Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds
Abstract
1. Introduction
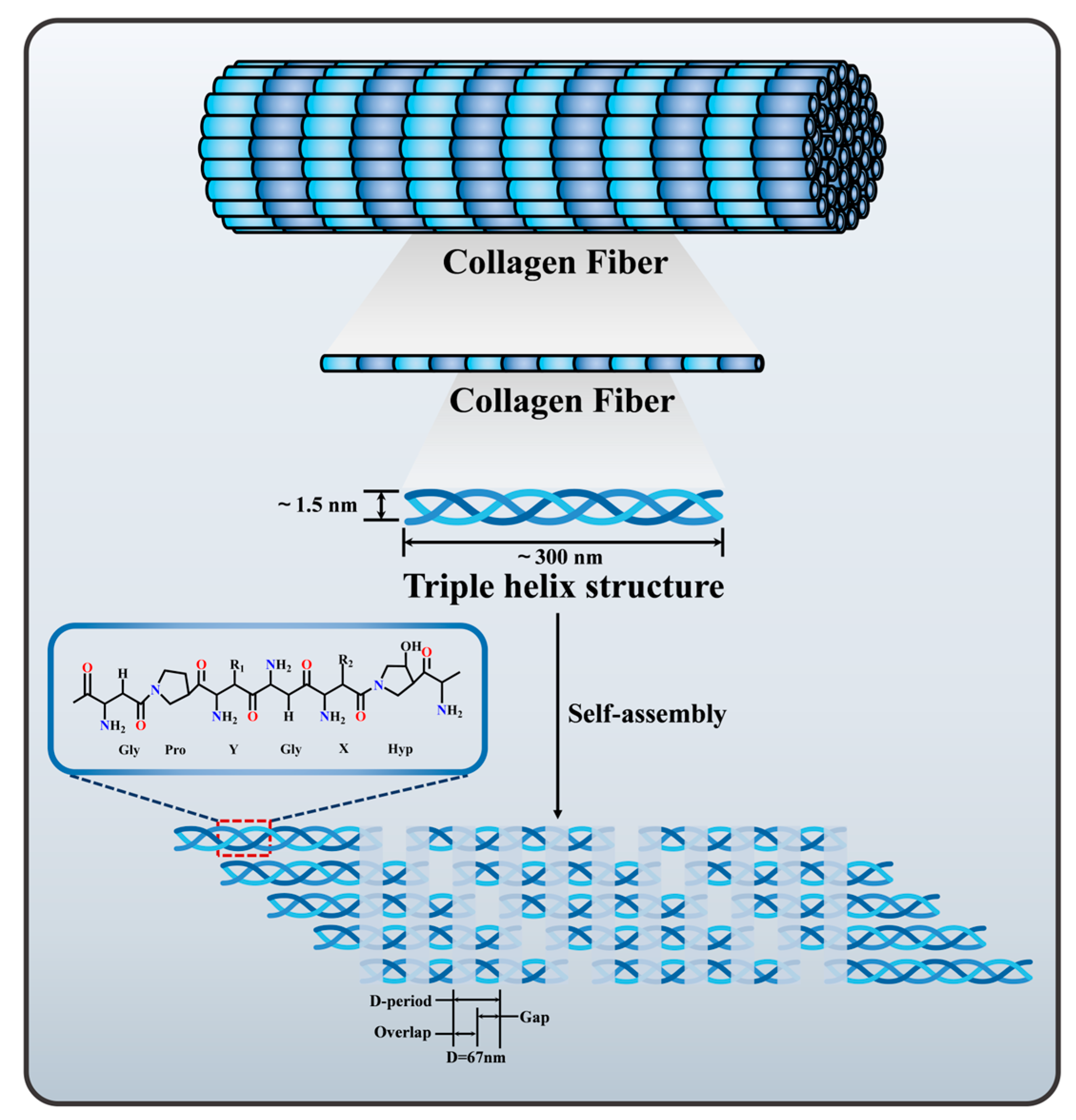
2. Collagen Sources, Structure and Properties
3. Effects of Collagen on Cellular Behavior
4. Collagen Scaffold Preparation
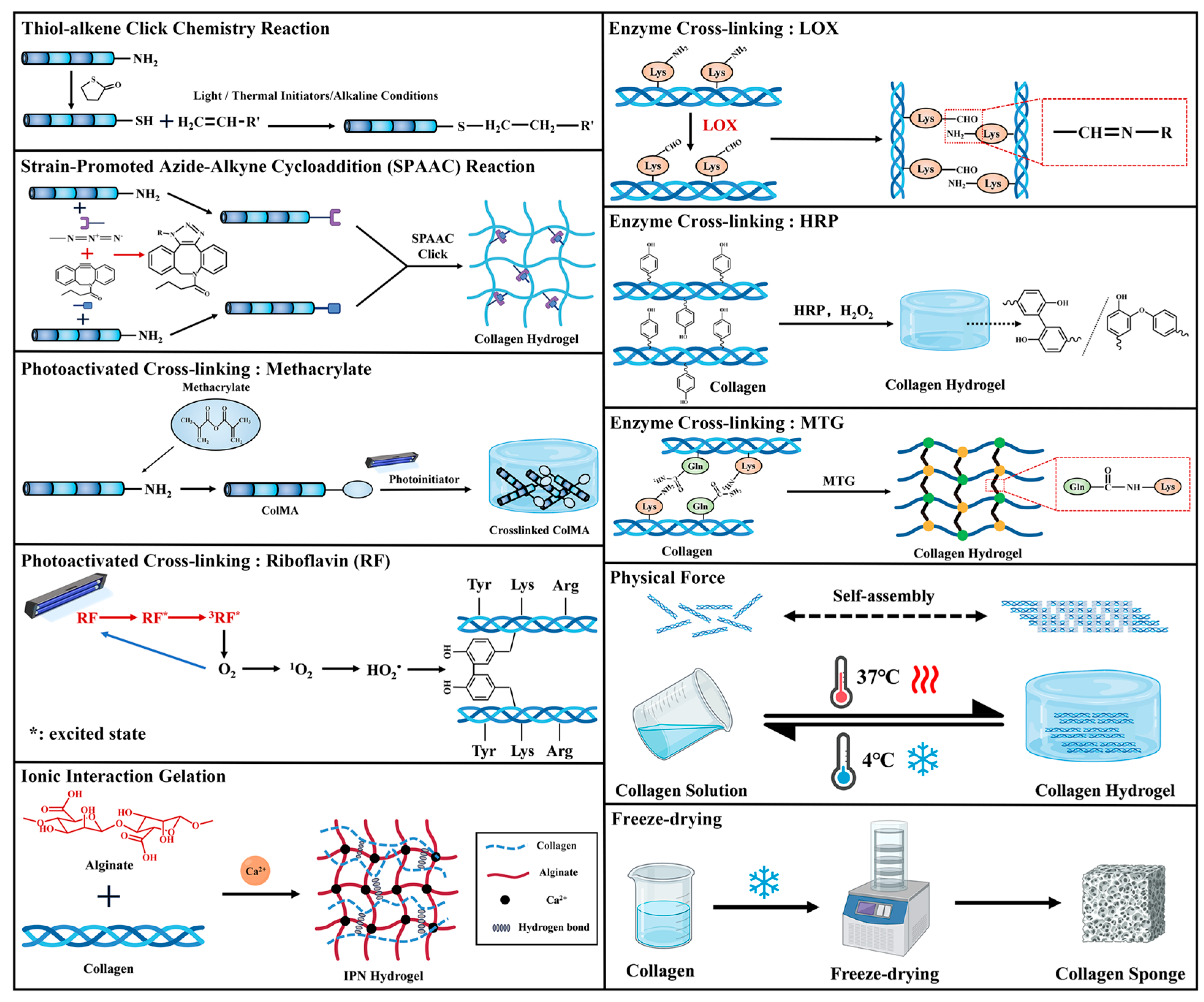
4.1. Chemical Cross-Linking
4.1.1. Click Chemistry
4.1.2. Photoactivated Cross-Linking
4.1.3. Enzyme Cross-Linking
4.2. Physical Force
4.3. Ionic Interaction Gelation
4.4. Freeze-Drying
5. Cell-Loaded Collagen Scaffolds for Tissue Engineering Applications
5.1. Skin Tissue Engineering
5.2. Neural Tissue Engineering
5.3. Bone/Cartilage Tissue Engineering
5.4. Cardiac Tissue Engineering
5.5. Liver Tissue Engineering
5.6. Other Tissue Engineering
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| MMPs | Matrix metalloproteinases |
| TIMPs | Tissue inhibitors of metalloproteinases |
| DDRs | Discoidin Domain Receptors |
| SPAAC | Strain-promoted azide-alkyne cycloaddition |
| COLMA | Collagen methacrylate |
| CSMA | Chondroitin sulfate methacrylate |
| Col-II-MA | Type II collagen methacrylamide |
| RF | Riboflavin |
| LOX | Lysine oxidase |
| MTG | Glutamine transaminase |
| HRP | Horseradish peroxidase |
| LCST | Low Critical Solution Temperature |
| UCST | Upper Critical Solution Temperature |
| SBF | Simulated body fluid |
| IPNs | Interpenetrating Polymer Networks |
| ESCs | Embryonic stem cells |
| BMSCs | Bone marrow mesenchymal stem cells |
| HUVECs | Human umbilical vein endothelial cells |
| HDPSCs | Human dental pulp stem cells |
| L929 | Mouse fibroblasts |
| MOVASs | Murine aortic vascular smooth muscle cells |
| IEC-6 | Rat intestinal epithelial cells |
| HCs | Human chondrocytes |
| MSC | Mesenchymal stem cell |
| NHDFs | Normal Human Dermal Fibroblasts |
| C2C12 | Murine myoblasts |
| IDG-SW3 | Murine bone cells |
| HS-5 | Human fibroblast cell line |
| MDA-MB-231 | Epithelial adenocarcinoma cells |
| hUC-MSCs | Human umbilical cord mesenchymal stem cells |
| MG-63 | Human osteosarcoma cells |
| U2OS | Human osteosarcoma clonal cells |
| HCT-116 | Human colon cancer cells |
| NSCs | Neural stem cells |
| PCL/Gel | Polycaprolactone/Gelatin |
| Col/Alg | Collagen/Alginate |
| ADSCs | Adipose-derived Stem Cells |
| CDRSs | Collagen dermal replacement scaffolds |
| EDS | Engineered dermal substitute |
| CNS | Central Nervous System |
| TBI | Traumatic brain injury |
| SCI | Spinal cord injury |
| mNSC | mouse Neural stem cell |
| NP | Nucleus pulposus |
| AF | Annulus fibrosus |
| IDD | Intervertebral Disc Degeneration |
| hbNSPCs | Human brain-derived neural stem/progenitor cells |
| hscNSPCs | Human spinal cord-derived neural stem/progenitor cells |
| ACSS | Aligned collagen sponge scaffold |
| HAP | Hydroxyapatite |
| hADSCs | Human adipose-derived stem cells |
| P-PCL | Phosphorus-modified polycaprolactone |
| AD-MSCs | Adipose-derived mesenchymal stem cells |
| ALP | Alkaline phosphatase |
| OBs | Osteoblasts |
| OCs | Osteoclast precursors |
| dECM | Decellularized extracellular matrix |
| ECM | Extracellular matrix |
| MI | Myocardial infarction |
| hiPSC | Human-induced pluripotent stem cell |
| APTES | 3-Aminopropyl-triethoxysilane |
| hCMs | Human ventricular cardiomyocytes |
| hCFs | Human cardiac fibroblasts |
| AMI | Acute myocardial infarction |
| FRESH | Freeform reversible embedding of suspended hydrogels |
| PCL | Polycaprolactone |
| PHHs | Primary human hepatocytes |
| HCs | Hepatocytes |
| ECs | Endothelial cells |
| hGFs | Human gingiva fibroblasts |
| HPLFs | Human periodontal membrane fibroblasts |
References
- Li, W.; Hu, J.; Chen, C.; Li, X.; Zhang, H.; Xin, Y.; Tian, Q.; Wang, S. Emerging advances in hydrogel-based therapeutic strategies for tissue regeneration. Regen. Ther. 2023, 24, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Armengol, E.; Hock, N.; Saribal, S.; To, D.; Summonte, S.; Veider, F.; Kali, G.; Bernkop-Schnurch, A.; Laffleur, F. Unveiling the potential of biomaterials and their synergistic fusion in tissue engineering. Eur. J. Pharm. Sci. 2024, 196, 106761. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Noro, J.; Vilaca-Faria, H.; Reis, R.L.; Pirraco, R.P. Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Aslam, M.A.; Bin Abdullah, M.F.; Hasan, A.; Shah, S.A.; Stojanović, G.M. Recent perspective of polymeric biomaterial in tissue engineering—A review. Mater. Today Chem. 2023, 34, 101818. [Google Scholar] [CrossRef]
- Patrawalla, N.Y.; Kajave, N.S.; Albanna, M.Z.; Kishore, V. Collagen and Beyond: A Comprehensive Comparison of Human ECM Properties Derived from Various Tissue Sources for Regenerative Medicine Applications. J. Funct. Biomater. 2023, 14, 363. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Kawazoe, N.; Yang, Y.; Chen, G. PLGA-collagen-ECM hybrid scaffolds functionalized with biomimetic extracellular matrices secreted by mesenchymal stem cells during stepwise osteogenesis-co-adipogenesis. J. Mater. Chem. B 2019, 7, 7195–7206. [Google Scholar] [CrossRef]
- Khatoon, F.; Narula, A.K.; Sehgal, P. Efficacy of collagen based biomaterials in diabetic foot ulcer wound healing. Eur. Polym. J. 2024, 217, 113345. [Google Scholar] [CrossRef]
- Sonwane, S.; Bonde, S.; Bonde, C.; Chandarana, C. Advances in gelatin-based scaffolds for tissue engineering applications: A review. J. Drug Deliv. Sci. Technol. 2025, 107, 106789. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, N.; Sun, H.; Hou, J.; Zhang, Y.; Wang, D.; Zhang, W.; Chen, Z. Advances in the development and optimization strategies of the hemostatic biomaterials. Front. Bioeng. Biotechnol. 2022, 10, 1062676. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, Y.D.; Zhang, Y.; Gu, T.W.; Peng, L.H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics 2023, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and Productive Source of Biopolymer Inspiration: Marine Collagens. Biomacromolecules 2021, 22, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Tapia, I.; Macouzet-Garduño, J.; Ramírez-Ruiz, F.; Vázquez-Vázquez, F.C.; Álvarez-Pérez, M.A.; Bucio-Galindo, L.; Piña-Barba, M.C. Bio-based composite membranes from fish scales: A novel approach to harnessing collagen and hydroxyapatite for tissue engineering applications. Biomed. Eng. Adv. 2025, 9, 100146. [Google Scholar] [CrossRef]
- Núñez-Tapia, I.A.; Vázquez-Vázquez, F.C.; Mendoza, O.F.; Bucio-Galindo, L.; Álvarez-Pérez, M.A.; Barba, M.C.P. Comparison of Commercial Collagen and Marine Collagen: Efficiency and Effects on the Formation of EDC/NHS-Crosslinked Membranes. Biomed. Mater. Devices 2024. [Google Scholar] [CrossRef]
- Yang, B.; O’Connell, G.D. Effect of collagen fibre orientation on intervertebral disc torsion mechanics. Biomech. Model. Mechanobiol. 2017, 16, 2005–2015. [Google Scholar] [CrossRef]
- Giacomini, F.; Baiao Barata, D.; Suk Rho, H.; Tahmasebi Birgani, Z.; van Blitterswijk, C.; Giselbrecht, S.; Truckenmuller, R.; Habibovic, P. Microfluidically Aligned Collagen to Maintain the Phenotype of Tenocytes In Vitro. Adv. Healthc. Mater. 2024, 13, e2303672. [Google Scholar] [CrossRef]
- Raju, V.; Koorata, P.K. Computational assessment on the impact of collagen fiber orientation in cartilages on healthy and arthritic knee kinetics/kinematics. Med. Eng. Phys. 2023, 117, 103997. [Google Scholar] [CrossRef]
- Moo, E.K.; Tanska, P.; Federico, S.; Al-Saffar, Y.; Herzog, W.; Korhonen, R.K. Collagen fibres determine the crack morphology in articular cartilage. Acta Biomater. 2021, 126, 301–314. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Tebyanian, H.; Soufdoost, R.S.; Motavallian, E.; Barkhordari, A.; Nourani, M.R. Extraction and Characterization of Collagen with Cost-Effective Method from Human Placenta for Biomedical Applications. World J. Plast. Surg. 2019, 8, 352–358. [Google Scholar] [PubMed]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, W.; Tang, K.; Kaplan, D.L. Collagen processing with mesoscale aggregates as templates and building blocks. Biotechnol. Adv. 2023, 63, 108099. [Google Scholar] [CrossRef]
- Penuela, L.; Negro, C.; Massa, M.; Repaci, E.; Cozzani, E.; Parodi, A.; Scaglione, S.; Quarto, R.; Raiteri, R. Atomic force microscopy for biomechanical and structural analysis of human dermis: A complementary tool for medical diagnosis and therapy monitoring. Exp. Dermatol. 2018, 27, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Fiala, T.; Heeb, R.; Vigliotti, L.; Wennemers, H. The Yin and Yang of How N-Terminal Acyl Caps Affect Collagen Triple Helices. Biomacromolecules 2023, 24, 3954–3960. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, K.; Tian, H.; Pei, Y. Collagen materials with oriented structure for biomedical applications. J. Polym. Sci. 2023, 62, 998–1019. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Reiss, M.J.; Han, Y.-P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2010, 147, 295–302. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Tang, W.; Hu, S.Q.; Fu, X.L.; Wu, H.; Shen, W.Q.; Chen, H.L. Effect of matrix metalloproteinases on the healing of diabetic foot ulcer: A systematic review. J. Tissue Viability 2023, 32, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Huang, Z.; Zhang, J.; Shi, G.; Cai, X.; Dou, R.; Tang, J.; Zhang, C.; Zhao, Y.; Chen, J. The potential of collagen-based materials for wound management. Mater. Today Chem. 2024, 41, 102295. [Google Scholar] [CrossRef]
- Hamaia, S.; Farndale, R.W. Integrin Recognition Motifs in the Human Collagens. In I Domain Integrins; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–142. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gong, H.; Cheng, L.; Zhang, D. Discoid Domain Receptors Signaling in Macrophages-Mediated Diseases. Int. J. Gen. Med. 2025, 18, 907–926. [Google Scholar] [CrossRef]
- Silva, M.E.; Hernandez-Andrade, M.; Abasolo, N.; Espinoza-Cruells, C.; Mansilla, J.B.; Reyes, C.R.; Aranda, S.; Esteban, Y.; Rodriguez-Calvo, R.; Martorell, L.; et al. DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. Int. J. Mol. Sci. 2023, 24, 1742. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Wang, C.Z. Exploring the Cellular and Molecular Mechanism of Discoidin Domain Receptors (DDR1 and DDR2) in Bone Formation, Regeneration, and Its Associated Disease Conditions. Int. J. Mol. Sci. 2023, 24, 14895. [Google Scholar] [CrossRef]
- Xu, H.; Bihan, D.; Chang, F.; Huang, P.H.; Farndale, R.W.; Leitinger, B. Discoidin domain receptors promote alpha1beta1-and alpha2beta1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS ONE 2012, 7, e52209. [Google Scholar] [CrossRef]
- Hou, G.; Vogel, W.; Bendeck, M.P. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J. Clin. Investig. 2001, 107, 727–735. [Google Scholar] [CrossRef]
- Afonso, P.V.; McCann, C.P.; Kapnick, S.M.; Parent, C.A. Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood 2013, 121, 1644–1650. [Google Scholar] [CrossRef][Green Version]
- Pedro, A.; Ruiz, G.J. Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices. Fibrogenesis Tissue Repair. 2012, 5, 3. [Google Scholar] [CrossRef]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.; Sahai, E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 2014, 15, 813–824. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.B.; Vachhani, B.; Iida, J. Cell Adhesion to Collagenous Matrices. Pept. Sci. 1996, 40, 371–381. [Google Scholar] [CrossRef]
- Clark, A.G.; Maitra, A.; Jacques, C.; Bergert, M.; Perez-Gonzalez, C.; Simon, A.; Lederer, L.; Diz-Munoz, A.; Trepat, X.; Voituriez, R.; et al. Self-generated gradients steer collective migration on viscoelastic collagen networks. Nat. Mater. 2022, 21, 1200–1210. [Google Scholar] [CrossRef]
- Riching, K.M.; Cox, B.L.; Salick, M.R.; Pehlke, C.; Riching, A.S.; Ponik, S.M.; Bass, B.R.; Crone, W.C.; Jiang, Y.; Weaver, A.M.; et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014, 107, 2546–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Cario, M. DDR1 and DDR2 in skin. Cell Adh Migr. 2018, 12, 386–393. [Google Scholar] [CrossRef]
- Olaso, E.; Labrador, J.P.; Wang, L.; Ikeda, K.; Eng, F.J.; Klein, R.; Lovett, D.H.; Lin, H.C.; Friedman, S.L. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J. Biol. Chem. 2002, 277, 3606–3613. [Google Scholar] [CrossRef]
- Kou, H.; Han, Q.; Xu, C.; Liao, L.; Hou, Y.; Wang, H.; Zhang, J. The fibrillogenesis influence the binding specificity of collagen to discoidin domain receptor 2 and cell behavior. Colloids Surf. B Biointerfaces 2025, 250, 114554. [Google Scholar] [CrossRef]
- Olaso, E.; Lin, H.-C.; Wang, L.-H.; Friedman, S.L. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair. 2011, 4, 5. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Song, C.; Yao, L.; Xiao, J. Recent progresses of collagen dressings for chronic skin wound healing. Collagen and Leather 2023, 5, 31. [Google Scholar] [CrossRef]
- San Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J. Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine. Bioengineering 2020, 8, 3. [Google Scholar] [CrossRef]
- Adam, J.; Singer, R.A.F.C. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Bao, M.; Xie, J.; Huck, W.T.S. Recent Advances in Engineering the Stem Cell Microniche in 3D. Adv. Sci. 2018, 5, 1800448. [Google Scholar] [CrossRef]
- Huang, D.; Li, Y.; Ma, Z.; Lin, H.; Zhu, X.; Xiao, Y.; Zhang, X. Collagen hydrogel viscoelasticity regulates MSC chondrogenesis in a ROCK-dependent manner. Sci. Adv. 2023, 9, eade9497. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Karaman, S.; Quartey, B.C.; Mohamed, W.K.E.; Mahtani, N.; Garcia-Sabate, A.; Teo, J. Collagen Fibril Orientation Instructs Fibroblast Differentiation Via Cell Contractility. Adv. Sci. 2023, 10, e2301353. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Long, X.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Wang, D.O.; Ikejima, T. Type I collagen promotes the migration and myogenic differentiation of C2C12 myoblasts via the release of interleukin-6 mediated by FAK/NF-kappaB p65 activation. Food Funct. 2020, 11, 328–338. [Google Scholar] [CrossRef]
- Duquette, D.; Dumont, M.-J. Comparative studies of chemical crosslinking reactions and applications of bio-based hydrogels. Polym. Bull. 2018, 76, 2683–2710. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of "Click" Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Deng, C.; Teng, X.; Suuronen, E.J.; Shen, Z.; Zhong, Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55–64. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhao, S.; Wang, X.; Liu, B.; Xu, H. Click Chemistry in Natural Product Modification. Front. Chem. 2021, 9, 774977. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Le, P.; Lai, K.; Fernandes-Cunha, G.M.; Myung, D. Simultaneous Interpenetrating Polymer Network of Collagen and Hyaluronic Acid as an In Situ-Forming Corneal Defect Filler. Chem. Mater. 2020, 32, 5208–5216. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Putra, I.; Koh, W.-G.; Myung, D. Tethering Growth Factors to Collagen Surfaces Using Copper-Free Click Chemistry_ Surface Characterization and in Vitro Biologica. ACS Appl. Mater. Interfaces 2017, 9, 23389–23399. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Wu, Z.; Cao, L. Photocrosslinked methacrylated natural macromolecular hydrogels for tissue engineering: A review. Int. J. Biol. Macromol. 2023, 246, 125570. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Chi, B.; Zhang, X.; Wang, Y.; Wu, J.; Huang, Q. Construction of a dual-component hydrogel matrix for 3D biomimetic skin based on photo-crosslinked chondroitin sulfate/collagen. Int. J. Biol. Macromol. 2024, 254, 127940. [Google Scholar] [CrossRef]
- Yang, K.; Sun, J.; Wei, D.; Yuan, L.; Yang, J.; Guo, L.; Fan, H.; Zhang, X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 8707–8718. [Google Scholar] [CrossRef] [PubMed]
- Tonndorf, R.; Gossla, E.; Aibibu, D.; Lindner, M.; Gelinsky, M.; Cherif, C. Wet spinning and riboflavin crosslinking of collagen type I/III filaments. Biomed. Mater. 2018, 14, 015007. [Google Scholar] [CrossRef]
- Fan, L.; Jung, O.; Herrmann, M.; Shirokikh, M.; Stojanovic, S.; Najman, S.; Körte, F.; Xiong, X.; Schenke-Layland, K.; Barbeck, M. Deciphering UVA/Riboflavin Collagen Crosslinking: A Pathway to Improve Biomedical Materials. Adv. Funct. Mater. 2024, 34, 2401742. [Google Scholar] [CrossRef]
- Lin, C.L.; Su, Y.W.; Chen, Y.W.; Kuo, C.H.; Tu, T.Y.; Tsai, J.C.; Shyong, Y.J. BMSC loaded photo-crosslinked hyaluronic acid/collagen hydrogel incorporating FG4592 for enhanced cell proliferation and nucleus pulposus differentiation. Int. J. Biol. Macromol. 2024, 273, 132828. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A novel smart injectable hydrogel prepared by microbial transglutaminase and human-like collagen: Its characterization and biocompatibility. Mater. Sci. Eng. C 2016, 68, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Lin, L.R.; Xu, L.Y.; Li, E.M. Reaction mechanism of lysyl oxidase-like 2 (LOXL2) studied by computational methods. J. Inorg. Biochem. 2020, 211, 111204. [Google Scholar] [CrossRef]
- Lopez-Jimenez, A.J.; Basak, T.; Vanacore, R.M. Proteolytic processing of lysyl oxidase-like-2 in the extracellular matrix is required for crosslinking of basement membrane collagen IV. J. Biol. Chem. 2017, 292, 16970–16982. [Google Scholar] [CrossRef]
- Ahmadian, M.; Khoshfetrat, A.B.; Khatami, N.; Morshedloo, F.; Rahbarghazi, R.; Hassani, A.; Kiani, S. Influence of gelatin and collagen incorporation on peroxidase-mediated injectable pectin-based hydrogel and bioactivity of fibroblasts. J. Biomater. Appl. 2021, 36, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zheng, M.; Liu, X.; Zhang, S.; Wang, X.; Chen, Y.; Hou, M.; Zhu, J. Feasibility Study of Tissue Transglutaminase for Self-Catalytic Cross-Linking of Self-Assembled Collagen Fibril Hydrogel and Its Promising Application in Wound Healing Promotion. ACS Omega 2019, 4, 12606–12615. [Google Scholar] [CrossRef]
- Tayabally, S.E.H.; Khan, A.A.; Abdallah, S.H.; Khattak, M.N.K.; Jayakumar, M.N.; Rani Samsudin, A.B. Increased strength in the Col-Tgel induces apoptosis in the human dental pulp stem cells: 3D culturing of human dental pulp stem cells at different strengths of collagen. Saudi J. Biol. Sci. 2022, 29, 2674–2682. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polym. Rev. 2022, 63, 574–612. [Google Scholar] [CrossRef]
- Yang, X.; Ahmad, K.; Yang, T.; Fan, Y.; Zhao, F.; Jiang, S.; Chen, P.; Hou, H. Collagen-based hydrogel sol-gel phase transition mechanism and their applications. Adv. Colloid Interface Sci. 2025, 340, 103456. [Google Scholar] [CrossRef]
- Du, M.; Zhao, E.; Li, J.; Yao, Y.; Wang, Y.; Chen, J.; Qu, C. Rapid sol-gel reversible thermosensitive collagen for 3D cell culture. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132813. [Google Scholar] [CrossRef]
- Rieu, C.; Parisi, C.; Mosser, G.; Haye, B.; Coradin, T.; Fernandes, F.M.; Trichet, L. Topotactic Fibrillogenesis of Freeze-casted Microridged Collagen Scaffolds for 3D Cell Culture. ACS Appl. Mater. 2019, 11, 14672–14683. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yang, Y. A review of sodium alginate-based hydrogels: Structure, mechanisms, applications, and perspectives. Int. J. Biol. Macromol. 2025, 292, 139151. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef] [PubMed]
- Brunel, L.G.; Long, C.M.; Christakopoulos, F.; Cai, B.; Johansson, P.K.; Singhal, D.; Enejder, A.; Myung, D.; Heilshorn, S.C. Interpenetrating networks of fibrillar and amorphous collagen promote cell spreading and hydrogel stability. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Bernero, M.; Zauchner, D.; Muller, R.; Qin, X.H. Interpenetrating network hydrogels for studying the role of matrix viscoelasticity in 3D osteocyte morphogenesis. Biomater. Sci. 2024, 12, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Ort, C.; Chen, Y.; Ghagre, A.; Ehrlicher, A.; Moraes, C. Bioprintable, Stiffness-Tunable Collagen-Alginate Microgels for Increased Throughput 3D Cell Culture Studies. ACS Biomater. Sci. Eng. 2021, 7, 2814–2822. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, Y.; Wang, Y.; Yao, M.; Zhang, K.; Chen, Z.; Yue, H.; Shi, J.; Guan, F.; et al. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021, 383, 809–821. [Google Scholar] [CrossRef]
- De, S.; Singh, N. Collagen-alginate 3D microscaffolds for studying cellular migration. Int. J. Biol. Macromol. 2023, 245, 125308. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Angulo, D.E.L.; Sobral, P.J.d.A. The Effect of Processing Parameters and Solid Concentration on the Microstructure and Pore Architecture of Gelatin-Chitosan Scaffolds Produced by Freeze-Drying. Mater. Res. 2016, 19, 839–845. [Google Scholar] [CrossRef]
- Katrilaka, C.; Karipidou, N.; Petrou, N.; Manglaris, C.; Katrilakas, G.; Tzavellas, A.N.; Pitou, M.; Tsiridis, E.E.; Choli-Papadopoulou, T.; Aggeli, A. Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering. Materials 2023, 16, 4425. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Reucroft, I.M.; Grota, M.C.; Shreiber, D.I. Production of Highly Aligned Collagen Scaffolds by Freeze-drying of Self-assembled, Fibrillar Collagen Gels. ACS Biomater. Sci. Eng. 2016, 2, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Khadivar, P.; Alipour, M. Synergic effect of bone marrow derived mesenchymal stem cells and differentiated keratinocytes-like cells with a novel cellulose and collagen nanoscaffold on wound healing in rats. Biomed. Pharmacother. 2023, 160, 114404. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Xie, X.; Lei, J.; Ge, L.; Yuan, L.; Li, D.; Mu, C. Controlling the Pore Structure of Collagen Sponge by Adjusting the Cross-Linking Degree for Construction of Heterogeneous Double-Layer Bone Barrier Membranes. ACS Appl. Bio Mater. 2020, 3, 2058–2067. [Google Scholar] [CrossRef]
- Lashkari, M.; Rahmani, M.; Yousefpoor, Y.; Ahmadi-Zeidabadi, M.; Faridi-Majidi, R.; Ameri, Z.; Salary, M.; Azizi, S.; Shahabi, A.; Rahi, A.; et al. Cell-based wound dressing: Bilayered PCL/gelatin nanofibers-alginate/collagen hydrogel scaffold loaded with mesenchymal stem cells. Int. J. Biol. Macromol. 2023, 239, 124099. [Google Scholar] [CrossRef]
- Yang, M.; He, S.; Su, Z.; Yang, Z.; Liang, X.; Wu, Y. Thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels for Enhancing Wound Healing by Encapsulating Mesenchymal Stem Cell Spheroids. ACS Omega 2020, 5, 21015–21023. [Google Scholar] [CrossRef]
- Liu, H.; Yang, R.; Zhao, S.; Zhou, F.; Liu, Y.; Zhou, Z.; Chen, L.; Xie, J. Collagen scaffolds derived from bovine skin loaded with MSC optimized M1 macrophages remodeling and chronic diabetic wounds healing. Bioeng. Transl. Med. 2023, 8, e10467. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Wang, X.; Chen, M.; Yao, B.; Dong, Y.; Li, X.; Yang, Q.; Tredget, E.E.; Xu, R.H.; et al. An Engineered Dermal Substitute with Mesenchymal Stem Cells Enhances Cutaneous Wound Healing. Tissue Eng. Part A 2023, 29, 491–505. [Google Scholar] [CrossRef]
- Bartlett, R.D.; Eleftheriadou, D.; Evans, R.; Choi, D.; Phillips, J.B. Mechanical properties of the spinal cord and brain: Comparison with clinical-grade biomaterials for tissue engineering and regenerative medicine. Biomaterials 2020, 258, 120303. [Google Scholar] [CrossRef]
- Wareham, L.K.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen in the central nervous system: Contributions to neurodegeneration and promise as a therapeutic target. Mol. Neurodegener. 2024, 19, 11. [Google Scholar] [CrossRef]
- Tan, H.X.; Borgo, M.P.D.; Aguilar, M.I.; Forsythe, J.S.; Taylor, J.M.; Crack, P.J. The use of bioactive matrices in regenerative therapies for traumatic brain injury. Acta Biomater. 2020, 102, 1–12. [Google Scholar] [CrossRef]
- Kim, J.T.; Cho, S.M.; Youn, D.H.; Hong, E.P.; Park, C.H.; Lee, Y.; Jung, H.; Jeon, J.P. Therapeutic effect of a hydrogel-based neural stem cell delivery sheet for mild traumatic brain injury. Acta Biomater. 2023, 167, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.J.; Chen, M.; Liu, X.Y.; Ma, K.; Xu, H.Y.; Deng, W.S.; Ye, Y.C.; Li, W.X.; Chen, X.Y.; et al. Collagen/heparan sulfate porous scaffolds loaded with neural stem cells improve neurological function in a rat model of traumatic brain injury. Neural Regen. Res. 2021, 16, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, L.; Mosley, G.E.; Laudier, D.; Gallate, Z.S.; Gansau, J.; Hoy, R.C.; Poeran, J.; Iatridis, J.C. Spatial mapping of collagen content and structure in human intervertebral disk degeneration. JOR Spine 2020, 3, e1129. [Google Scholar] [CrossRef]
- Hu, H.; Hu, R.; Fu, X.; Wang, Y.; Zhang, Y.; Chen, S.; Wang, T.; Cui, S.; Wan, Y.; Guo, W.; et al. An injectable anti-vascularization functionalized hydrogel for degenerative nucleus pulposus repair. J. Mater. Sci. Technol. 2024, 203, 143–154. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.; Zeng, S.; Hu, X.; Wu, Y.; Zhou, J.; Yuan, L.; Zhang, Q.; Xiang, C.; Feng, Z. Menstrual blood-derived mesenchymal stem cells combined with collagen I gel as a regenerative therapeutic strategy for degenerated disc after discectomy in rats. Stem Cell Res. Ther. 2024, 15, 75. [Google Scholar] [CrossRef]
- Mneimneh, A.T.; Mehanna, M.M. Collagen-based scaffolds: An auspicious tool to support repair, recovery, and regeneration post spinal cord injury. Int. J. Pharm. 2021, 601, 120559. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, D.; Shen, H.; Zhao, Y.; Xu, B.; Fan, Y.; Sun, Z.; Chen, B.; Xue, W.; Shi, Y.; et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 2020, 8, 5145–5156. [Google Scholar] [CrossRef]
- Jiang, J.P.; Liu, X.Y.; Zhao, F.; Zhu, X.; Li, X.Y.; Niu, X.G.; Yao, Z.T.; Dai, C.; Xu, H.Y.; Ma, K.; et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen. Res. 2020, 15, 959–968. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Zhang, X.; Wu, T.; Huang, K.; Wang, B.; Liao, J. Self-healing hydrogels for bone defect repair. RSC Adv. 2023, 13, 16773–16788. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Aslam, M.A.; Bin Abdullah, M.F.; Stojanovic, G.M. Current Perspectives of Protein in Bone Tissue Engineering: Bone Structure, Ideal Scaffolds, Fabrication Techniques, Applications, Scopes, and Future Advances. ACS Appl. Bio Mater. 2024, 7, 5082–5106. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, R.; Tang, W.; Yang, F.; Tian, H.; Peng, S.; Pan, H.; Shuai, C. Structural and Functional Adaptive Artificial Bone: Materials, Fabrications, and Properties. Adv. Funct. Mater. 2023, 33, 2214726. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Li, R.; Xu, S.; Guo, Y.; Cao, C.; Xu, J.; Hao, L.; Luo, S.; Chen, X.; Du, Y.; Li, Y.; et al. Application of collagen in bone regeneration. J. Orthop. Transl. 2025, 50, 129–143. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D bioprinting of HAP/collagen scaffold in gelation bath for bone tissue engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Perut, F.; Ciapetti, G.; Baldini, N.; Vitale-Brovarone, C. Osteoblast and osteoclast activity on collagen-based 3D printed scaffolds enriched with strontium-doped bioactive glasses and hydroxyapatite nanorods for bone tissue engineering. Biomed. Mater. 2024, 19, 065007. [Google Scholar] [CrossRef]
- Ramírez-Ruiz, F.; Núñez-Tapia, I.; Piña-Barba, M.C.; Alvarez-Pérez, M.A.; Guarino, V.; Serrano-Bello, J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering 2025, 12, 46. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Li, Y.; Gao, A.; Zheng, Z.; Wei, J.; Yang, H.; Ping, H.; Xie, H.; Wang, H.; et al. Intrafibrillar calcium carbonate mineralization of electrospinning polyvinyl alcohol/collagen films with improved mechanical and bioactive properties. J. Mater. Chem. B 2025, 13, 312–325. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 2863–2870. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Roshangar, L.; Aghanejad, A.; Davaran, S. A bioactive porous scaffold containing collagen/ phosphorous-modified polycaprolactone for osteogenesis of adipose-derived mesenchymal stem cells. Eur. Polym. J. 2022, 171, 111220. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Chen, M.; Liu, Y.; Cui, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Childhood Cartilage ECM Enhances the Chondrogenesis of Endogenous Cells and Subchondral Bone Repair of the Unidirectional Collagen–dECM Scaffolds in Combination with Microfracture. ACS Appl. Mater. Interfaces 2021, 13, 57043–57057. [Google Scholar] [CrossRef]
- Yu, S.; Shu, X.; Chen, L.; Wang, C.; Wang, X.; Jing, J.; Yan, G.; Zhang, Y.; Wu, C. Construction of ultrasonically treated collagen/silk fibroin composite scaffolds to induce cartilage regeneration. Sci. Rep. 2023, 13, 20168. [Google Scholar] [CrossRef] [PubMed]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef]
- Liu, T.; Hao, Y.; Zhang, Z.; Zhou, H.; Peng, S.; Zhang, D.; Li, K.; Chen, Y.; Chen, M. Advanced Cardiac Patches for the Treatment of Myocardial Infarction. Circulation 2024, 149, 2002–2020. [Google Scholar] [CrossRef] [PubMed]
- Tashakori-Miyanroudi, M.; Rakhshan, K.; Ramez, M.; Asgarian, S.; Janzadeh, A.; Azizi, Y.; Seifalian, A.; Ramezani, F. Conductive carbon nanofibers incorporated into collagen bio-scaffold assists myocardial injury repair. Int. J. Biol. Macromol. 2020, 163, 1136–1146. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Schiffer, M.; Carls, E.; Angeloni, M.; Kolesnik-Gray, M.; Schruefer, S.; Schubert, D.W.; Ferrazzi, F.; Krstic, V.; Fleischmann, B.K.; et al. Electrically Conductive Collagen-PEDOT:PSS Hydrogel Prevents Post-Infarct Cardiac Arrhythmia and Supports hiPSC-Cardiomyocyte Function. Adv. Mater. 2024, 36, e2403642. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki, N.; Simchi, A. Conductive, injectable, and self-healing collagen-hyaluronic acid hydrogels loaded with bacterial cellulose and gold nanoparticles for heart tissue engineering. Int. J. Biol. Macromol. 2024, 280, 135749. [Google Scholar] [CrossRef]
- Kim, K.S.; Joo, H.J.; Choi, S.C.; Kim, J.H.; Park, C.Y.; Song, M.H.; Noh, J.M.; Cha, J.J.; Hong, S.J.; Ahn, T.H.; et al. Transplantation of 3D bio-printed cardiac mesh improves cardiac function and vessel formation via ANGPT1/Tie2 pathway in rats with acute myocardial infarction. Biofabrication 2021, 13, 045014. [Google Scholar] [CrossRef]
- Das, S.; Kim, S.-W.; Choi, Y.-J.; Lee, S.; Lee, S.-H.; Kong, J.-S.; Park, H.-J.; Cho, D.-W.; Jang, J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019, 95, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Nadi, A.; Moradi, L.; Ai, J.; Asadpour, S. Stem Cells and Hydrogels for Liver Tissue Engineering: Synergistic Cure for Liver Regeneration. Stem Cell Rev. Rep. 2020, 16, 1092–1104. [Google Scholar] [CrossRef]
- Nair, D.G.; Weiskirchen, R. Recent Advances in Liver Tissue Engineering as an Alternative and Complementary Approach for Liver Transplantation. Curr. Issues Mol. Biol. 2023, 46, 262–278. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Morelli, S.; D’Amora, U.; Piscioneri, A.; Oliviero, M.; Scialla, S.; Coppola, A.; De Pascale, D.; Crocetta, F.; De Santo, M.P.; Davoli, M.; et al. Methacrylated chitosan/jellyfish collagen membranes as cell instructive platforms for liver tissue engineering. Int. J. Biol. Macromol. 2024, 281, 136313. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Gibeley, S.B.; Xu, C.; Xiao, Y.; Celik, O.; Ginsberg, H.N.; Leong, K.W. Engineering liver microtissues for disease modeling and regenerative medicine. Adv. Funct. Mater. 2020, 30, 1909553. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X.; Zhao, H.; Xia, S.; Liu, W.; Bai, H.; Lv, F.; Zheng, X.; Huang, Y.; Gu, Q.; et al. Synthesis of easily-processable collagen bio-inks using ionic liquid for 3D bioprinted liver tissue models with branched vascular networks. Sci. China Chem. 2023, 66, 1489–1499. [Google Scholar] [CrossRef]
- Keisuke Harada, T.M.; Miyamoto, S.; Sugimoto, S.; Ikeda, S.; Takeda, H.; Mochizuki, Y.; Hirata, K. Rapid formation of hepatic organoid in collagen sponge by rat small hepatocytes and hepatic nonparenchymal cells. J. Hepatol. 2003, 39, 716–723. [Google Scholar] [CrossRef]
- Nishida, Y.; Taniguchi, A. A three-dimensional collagen-sponge-based culture system coated with simplified recombinant fibronectin improves the function of a hepatocyte cell line. In Vitro Cell Dev. Biol. Anim. 2016, 52, 271–277. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Piña-Barba, C.; Núñez-Tapia, I.A.; Hernandez-Santillan, M.; Hernández-Barragán, A.; Gutiérrez-Reyes, G. Collagen matrix scaffold as vehicle of WP1066, STAT-3 inhibitors, in an in vitro hepatocellular model. Ann. Hepatol. 2024, 29, 101403. [Google Scholar] [CrossRef]
- Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core-shell bioprinting of vascularizedin vitroliver sinusoid models. Biofabrication 2022, 14, 045019. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-Gonzalez, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Wei, S.Y.; Chen, P.Y.; Hsieh, C.C.; Chen, Y.S.; Chen, T.H.; Yu, Y.S.; Tsai, M.C.; Xie, R.H.; Chen, G.Y.; Yin, G.C.; et al. Engineering large and geometrically controlled vascularized nerve tissue in collagen hydrogels to restore large-sized volumetric muscle loss. Biomaterials 2023, 303, 122402. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Jin, S.; Kim, S.; Son, D.; Shin, M. Tyramine-Functionalized Alginate-Collagen Hybrid Hydrogel Inks for 3D-Bioprinting. Polymers 2022, 14, 3173. [Google Scholar] [CrossRef]
- Unterman, S.; Freiman, A.; Beckerman, M.; Abraham, E.; Stanley, J.R.L.; Levy, E.; Artzi, N.; Edelman, E. Tuning of Collagen Scaffold Properties Modulates Embedded Endothelial Cell Regulatory Phenotype in Repair of Vascular Injuries In Vivo. Adv. Healthc. Mater. 2015, 4, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, L.; Hu, X.; Yao, S.; Hao, Z.; Zhou, J.; Li, X.; Lu, H.; He, J.; Wang, L.; et al. 3D Bioprinting of Neurovascular Tissue Modeling with Collagen-Based Low-Viscosity Composites. Adv. Healthc. Mater. 2023, 12, e2300004. [Google Scholar] [CrossRef]
- Enrico, A.; Voulgaris, D.; Ostmans, R.; Sundaravadivel, N.; Moutaux, L.; Cordier, A.; Niklaus, F.; Herland, A.; Stemme, G. 3D Microvascularized Tissue Models by Laser-Based Cavitation Molding of Collagen. Adv. Mater. 2022, 34, e2109823. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Jiang, L.; Zeng, Y.; Han, Y.; Sha, C.; Xie, X.; Li, H.; Zhou, J.; Lin, W. 3D bioprinting of collagen-based materials for oral medicine. Collagen Leather 2023, 5, 23. [Google Scholar] [CrossRef]
- Gelin, A.; Masson-Meyers, D.; Amini, F.; Moharamzadeh, K.; Tayebi, L. Collagen: The superior material for full-thickness oral mucosa tissue engineering. J. Oral Biosci. 2024, 66, 511–518. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Lai, C.-C.; Lin, D.-J. Collagen Scaffolds Laden with Human Periodontal Ligament Fibroblasts Promote Periodontal Regeneration in SD Rat Model. Polymers 2023, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cabrerizo, P.; Saludas, L.; Prosper, F.; Abizanda, G.; Echanove-Gonzalez de Anleo, M.; Ruiz-Villalba, A.; Garbayo, E.; Blanco-Prieto, M.J. Development of an injectable alginate-collagen hydrogel for cardiac delivery of extracellular vesicles. Int. J. Pharm. 2022, 629, 122356. [Google Scholar] [CrossRef]
- Fu, C.; Fan, Y.; Liu, G.; Li, W.; Ma, J.; Xiao, J. One-step fabrication of an injectable antibacterial collagen hydrogel with in situ synthesized silver nanoparticles for accelerated diabetic wound healing. Chem. Eng. J. 2024, 480, 148288. [Google Scholar] [CrossRef]

| Method | Scaffold | Cross-Linking Agent or Influencing Factor | Encapsulated Cells | Reference |
|---|---|---|---|---|
| Click Chemistry | Thiolated-collagen injectable hydrogel | Thiol-alkene click chemistry | BMSCs | [61] |
| Thiolated-collagen injectable hydrogel | Thiol-alkene click chemistry | BMSCs, HUVEC | [62] | |
| Hyaluronic acid/collagen hydrogel | SPAAC | Corneal epithelial cells | [64] | |
| EGF/collagen scaffolds | SPAAC | Corneal epithelial cells | [65] | |
| Photoactivated Cross-linking | CSMA-COLMA hydrogel | Methacrylic anhydride | Keratinocytes, Fibroblasts | [67] |
| Col-II-MA hydrogel | Methacrylic anhydride | BMSCs | [68] | |
| Collagen scaffolds | Riboflavin | Fibroblasts | [70] | |
| Collagen/hyaluronic acid scaffolds | Riboflavin | BMSCs | [71] | |
| Enzyme Cross-linking | Collagen-hyaluronic acid hydrogel | HRP | HMEC, Fibroblasts | [73] |
| Collagen fibril hydrogel | MTG | Fibroblasts | [77] | |
| Transglutaminase-cross-linked collagen hydrogels (Col-Tgel) | MTG | HDPSCs | [78] | |
| Physical Force | Rapid sol-gel reversible thermosensitive collagen (RRTC) hydrogel | pH/Temperature | L929, HUVEC, Mouse myeloma cells (Sp2/0), MOVAS, IEC-6, HC | [81] |
| Collagen hydrogel | pH/Temperature | MSC | [56] | |
| 3D biomimetic collagen scaffolds | pH/Temperature | NHDFs, C2C12 | [82] | |
| Ionic Interaction Gelation | Alginate-collagen interpenetrating network (IPN) hydrogel | CaCl2 | IDG-SW3 | [86] |
| Collagen-alginate microgels | CaCl2 | HS-5, MDA-MB-231 | [87] | |
| Sodium alginate/collagen hydrogel | CaCl2 | hUC-MSCs | [88] | |
| Collagen-alginate 3D microscaffolds | CaCl2 | MG-63, MDA-MB-231, U2OS, HCT-116 | [89] | |
| Freeze-drying | Cellulose and collagen nano-scaffold | Freeze-drying | BMSCs and differentiated keratinocytes | [94] |
| Double-layer collagen sponge | Freeze-drying | C2C12/MC3T3-E1 | [95] |
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Click Chemistry |
|
| [60,61,62,65] |
| Photoactivated Cross-linking |
|
| [66,69,70,71] |
| Enzyme Cross-linking |
|
| [73,74,75,77] |
| Physical Force |
|
| [56,79,81] |
| Ionic Interaction Gelation |
|
| [83,84,85,86,89] |
| Freeze-drying |
|
| [90,91,92,93,94] |
| Tissue/Organ to Be Regenerated | Scaffold | Encapsulated Cells | Key Findings | Reference |
|---|---|---|---|---|
| Skin/Wound Healing | Bilayered PCL/gelatin nanofibers-alginate/collagen hydrogel | ADSCs | The bilayer scaffold containing ADSCs reduced inflammation and improved re-epithelialization and collagen reorganization. | [96] |
| A novel cellulose and collagen nano-scaffold | Bone marrow-derived mesenchymal stem cells and differentiated keratinocyte-like cells | Cell therapy did not induce inflammation; combining scaffold and cellular therapy enhanced collagen deposition. | [94] | |
| Thermosensitive injectable chitosan/collagen/β-glycerophosphate composite hydrogels | MSCs | The combined treatment accelerated the wound closure in diabetic mice by enhanced vascularization and paracrine effects. | [97] | |
| Collagen dermal-replacement scaffolds | BMSCs | CBS-MSCs facilitated the noncontractile and re-epithelialization processes, as well as granulation tissue regeneration and neovascularization, in chronic diabetic wounds. | [98] | |
| A collagen–glycosaminoglycan matrix to form a dermis-like tissue sheet | MSCs | Prolonged MSC engraftment is associated with accelerated wound re-epithelialization and healing, accompanied by increased macrophage recruitment and angiogenesis. | [99] | |
| Nerve | Collagen-fibrin hydrogel | mNSC | Enhanced cognitive function through the reconstruction of the damaged cortex. | [103] |
| Collagen/heparan sulfate porous scaffolds | NSCs | Enhanced regeneration of neurons, nerve fibers, synapses, and myelin sheaths in injured brain tissue, reduced brain edema and apoptosis, and substantially recovered motor and cognitive functions. | [104] | |
| Methacrylate hyaluronic acid/collagen hydrogel | MSCs | Maintained disc height, protected NP, and alleviated vascularization and inflammation. | [106] | |
| Type I collagen hydrogel | MSCs | It effectively prevents disc degeneration and inhibits cell apoptosis following discectomy. | [107] | |
| Collagen sponge scaffold | hbNSPCs hscNSPCs | Effectively promoted long-term cell survival and neuronal differentiation and improved the SCI microenvironment by reducing inflammation and glial scar formation. | [109] | |
| Collagen/silk fibroin scaffold | NSCs | Significantly increased the amplitude of motor-evoked potentials, as well as improved continuity and cavity filling within the injured spinal cord. | [110] | |
| Bone/Cartilage | HAP/collagen scaffold | BMSCs | BMSCs on the scaffold remained viable and continued to proliferate, exhibiting high alkaline phosphatase expression. | [117] |
| Collagen-HAP scaffold | hADSCs | Capable of recruiting host cells to undergo osteogenic differentiation while promoting bone enlargement and the formation of vascular elements. | [118] | |
| Collagen/strontium-doped bioactive glasses/HAP nanorods scaffold | OBs | Positively influenced cell proliferation and metabolic activity, enhancing alkaline phosphatase activity in osteoblasts and reducing osteoclast differentiation. | [119] | |
| Collagen/PVA-CaCO₃ membranes | BMSCs | Effectively promoted BMSC adhesion, proliferation, and osteogenic differentiation. | [121] | |
| Collagen/PLGA/HAP fibrous scaffolds | MSCs | Collagen coating enhanced cell–membrane interactions. | [122] | |
| Collagen/phosphorous-modified polycaprolactone porous scaffold | AD-MSCs | Facilitates cell adhesion, proliferation, and upregulation of osteogenic marker genes, thereby inducing osteogenic differentiation of stem cells. | [123] | |
| Unidirectional collagen-dECM scaffolds | BMSCs | Highlights the immature cartilage and dECM at different developmental stages, which result in the diversified effects of BMSCs. | [124] | |
| Collagen/silk fibroin scaffold | ADSCs | Stimulates chondrogenic differentiation of stem cells and enhances cartilage regeneration. | [125] | |
| Cardiac | Collagen-PEDOT–PSS hydrogel | Human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes | Facilitates partial regeneration of cardiac muscle, improving contractility, calcium handling, and conduction efficiency. | [129] |
| Collagen-hyaluronic acid hydrogels | Human embryonic stem cell-derived cardiomyocytes | Exhibits heat sensitivity, self-repair capability, and electrical conductivity, facilitating myocardial regeneration. | [130] | |
| Gelatin-methacryloyl–collagen hydrogel | hCM, hCF | Vessel formation and stabilization, reduced fibrosis, increased left ventricle thickness, and enhanced cardiac function. | [131] | |
| Collagen bio-ink | Human cardiomyocytes | The printed heart model demonstrates synchronized contraction, directed action potential propagation, and wall thickening during peak ventricular contraction. | [133] | |
| Liver | Collagen hydrogel | HCs, HUVEC, Human lung fibroblasts | Constructs liver tissue structures that facilitate interaction between heterogeneous cells. | [136] |
| Methacrylated chitosan/jellyfish collagen membranes | Hepatocytes | Provides an optimal microenvironment for liver cells. | [137] | |
| Norbornene-functionalized collagen (Col-Nor) hydrogel | HUVEC | Development of a three-dimensional (3D) liver tissue model incorporating branching vascular networks. | [139] | |
| Demineralized collagen sponge | SHs, NPCs | Hepatic organoids can be rapidly reconstructed in a collagen sponge by rat SHs and NPCs. | [140] | |
| Demineralized collagen sponge | HepG2 | The collagen sponge can be utilized for drug metabolism studies, toxicity testing, and therapeutic agent screening. | [141] | |
| HAP-mineralized collagen sponges | HEPA1-6, HepG2 | Collagen matrix scaffold as a vehicle in an in vitro hepatocellular model. | [142] | |
| Alginate/methylcellulose bio-ink–collagen/fibronectin bio-ink | HepG2, HUVEC, Human dermal fibroblasts | Development of an In vitro Triple-Culture Model with Complex Hepatic Sinusoids. | [143] | |
| Tendon | Collagen hydrogel | HUVEC, MSCs | Development of large vascularized neural tissue structures for repairing volumetric muscle loss and restoring muscle function. | [145] |
| Vascular | Tyramine-functionalized alginate-collagen hybrid hydrogel | Fibroblast cells | Three-dimensional (3D) printed vascular extracellular matrix (ECM) mimetic scaffolds for supporting tissue regeneration. | [146] |
| Collagen scaffold | ECs | It prevents in vivo cell inactivation, reduces injury-induced inflammation, and enhances endothelialization and smooth muscle proliferation. | [147] | |
| Alginate/collagen hydrogels | Mouse brain microvascular endothelial cells (BEND.3), Astrocytes | A 3D hollow coaxial neurovascular model is fabricated. | [148] | |
| Collagen hydrogels | HUVEC | Developing organ-on-a-chip and 3D tissue models with complex microvasculature. | [149] | |
| Stomatology | Collagen/strontium-doped calcium silicate scaffold | hGF | Guided periodontal regeneration. | [152] |
| Collagen/riboflavin hydrogels | HPLFs | Well-oriented periodontal ligament and alveolar bone regeneration. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Feng, T.; Xie, Y.; Swamiappan, S.; Zhou, Y.; Zhou, Y.; Zhou, H.; Peng, X. Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds. Int. J. Mol. Sci. 2025, 26, 4009. https://doi.org/10.3390/ijms26094009
He Q, Feng T, Xie Y, Swamiappan S, Zhou Y, Zhou Y, Zhou H, Peng X. Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds. International Journal of Molecular Sciences. 2025; 26(9):4009. https://doi.org/10.3390/ijms26094009
Chicago/Turabian StyleHe, Qiming, Tao Feng, Yingyan Xie, Sathiskumar Swamiappan, Yue Zhou, Yanfang Zhou, Hui Zhou, and Xinsheng Peng. 2025. "Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds" International Journal of Molecular Sciences 26, no. 9: 4009. https://doi.org/10.3390/ijms26094009
APA StyleHe, Q., Feng, T., Xie, Y., Swamiappan, S., Zhou, Y., Zhou, Y., Zhou, H., & Peng, X. (2025). Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds. International Journal of Molecular Sciences, 26(9), 4009. https://doi.org/10.3390/ijms26094009







