Mechanosignaling in Osteoporosis: When Cells Feel the Force
Abstract
:1. Introduction
2. Mechanosensing of Bone Cells
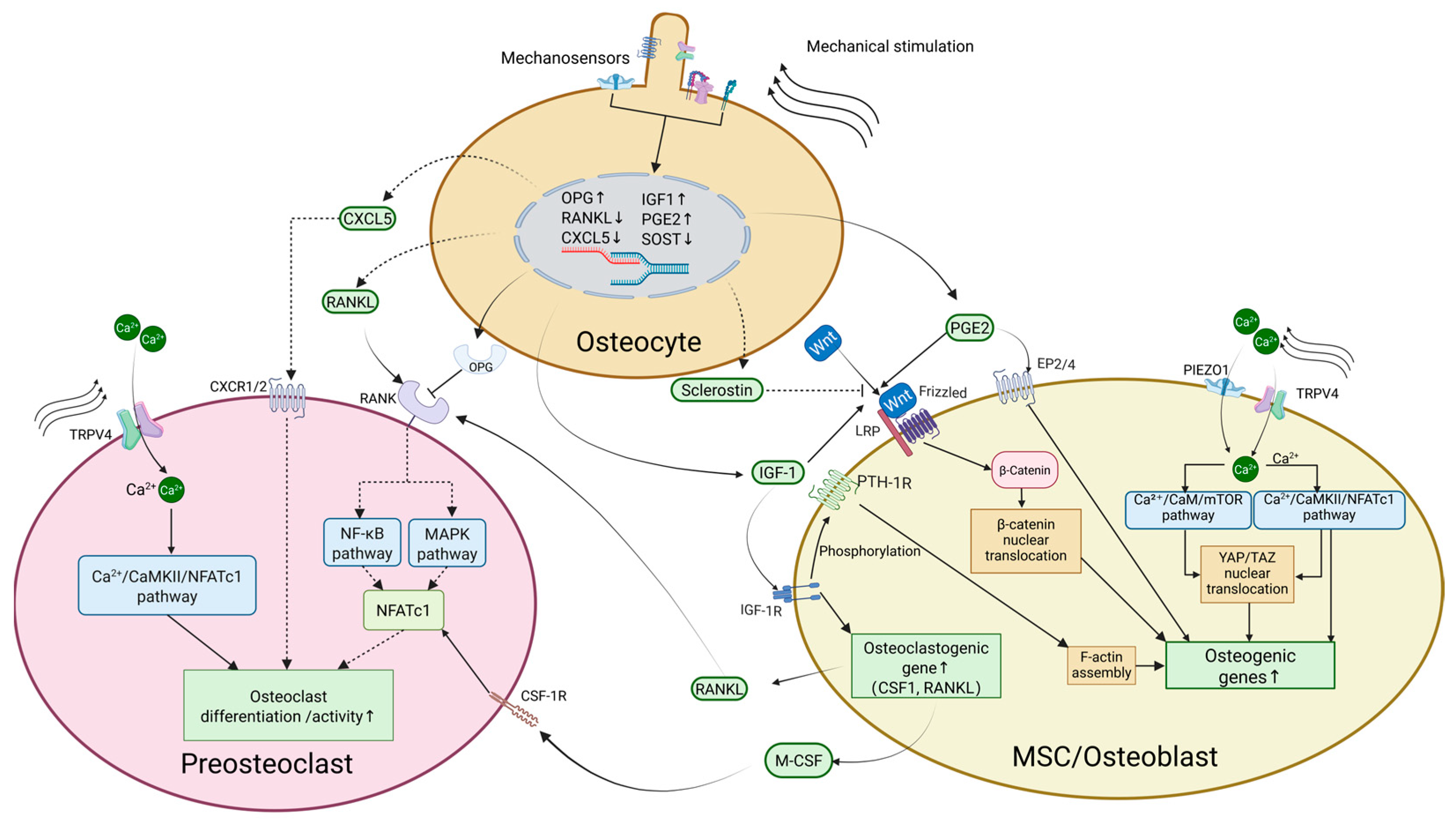
2.1. Osteocytes
2.2. Osteoblasts
2.3. Osteoclasts
3. Mechanosensitive Structures Mediating Bone Mechanotransduction
3.1. Extracellular Matrix (ECM) and Pericellular Matrix (PCM)
3.1.1. ECM Stiffness and Osteocyte Mechanosensitivity
3.1.2. ECM Stiffness and Regulation of Osteoblast and Osteoclast Function and Differentiation
3.1.3. Effects of PCM Components on Osteocyte Mechanotransduction
3.2. Integrins and Connexons
3.3. Mechanosensitive Ion Channels
3.3.1. Piezo Channels
3.3.2. TRPV4 Channels
3.4. Primary Cilia
4. Mechanosignaling in Osteoporosis
4.1. Disuse Osteoporosis
4.1.1. Effects of Unloading on Bone Cells
4.1.2. Muscle–Bone Interaction
4.2. Postmenopausal Osteoporosis
4.3. Senile Osteoporosis
4.4. Endocrinological Causes
4.4.1. Diabetes Mellitus
4.4.2. Primary Hyperparathyroidism
4.5. Glucocorticoid-Related Osteoporosis
5. Potential Treatment Strategies Targeting Mechanosignaling
5.1. Primary Cilia Modulators
5.2. Ion Channel Modulators
5.3. Cx43 Hemichannels
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A2BAR | A2B adenosine receptor |
| AC6 | Adenylyl cyclase 6 |
| ACP5 | Acid phosphatase 5, tartrate resistant |
| AGEs | Advanced glycation end-products |
| AKT | Protein kinase b |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| ATP2A2 | ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 |
| BGLAP | Bone gamma-carboxyglutamate protein |
| BMD | Bone mineral density |
| BMP | bone morphogenetic protein |
| BMSC | Bone-marrow-derived mesenchymal stem cell |
| CaM | Calmodulin |
| CaMKII | Calcium/calmodulin-dependent protein kinase ii |
| cAMP | Cyclic adenosine monophosphate |
| CFL1 | Cofilin 1 |
| CHOP | C/EBP homologous protein |
| CKO | Conditional knockout |
| CNN1 | Calponin 1 |
| COL7A1 | Collagen type VII alpha 1 chain |
| COL8A1 | Collagen type VIII alpha 1 chain |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP response element binding protein |
| Cx43 | Connexin-43 |
| CXCL5 | C-X-C motif chemokine ligand 5 |
| DLX5 | Distal-less homeobox 5 |
| DMP1 | Dentin matrix acidic phosphoprotein 1 |
| DRP1 | Dynamin-related protein 1 |
| ECM | Extracellular matrix |
| EP | Prostaglandin e2 receptor |
| ER | Estrogen receptors |
| ERK | Extracellular signal-regulated kinase |
| F-actin | Filamentous actin |
| FAK | Focal adhesion kinase |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit |
| FSS | Fluid shear stress |
| GC | Glucocorticoid |
| Gli | GLI family zinc finger protein |
| GPER1 | G-protein-coupled estrogen receptor 1 |
| HP | Hydrostatic pressure |
| IFT | Intraflagellar transport |
| IFT88 | Intraflagellar transport 88 |
| IGF-1 | Insulin-like growth factor 1 |
| IGF1R | Insulin-like growth factor 1 receptor |
| IL | Interleukin |
| IRE1 | Inositol-requiring enzyme 1 |
| ITGA3 | Integrin subunit alpha 3 |
| ITGA5 | Integrin subunit alpha 5 |
| ITGAV | Integrin subunit alpha V |
| ITGB1 | Integrin subunit beta 1 |
| ITGB3 | Integrin subunit beta 3 |
| JAK | Janus kinase |
| KIF3A | Kinesin family member 3A |
| LAMA3 | Laminin subunit alpha 3 |
| LCS | Lacuna-canaliculi system |
| LRP5/6 | Lipoprotein receptor-related proteins 5/6 |
| MAPK | Mitogen-activated protein kinase |
| M-CSF | Macrophage colony-stimulating factor |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cells |
| mTOR | Mechanistic target of rapamycin |
| NFATc1 | Nuclear factor of activated T cells cytoplasmic 1 |
| NICD | Notch intracellular domain |
| NMD4 | Nuclear matrix protein 4 |
| NO | Nitric oxide |
| NOTCH3 | Notch receptor 3 |
| NOX | NADPH oxidase |
| OPG | Osteoprotegerin |
| Panx1 | Pannexin-1 |
| PCM | Pericellular matrix |
| PGE2 | Prostaglandin E2 |
| PHPT | Primary hyperparathyroidism |
| PI3K | Phosphatidylinositol-3-kinase |
| PIEZO1 | Piezo-type mechanosensitive ion channel component 1 |
| PKA | Protein kinase A |
| PKB | Protein kinase B |
| PKC | Protein kinase C |
| PTH | Parathyroid hormone |
| PTH1R | Parathyroid hormone 1 receptor |
| PXN | Paxillin |
| RANK | Receptor activator of nuclear factor kappa-B |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RHOA | Ras homolog family member A |
| ROCK | Rho-associated coiled-coil containing protein kinase |
| ROS | Reactive oxygen species |
| RUNX2 | Runx family transcription factor 2 |
| SCI | Spinal cord injury |
| SGK1 | Serum/glucocorticoid-regulated kinase 1 |
| SMAD | Smad family member |
| SMG | Simulated microgravity |
| SOST | Sclerostin |
| SP7 | Sp7 transcription factor |
| SPP1 | Secreted phosphoprotein 1 |
| SRC | Proto-oncogene tyrosine-protein kinase Src |
| STIM1 | Stromal interaction molecule 1 |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TCA | Tricarboxylic acid |
| TEAD | TEA domain transcription factor |
| TGF-β | Transforming growth factor beta |
| TIMP3/4 | TIMP metallopeptidase inhibitor 3/4 |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| WHO | World Health Organization |
| YAP | Yes-associated protein |
| YAP1 | Yes1-associated transcriptional regulator |
| ZNF384 | Zinc finger protein 384 |
References
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Teufel, S.; Hartmann, C. Wnt-signaling in skeletal development. Curr. Top. Dev. Biol. 2019, 133, 235–279. [Google Scholar] [CrossRef]
- Carroll, M.; Alliston, T.; Dole, N. The Multifaceted Effects of Osteocytic TGF beta Signaling on the Skeletal and Extraskeletal Functions of Bone. Curr. Osteoporos. Rep. 2023, 21, 414–425. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sassi, A.; Wu, Y.; Seaman, K.; Tang, W.; Song, X.; Bienenstock, R.; Yokota, H.; Sun, Y.; Geng, F.; et al. Mechanotransduction pathways regulating YAP nuclear translocation under Yoda1 and vibration in osteocytes. Bone 2025, 190, 117283. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.; Barrett-Connor, E.; Black, D.; Bonjour, J.P.; et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yang, Y.; Jung, H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.W.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Wang, L.P.; Ge, L.H.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, X.; Gao, X.; Tong, J.; Zhang, J. The calcium transient characteristics induced by fluid shear stress affect the osteoblast proliferation. Exp. Cell Res. 2018, 362, 51–62. [Google Scholar] [CrossRef]
- McAllister, T.N.; Du, T.; Frangos, J.A. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone-marrow-derived preosteoclast-like cells. Biochem. Biophys. Res. Commun. 2000, 270, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bian, X.Y.; Liu, C.L.; Wang, S.R.; Guo, M.M.; Tao, Y.J.; Huo, B. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium 2018, 71, 45–52. [Google Scholar] [CrossRef]
- Noble, B.S. The osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 106–111. [Google Scholar] [CrossRef]
- Knothe Tate, M.L.; Adamson, J.R.; Tami, A.E.; Bauer, T.W. The osteocyte. Int. J. Biochem. Cell Biol. 2004, 36, 1–8. [Google Scholar] [CrossRef]
- Cowin, S.C.; Cardoso, L. Blood and interstitial flow in the hierarchical pore space architecture of bone tissue. J. Biomech. 2015, 48, 842–854. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef]
- Amjadi-Moheb, F.; Akhavan-Niaki, H. Wnt signaling pathway in osteoporosis: Epigenetic regulation, interaction with other signaling pathways, and therapeutic promises. J. Cell. Physiol. 2019, 234, 14641–14650. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, H.; Song, X.; Zhao, J.; Xu, D.; Liu, M. The Wnt/β-catenin signaling pathway inhibits osteoporosis by regulating the expression of TERT: An in vivo and in vitro study. Aging 2023, 15, 11471–11488. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.-W.; Liang, M.; Wang, X.-N.; Fan, Y.-B. The Response of wnt/ß-Catenin Signaling Pathway in Osteocytes Under Simulated Microgravity. Microgravity Sci. Technol. 2015, 27, 473–483. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, N.; Wang, Q.; Feng, J.; Sun, D.; Zhang, Q.; Huang, J.; Wen, Q.; Hu, R.; Wang, L.; et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J. Bone Miner. Res. 2019, 34, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Crane, J.L.; Xie, L.; Xian, L.; Xie, H.; Cao, X. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Centrella, M.; Canalis, E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology 1989, 124, 1247–1253. [Google Scholar] [CrossRef]
- Wang, Y.; Nishida, S.; Elalieh, H.Z.; Long, R.K.; Halloran, B.P.; Bikle, D.D. Role of IGF-I signaling in regulating osteoclastogenesis. J. Bone Miner. Res. 2006, 21, 1350–1358. [Google Scholar] [CrossRef]
- Ru, Y.; Gu, H.; Sun, L.; Zhang, W.; Wang, L. Mechanical Stretch-Induced ATP Release from Osteocytes Promotes Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Discov. Med. 2024, 36, 494–508. [Google Scholar] [CrossRef]
- Ciciarello, M.; Zini, R.; Rossi, L.; Salvestrini, V.; Ferrari, D.; Manfredini, R.; Lemoli, R.M. Extracellular purines promote the differentiation of human bone-marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev. 2013, 22, 1097–1111. [Google Scholar] [CrossRef]
- Carroll, S.H.; Ravid, K. Differentiation of mesenchymal stem cells to osteoblasts and chondrocytes: A focus on adenosine receptors. Expert Rev. Mol. Med. 2013, 15, e1. [Google Scholar] [CrossRef]
- Lisowska, B.; Kosson, D.; Domaracka, K. Lights and shadows of NSAIDs in bone healing: The role of prostaglandins in bone metabolism. Drug Des. Devel. Ther. 2018, 12, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Riquelme, M.A.; Guda, T.; Tu, C.; Xu, H.; Gu, S.; Jiang, J.X. Connexin hemichannels with prostaglandin release in anabolic function of bone to mechanical loading. Elife 2022, 11, e74365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Z.; Wu, J.W.; Acosta, F.M.; Xu, H.Y.; Jiang, J.X. Connexin 43 hemichannels and prostaglandin E-2 release in anabolic function of the skeletal tissue to mechanical stimulation. Front. Cell Dev. Biol. 2023, 11, 1151838. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, R.; Koide, M.; Udagawa, N.; Kobayashi, Y. Positive and Negative Regulators of Sclerostin Expression. Int. J. Mol. Sci. 2022, 23, 4895. [Google Scholar] [CrossRef]
- Minamizaki, T.; Yoshiko, Y.; Kozai, K.; Aubin, J.E.; Maeda, N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone 2009, 44, 1177–1185. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Matsugaki, A.; Nakano, T. Control of osteoblast arrangement by osteocyte mechanoresponse through prostaglandin E2 signaling under oscillatory fluid flow stimuli. Biomaterials 2021, 279, 121203. [Google Scholar] [CrossRef]
- Blackwell, K.A.; Raisz, L.G.; Pilbeam, C.C. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010, 21, 294–301. [Google Scholar] [CrossRef]
- Ozawa, H.; Imamura, K.; Abe, E.; Takahashi, N.; Hiraide, T.; Shibasaki, Y.; Fukuhara, T.; Suda, T. Effect of a continuously applied compressive pressure on mouse osteoblast-like cells (MC3T3-E1) in vitro. J. Cell. Physiol. 1990, 142, 177–185. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhang, Q.; Zhao, R.; Setterberg, R.B.; Zeng, Q.Q.; Ma, Y.F.; Jee, W.S. Continuous infusion of PGE2 is catabolic with a negative bone balance on both cancellous and cortical bone in rats. J. Musculoskelet. Neuronal Interact. 2007, 7, 372–381. [Google Scholar]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Canalis, E.; Zanotti, S.; Schilling, L.; Eller, T.; Yu, J. Activation of Notch3 in osteoblasts/osteocytes causes compartment-specific changes in bone remodeling. J. Biol. Chem. 2021, 296, 100583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, Y.; He, L.; Geng, B.; Lu, F.; He, J.; Yi, Q.; Liu, X.; Zhang, K.; Wang, L.; et al. Piezo1-mediated fluid shear stress promotes OPG and inhibits RANKL via NOTCH3 in MLO-Y4 osteocytes. Channels 2022, 16, 127–136. [Google Scholar] [CrossRef]
- Tirado-Cabrera, I.; Martin-Guerrero, E.; Heredero-Jimenez, S.; Ardura, J.A.; Gortazar, A.R. PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion. J. Cell. Physiol. 2022, 237, 3927–3943. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Spatz, J.M.; Wein, M.N.; Gooi, J.H.; Qu, Y.; Garr, J.L.; Liu, S.; Barry, K.J.; Uda, Y.; Lai, F.; Dedic, C.; et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. J. Biol. Chem. 2015, 290, 16744–16758. [Google Scholar] [CrossRef]
- Ryder, K.D.; Duncan, R.L. Parathyroid hormone enhances fluid shear-induced [Ca2+](i) signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J. Bone Miner. Res. 2001, 16, 240–248. [Google Scholar] [CrossRef]
- Sun, J.Q.; Liu, X.F.; Tong, J.; Sun, L.J.; Xu, H.; Shi, L.; Zhang, J.B. Fluid shear stress induces calcium transients in osteoblasts through depolarization of osteoblastic membrane. J. Biomech. 2014, 47, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Zhang, C.N.; Bowman, H.H.; Stambough, J.B.; Stronach, B.M.; Mears, S.C.; Barnes, L.C.; Ambrogini, E.; Xiong, J.H. Piezo1 opposes age-associated cortical bone loss. Aging Cell 2023, 22, e13846. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Stavenschi, E.; Labour, M.N.; Hoey, D.A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J. Biomech. 2017, 55, 99–106. [Google Scholar] [CrossRef]
- Jin, J.; Jaspers, R.T.; Wu, G.; Korfage, J.A.M.; Klein-Nulend, J.; Bakker, A.D. Shear Stress Modulates Osteoblast Cell and Nucleus Morphology and Volume. Int. J. Mol. Sci. 2020, 21, 8361. [Google Scholar] [CrossRef] [PubMed]
- Gardinier, J.D.; Majumdar, S.; Duncan, R.L.; Wang, L.Y. Cyclic Hydraulic Pressure and Fluid Flow Differentially Modulate Cytoskeleton Re-Organization in MC3T3 Osteoblasts. Cell. Mol. Bioeng. 2009, 2, 133–143. [Google Scholar] [CrossRef]
- Cao, B.; Dai, X.; Wang, W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca(2+) -calcineurin-NFATc1 pathway. J. Cell. Physiol. 2019, 234, 6831–6841. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Hu, M.; Long, M.; Zhang, D.; Huo, B. Fluid flow-induced calcium response in osteoclasts: Signaling pathways. Ann. Biomed. Eng. 2014, 42, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Bratengeier, C.; Liszka, A.; Hoffman, J.; Bakker, A.D.; Fahlgren, A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. 2020, 34, 3755–3772. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.Y.; Sun, Q.; Huo, B. Gradient fluid shear stress regulates migration of osteoclast precursors. Cell Adhes. Migr. 2019, 13, 183–191. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Q.; Ye, C.Y.; Li, T.Y.; Jiao, F.; Gao, Y.; Huo, B. Finite element analysis on mechanical state on the osteoclasts under gradient fluid shear stress. Biomech. Model. Mechanobiol. 2022, 21, 1067–1078. [Google Scholar] [CrossRef]
- Alford, A.I.; Kozloff, K.M.; Hankenson, K.D. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 2015, 65, 20–31. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Varma, S.; Orgel, J.P.; Schieber, J.D. Nanomechanics of Type I Collagen. Biophys. J. 2016, 111, 50–56. [Google Scholar] [CrossRef]
- McNamara, L.M.; Majeska, R.J.; Weinbaum, S.; Friedrich, V.; Schaffler, M.B. Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. 2009, 292, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, C.; Wang, Q.; Cai, L.; Du, W.; Li, X.; Zhou, X.; Xie, J. Extracellular Matrix Elasticity Regulates Osteocyte Gap Junction Elongation: Involvement of Paxillin in Intracellular Signal Transduction. Cell Physiol. Biochem. 2018, 51, 1013–1026. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef]
- Du, G.; Chen, W.; Li, L.; Zhang, Q. The potential role of mechanosensitive ion channels in substrate stiffness-regulated Ca(2+) response in chondrocytes. Connect. Tissue Res. 2022, 63, 453–462. [Google Scholar] [CrossRef]
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 13–25. [Google Scholar] [CrossRef]
- Pavalko, F.M.; Norvell, S.M.; Burr, D.B.; Turner, C.H.; Duncan, R.L.; Bidwell, J.P. A model for mechanotransduction in bone cells: The load-bearing mechanosomes. J. Cell. Biochem. 2003, 88, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS ONE 2015, 10, e0135519. [Google Scholar] [CrossRef]
- Mullen, C.A.; Haugh, M.G.; Schaffler, M.B.; Majeska, R.J.; McNamara, L.M. Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J. Mech. Behav. Biomed. Mater. 2013, 28, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, J.; Zhou, C.; Lai, W. Substrate stiffness regulates the differentiation profile and functions of osteoclasts via cytoskeletal arrangement. Cell Prolif. 2022, 55, e13172. [Google Scholar] [CrossRef]
- Burra, S.; Nicolella, D.P.; Francis, W.L.; Freitas, C.J.; Mueschke, N.J.; Poole, K.; Jiang, J.X. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc. Natl. Acad. Sci. USA 2010, 107, 13648–13653. [Google Scholar] [CrossRef]
- Burra, S.; Nicolella, D.P.; Jiang, J.X. Dark horse in osteocyte biology: Glycocalyx around the dendrites is critical for osteocyte mechanosensing. Commun. Integr. Biol. 2011, 4, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.L.; Yu, K.; Zhu, J.; Vinson, B.N.; Roberts, R.L.; Montesinos Cartagena, M.; Johnson, M.H.; Wang, L.; Isales, C.M.; Hamrick, M.W.; et al. Decreased pericellular matrix production and selection for enhanced cell membrane repair may impair osteocyte responses to mechanical loading in the aging skeleton. Aging Cell 2020, 19, e13056. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Cohen, I.R.; Grässel, S.; Murdoch, A.D. The biology of perlecan: The multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 1994, 302 Pt 3, 625–639. [Google Scholar] [CrossRef]
- Wang, L. Solute Transport in the Bone Lacunar-Canalicular System (LCS). Curr. Osteoporos. Rep. 2018, 16, 32–41. [Google Scholar] [CrossRef]
- Wang, B.; Lai, X.; Price, C.; Thompson, W.R.; Li, W.; Quabili, T.R.; Tseng, W.J.; Liu, X.S.; Zhang, H.; Pan, J.; et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 2014, 29, 878–891. [Google Scholar] [CrossRef]
- Pei, S.; Parthasarathy, S.; Parajuli, A.; Martinez, J.; Lv, M.; Jiang, S.; Wu, D.; Wei, S.; Lu, X.L.; Farach-Carson, M.C.; et al. Perlecan/Hspg2 deficiency impairs bone’s calcium signaling and associated transcriptome in response to mechanical loading. Bone 2020, 131, 115078. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, T.L.; Zhong, Y.M.; Chen, M.J.; Yao, Q.; Chen, D.; Shao, Z.W.; Xiao, G.Z. Roles of focal adhesion proteins in skeleton and diseases. Acta Pharm. Sin. B 2023, 13, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos. Rep. 2019, 17, 195–206. [Google Scholar] [CrossRef]
- Thi, M.M.; Suadicani, S.O.; Schaffler, M.B.; Weinbaum, S.; Spray, D.C. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require alpha(V)beta(3) integrin. Proc. Natl. Acad. Sci. USA 2013, 110, 21012–21017. [Google Scholar] [CrossRef]
- Batra, N.; Burra, S.; Siller-Jackson, A.J.; Gu, S.M.; Xia, X.C.; Weber, G.F.; DeSimone, D.; Bonewald, L.F.; Lafer, E.M.; Sprague, E.; et al. Mechanical stress-activated integrin alpha 5 beta 1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. USA 2012, 109, 3359–3364. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Gu, S.M.; Hua, R.; Jiang, J.A.X. Mechanotransduction via the coordinated actions of integrins, PI3K signaling and Connexin hemichannels. Bone Res. 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, T.L.; Yang, D.Z.; Wang, Y.S.; Li, Z.J.; Yan, Q.N.; Zhang, P.J.; Chen, Z.C.; Lin, S.X.; Gao, H.Q.; et al. Osteocyte beta 1 integrin loss causes low bone mass and impairs bone mechanotransduction in mice. J. Orthop. Transl. 2022, 34, 60–72. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Z.C.; Yang, D.Z.; He, T.L.; Xu, Z.; Zhang, P.J.; Chen, D.; Yi, W.H.; Xiao, G.Z. Osteocyte β3 integrin promotes bone mass accrual and force-induced bone formation in mice*. J. Orthop. Transl. 2023, 40, 58–71. [Google Scholar] [CrossRef]
- Qian, A.; Hu, L.; Di, S.; Gao, X.; Meng, R.; Shang, P. Roles of osteocytes in mechanosensation. Chin. J. Aerosp. Med. 2010, 21, 149–154. [Google Scholar]
- Zappala, A.; Romano, I.R.; D’Angeli, F.; Musumeci, G.; Lo Furno, D.; Giuffrida, R.; Mannino, G. Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components. Int. J. Mol. Sci. 2023, 24, 4156. [Google Scholar] [CrossRef]
- Lecanda, F.; Warlow, P.M.; Sheikh, S.; Furlan, F.; Steinberg, T.H.; Civitelli, R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000, 151, 931–944. [Google Scholar] [CrossRef]
- Davis, H.M.; Aref, M.W.; Aguilar-Perez, A.; Pacheco-Costa, R.; Allen, K.; Valdez, S.; Herrera, C.; Atkinson, E.G.; Mohammad, A.; Lopez, D.; et al. Cx43 overexpression in osteocytes prevents osteocyte apoptosis and preserves cortical bone quality in aging mice. JBMR Plus 2018, 2, 206–216. [Google Scholar] [CrossRef]
- Joiner, D.M.; Tayim, R.J.; McElderry, J.D.; Morris, M.D.; Goldstein, S.A. Aged Male Rats Regenerate Cortical Bone with Reduced Osteocyte Density and Reduced Secretion of Nitric Oxide After Mechanical Stimulation. Calcif. Tissue Int. 2014, 94, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Riquelme, M.A.; Hua, R.; Acosta, F.M.; Gu, S.; Jiang, J.X. Connexin 43 hemichannels regulate mitochondrial ATP generation, mobilization, and mitochondrial homeostasis against oxidative stress. Elife 2022, 11, e82206. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Burra, S.; Kar, R.; Lampe, P.D.; Jiang, J.X. Mitogen-activated Protein Kinase (MAPK) Activated by Prostaglandin E2 Phosphorylates Connexin 43 and Closes Osteocytic Hemichannels in Response to Continuous Flow Shear Stress. J. Biol. Chem. 2015, 290, 28321–28328. [Google Scholar] [CrossRef]
- Batra, N.; Riquelme, M.A.; Burra, S.; Jiang, J.X. 14-3-3 theta facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J. Cell Sci. 2014, 127, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Savadipour, A.; Palmer, D.; Ely, E.V.; Collins, K.H.; Garcia-Castorena, J.M.; Harissa, Z.; Kim, Y.S.; Oestrich, A.; Qu, F.N.; Rashidi, N.; et al. The role of PIEZO ion channels in the musculoskeletal system. Am. J. Physiol.-Cell Physiol. 2023, 324, C728–C740. [Google Scholar] [CrossRef]
- Liu, N.; Lu, W.; Dai, X.; Qu, X.; Zhu, C. The role of TRPV channels in osteoporosis. Mol. Biol. Rep. 2022, 49, 577–585. [Google Scholar] [CrossRef]
- Qin, L.; He, T.L.; Chen, S.; Yang, D.Z.; Yi, W.H.; Cao, H.L.; Xiao, G.Z. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Liu, Y.A.; Huang, C.J.; Yen, M.H.; Tseng, C.T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015, 5, 16522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.D.; Li, X.F.; Wu, L.; Qin, Y.X. Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. 2021, 9, 16. [Google Scholar] [CrossRef]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef]
- Knecht, R.S.; Bucher, C.H.; Van Linthout, S.; Tschope, C.; Schmidt-Bleek, K.; Duda, G.N. Mechanobiological Principles Influence the Immune Response in Regeneration: Implications for Bone Healing. Front. Bioeng. Biotechnol. 2021, 9, 614508. [Google Scholar] [CrossRef]
- Zeng, Y.; Riquelme, M.A.; Hua, R.; Zhang, J.R.; Acosta, F.M.; Gu, S.M.; Jiang, J.X. Mechanosensitive piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J.H. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. TRPV4 antagonists: A patent review (2015–2020). Expert. Opin. Ther. Pat. 2021, 31, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.S.; Monsen, J.; Ahmed, S.; Huang, Y.M.; Hoey, D.A.; Nowlan, N.C. Mechanoregulatory role of TRPV4 in prenatal skeletal development. Sci. Adv. 2023, 9, eade2155. [Google Scholar] [CrossRef] [PubMed]
- Comellas, E.; Farkas, J.E.; Kleinberg, G.; Lloyd, K.; Mueller, T.; Duerr, T.J.; Munoz, J.J.; Monaghan, J.R.; Shefelbine, S.J. Local mechanical stimuli correlate with tissue growth in axolotl salamander joint morphogenesis. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220621. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Kang, S.S.; Shin, S.H.; Auh, C.K.; Chun, J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012, 44, 707–722. [Google Scholar] [CrossRef]
- Hu, K.; Sun, H.; Gui, B.; Sui, C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2017, 91, 841–848. [Google Scholar] [CrossRef]
- Williams, K.M.; Leser, J.M.; Gould, N.R.; Joca, H.C.; Lyons, J.S.; Khairallah, R.J.; Ward, C.W.; Stains, J.P. TRPV4 calcium influx controls sclerostin protein loss independent of purinergic calcium oscillations. Bone 2020, 136, 115356. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960. [Google Scholar] [CrossRef]
- Nishimura, H.; Kawasaki, M.; Tsukamoto, M.; Menuki, K.; Suzuki, H.; Matsuura, T.; Baba, K.; Motojima, Y.; Fujitani, T.; Ohnishi, H.; et al. Transient receptor potential vanilloid 1 and 4 double knockout leads to increased bone mass in mice. Bone Rep. 2020, 12, 100268. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, F.; Mizuno, A.; Hayata, T.; Nakashima, K.; Heller, S.; Ushida, T.; Sokabe, M.; Miyasaka, N.; Suzuki, M.; Ezura, Y.; et al. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J. Cell. Physiol. 2008, 216, 47–53. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Sittichokechaiwut, A.; Reilly, G.C. Osteocytes and Primary Cilia. Curr. Osteoporos. Rep. 2023, 21, 719–730. [Google Scholar] [CrossRef]
- Ding, D.; Yang, X.; Luan, H.Q.; Wu, X.T.; Sun, L.W.; Fan, Y.B. The microgravity induces the ciliary shortening and an increased ratio of anterograde/retrograde intraflagellar transport of osteocytes. Biochem. Biophys. Res. Commun. 2020, 530, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.A.; Murphy, P. The Primary Cilium on Cells of Developing Skeletal Rudiments; Distribution, Characteristics and Response to Mechanical Stimulation. Front. Cell Dev. Biol. 2021, 9, 725018. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhu, D.; Yang, T.Y.; Cheng, L.; Sun, J.; Tan, L. Crosstalk between the COX2-PGE2-EP4 signaling pathway and primary cilia in osteoblasts after mechanical stimulation. J. Cell. Physiol. 2021, 236, 4764–4777. [Google Scholar] [CrossRef]
- Moore, E.R.; Zhu, Y.X.; Ryu, H.S.; Jacobs, C.R. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res. Ther. 2018, 9, 229. [Google Scholar] [CrossRef]
- Sutton, M.M.; Duffy, M.P.; Verbruggen, S.W.; Jacobs, C.R. Osteoclastogenesis requires primary cilia disassembly and can be inhibited by promoting primary cilia formation pharmacologically. Cells Tissues Organs 2023, 213, 235–244. [Google Scholar] [CrossRef]
- Lacey, S.E.; Pigino, G. The intraflagellar transport cycle. Nat. Rev. Mol. Cell Biol. 2024, 26, 175–192. [Google Scholar] [CrossRef]
- Klena, N.; Pigino, G. Structural Biology of Cilia and Intraflagellar Transport. Annu. Rev. Cell Dev. Biol. 2022, 38, 103–123. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.X.; Dong, H.; Yu, J.H.; Yan, Y.; Wu, X.G.; Wang, Y.Q.; Li, P.C.; Wei, X.C.; Chen, W.Y. Mechanosensation of osteocyte with collagen hillocks and primary cilia under pressure and electric field stimulation. Acta Mech. Sin. 2022, 38, 621569. [Google Scholar] [CrossRef]
- Johnson, G.P.; Fair, S.; Hoey, D.A. Primary cilium-mediated MSC mechanotransduction is dependent on Gpr161 regulation of hedgehog signalling. Bone 2021, 145, 115846. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Tian, R.; Yang, X.; Ren, Z.; Jing, Z.C.; Wu, X.T.; Sun, L.W. The impact of ciliary length on the mechanical response of osteocytes to fluid shear stress. Nitric Oxide 2024, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, Y.H.; Zhu, B.Y.; Shao, J.L.; Ma, H.P.; Xian, C.J.; Chen, K.M. Sinusoidal Electromagnetic Fields Increase Peak Bone Mass in Rats by Activating Wnt10b/β-Catenin in Primary Cilia of Osteoblasts. J. Bone Miner. Res. 2019, 34, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.S.; Kumar, V.; Das, S.L. Classification of Osteoporosis. Indian J. Orthop. 2023, 57, 49–54. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary Osteoporosis. Endocr. Rev. 2022, 43, 240–313. [Google Scholar] [CrossRef]
- Khinda, R.; Valecha, S.; Kumar, N.; Walia, J.P.S.; Singh, K.; Sethi, S.; Singh, A.; Singh, M.; Singh, P.; Mastana, S. Prevalence and Predictors of Osteoporosis and Osteopenia in Postmenopausal Women of Punjab, India. Int. J. Environ. Res. Public Health 2022, 19, 2999. [Google Scholar] [CrossRef]
- Meng, S.; Tong, M.; Yu, Y.; Cao, Y.; Tang, B.; Shi, X.; Liu, K. The prevalence of osteoporotic fractures in the elderly in China: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 536. [Google Scholar] [CrossRef]
- Liu, X.; Chen, F.; Liu, L.; Zhang, Q. Prevalence of osteoporosis in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 2023, 23, 1. [Google Scholar] [CrossRef]
- Si, Y.; Wang, C.; Guo, Y.; Xu, G.; Ma, Y. Prevalence of Osteoporosis in Patients with Type 2 Diabetes Mellitus in the Chinese Mainland: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2019, 48, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Ejlsmark-Svensson, H.; Bislev, L.S.; Lajlev, S.; Harsløf, T.; Rolighed, L.; Sikjaer, T.; Rejnmark, L. Prevalence and Risk of Vertebral Fractures in Primary Hyperparathyroidism: A Nested Case-Control Study. J. Bone Miner. Res. 2018, 33, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Bahar, T.; Rahman, S.; Gomes, L.C.; Hossain, M.M.; Chowdhury, Z.Z.; Hossain, A. Frequency of Osteoporosis in Iatrogenic Cushing’s syndrome: Scenario of outpatient department in urban hospitals. Bangladesh J. Med. 2020, 31, 18–21. [Google Scholar] [CrossRef]
- Barbosa, A.P.; Mascarenhas, M.R.; Bicho, M.; Janeiro, J.; Oliveira, A.G. The main autoimmune and nonautoimmune etiologies of endogenous hyperthyroidism do not seem to influence the increased prevalence of morphometric vertebral fractures and osteoporosis in Portuguese men. Osteoporos. Sarcopenia 2017, 3, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Lee, J.A.; Kang, S.Y.; Kim, Y.S.; Sunwoo, S.; Kim, B.S.; Yook, J.H. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer 2018, 21, 720–727. [Google Scholar] [CrossRef]
- Tárraga López, P.J.; López, C.F.; de Mora, F.N.; Montes, J.A.; Albero, J.S.; Mañez, A.N.; Casas, A.G. Osteoporosis in patients with subclinical hypothyroidism treated with thyroid hormone. Clin. Cases Miner. Bone Metab. 2011, 8, 44–48. [Google Scholar]
- Najar, M.S.; Mir, M.M.; Muzamil, M. Prevalence of osteoporosis in patients with chronic kidney disease (stages 3–5) in comparison with age- and sex-matched controls: A study from Kashmir Valley Tertiary Care Center. Saudi. J. Kidney Dis. Transpl. 2017, 28, 538–544. [Google Scholar] [CrossRef]
- Aggarwal, H.K.; Jain, D.; Yadav, S.; Kaverappa, V. Bone mineral density in patients with predialysis chronic kidney disease. Ren. Fail. 2013, 35, 1105–1111. [Google Scholar] [CrossRef]
- Fidan, N.; Inci, A.; Coban, M.; Ulman, C.; Kursat, S. Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. J. Investig. Med. 2016, 64, 861–866. [Google Scholar] [CrossRef]
- Ganji, R.; Moghbeli, M.; Sadeghi, R.; Bayat, G.; Ganji, A. Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: A systematic review. Nutr. J. 2019, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Smyth, N.D.; Murphy, A.; Macnaughton, D.; O’Keefe, S.J.; Conlon, K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, Y.; Bi, Y.F.; Wang, X.B. Prevalence and risk factors of osteoporosis detected by dual-energy X-ray absorptiometry among Chinese patients with primary biliary cholangitis. World J. Gastroenterol. 2023, 29, 4580–4592. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, R.; Takahashi, T.; Saito, Y.; Nakatsuka, R.; Imamura, H.; Motoori, M.; Makari, Y.; Takeno, A.; Kishi, K.; Adachi, S.; et al. Analysis of the risk factors for osteoporosis and its prevalence after gastrectomy for gastric cancer in older patients: A prospective study. Surg. Today 2023, 53, 435–442. [Google Scholar] [CrossRef]
- Lo, B.; Holm, J.P.; Vester-Andersen, M.K.; Bendtsen, F.; Vind, I.; Burisch, J. Incidence, Risk Factors and Evaluation of Osteoporosis in Patients With Inflammatory Bowel Disease: A Danish Population-Based Inception Cohort With 10 Years of Follow-Up. J. Crohn’s Colitis 2020, 14, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Feng, Y.; Zeng, Y. Iron Deficiency and Iron Deficiency Anemia: Potential Risk Factors in Bone Loss. Int. J. Mol. Sci. 2023, 24, 6891. [Google Scholar] [CrossRef]
- Anagnostis, P.; Vakalopoulou, S.; Slavakis, A.; Charizopoulou, M.; Kazantzidou, E.; Chrysopoulou, T.; Vyzantiadis, T.A.; Moka, E.; Agapidou, A.; Garipidou, V. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb. Haemost. 2012, 107, 545–551. [Google Scholar] [CrossRef]
- Sakalová, A.; Herrmann, Z.; Gazová, S.; Chabronová, I.; Dedík, L.; Mistrík, M.; Hrubisko, M. Osteoporosis in multiple myeloma. Vnitr. Lek. 1998, 44, 649–653. [Google Scholar]
- Turgutkaya, A.; Yavaşoğlu, İ.; Şahin, T.; Bolaman, A.Z. Investigation of the qualification of radiological techniques to detect osteolytic lesions, fractures, and osteoporosis in multiple myeloma patients. Hematol. Transfus. Cell Ther. 2021, 43, S17–S18. [Google Scholar] [CrossRef]
- Vaucher, M.; Gonzalez Rodriguez, E.; Efthymiou, A.; Sagez, J. Systemic mastocytosis and bone impact. Rev. Med. Suisse. 2023, 19, 591–595. [Google Scholar] [CrossRef]
- van der Veer, E.; van der Goot, W.; de Monchy, J.G.; Kluin-Nelemans, H.C.; van Doormaal, J.J. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy 2012, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Eischen, M.; Nigon, D.; Apoil, P.A.; Mailhol, C.; Tournier, E.; Laurent, C.; Hanssens, K.; Hermine, O.; Paul, C.; et al. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone 2017, 105, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, I.; Paruk, F.; Tabra, S.; Maatallah, K.; Bouziane, A.; Abouqal, R.; El Maidany, Y.; El Maghraoui, A.; Kalla, A.A. Prevalence of glucocorticoid-induced osteoporosis among rheumatology patients in Africa: A systematic review and meta-analysis. Arch. Osteoporos. 2023, 18, 59. [Google Scholar] [CrossRef]
- Rajha, H.E.; Abdelaal, R.; Charfi, K.; Alemadi, A.O.; Al-Sheraim, A.S.; Al-Maadid, M.A.; Louati, Y.; Doi, S.; Khaled, S.M. Examining depression, antidepressants use, and class and their potential associations with osteoporosis and fractures in adult women: Results from ten NHANES cohorts. J. Affect. Disord. 2025, 369, 1223–1232. [Google Scholar] [CrossRef]
- Mahitthiharn, K.; Kovindha, A.; Kaewchur, T.; Morse, L.R.; Pattanakuhar, S. Prevalence and influencing factors of spinal cord injury-related osteoporosis and fragility fractures in Thai people with chronic spinal cord injury: A cross-sectional, observational study. J. Spinal Cord Med. 2023, 46, 458–465. [Google Scholar] [CrossRef]
- Pelletier, C.A.; Dumont, F.S.; Leblond, J.; Noreau, L.; Giangregorio, L.; Craven, B.C. Self-report of one-year fracture incidence and osteoporosis prevalence in a community cohort of canadians with spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2014, 20, 302–309. [Google Scholar] [CrossRef]
- Ward, L.M.; Hadjiyannakis, S.; McMillan, H.J.; Noritz, G.; Weber, D.R. Bone Health and Osteoporosis Management of the Patient With Duchenne Muscular Dystrophy. Pediatrics 2018, 142, S34–S42. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Schneider, V.; Shackelford, L.; West, S.; Oganov, V.; Bakulin, A.; Voronin, L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal Interact. 2000, 1, 157–160. [Google Scholar]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H.; Stewart, C.A.; Ashford, R.; Vigil, D. Regional osteoporosis in women who have a complete spinal cord injury. J. Bone Jt. Surg. Am. 2001, 83, 1195–1200. [Google Scholar] [CrossRef]
- Leblanc, A.D.; Schneider, V.S.; Evans, H.J.; Engelbretson, D.A.; Krebs, J.M. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 1990, 5, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, C.; Vico, L. Pathophysiology of bone loss in disuse osteoporosis. Jt. Bone Spine 2011, 78, 572–576. [Google Scholar] [CrossRef]
- Xu, H.J.; Wu, F.; Zhang, H.Y.; Yang, C.; Li, K.; Wang, H.L.; Yang, H.H.; Liu, Y.; Ding, B.; Tan, Y.J.; et al. Actin cytoskeleton mediates BMP2-Smad signaling via calponin 1 in preosteoblast under simulated microgravity. Biochimie 2017, 138, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Li, S.; Wu, X.T.; Yang, X.; Sun, L.W. Contribution of endoplasmic reticulum stress response to the mechanosensitivity alteration in osteocytes under simulated microgravity. Acta Astronaut. 2022, 191, 522–527. [Google Scholar] [CrossRef]
- McCutcheon, S.; Majeska, R.J.; Spray, D.C.; Schaffler, M.B.; Vazquez, M. Apoptotic Osteocytes Induce RANKL Production in Bystanders via Purinergic Signaling and Activation of Pannexin Channels. J. Bone Miner. Res. 2020, 35, 966–977. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; Block, D.; Toma, C.D.; Gerstenfeld, L.C. Spaceflight effects on cultured embryonic chick bone cells. J. Bone Miner. Res. 2000, 15, 1099–1112. [Google Scholar] [CrossRef]
- Zhivodernikov, I.; Ratushnyy, A.; Buravkova, L. Simulated Microgravity Remodels Extracellular Matrix of Osteocommitted Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2021, 22, 5428. [Google Scholar] [CrossRef]
- Buravkova, L.; Larina, I.; Andreeva, E.; Grigoriev, A. Microgravity Effects on the Matrisome. Cells 2021, 10, 2226. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef]
- Andreeva, E.; Matveeva, D.; Zhidkova, O.; Zhivodernikov, I.; Kotov, O.; Buravkova, L. Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life 2022, 12, 1343. [Google Scholar] [CrossRef]
- Wu, X.T.; Yang, X.; Tian, R.; Li, Y.H.; Wang, C.Y.; Fan, Y.B.; Sun, L.W. Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 2022, 36, e22114. [Google Scholar] [CrossRef]
- Deng, A.F.; Wang, F.X.; Wang, S.C.; Zhang, Y.Z.; Bai, L.; Su, J.C. Bone-organ axes: Bidirectional crosstalk. Mil. Med. Res. 2024, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, J.; Chen, X.; Tong, X.; Xu, J.; Zou, J. The Role of Irisin in Exercise-Mediated Bone Health. Front. Cell Dev. Biol. 2021, 9, 668759. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar]
- Xu, Q.; Cui, Y.; Luan, J.; Zhou, X.; Li, H.; Han, J. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem. Biophys. Res. Commun. 2018, 498, 32–37. [Google Scholar] [CrossRef]
- Covington, J.D.; Tam, C.S.; Bajpeyi, S.; Galgani, J.E.; Noland, R.C.; Smith, S.R.; Redman, L.M.; Ravussin, E. Myokine Expression in Muscle and Myotubes in Response to Exercise Stimulation. Med. Sci. Sports Exerc. 2016, 48, 384–390. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Lara-Castillo, N. Estrogen Signaling in Bone. Appl. Sci. 2021, 11, 4439. [Google Scholar] [CrossRef]
- Voisin, M.; McNamara, L.M. Differential beta(3) and beta(1) Integrin Expression in Bone Marrow and Cortical Bone of Estrogen Deficient Rats. Anat. Rec.-Adv. Integr. Anat. Evol. Biol. 2015, 298, 1548–1559. [Google Scholar] [CrossRef]
- Lewis, K.J.; Cabahug-Zuckerman, P.; Boorman-Padgett, J.F.; Basta-Pljakic, J.; Louie, J.; Stephen, S.; Spray, D.C.; Thi, M.M.; Seref-Ferlengez, Z.; Majeska, R.J.; et al. Estrogen depletion on In vivo osteocyte calcium signaling responses to mechanical loading. Bone 2021, 152, 116072. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Estrogen deficiency impairs integrin alpha(v)beta(3)-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci. Rep. 2019, 9, 4654. [Google Scholar] [CrossRef]
- Vinson, B.; Xu, J.; Ding, K.; Elsalanty, M.; Isales, C.M.; McGee-Lawrence, M.E. Estrogen deficiency from ovariectomy enhances the formation of osteocyte plasma membrane disruptions from treadmill exercise in vivo. FASEB J. 2019, 33, 326-3. [Google Scholar] [CrossRef]
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, Calcium, Parathyroid Hormone, and Sex Steroids in Bone Health and Effects of Aging. J. Osteoporos. 2020, 2020, 9324505. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Lai, S.; Huang, Z.; Cai, G.; Zhao, K.; Gao, J.; Wu, Z.; Zhong, Z. Accumulation of advanced oxidation protein products contributes to age-related impairment of gap junction intercellular communication in osteocytes of male mice. Bone Jt. Res. 2022, 11, 413–425. [Google Scholar] [CrossRef]
- Vilaca, T.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone 2020, 137, 115457. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torres, M.; Jódar, E.; Escobar-Jiménez, F.; López-Ibarra, P.J.; Luna, J.D. Bone mineral density measured by dual X-ray absorptiometry in Spanish patients with insulin-dependent diabetes mellitus. Calcif. Tissue Int. 1996, 58, 316–319. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012, 27, 319–332. [Google Scholar] [CrossRef]
- Rubin, M.R.; Patsch, J.M. Assessment of bone turnover and bone quality in type 2 diabetic bone disease: Current concepts and future directions. Bone Res. 2016, 4, 16001. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.-J.; Wang, Z.-Z.; Ding, D.; Shi, J.-W.; Wu, X.-T.; Sun, L.-W.; Fan, Y.-B. Effects of advanced glycation end products on osteocytes mechanosensitivity. Biochem. Biophys. Res. Commun. 2021, 568, 151–157. [Google Scholar] [CrossRef]
- Cavati, G.; Pirrotta, F.; Merlotti, D.; Ceccarelli, E.; Calabrese, M.; Gennari, L.; Mingiano, C. Role of Advanced Glycation End-Products and Oxidative Stress in Type-2-Diabetes-Induced Bone Fragility and Implications on Fracture Risk Stratification. Antioxidants 2023, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Maung, S.; Wang, X.; Basta-Pljakic, J.; Schaffler, M.; Spray, D.; Suadicani, S.; Thi, M. Elevation of glucose to levels associated with type I diabetes impairs bone cell mechanosignaling (1180.10). FASEB J. 2014, 28, 1180-10. [Google Scholar] [CrossRef]
- Seref-Ferlengez, Z.; Maung, S.; Schaffler, M.B.; Spray, D.C.; Suadicani, S.O.; Thi, M.M. P2X7R-Panx1 Complex Impairs Bone Mechanosignaling under High Glucose Levels Associated with Type-1 Diabetes. PLoS ONE 2016, 11, e0155107. [Google Scholar] [CrossRef]
- Ejlsmark-Svensson, H.; Rolighed, L.; Harsløf, T.; Rejnmark, L. Risk of fractures in primary hyperparathyroidism: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.R.; Williams, K.M.; Joca, H.C.; Torre, O.M.; Lyons, J.S.; Leser, J.M.; Srikanth, M.P.; Hughes, M.; Khairallah, R.J.; Feldman, R.A.; et al. Disparate bone anabolic cues activate bone formation by regulating the rapid lysosomal degradation of sclerostin protein. Elife 2021, 10, e64393. [Google Scholar] [CrossRef]
- Sebastian, A.; Loots, G.G. Transcriptional control of Sost in bone. Bone 2017, 96, 76–84. [Google Scholar] [CrossRef]
- Kramer, I.; Keller, H.; Leupin, O.; Kneissel, M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol. Metab. 2010, 21, 237–244. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Tyagi, A.M.; Vaccaro, C.; Hsu, E.; Adams, J.; Bellido, T.; Weitzmann, M.N.; Pacifici, R. IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. J. Bone Miner. Res. 2019, 34, 349–360. [Google Scholar] [CrossRef]
- Qing, H.; Ardeshirpour, L.; Pajevic, P.D.; Dusevich, V.; Jähn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar] [CrossRef]
- Ma, C.C.; Xu, S.Q.; Gong, X.; Wu, Y.; Qi, S.; Liu, W.; Xu, J.H. Prevalence and risk factors associated with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Arch. Osteoporos. 2017, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Etani, Y.; Noguchi, T.; Miura, T.; Kurihara, T.; Fukuda, Y.; Hamada, H.; Uemura, K.; Takashima, K.; Tamaki, M. The pivotal role of the Hes1/Piezo1 pathway in the pathophysiology of glucocorticoid-induced osteoporosis. JCI Insight 2024, 9, e179963. [Google Scholar] [CrossRef]
- dos Santos, C.V.; Vieira Neto, L.; Madeira, M.; Alves Coelho, M.C.; de Mendonça, L.M.; Paranhos-Neto Fde, P.; Lima, I.C.; Gadelha, M.R.; Farias, M.L. Bone density and microarchitecture in endogenous hypercortisolism. Clin. Endocrinol. 2015, 83, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Sun, J.; Han, X.; Liu, H.; Li, J.; Du, J.; Feng, W.; Liu, B.; Cui, J.; Guo, J.; et al. Immunolocalization of MMP 2, 9 and 13 in prednisolone induced osteoporosis in mice. Histol. Histopathol. 2016, 31, 647–656. [Google Scholar] [CrossRef]
- Umrath, F.; Pfeifer, A.; Cen, W.; Danalache, M.; Reinert, S.; Alexander, D.; Naros, A. How osteogenic is dexamethasone?-effect of the corticosteroid on the osteogenesis, extracellular matrix, and secretion of osteoclastogenic factors of jaw periosteum-derived mesenchymal stem/stromal cells. Front. Cell Dev. Biol. 2022, 10, 953516. [Google Scholar] [CrossRef]
- Strokotova, A.V.; Grigorieva, E.V. Glucocorticoid Effects on Proteoglycans and Glycosaminoglycans. Int. J. Mol. Sci. 2022, 23, 15678. [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2021, 9, 770143. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, L.; Wang, X.; Zheng, Q.; Zhan, F.; Zhou, L.; Dong, Y.; Xiong, Y.; Yi, P.; Xu, G.; et al. Dexamethasone upregulates macrophage PIEZO1 via SGK1, suppressing inflammation and increasing ROS and apoptosis. Biochem. Pharmacol. 2024, 222, 116050. [Google Scholar] [CrossRef]
- Ding, D.; Yang, X.; Luan, H.Q.; Wu, X.T.; He, C.; Sun, L.W.; Fan, Y.B. Pharmacological Regulation of Primary Cilium Formation Affects the Mechanosensitivity of Osteocytes. Calcif. Tissue Int. 2020, 107, 625–635. [Google Scholar] [CrossRef]
- Spasic, M.; Duffy, M.P.; Jacobs, C.R. Fenoldopam Sensitizes Primary Cilia-Mediated Mechanosensing to Promote Osteogenic Intercellular Signaling and Whole Bone Adaptation. J. Bone Miner. Res. 2022, 37, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Spasic, M.; Jacobs, C.R. Lengthening primary cilia enhances cellular mechanosensitivity. Eur. Cells Mater. 2017, 33, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, C.; López-Rivera, E.; García-Rama, C.; Saura, M.; Martínez-Ruíz, A.; Lizarbe, T.R.; Martín-de-Lara, F.; Lamas, S. Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J. Cell Sci. 2006, 119, 1896–1902. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Ferradaes, T.M.; Riffault, M.; Hoey, D.A. Ciliotherapy Treatments to Enhance Biochemically- and Biophysically-Induced Mesenchymal Stem Cell Osteogenesis: A Comparison Study. Cell. Mol. Bioeng. 2019, 12, 53–67. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, H.; Chen, W.; Liu, Y.; Cao, Y.; Pei, H.; Ming, C.; Shan, C.; Chen, X.; Dai, Z.; et al. The Critical Role of The Piezo1/β-catenin/ATF4 Axis on The Stemness of Gli1(+) BMSCs During Simulated Microgravity-Induced Bone Loss. Adv. Sci. 2023, 10, e2303375. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Wang, X.; Acosta, F.M.; Zhang, J.; Chavez, J.; Gu, S.; Zhao, P.; Xiong, W.; Zhang, N.; Li, G.; et al. Antibody-activation of connexin hemichannels in bone osteocytes with ATP release suppresses breast cancer and osteosarcoma malignancy. Cell Rep. 2024, 43, 114377. [Google Scholar] [CrossRef]
- Zhao, D.; Tu, C.; Zhang, L.; Guda, T.; Gu, S.; Jiang, J.X. Activation of connexin hemichannels enhances mechanosensitivity and anabolism in disused and aged bone. JCI Insight 2024, 9, e177557. [Google Scholar] [CrossRef]


| Types of Osteoporosis | Prevalence Rates |
|---|---|
| Primary Osteoporosis | |
| Type I (postmenopausal osteoporosis) | 29.13–30.50% [25,131] |
| Type II (senile osteoporosis) | 18.9% [132] |
| Secondary Osteoporosis | |
| Endocrine disorders | |
| 27.67–37.8% [133,134] 29% [135] 17.69% [136] 29.3–37.5% [137,138] 9.4% [139] |
| Renal disorders | |
| 21.3–31.8% [140,141,142] |
| Gastrointestinal Causes | |
| 14.4% [143] 23.4% [144] 45.5% [145] 38.2% [146] 14.2% [147] |
| Hemato-Oncological Causes | |
| 2.27% [148] 58.7% [149] 24.5–83% [150,151] 18–40% [152,153,154] |
| Drug-Induced | |
| 47.7% [155] |
| 23.5% [156] |
| Disuse | |
| 21.5–43.8% [157,158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Danalache, M.; Liang, C.; Alexander, D.; Umrath, F. Mechanosignaling in Osteoporosis: When Cells Feel the Force. Int. J. Mol. Sci. 2025, 26, 4007. https://doi.org/10.3390/ijms26094007
Chen N, Danalache M, Liang C, Alexander D, Umrath F. Mechanosignaling in Osteoporosis: When Cells Feel the Force. International Journal of Molecular Sciences. 2025; 26(9):4007. https://doi.org/10.3390/ijms26094007
Chicago/Turabian StyleChen, Nuo, Marina Danalache, Chen Liang, Dorothea Alexander, and Felix Umrath. 2025. "Mechanosignaling in Osteoporosis: When Cells Feel the Force" International Journal of Molecular Sciences 26, no. 9: 4007. https://doi.org/10.3390/ijms26094007
APA StyleChen, N., Danalache, M., Liang, C., Alexander, D., & Umrath, F. (2025). Mechanosignaling in Osteoporosis: When Cells Feel the Force. International Journal of Molecular Sciences, 26(9), 4007. https://doi.org/10.3390/ijms26094007






