared in an indoor recirculation aquaculture system for one year. Vet. Med. 2022, 67, 138–149. [Google Scholar] [CrossRef]
- Zapata, A.G. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Levy-Pereira, N.; Carriero, M.M.; Yasui, G.S.; Meira, C.M.; de Sousa, R.L.M.; Maia, A.A.M.; Senhorini, J.A.; Pilarski, F. Effects of triploid induction on innate immunity and hematology in Astyanax altiparanae. Fish Shellfish. Immunol. 2021, 116, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cadonic, I.G.; Heath, J.W.; Dixon, B.; Craig, P.M. Diploid and triploid Chinook salmon (Oncorhynchus tshawytscha) have altered microRNA responses in immune tissues after infection with Vibrio anguillarum. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2023, 48, 101121. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, W.; Abbas, K.; Zhou, X.; Yang, Y.; Diana, J.S.; Wang, H.; Wang, H.; Li, Y.; Sun, Y. Haematological characterization of loach Misgurnus anguillicaudatus: Comparison among diploid, triploid and tetraploid specimens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 1001–1008. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Kolman, R.; Woznicki, P. Induction of meiotic gynogenesis in sterlet (Acipenser ruthenus) using UV-irradiated bester sperm. Aquaculture 2007, 264, 54–58. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Kujawa, R. Protective Right for a Utility Model—Device for Thermal Shock and Incubation of Sturgeon Fish Eggs. Patent Number 72570, 1 May 2022. [Google Scholar]
- Fopp-Bayat, D.; Nitkiewicz, A.; Gomułka, P. The Effect of Cryopreserved Sperm on the Early Development, Survival, and Growth of Intergeneric Sterbel Hybrids (Acipenser ruthenus × Huso huso). Int. J. Mol. Sci. 2024, 25, 5784. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.; Tian, Z.; Sun, A.; Dong, Y.; Dong, T.; Hu, H. Effects of 11-ketotestosterone on development of the previtellogenic ovary in the sterlet, Acipenser ruthenus. Front. Endocrinol. 2020, 11, 115. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum Information for publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Svobodová, Z.; Pravda, D.; Paláčková, J. Unified Methods of Haematological Examination of Fish; Edition Methods; Research Institute of Fish Culture and Hydrobiology: Vodňany, Czech Republic, 1991; Volume 22, 31p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).